Abstract
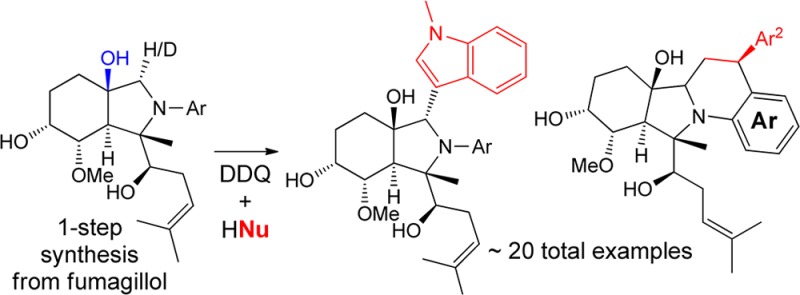
An efficient, two-step construction of highly complex alkaloid-like compounds from the natural product fumagillol is described. This approach, which mimics a biosynthetic cyclase/oxidase sequence, allows for rapid and efficient structure elaboration of the basic fumagillol scaffold with a variety of readily available coupling partners. Mechanistic experiments leading to the discovery of an oxygen-directed oxidative Mannich reaction are also described.
The use of abundant natural products as synthetic launching points for diversity-oriented synthesis (DOS) has garnered significant attention recently, as highly complex compounds can be rapidly prepared for biological evaluation.1 Importantly, this approach utilizes the inherent complexity of the natural product which may be “structurally remodeled”2a into a new architecture with unique biological activity in comparison to the parent natural product. Exemplary natural products include semisynthetic steroids,2b bryonolic acid,2c adrenosterone,2d cinchonine,2d gibberelic acid,2d and steviol.2e
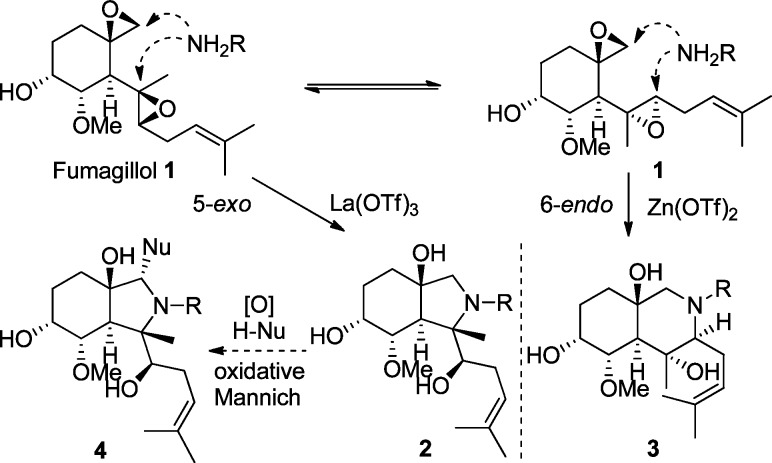
We recently reported that the natural product fumagillol 1 can undergo controlled 5-exo- or 6-endo-bis-epoxide opening/cyclization, depending on the metal-catalyst additive, leading to highly complex alkaloidal perhydroisoindoles 2 and perhydroisoquinolines 3 (Scheme 1).2a Due to the efficiency of these reactions, we considered further processing of this scaffold to increase diversity and complexity for biological evaluation. Herein we report a two-step diversity-oriented synthesis3 strategy using cascade cyclizations and oxidative Mannich reactions,4 reminiscent of natural product biosynthesis5 (“cyclase” followed by “oxidase” as coined by Baran6), yielding alkaloid-like structures 4 with high levels of complexity (Scheme 1). Mechanistic experiments leading to the discovery of an oxygen-directed Mannich reaction are also outlined.
Scheme 1. Fumagillol Bis-Epoxide Opening and Oxidative Mannich Strategy.
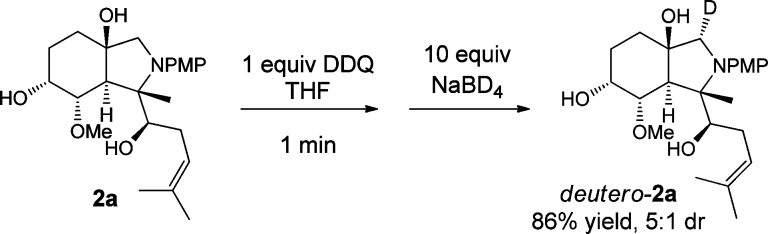
Following our previously reported preparation of fumagillol–anisidine adducts 2a,2a,7 we began oxidative coupling studies on this scaffold. An oxidation screen led to the discovery of a rapid (<1 min, 100% conversion as monitored by UPLC analysis7) amine-to-iminium oxidation of scaffold 2a when 1 equiv of DDQ was added in THF (Scheme 2).8,9 The site of oxidation was confirmed by a quench with sodium borodeuteride, stereoselectively yielding α-deutero-2a as the sole product in 83% isolated yield.
Scheme 2. Discovery of a Chemoselective Oxidation.
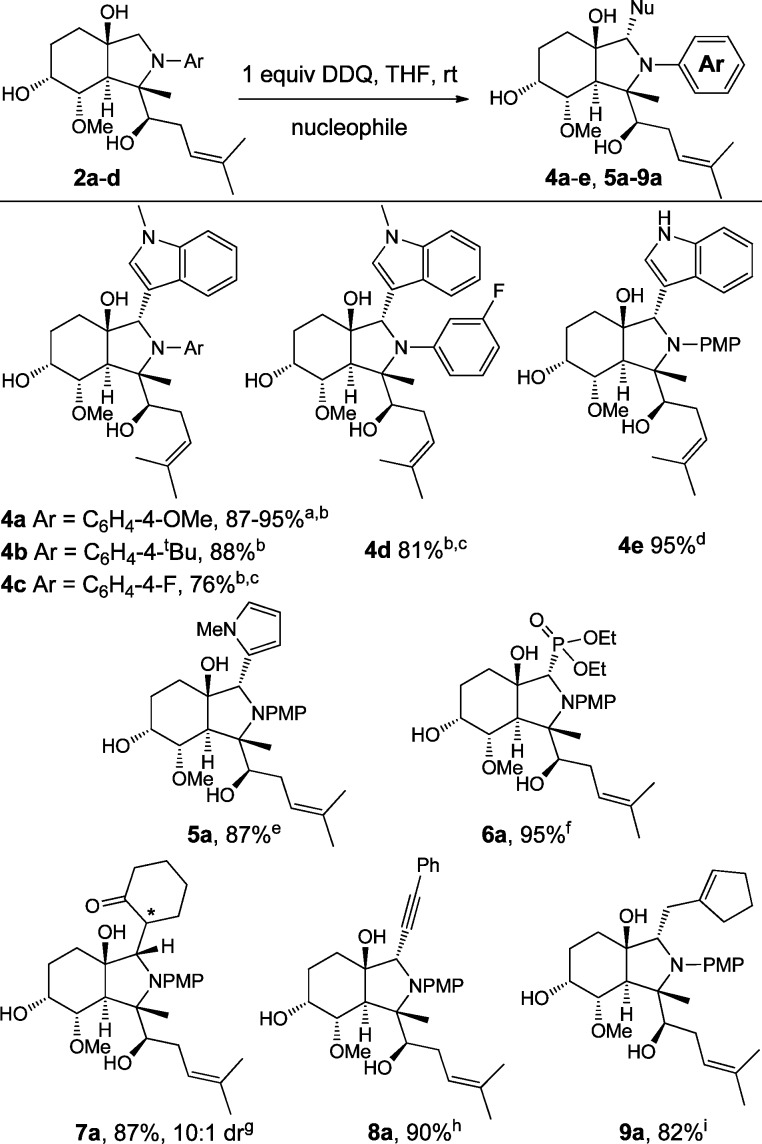
The scope of the oxidative coupling of fumagillol–aniline adducts 2 with various nucleophiles was next examined (Figure 1). Substrates 2a–2c underwent clean oxidation and subsequent nucleophilic addition with N-methylindole to afford adducts 4a–4c, respectively, in good yields.9 Similarly, substrates substituted with a meta-electron withdrawing group on the aniline subunit underwent chemoselective oxidation adjacent to nitrogen and were found to be suitable for the methodology (2d → 4d, 81% yield). In general, substrates containing electron-withdrawing groups required heating (60 °C) to enable oxidation in a timely fashion (3 h). In addition to N-methylindole as the nucleophilic partner, simple indole was also compatible with the methodology and could be oxidatively coupled to scaffold 2a to afford adduct 4e. To illustrate the practicality of the couplings, product 4a could be prepared on reaction scales from 50 to 750 mg. Thus, both the bis-epoxide opening and the oxidative coupling steps are applicable to near gram scale synthesis.
Figure 1.
Scope of the oxidative coupling. aMultiple runs, 50–750 mg scale. bNucleophile = N-methylindole (1.1 equiv). cInitial oxidation required heating at 60 °C for 1–3 h to complete. Once complete, indole (1.1 equiv) was added and the reaction was stirred until coupling was complete as monitored by UPLC. dNucleophile = indole (1.1 equiv). eNucleophile = N-methylpyrrole (1.1 equiv). fNucleophile = diethylphosphite (10 equiv). gNucleophile = cyclohexane silylenol ether (1.5 equiv); the α-stereocenter could not be unambiguously assigned. hNucleophile = phenylacetylene (1.5 equiv) + Cul (10 mol %) and Et3N (1.1 qeuiv). INucleophile = exomethylenecyclopentene (10 equiv). [DDQ = 2,3-dichloro-5,6-dicyano-p-benzoquinone, Ar = aryl, PMP = p-methoxyphenyl].
Highly efficient oxidative couplings of remodeled scaffold 2a with other nucleophiles were also found to be successful (Figure 1, products 5–9). Under the optimized conditions, oxidative couplings with N-methylpyrrole, diethyl phosphite,10 cyclohexanone silyl enol ether,11 phenylacetylene,12 and methylenecyclopentane11 produced products 5a–9a, respectively.
The use of styrenes as nucleophilic partners in the oxidative couplings yielded Povarov products 10 (Figure 2).13 Importantly, this oxidative Povarov reaction allowed for additional structural complexity to be achieved by constructing multiple bonds in a single transformation. Simple styrenic nucleophiles including styrene and p-methoxystyrene underwent Povarov cycloaddition with the in situ generated iminum ion. Adducts 10a–c were isolated in good to excellent yields with the electronically differentiated fumagillol scaffolds 2b and 2c containing p-methoxy and p-fluoro groups, respectively. In all cases, the diastereoselectivity of the newly formed benzylic stereocenter was modest (dr = 2.5–3:1). However, in the case of 10b–c, the products could be diastereomerically enriched (to >20:1 dr) by recrystallization, allowing for stereochemistry determination by NOE analysis.7 By utilizing indene, the highly complex adduct 10d was prepared as a single diastereomer. Unfortunately, the trans-styrene derivative anethole did not react with the in situ generated iminium (Figure 2, note b).
Figure 2.
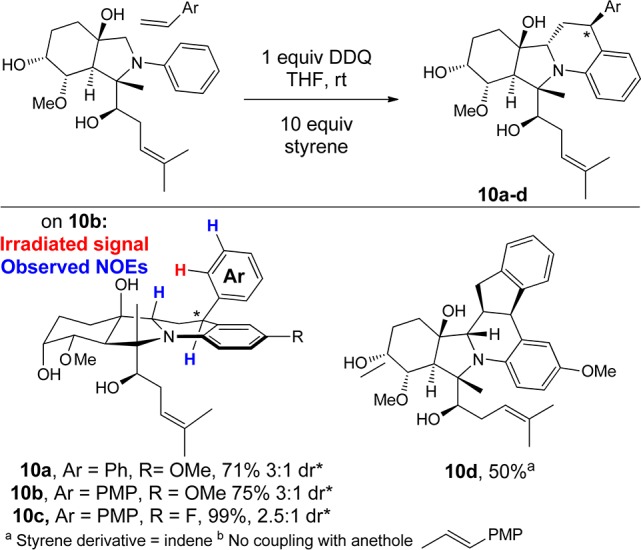
Oxidative Povarov cyclization.
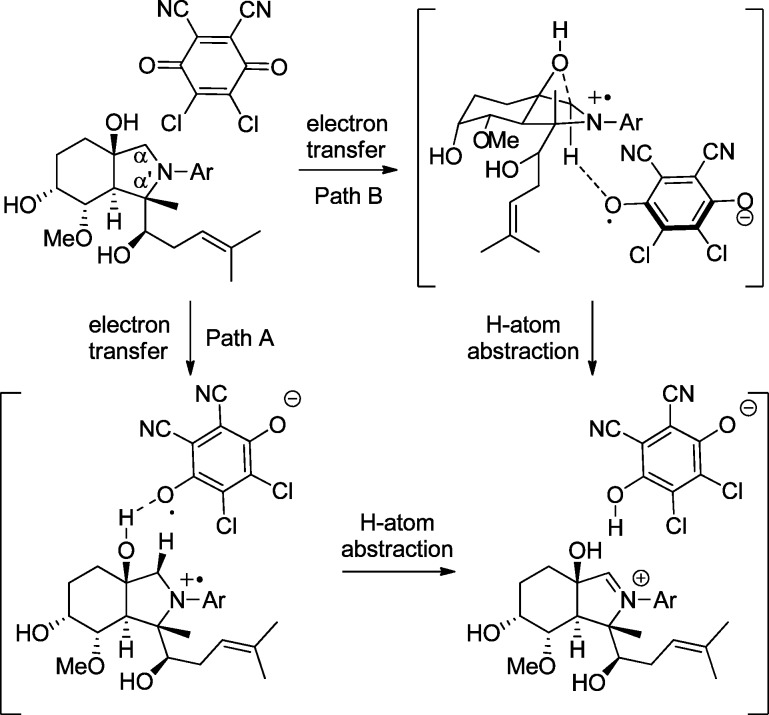
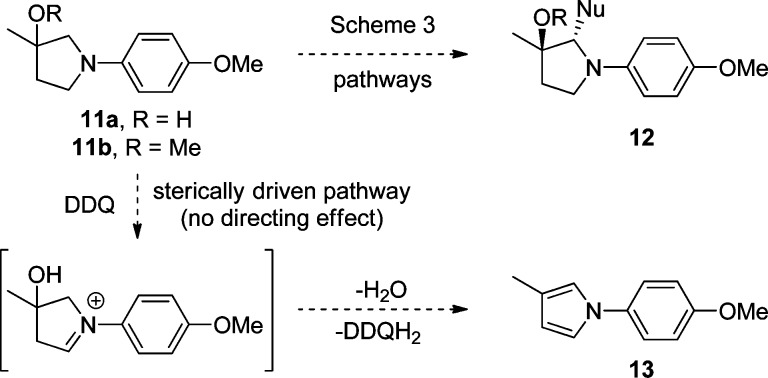
Having defined a wide scope for our oxidative coupling methodology, the possibility for an “oxygen”-directed oxidation mechanism for the initial DDQ conversion of the aniline to an iminium intermediate was next examined. Two possible mechanisms by which the hydroxy group could influence the oxidation were envisioned (Scheme 3): the hydroxyl group could function (1) as a general acid catalyst for DDQ activation and direction (pathway A) or (2) as a neighboring nucleophile (pathway B via anchimeric assistance) to facilitate formal hydride delivery to the DDQ oxidant. Since the α′-position of the fumagillol substrate is fully substituted and therefore cannot undergo oxidation, it was not clear whether there was also a hydroxy-directing, regioselectivity effect14 in addition to the diastereocontrol in nucleophilic addition to the iminium ion intermediate.
Scheme 3. Possible Pathways for Oxygen-Directed Oxidation.
As shown in Scheme 4, if there is an intrinsic hydroxyl-directing effect, one would expect products of type 12 to arise from either the simplified hydroxy (11a) and/or ether (11b) substrates thus allowing for development of a general methodology toward vicinally chiral pyrrolidinols. However, if there is no directing effect, a sterically driven oxidation would be expected, likely resulting in the formation of pyrrole 13.8e Thus, the directed oxidation would produce compounds of increased complexity containing vicinal stereocenters whereas the steric oxidation would result in a loss of complexity, likely leading to N-arylpyrrole 13via enamine formation and dehydration.15
Scheme 4. Possible Outcomes for Oxidative Mannich Reaction of 11.
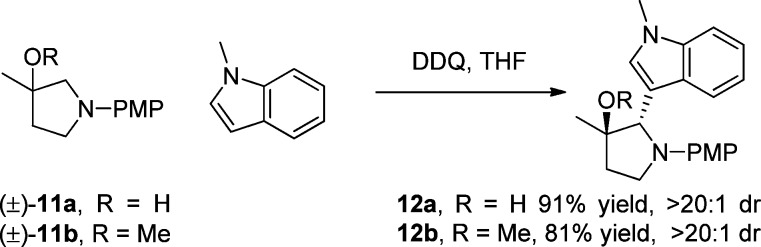
We were pleased to find that the simplified substrate 11a indeed underwent the oxidative Mannich reaction with N-methylindole in the direction of the hydroxy group affording adduct 12a in 91% yield as a single diastereomer (Scheme 5). This result confirmed that there is an oxygen-directing effect; however it does not distinguish between the H-bonding and anchimeric-assisted mechanisms (Paths A and B, Scheme 3). Interestingly, ether substrate 11b also underwent directed oxidative Mannich reaction with N-methylindole in good yield producing 12b as the sole product, suggesting that preorganization of the DDQ and the substrate through a hydrogen bond is not required, thus supporting the anchimeric-assisted mechanism (Pathway B).
Scheme 5. A Simplified Variant.
Standard conditions: 1 equiv DDQ, THF (0.5M), 5 equiv, indole derivative, 12 h, rt.
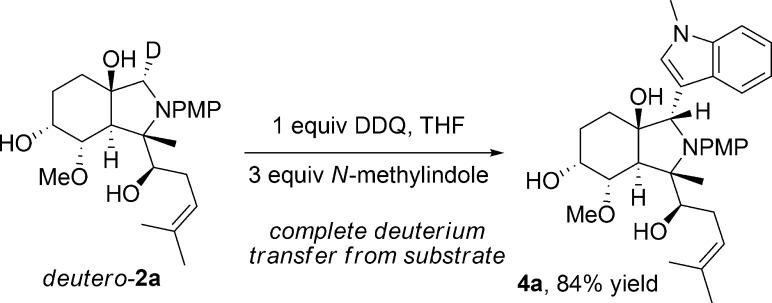
The nature of the oxygen-directed oxidative Mannich reaction was further explored using a deuterium labeling experiment.16 Stereoselective oxidation was observed on the deuterated-fumagillol scaffold deutero-2a wherein complete deuterium transfer to the DDQ oxidant was observed and deuterium-free product 4a was isolated in high yield following nucleophilic quench with N-methylindole (Scheme 6). This result is in line with the hypothesis that the oxygen atom activates its anti-hydrogen toward transfer via anchimeric, σ*-orbital overlap (Pathway B, Scheme 3).14a
Scheme 6. Deuterium Labeling Study.
In conclusion, we have outlined a rapid approach for the construction of highly complex alkaloid-like “unnatural products” from the natural product scaffold fumagillol utilizing a cascade of bis-epoxide opening (“structure remodeling”) followed by a diversifying chemo- and stereoselective oxidative Mannich/coupling protocol. Mechanistic experiments including a deuterium labeling study as well as oxidations with simpler pyrrolidine substrates were used to define the nature of the oxygen-directed oxidative Mannich reaction and support an “anchimeric-assisted” mechanism. Further studies including additional applications and biological evaluation of alkaloid-like products are currently in progress and will be reported in due course.
Acknowledgments
Financial support from the National Institutes of Health (P50 GM067041) is gratefully acknowledged. We thank Professors Scott Schaus (Boston University) and Corey Stephenson (University of Michigan) for helpful discussions. We thank Madeline Weber (CMLD-BU) for assistance with preparative HPLC purification and Gina Kim for full spectroscopic analysis of fumagillol.
Supporting Information Available
Experimental procedures, compound characterization, and 1H/13C NMR reprints. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Morrison K. C.; Hergenrother P. J. Nat. Prod. Rep. 2014, 31, 6–14. [DOI] [PubMed] [Google Scholar]
- a Balthaser B. R.; Maloney M. C.; Beeler A. B.; Porco J. A. Jr.; Snyder J. K. Nat. Chem. 2011, 3, 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kumar N.; Kiuchi M.; Tallarico J. A.; Schreiber S. L. Org. Lett. 2005, 7, 2535–2538. [DOI] [PubMed] [Google Scholar]; c Ignatenko V. A.; Han Y.; Tochtrop G. P. J. Org. Chem. 2012, 78, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Huigens R. W.; Morrison K. C.; Hicklin R. W.; Flood T. A.; Richter M. F.; Hergenrother P. J. Nat. Chem. 2013, 5, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hutt O. E.; Doan T. L.; Georg G. I. Org. Lett. 2013, 15, 1602–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Schwarz O.; Jakupovic S.; Ambrosi H.-D.; Haustedt L. O.; Mang C.; Müller-Kuhrt L. J. Comb. Chem. 2007, 9, 1104–1113. [DOI] [PubMed] [Google Scholar]; g Sumskaya Y. G.; Whitney P. S. III; Bergmeier S. C.; McMills M. C.; Priestley N. D.; Wright D. L. ARKIVOC 2011, 144–166. [Google Scholar]; h Kesavan S.; Marcaurelle L. A. Nat. Chem. Biol. 2013, 9, 210–213. [DOI] [PubMed] [Google Scholar]; i Li J.; Cisar J. S.; Zhou C.-Y.; Vera B.; Williams H.; Rodríguez A. D.; Cravatt B. F.; Romo D. Nat. Chem. 2013, 5, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a O’Connor C. J.; Beckmann H. S. G.; Spring D. R. Chem. Soc. Rev. 2012, 41, 4444–4456. [DOI] [PubMed] [Google Scholar]; b Ganem B. Acc. Chem. Res. 2009, 42, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tan D. S. Nat. Chem. Biol. 2005, 1, 74–84. [DOI] [PubMed] [Google Scholar]; d Spring D. R. Org. Bio. Mol. Chem. 2003, 1, 3867–3870. [DOI] [PubMed] [Google Scholar]; e Schreiber S. L. Science 2000, 287, 1964–1969. [DOI] [PubMed] [Google Scholar]
- a Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215–1292. [DOI] [PubMed] [Google Scholar]; b Liu C.; Zhang H.; Shi W.; Lei A. Chem. Rev. 2011, 111, 1780–1824. [DOI] [PubMed] [Google Scholar]; c Sun C.-L.; Li B.-J.; Shi Z.-J. Chem. Rev. 2010, 111, 1293–1314. [DOI] [PubMed] [Google Scholar]; d Li C.-J. Acc. Chem. Res. 2009, 42, 335–344. [DOI] [PubMed] [Google Scholar]; e Murahashi S.-I.; Zhang D. Chem. Soc. Rev. 2008, 37, 1490–1501. [DOI] [PubMed] [Google Scholar]
- a Ames B. D.; Haynes S. W.; Gao X.; Evans B. S.; Kelleher N. L.; Tang Y.; Walsh C. T. Biochemistry 2011, 50, 8756–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ames B. D.; Liu X.; Walsh C. T. Biochemistry 2010, 49, 8564–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ishihara Y.; Baran P. S. Synlett 2010, 1733–1745. [Google Scholar]; b Chen K.; Baran P. S. Nature 2009, 459, 824–828. [DOI] [PubMed] [Google Scholar]; c Foo K.; Usui I.; Götz D. C. G.; Werner E. W.; Holte D.; Baran P. S. Angew. Chem., Int. Ed. 2012, 51, 11491–11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for complete details.
- DDQ is a common oxidant in various cross-dehydrogenative coupling (CDC) reactions; see:; a Zhang Y.; Li C.-J. Angew. Chem., Int. Ed. 2006, 45, 1949–1952. [DOI] [PubMed] [Google Scholar]; b Zhang Y.; Li C.-J. J. Am. Chem. Soc. 2006, 128, 4242–4243. [DOI] [PubMed] [Google Scholar]; c Tu W.; Liu L.; Floreancig P. E. Angew. Chem., Int. Ed. 2008, 47, 4184–4187. [DOI] [PubMed] [Google Scholar]; d Zhai L.; Shukla R.; Rathore R. Org. Lett. 2009, 11, 3474–3477. [DOI] [PubMed] [Google Scholar]; e Liu L.; Floreancig P. E. Org. Lett. 2010, 12, 4686–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Brizgys G. J.; Jung H. H.; Floreancig P. E. Chem. Sci. 2012, 3, 438–442. [Google Scholar]; g Clausen D. J.; Floreancig P. E. J. Org. Chem. 2012, 77, 6574–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For select examples of oxidative Mannich reactions, see:; a Catino A. J.; Nichols J. M.; Nettles B. J.; Doyle M. P. J. Am. Chem. Soc. 2006, 128, 5648–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang F.; Li J.; Xie J.; Huang Z.-Z. Org. Lett. 2010, 12, 5214–5217. [DOI] [PubMed] [Google Scholar]; c Condie A. G.; González-Gómez J. C.; Stephenson C. R. J. J. Am. Chem. Soc. 2010, 132, 1464–1465. [DOI] [PubMed] [Google Scholar]; d Freeman D. B.; Furst L.; Condie A. G.; Stephenson C. R. J. Org. Lett. 2011, 14, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Allen J. M.; Lambert T. H. J. Am. Chem. Soc. 2011, 133, 1260–1262. [DOI] [PubMed] [Google Scholar]
- Alagiri K.; Devadig P.; Prabhu K. R. Chem.—Eur. J. 2012, 18, 5160–5164. [DOI] [PubMed] [Google Scholar]
- Huang L.; Zhang X.; Zhang Y. Org. Lett. 2009, 11, 3730–3733. [DOI] [PubMed] [Google Scholar]
- a Su W.-K.; Yu J.-B.; Li Z.-H.; Jiang Z.-J. J. Org. Chem. 2011, 76, 9144–9150. [DOI] [PubMed] [Google Scholar]; b Zhao L.; Li C.-J. Angew. Chem., Int. Ed. 2008, 47, 7075–7078. [DOI] [PubMed] [Google Scholar]
- a Richter H.; García Mancheño O. Org. Lett. 2011, 13, 6066–6069. [DOI] [PubMed] [Google Scholar]; b Rohlmann R.; Stopka T.; Richter H.; García Mancheño O. J. Org. Chem. 2013, 78, 6050–6064. [DOI] [PubMed] [Google Scholar]; c Jia X.; Wang Y.; Peng F.; Huo C.; Yu L.; Liu J.; Wang X. J. Org. Chem. 2013, 78, 9450–9456. [DOI] [PubMed] [Google Scholar]
- a Cicchi S.; Goti A.; Brandi A. J. Org. Chem. 1995, 60, 4743–4748. [Google Scholar]; b Goti A.; Cicchi S.; Fedi V.; Nannelli L.; Brandi A. J. Org. Chem. 1997, 62, 3119–3125. [DOI] [PubMed] [Google Scholar]; c Archibald G.; Lin C.-P.; Boyd P.; Barker D.; Caprio V. J. Org. Chem. 2012, 77, 7968–7980. [DOI] [PubMed] [Google Scholar]
- Takasu N.; Oisaki K.; Kanai M. Org. Lett. 2013, 15, 1918–1921. [DOI] [PubMed] [Google Scholar]
- For deuterium-labeling studies of DDQ-mediated oxidative Prins cyclizations, see:Jung H. H.; Floreancig P. E. Tetrahedron 2009, 65, 10830–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.