Abstract
Background
Classical fear conditioning is commonly used to study the biology of fear, anxiety and memory. Previous research demonstrated that delay conditioning requires a neural circuit involving the amygdala, but not usually the hippocampus. Trace and contextual fear conditioning require the amygdala and hippocampus. While these paradigms were developed primarily using rat models, they are increasingly being used in mice.
New Method
The current studies develop trace fear conditioning and control paradigms to allow for the assessment of trace and delay fear conditioning in C57BL/6N mice. Our initial protocol yielded clear delay and contextual conditioning. However, trace conditioning failed to differentiate from an unpaired group and was not hippocampus-dependent. These results suggested that the protocol needed to be modified to specifically accommodate trace conditioning the mice. In order to reduce unconditioned freezing and increase learning, the final protocol was developed by decreasing the intensity of the tone and by increasing the inter-trial interval.
Results
Our final protocol produced trace conditioned freezing that was significantly greater than that followed unpaired stimulus exposure and was disrupted by hippocampus lesions.
Comparison with Existing Methods
A review of the literature produced 90 articles using trace conditioning in mice. Few of those articles used any kind of behavioral control group, which is required to rule out non-associative factors causing fearful behavior. Fewer used unpaired groups involving tones and shocks within a session, which is the optimal control group.
Conclusions
Our final trace conditioning protocol can be used in future studies examining genetically modified C57BL/6N mice.
Keywords: behavior, fear, associative learning, mouse, conditioning
1.0 Introduction
Variants of classical fear conditioning are being increasingly used to examine the neurobiology of learning and memory in addition to fear and anxiety. Different variants require different neural circuits. For example, “delayed” fear conditioning involves a previously neutral conditioned stimulus (CS) that overlaps with an aversive unconditioned stimulus (US) such that the CS comes to induce fearful behaviors. This task appears to rely upon a fairly constrained neural circuit primarily involving the amygdala (Davis, 1992; LeDoux et al., 1990; Maren and Fanselow, 1996). In contrast, “trace” fear conditioning involves non-overlapping stimuli and requires an intact hippocampus, anterior cingulate and prefrontal cortex as well as associated circuitry (Chowdhury et al., 2005; Han et al., 2003; Misane et al., 2005; Runyan et al., 2004).
Although these tasks have been extensively worked out in rats (Burman and Gewirtz, 2004; McEchron et al., 1998; Quinn et al., 2002), they are increasingly used with mice to examine the effects of targeted genetic manipulations (i.e. Crestani et al., 2002; Zhao et al., 2005). Due to differences between species (as well as between strains within a species), it is not always simple to adapt these tasks to new animal models. For example, classical fear conditioning experiments attempt to examine changes in fearful behaviors following pairing of the CS and US. These behavioral changes are presumed to be due to an association being formed between the stimuli. However, due to the aversive nature of the US and the novelty of both the CS and US, fearful behaviors can emerge to the CS despite the absence of an association (termed pseudo-conditioning). Pseudo-conditioning is an alteration in stimulus-elicited behavior that can appear similar to the anticipated conditioned response, but can be accounted for by non-associative mechanisms. Determining that a biological manipulation affected fear conditioning, and not pseudo-conditioning, typically involves the use of unpaired (Gormezano et al., 1983; Papini and Bitterman, 1990) or random control groups (Rescorla, 1967), in which mice are exposed to both the CS and US, but are unable to form an association between them. Any behavioral change would then be due to the association being formed and not merely exposure to the stimuli.
Unfortunately, behavioral control groups in studies of mouse trace fear conditioning are often missing or inadequate, precluding a clear interpretation of the biological manipulation or measurement. A PubMed search and subsequent literature review yielded about 90 studies examining trace fear conditioning in mice. Of these, only 7 include any type of behavioral control group. Moreover, some of these studies present only CSs or USs (but not both) as the control group (Han et al., 2003; Reich et al., 2008). Although this rules out some alternate interpretations of increased fearful behaviors to the CS (sensitization and habituation), it does not preclude pseudo-conditioning. In order to address this problem Smith et al. (2007) conducted a large multi-strain study to develop protocols that would reduce non-associative freezing. They found that many strains produced large amounts of non-associative freezing unless the CSs and USs were presented on separate days, resulting in a final protocol in which the unpaired groups receive CSs on 1 day and USs on day 2 (Hwang et al., 2010; Smith et al., 2007). Although this protocol successfully reduces freezing in the unpaired group, it unfortunately does not rule out pseudo-conditioning as a source of freezing in their “trace conditioned” subjects because the cues are presented on separate days.
As far as we could find, only 4 previous studies examining trace conditioning have utilized control groups in which CSs and USs are presented in the same session (Huang et al., 2010; Huerta et al., 2000; Misane et al., 2005; Stiedl and Spiess, 1997), a pre-requisite for ruling out pseudo-conditioning. Two of those studies created the control by simply extending the trace interval (Misane et al., 2005; Stiedl and Spiess, 1997), essentially creating an explicitly unpaired situation which may cause some inhibitory learning (Rescorla, 1969, 1967). It should be noted that studies using trace eyelid conditioning do not appear to suffer from similar difficulties (Chau et al., 2013; Tseng et al., 2004), nor do studies utilizing the more common “delayed” fear conditioning (Contarino et al., 2002; Paylor et al., 1994). The current series of experiments attempted to develop trace and delayed conditioning protocols in which increases in freezing in “paired” groups could be clearly differentiated from subjects received “unpaired” stimuli in the same session. We then used hippocampus lesions to ensure that our “trace” conditioning was indeed hippocampus-dependent. These protocols can then be used in future studies examining genetic or pharmacological manipulations in mice.
2.0 Methods
2.1 Subjects
Adult male C57BL/6NCrl mice were purchased from Charles River. Mice arrived at 60 days of age and were allowed to acclimate for approximately one to two weeks. The mice were housed in groups of 2–4, in clear polypropylene high density mouse cages (Innovive San Diego, CA) in the University of New England vivarium, which operates under NIH rules of conduct. All mice had free access to food and water, and were maintained on a 12-h light/dark cycle with lights on at 7:00 am. Experiment 1 used 32 mice, divided into delay (n= 10), trace (n= 10), and unpaired (n= 12) conditioning. Experiment 2 used 35 mice divided into delay conditioning (11 lesion and 8 sham) and trace conditioning (9 lesion and 7 sham). For Experiment 3, 39 mice were used in delay (n= 9), trace (n= 13), and unpaired (n= 17) conditions. For Experiment 4, 36 mice were used in delay (n= 11), trace (n= 10), and unpaired (n= 15) conditions. For Experiment 5, 34 mice were used in delay conditioning (9 sham and 8 lesion) and trace conditioning (8 sham 9 lesion).
2.2 Apparatus
The conditioning apparatus used were four Startfear chambers (Harvard Apparatus/Panlab model #58722) that measure animal movement through a high sensitivity weight transducer system. The chambers were equipped with a shock-delivery and weight-sensitive grid floor, a dim light and speakers. The chamber could be transformed into multiple contextual configurations, including alternating between a black cubic shape (Context A) and a white cylindrical shape (Context B). Training occurred with a metal grid floor, whereas testing in the alternate context utilized a smooth silicone floor. Odors also differed between contexts, with 70% ethanol scenting context A and1% ammonia hydroxide scenting context B. Animals were allowed to move freely throughout the apparatus while cage displacement was recorded as changes in relative pressure on the corners of the chamber connected to the load cell unit, digitized, low-pass filtered (cutoff at 0.2 Hz) and recorded by the accompanying Startfear “Freezing” computer software. The equipment was calibrated such that 20 g of force produced a reading of “100” units with the gain on the hardware set to 8000. The software amplified the signal again (gain = 8) and freezing behavior was defined as an absence of cage displacement above a threshold of “4” for a minimum of 500 ms. Activity was sampled every 50 ms.
2.3 Behavioral testing
Mice were randomly assigned to one of three conditioning groups (delay, trace, or unpaired). Each experiment lasted 3 days. The same experimenter performed the three-day behavioral protocol between 8:00 a.m. and 11:00 a.m. each day. On each day of testing, all ambient sound was muted and the home cage was brought into the lab. Mice were weighed, labeled and placed into individual transport cages (24 cm × 18 cm × 13 cm and made of clear plastic). The mice were then transported approximately 7.5–9 m to the testing room and set next to the Startfear conditioning chambers for a 5 min resting period, after which they were placed into individual Startfear conditioning chambers. After each test, all mice were returned to their home cages. The experimental chambers were then cleaned thoroughly using Sparkleen and water, followed by a thorough washing with either ethanol (Context A) or ammonia (Context B).
2.3.1 Day 1: training
On day 1, animals in the delay conditioning groups were pre-exposed to the experimental chamber for 5 min. This was followed by 5 conditioning trials in which a 10-s 70-dB (Experiments 1 & 2) or 67-dB (Experiments 3–5) 4-kHz tone co-terminated with a 2-s 0.3-mA aversive footshock. Trials were separated by randomly chosen intervals (mean = 2.5 min, range = 1.5–3.5 min for Experiments 1–3 and mean = 3.5 min, range = 2.5–4.5 min for Experiments 4 and 5). Animals in the trace conditioned group were also pre-exposed to an experimental chamber for 5 min, then exposed to 10 trials in which 10-s tones were followed 20 s later by 2 s 0.3 mA footshocks. Animals in the “unpaired training” condition were also pre-exposed to an experimental chamber for 5 min. However, the subsequent 10 auditory tones and 10 aversive stimuli were presented in random order. Any two stimuli were separated by a randomly chosen interval between 45 and 105 s (mean = 75 s) for Experiments 1–3 and 75 s to 135 s for Experiments 4 and 5. This procedure was designed to prevent any associative learning from occurring. Although our software continuously records movement, due to the confounding influence of footshock on freezing to the tone, we only report freezing for the 5 min of context exposure prior to the onset of the aversive stimulus. Moreover, to examine any effects of hippocampus lesions on activity, we also report average cage displacement for this habituation period in Experiment 5.
2.3.2 Day 2: context fear test
The following day, mice were returned to the same experimental chamber with the same contextual configuration for 5 min. Freezing behavior was recorded.
2.3.3 Day 3: tone fear test
On the third day, mice were placed into a novel chamber, which was in the opposite contextual configuration, for 5 min during which freezing was record. This was followed by 10 exposures to the 10-s 4-kHz tone, separated by 20 s intervals. The software reports freezing behavior to the tone as percent freezing across all 10 trials.
2.4 Surgery
After the acclimation period to the animal colony, mice were placed into a transport cage and anesthetized using an intraperitoneal injection of a ketamine/xylazine cocktail (80 mg/kg ketamine + 6 mg/kg xylazine) followed by a subcutaneous injection of buprenorphine (.05–.10 mg/kg). Once the drugs had taken effect, the scalp was shaved, cleaned with betadine, and the mice were placed into a stereotaxic frame (World Precision Instruments, #502650) with a neonatal rat/mouse attachment (KOPF #270). Small holes were then drilled above the hippocampus, and an electrode was lowered to the proper coordinates (−1.8, ±2.0, −2.2) from bregma (Frankland, et al. 1998). Bilateral hippocampal lesions were made by passing a 2.7-mA current for or 5 s, using a direct current stimulus isolator (#A360 World Precision Instruments), through an electrode (125 um PT/IR microelectrode, FHC, Bowdoin, ME). The electrodes were previously used for recording experiments and were prepared by trimming the electrode tip prior to removing the epoxy coating and exposing a new 1 mm blunt tip. For sham mice the electrode was lowered to the same location, but no current was administered. The incision site was then closed and mice were placed into their individual transport cages, which were placed on a heating pad set at low. Mice were left on the heating pad until fully recovered from the anesthetics. Mice were then placed back in the animal colony for another week to recover before being tested. Mice were checked on daily to monitor the incision and overall health of the animal.
2.5 Histology
To characterize the extent of the lesions, brains were collected within 36 hours of testing. Mice were overdosed with euthanasia solution (pentobarbital), then perfused intracardially using 0.9% saline solution followed by 10% formalin. The brains were stored at 4°C in 10% formalin overnight prior to being switched the following day to 30% sucrose. Brains were then sliced using a freezing microtome, and every sixth slice was kept and mounted on to slides. After drying, the slides were Nissl stained using cresyl violet and cover slipped with Permount. Slides were then examined under a microscope and reconstructed by hand using a mouse brain atlas (Figures 1). Based on pilot work in which no substantial damage was observed in sham mice full histology was only carried out on lesioned subjects.
Figure 1.
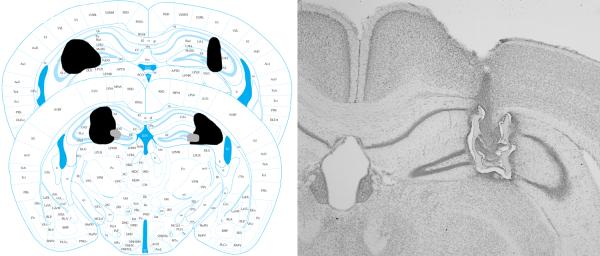
Left: Reconstructions of the largest (black) and smallest (grey) lesions that were included in the data analysis for Experiment 5. Figures from Franklin and Paxinos (2008) used with permission. Right: Photomicrograph of a representative lesion.
2.6 Statistics and Data Analysis
For Experiments 1, 2 & 3, a 3 (behavioral condition) × 4 (test session) mixed-model repeated measures ANOVA was used to analyze the data. For Experiment 4, a 2 (behavioral condition) × 2 (lesion vs. sham) × 4 (test session) mixed model repeated measures ANOVA was conducted. These analyses were followed by one-way ANOVAs looking at contextual and auditory freezing separately. Planned comparisons and Tukey's HSD post-hoc tests were used as appropriate.
In Experiment 1, 1 mouse was excluded from the delay conditioned group for being a statistical outlier (more than 2.5 SD from the mean). For Experiment 2, 1 trace conditioned mouse was excluded for procedural error. For Experiment 3, 4 mice (1 delay and 1 trace and 2 unpaired) were excluded for being a statistical outlier. For Experiment 4, 1 trace conditioned sham surgery mouse and 1 trace condition lesion surgery mouse were excluded from the statistical analysis due to being a statistical outlier. 1 delay conditioned lesion surgery mouse was excluded for inadequate lesion size. 1 trace conditioned lesion surgery mouse was excluded for a technical malfunction with the Startfear chamber.
3.0 Results
3.1 Experiment 1: Conditioning with a 70-db tone and a 2.5-min inter-trial interval
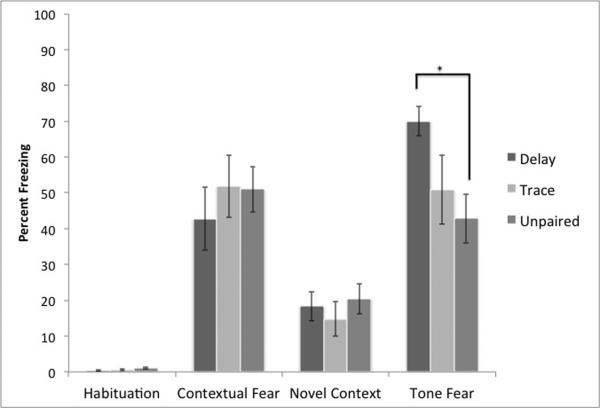
Our initial protocol was slightly modified from a protocol we piloted for successful delay conditioning and used a relatively loud CS and short inter-trial interval (see Figure 2). We anticipated that both delay and trace conditioning would yield higher levels of freezing to the auditory cue than the unpaired group, whereas contextual freezing should be similar. Indeed, the mixed-model repeated measures ANOVA demonstrated the expected significant main effect of test session F (3,84)= 70.26, p< .01 and a significant interaction between condition and test session F (6,84)= 2.25, p< .05. There was no main effect of condition F (2,28)= 0.34, p> .10. Indeed, a follow-up one-way ANOVA on freezing to the auditory cue showed that tone test session found a significant effect F (2,28)=3.46, p< .05. whereas there was no effect of behavioral condition on contextual freezing F(2,28)=.38, p>.10, as expected. Post-hoc tests found significant differences between delay and unpaired conditioning, but not between any other groups. This suggests that this protocol fails to produce clear trace conditioning
Figure 2.
Results from our initial conditioning protocols (Experiment 1). As expected, all animals show evidence of contextual fear conditioning. However, only delay conditioning produces specific evidence of freezing above the levels of unpaired controls (* = significantly different p<.05).
3.2 Experiment 2: intermediate protocol - conditioning with a 67-dB tone and a 2.5-min inter-trial interval
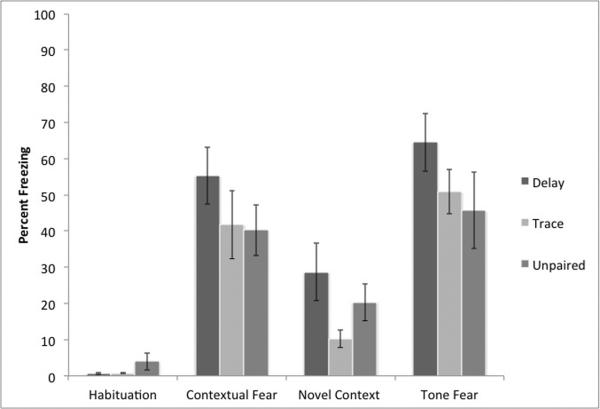
Our previous protocol failed to induce differential conditioning in trace and unpaired conditioning. This could be due to low levels of freezing in the trace conditioning group or the high level of freezing in the unpaired group. This protocol attempts to reduce the innate anxiogenic properties of the CS by reducing its intensity, thereby reducing freezing in the control group (Figure 3). However, a mixed-model repeated-measures ANOVA demonstrates a significant main effect of test session F (3,60) = 55.83 p< .01, but not of condition F (2,20)= 0.18, p> .10. The interaction between condition and test session was also not significant F (6,60)= 1.14, p> .10, suggesting that we failed to demonstrate clear delay or trace conditioning. This was confirmed by a separate one-way ANOVA on freezing to the tone F (2,20)= 1.40, p> .10 and freezing to the context F (2,20) = 1.10, p>10, both of which failed to find a significant difference.
Figure 3.
Results from our intermediate conditioning protocols (Experiment 2). As expected, all animals show evidence of contextual fear conditioning. However, no conditioning protocol produces specific evidence of freezing above the levels of unpaired controls.
3.3 Experiment 3: final protocol - conditioning with a 67-dB tone and a 3.5-min inter-trial interval
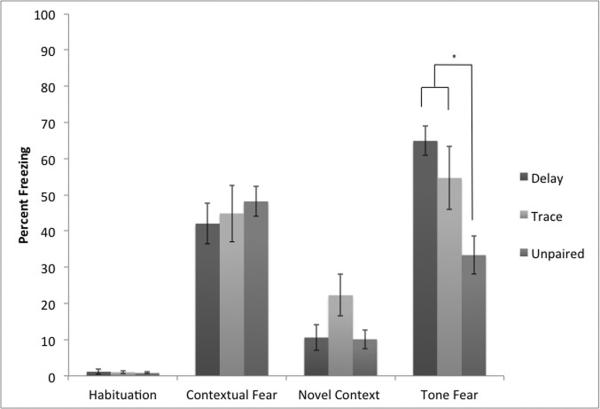
Our intermediate protocol somewhat reduced freezing in the unpaired group, but also failed to produce conditioning using either delay or trace protocols. This protocol attempted to strengthen conditioning and lower freezing in the unpaired groups by increasing the inter-trial interval (ITI; Figure 4). Increasing the ITI should increase CS learning and lower the associative value of the context (Detert et al., 2008; Rescorla and Durlach, 1987). A mixed-model repeated measures ANOVA demonstrated the success of this protocol. There was a significant main effect of test session F (3,87)= 90.80, p< .01 and a significant interaction between condition and test session F (6,87)= 4.68, p< .01. There was a trend towards a significant effect of condition F (2,29)= 0.19, p= 0.08. A follow-up one-way ANOVA on freezing to the tone showed a significant effect of condition F (2, 29)= 7.75, p< .01. A Tukey's HSD post-hoc test found that the interactions between delay and trace were not significantly different but both differed from the unpaired group. There was no effect of condition on contextual freezing F(2,29) = 0.32, p>.10.
Figure 4.
Results from our final conditioning protocols (Experiment 3). As expected, all animals show evidence of contextual fear conditioning. In addition, both trace and delay conditioning produce specific evidence of freezing above the levels of unpaired controls (* = significantly different p<.05).
3.4 Experiment 4: Conditioning with a a 67-dB tone and a 3.5-min inter-trial interval after hippocampus lesions
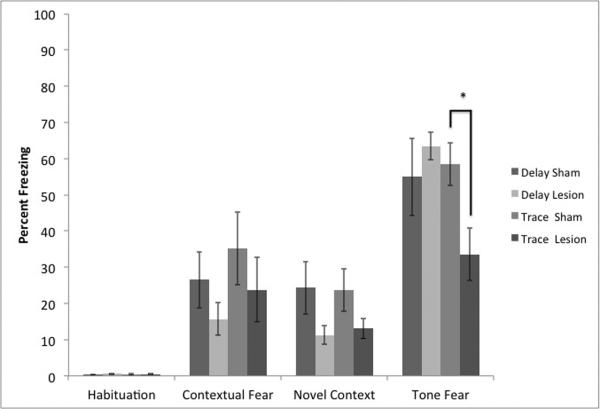
Our final protocol appeared to produce clear evidence of specific delay and trace fear conditioning (Figure 5). However, we wanted to ensure that freezing to the auditory cue following trace conditioning depended upon an intact hippocampus. Indeed the ANOVAs to test the effect of the lesions on trace and delay conditioning yielded a trend towards a main effect of lesion type F(1,30)= 3.13, p= 0.09. There was also a significant effect of test session F(3,90)= 70.87, p<.01. There was a significant interaction between test session and behavioral task F(3,90)=3.25, p<.05 and a significant 3-way interaction F(3,90)=2.95, p<.05. A follow up 2 (lesion) × 2(task) ANOVA on contextual freezing yielded no significant effects or interactions. However, a similar ANOVA examining novel context freezing did produce a significant effect of lesion type F(1,1) = 5.25, p<.05, but no effect of behavioral task nor an interaction. A similar ANOVA auditory freezing yielded a significant interaction F(1,30)=4.81, p<.05. Planned contrasts confirmed our expectations that trace, but not delay, conditioning was disrupted by the lesions (p<.05).
Figure 5.
Results from hippocampus lesions on our final conditioning protocols (Experiment 4). Hippocampus lesions disrupted freezing to the auditory cue following trace conditioning, but not delay conditioning (*=p<.05) confirming that this protocol produces hippocampus-dependent trace fear conditioning in mice. In addition, freezing to the novel context, but not the conditioning context, was significantly disrupted by the lesions.
In addition, to examine any potential effects of the lesions on locomotor activity, which could complicate our interpretation of the freezing data, we compared average cage displacement during the first 5 min of context exposure (habituation) as a function of surgery condition. Our sham subjects (10.92 +/− 0.43) significantly differed from our lesioned subjects (9.78 +/− 0.32) t = 2.15, p<.05., df = 29.67. It is important to note, however, that the lesioned subjects showed reduced cage displacement, inconsistent with the reduction of conditioned freezing noted above, but consistent with the slight increase in unconditioned freezing behavior we observe during the habituation period in the lesioned subjects.
4.0 Discussion
The purpose of these studies was to develop and validate trace and delayed fear conditioning protocols that can then be used to assess learning and memory in genetically modified mice with a predominate C57BL/6 background. Although our initial protocol failed to achieve this, by altering CS intensity and the inter-trial interval we were able to produce viable protocols that differentiated from the key control group. Moreover, hippocampus lesions disrupted trace conditioning, but not delay conditioning or freezing due to non-associative factors.
4.1 Pseudo-conditioning and behavioral control groups
Most studies examining the effects of genetic manipulations on fear conditioning seek to determine the molecules and intracellular pathways involved in learning, memory or anxiety. Comparing subjects that undergo conditioning with behavioral control groups is critical to that effort in order to rule out effects on non-associative factors, such as stress or arousal, that may have been influencing behavior and been affected by the genetic manipulations. Which control groups are the best to use in classical conditioning experiments has historically been somewhat controversial. Some researchers support the use of explicitly unpaired control groups to avoid any potential excitatory learning (Gormezano et al., 1983; Papini and Bitterman, 1990). Others propose that truly random control groups are best, to avoid any potential inhibitory learning caused by the negative correlation in explicitly unpaired groups (Rescorla, 1967). We have attempted to use features of both by using a control group in which the stimuli cannot overlap, but occur in a random order and with variable intervals between them. Presenting the stimuli in a random order, but separated by large gaps, should prevent any excitatory associations from being formed. Indeed, although our final protocol does produce some pseudoconditioning, it also supports statistically more conditioning, in both the delay and trace configurations
We are not the first group to use unpaired control groups in mice. At least two previous groups have run unpaired groups in which tones and shocks were presented in random (or pseudo-random) order (Huang et al., 2010; Huerta et al., 2000). In both of these studies, the unpaired groups produced substantial freezing to the tone, albeit less than that of paired groups. This is particular troublesome for experiments that fail to include behavioral control groups as the pseudo-conditioned increase in freezing to the tone could easily be mistaken for associative learning. The current data from our final protocol are quite similar to these studies in demonstrating that paired training produces slightly less than double the freezing of unpaired groups. Some unpaired freezing to the tone is likely inevitable and should be expected due to its role as part of the aversive context predicting shock presentation. Our work is novel in that we identify several factors that contribute to successful conditioning (CS intensity and ITI) and validate our protocol using hippocampus lesions.
Previous work conducting similar protocol development in mice demonstrated that non-associative freezing levels were high in a variety of mouse strains (Hwang et al., 2010; Smith et al., 2007). This group landed upon a protocol in which tones and shocks are presented on separate days. This innovative idea was successful in reducing freezing in the unpaired group allowing a very clear differentiation between trace and unpaired groups. It's less clear, however, that this type of procedure rules out non-associative, pseudo-conditioning, factors as the cause of the increased freezing in the trace conditioned subjects. Although the causes of pseudo-conditioning are likely varied, if specifically unpaired groups in which the stimuli are separated by long intervals within a single session produce similar freezing to trace conditioned subjects, it calls into question the nature of the trace conditioning. It is clearly preferable to have unpaired groups in which the stimuli are presented within a session.
It is likely that the choice of the C57BL/6NCrl strain influenced the amount of generalized contextual fear and pseudo-conditioning observed in the current experiments. In comparisons of several C57BL/6 substrains, the “NCrl” substrain used in the current experiments demonstrated significantly more freezing in a novel context than the “J” substrain (Bryant et al., 2008; Radulovic et al., 1998; Stiedl et al., 1999). Whether these differences translate into a greater propensity to demonstrate pseudo-conditioning is unknown. However, as our final protocol produces significantly greater freezing in paired versus unpaired subjects in the substrain that produces greater generalized and non-specific freezing, our protocol would likely be effective with the “J” substrain also.
4.2 Effects of hippocampus damage
It was previously shown that trace fear conditioning in mice is hippocampus-dependent (Chowdhury et al., 2005). Other studies make similar points using pharmacological or genetic manipulations (Huerta et al., 2000; Misane et al., 2005; Wanisch et al., 2005). The current data in Experiment 4 demonstrated that hippocampus lesions reduce freezing to the auditory cue after trace conditioning down to the same level as unpaired training, without affecting delay conditioning. Because delay conditioning remained intact, we can rule out motoric or sensory changes as accounting for the deficits we observed.
Although our lesions significantly disrupted freezing to the novel context, they failed to significantly reduce freezing to the conditioning context in Experiment 5. It is only somewhat surprising that the hippocampus lesions failed to disrupt conditioned contextual freezing. It has been previously demonstrated that pre-training lesions are not always effective at disrupting contextual fear conditioning (Anagnostaras et al., 2001), which is one of the limitations of the contextual fear paradigm. In addition, other studies have shown that the contextual freezing concomitant with trace conditioning versus delay conditioning are differentially disrupted by hippocampus damage (Quinn et al., 2002), although the pattern in that paper is opposite to ours. Moreover, increasing the ITI for Experiments 3 and 4 was likely to increase specific auditory freezing while reducing contextual freezing, thus masking any potential effect of the lesions (Detert et al., 2008; Rescorla and Durlach, 1987). It is important to note that the goal of our project was to assess trace fear conditioning relative to delay fear conditioning. Our initial protocol was perfectly adequate to make the more common cued vs. contextual fear conditioning comparison.
Because hippocampus lesion appear to disrupt only freezing caused by specific trace conditioning and not delay conditioning or pseudo-conditioned freezing, it seems likely that previous studies examining localized hippocampus damage were likely disrupting specific conditioning. However, this conclusion should not generalize to genetic or pharmacological manipulations that have less anatomical specificity or which may appear to disrupt trace fear conditioning by instead affecting stress, anxiety, or arousal systems. Therefore, it is critical that conditioning protocols are validated within the specific species being examined and that the effects of manipulations on non-specific freezing are assessed.
5.0 Conclusion
Overall, these studies discovered and validated a procedure that can be used to study trace fear conditioning in mice. A prerequisite of such studies is that conditioning produces greater freezing than unpaired groups. In addition, we demonstrate that our final protocol is susceptible to disruption by hippocampus lesions.
Highlights
Many studies of trace fear conditioning in mice fail to use proper controls.
Pseudo-conditioning of fear is especially common in mice
Altering CS intensity and the inter-trial interval (ITI) alter conditioning
A 3.5-min ITI and 67-dB 4-kHz tone produce robust trace conditioning.
Hippocampus lesions significantly impair our trace conditioning protocol.
Acknowledgements
We'd like to thank the University of New England College of Arts and Sciences and Center for Excellence in the Neurosciences for their generous support. MAB is supported in part by NIH grant R15MH093950. LL is supported in part by NIH grant P20GM103643.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. Journal of neurogenetics. 2008;22:315–31. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Timing of fear expression in trace and delay conditioning measured by fear-potentiated startle in rats. Learning & memory (Cold Spring Harbor, N.Y.) 2004;11:205–12. doi: 10.1101/lm.66004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau LS, Davis AS, Galvez R. Neocortical synaptic proliferation following forebrain-dependent trace associative learning. Behavioral neuroscience. 2013;127:285–92. doi: 10.1037/a0031890. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral neuroscience. 2005;119:1396–402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Contarino A, Baca L, Kennelly A, Gold LH. Automated assessment of conditioning parameters for context and cued fear in mice. Learning & memory (Cold Spring Harbor, N.Y.) 2002;9:89–96. doi: 10.1101/lm.43002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:0–8980. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends in Pharmacological Sciences. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Detert JA, Kampa ND, Moyer JR., Jr Differential effects of training intertrial interval on acquisition of trace and long-delay fear conditioning in rats. Behavioral neuroscience. 2008;122:1318–27. doi: 10.1037/a0013512. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates, Compact. 3rd ed Academic Press; 2008. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall B. Twenty years of classical conditioning with the rabbit. Progress in psychobiology and physiological psychology. 1983;10:197–275. [Google Scholar]
- Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13087–92. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Chiang YW, Liang KC, Thompson RF, Liu IY. Extra-cellular signal-regulated kinase 1/2 (ERK1/2) activated in the hippocampal CA1 neurons is critical for retrieval of auditory trace fear memory. Brain research. 2010;1326:143–51. doi: 10.1016/j.brainres.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:0–473. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Hwang YK, Song JC, Han SH, Cho J, Smith DR, Gallagher M, Han JS. Differences in hippocampal CREB phosphorylation in trace fear conditioning of two inbred mouse strains. Brain research. 2010;1345:156–63. doi: 10.1016/j.brainres.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:0–237. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–46. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–26. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Papini MR, Bitterman ME. The role of contingency in classical conditioning. Psychological review. 1990;97:396–403. doi: 10.1037/0033-295x.97.3.396. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behavioral neuroscience. 1994;108:0–810. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Generalization of fear responses in C57BL/6N mice subjected to one-trial foreground contextual fear conditioning. Behavioural brain research. 1998;95:179–89. doi: 10.1016/s0166-4328(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. Journal of psychopharmacology (Oxford, England) 2008;22:769–77. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian Conditioned Inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological review. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Durlach PJ. The Role of Context in Intertrial Interval Effects in Autoshaping. The Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology. 1987;39:35–48. [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1288–95. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learning & memory (Cold Spring Harbor, N.Y.) 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Sananbenesi F, Spiess J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behavioural brain research. 1999;104:1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Spiess J. Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6N mice. Behavioral neuroscience. 1997;111:703–11. doi: 10.1037//0735-7044.111.4.703. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Tang J, Mederer A, Wotjak CT. Trace fear conditioning depends on NMDA receptor activation and protein synthesis within the dorsal hippocampus of mice. Behavioural brain research. 2005;157:63–9. doi: 10.1016/j.bbr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7385–92. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]