Abstract
The recommended anticoagulation regimen during continuous-flow axial left ventricular assist device (LVAD) support is aspirin and warfarin with a targeted international normalized ratio of 2.0–3.0. We report two patients in whom recurrent gastrointestinal bleeding during LVAD support necessitated discontinuation of this anti-thrombotic regimen for a year or more. Despite this, neither patients developed thrombotic complications during 29 patient-months of follow-up. An acquired von Willebrand factor (VWF) abnormality reflected by the absence or decreased abundance of the highest molecular weight multimers was demonstrated in both patients. The gold standard test for platelet function, light transmission platelet aggregometry was measured in one patient and was normal, indicative that the predominant abnormality in the coagulation profile of these patients is an acquired VWF syndrome. Clinical trials are required to address the question whether it is safe to discontinue anticoagulation in LVAD patients with acquired VWF abnormalities.
Keywords: Heart failure, Ventricular assist device, Anticoagulation, Gastrointestinal bleeding
1. Introduction
Left ventricular assist devices (LVAD) are increasingly being used to bridge patients to cardiac transplantation and as permanent therapy for selected patients with end-stage heart failure. Patient survival since the introduction of continuous-flow LVADs has improved considerably and is reported as 58% at two years [1]. The advantage of continuous-flow devices compared to the pulsatile-flow LVAD is that they are more durable, but a disadvantage is the suggested requirement for systemic anticoagulation. The recommended antithrombotic regimen is aspirin and warfarin with a targeted international normalized ratio (INR) of 2.0–3.0 [2]. However, there appears to be an increased risk of gastrointestinal (GI) bleeding after implantation that may necessitate discontinuation of antithrombotic therapy [3, 4]. The long-term follow-up of such patients has not been well described. We report two patients in whom despite discontinuation of antithrombotic therapy for a year or more, continued to have normal device function without thromboembolic complications.
2. Case 1
A 74-year-old male with a history of ischemic cardiomyopathy underwent HeartMate XVE implantation. Approximately a month later, due to device failure, he underwent an exchange with HeartMate II implantation. His antithrombotic regimen consisted of warfarin and aspirin. He developed multi-drug resistant Pseudomonas aeruginosa bacteremia and was placed on chronic ceftazidime therapy. Approximately eight months after HeartMate II implantation, due to recurrent GI bleeding with no active bleeding sites identified, warfarin and aspirin were discontinued. Despite discontinuation, within a year the patient presented with recurrent melena and anemia on two separate occasions. The patient developed progressive debility, was bed-ridden and had difficulty in controlling ongoing bacteremia and infection. Approximately 21 months after HeartMate II device implantation, hemodialysis was with-held as per patient and family wishes, and he expired. This patient had no thrombotic complications and had normal device function for a year after warfarin and aspirin were discontinued. His INR remained within normal limits; however, he had borderline thrombocytopenia (120,000–140,000/μl). His D-dimer was elevated at 1010 (normal ≤250) suggestive of an acute phase reactant, and factor VIII coagulant activity was normal. The von Willebrand factor (VWF) multimer analysis revealed subtle loss of the highest molecular weight multimers, but no increased abundance of lower molecular weight VWF multimers suggesting an acquired VWF abnormality.
3. Case 2
A 59-year-old female with idiopathic dilated cardiomyopathy underwent implantation of a HeartMate II device with a bioprosthetic tricuspid valve replacement. She had significant renal dysfunction preoperatively and became dialysis dependent postoperatively. She developed recurrent GI bleeding on warfarin and aspirin therapy after LVAD implantation. Despite undergoing extensive endoscopy no source of active bleeding was found and was presumed to be due to vascular ectasia in the small bowel. Approximately seven weeks after implantation, warfarin and aspirin therapy was therefore discontinued. She was admitted several times for recurrent GI bleeding despite discontinuation of antithrombotic therapy. Her INR has been within normal limits; however, she too had a mild thrombocytopenia (120,000–140,000/μl) and elevated D-dimer (1600 ngyml). Platelet function was assessed by PFA-100 and light transmission aggregometry. She had prolonged PFA-100 epinephrine and ADP cartridge closure times. Her platelet aggregation responses to arachidonic acid, ADP, epinephrine, collagen, and ristocetin were all within normal range. Therefore, the prolonged PFA-100 closure times were most likely attributed to acquired VWS.
Her fibrinogen was normal at 296 mgydl (200–430), soluble fibrin monomers were negative, and D-dimer was elevated at 1600. Coagulation factors II, V, VII, VIII, and XIII were all normal. Activated factor X was mildly reduced at 45% (60–140) likely related to recent warfarin use. The VWF multimer analysis revealed marked decrease in abundance of the highest molecular weight multimers (Fig. 1). She had no thrombotic complications and the device functions normally, 18 months after antithrombotic therapy has been discontinued. The VWF profile of both patients is outlined in the Table 1.
Fig. 1.
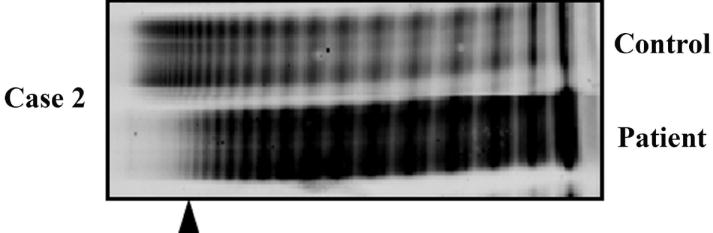
VWF multimer analysis. Patients’ plasma samples were analyzed by VWF multimer electrophoresis. (Delete: Patient 1 had subtle loss highest molecular weight VWF multimer, while) Patient 2, who had significant loss of VWF multimer as compared to control and indicated by the arrow head, is displayed. VWF, von Willebrand factor.
Table 1.
The von Willebrand factor (VWF) profile in two patients with continuous axial flow left ventricular assist devices.
| VWF Ristocetin cofactor Normal values (55-200%) | VWF antigen Normal values (55-200%) | VWF Ristocetin activity/antigen ratio. Normal values ≥ 0.7 |
|---|---|---|
| Patient 1: 177% | 169% | 1.1 |
| Patient 2: 210% | 247% | 0.8 |
4. Discussion
The recommendations for continuous-flow devices include warfarin and aspirin therapy to prevent thrombotic complications. However, as the durability of LVADs and survival of these patients improve; complications, such as GI bleeding are becoming increasingly recognized. Bleeding complications were a major cause of death in the recently reported HeartMate II destination therapy trial [1]. Non-fatal hemorrhagic complications have been reported in single center experiences describing GI bleeding occurring in 15–40% of HeartMate II patients [3, 4].
The occurrence of bleeding complications may require discontinuation of anticoagulation therapy. The proposed risk incurred by patients who have had anticoagulation discontinued is device thrombosis. Patients with HeartMate II devices despite being maintained with a lower INR seem to have an acceptable thromboembolic risk during a mean follow-up period of 7.2±5.2 months [4]. However, long-term follow-up of patients with HeartMate II in whom warfarin and aspirin have been discontinued has not been reported. Our experience describes two patients who had significant, recurrent GI bleeds with no thrombotic complications, a year or more following discontinuation of antithrombotic therapy.
The most likely explanation for the patient’s bleeding risk and favorable thrombotic profile is the acquired VWF abnormality. Patients with chronic renal failure are also at an increased risk for both GI and procedure related bleeding complications [5] primarily due to platelet dysfunction [6]. However, dialysis which was being done in both patients does partially correct this bleeding tendency [6] and platelet dysfunction alone would not explain the lack of thrombosis due to warfarin being discontinued. Furthermore, light transmission platelet aggregometry performed in patient 2 was normal providing no conclusive laboratory evidence of acquired – platelet dysfunction. Coagulation abnormalities due to acquired – VW syndrome have been described with the HeartMate II device [7]. Both the cases reported had absent or reduced VWF high molecular weight multimers. This reduction may be due to accelerated proteolysis caused by shear stress with the HeartMate II device, similar to the mechanism for VWF abnormalities in severe aortic stenosis [8]. The VWF ristocetin activity/antigen ratios of both cases were normal as shown in the Table 1, indicating that the current VWF activity test by ristocetin may not be sensitive enough to identify such an acquired – VWF abnormality.
These cases therefore raise the possibility that anticoagulation may not be required for selected patients with HeartMate II devices who have acquired VWS. The textured inner surface of the inflow conduit and outflow graft and the polished surfaces of the pump rotor, duct, inlet and outlet stator may also help reduce the thrombogenicity of the HeartMate II device. However, clinical trials will be required to address the use of an altered anticoagulation regimen in these patients.
Acknowledgments
We thank Kasey L. Muetzel for her assistance with the preparation of this manuscript.
Footnotes
Grant support: This study was supported, in part by HL 84904 (Heart Failure Clinical Research Network), and NIH grants UL1RR24150 (N.L. Pereira).
References
- 1.HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 3.Stern DR, Kazam J, Shariff S, Edwards P, McAllister N, Maybaum S, Bello R, D’Allessandro D, Goldstein D. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2009;25:352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 4.John R, Lee S. The biological basis of thrombosis and bleeding in patients with ventricular assist devices. J Cardiovasc Trans Res. 2009;2:63–70. doi: 10.1007/s12265-008-9072-7. [DOI] [PubMed] [Google Scholar]
- 5.Gangji AS, Shoal AS, Treleaven D, Crowther MA. Bleeding in patients with renal insufficiency: a practical guide to clinical management. Thromb Res. 2006;118:423–428. doi: 10.1016/j.thromres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G. Bleeding in renal failure. Lancet. 1988;331:1205–1208. doi: 10.1016/s0140-6736(88)92019-3. [DOI] [PubMed] [Google Scholar]
- 7.Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold-Herz M, Schlensak C, Budde U, Zieger B. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]


