Abstract
Cervical cancer is the most common genital malignancy and the high-risk human papillomaviruses (HPV type 16, 18 and 31, and so on) are major agents for its cause. A key switch for the onset of cervical cancers by HPVs is the cellular degradation of the tumor-suppressor p53 that is mediated by the HPV-generated E6 protein. E6 forms a complex with the E3 ubiquitin-ligase E6-associated protein (E6AP) leading to p53 degradation. The components that control E6 expression and the mechanisms for regulation of the expression in host cells remain undefined. Here we show that the nuclear noncanonical poly(A) polymerase (PAP) speckle targeted PIPKIα regulated PAP (Star-PAP) controls E6 mRNA polyadenylation and expression and modulates wild-type p53 levels as well as cell cycle profile in high-risk HPV-positive cells. In the absence of Star-PAP, treatment of cells with the chemotherapeutic drug VP-16 dramatically reduced E6 and increased p53 levels. This diminished both cell proliferation and anchorage-independent growth required for cancer progression, indicating a synergism between VP-16 treatment and the loss of Star-PAP. This identifies Star-PAP as a potential drug target for the treatment of HPV-positive cancer cells. These data provide a mechanistic basis for increasing the sensitivity and efficiency of chemotherapy in the treatment of cancers that have low levels of wild-type p53.
Keywords: p53, HPV E6, VP-16, Star-PAP, polyadenylation, cell growth and proliferation
INTRODUCTION
TP53 is one of the most frequently mutated genes in human cancers, and its protein product p53 controls cell cycle progression and apoptosis, and therefore is critical for cell proliferation and cancer progression. However, only wild type and not mutant p53 is capable of suppressing cell growth1,2 and the wild-type p53 stability and levels are low in high-risk cervical cancer cells because of E6/E6-associated protein (E6AP)-mediated degradation.3 The high-risk human papillomavirus (HPV) E6/E7 early viral sequence merges into the host cell genome with integrated 5′-promoter and 3′-end host sequence preceded by the standard hexanucleotide polyadenylation signal (AATAAA).4,5 E6 expression in host cells eliminates the cellular protective response to genotoxic stress such as DNA damage by interfering with p53-mediated cell cycle arrest.6 Chemotherapeutic drugs such as VP-16 (DNA damage agent) and cisplatin (alkylating agent and DNA crosslinker) could restore p53 levels by decreasing E6 expression and usurp the E6/E6AP-mediated proteosomal degradation pathway in these cells.7,8 As restoration of the expression of the wild-type p53 leads to enhanced cell death of cancer cells with DNA damage, this has been proposed as a strategy for cancer therapy.9–12
The nuclear speckle targeted PIPKIα regulated poly(A) polymerase, Star-PAP, is a non-canonical poly(A) polymerase (PAP) that selectively controls gene expression by processing pre-mRNAs.13–15 Star-PAP is incorporated into transcriptional and 3′-end processing complexes that are distinct from those of the canonical PAP and are required for 3′-end cleavage and polyadenylation of select target pre-mRNAs.13–15 The specificity and activity of Star-PAP toward genes are controlled by signaling pathways through the assembly of specific signaling molecules into the Star-PAP complex.15 Downstream of signaling, Star-PAP is regulated by specific interactions with protein kinases including PKCδ, CKIα and CKIε and the associated phosphoinositide kinase PIPKIα, which generates phosphatidylinositol 4,5-bisphosphate (PI4,5P2).13,15–17 PI4,5P2 acts as a lipid messenger that directly stimulates Star-PAP’s PAP activity13,15–17 and the associated kinases regulate Star-PAP gene specificity.15 Previously, we showed that Star-PAP is a DNA damage sensitive controller of the expression of the pro-apoptotic gene BIK and VP-16 could potentiate the PAP activity of Star-PAP required for mRNA 3′-end processing.15 This study identified Star-PAP as a direct upstream mediator of HPV E6 expression, which controls p53 levels critical for the cellular response to DNA damage signals and cell survival. Importantly, downregulation of Star-PAP greatly improved the cytotoxicity of VP-16 and increased the mortality of the high-risk HPV-positive cells.
RESULTS AND DISCUSSION
Star-PAP controls E6 and p53 expression in high-risk HPV-positive cervical cancer cells downstream of DNA damage
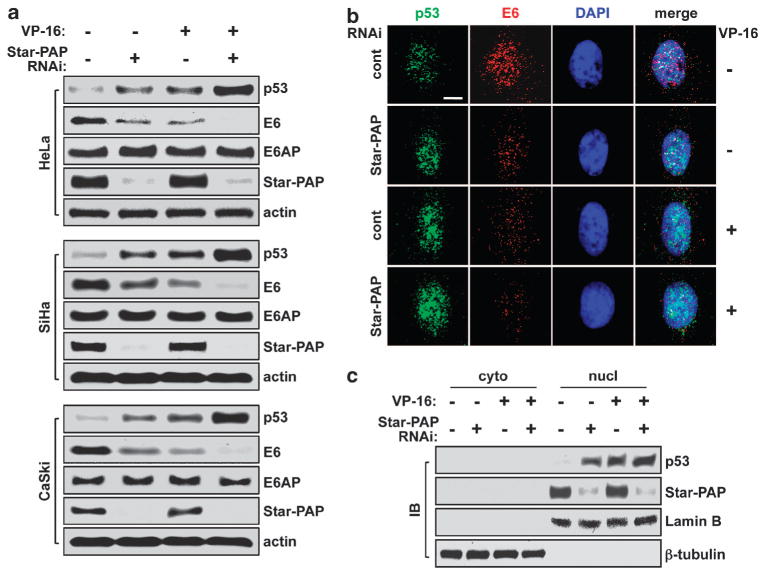
In normal cells, p53 levels are regulated by Mdm2-mediated proteosomal degradation. In high-risk HPV-positive cancerous cells, p53 expression is low and modulated by the E6 and E6AP-controlled destruction pathway.18 During the study of Star-PAP regulation of specific gene expression in response to DNA damage in HeLa cells (HPV-18-positive), we found that p53 levels were remarkably increased when Star-PAP is downregulated by small interfering RNA knockdown and this is accompanied by a loss of E6 protein (Figure 1a). VP-16 treatment (DNA damage) also diminished E6 and increased p53 (Figure 1a and Supplementary Figure 1a) consistent with reported previously.19 Strikingly, Star-PAP knockdown-coupled VP-16 treatment resulted in a dramatic loss of E6 protein and a synergistic increase in p53 levels whereas E6AP protein levels were not affected (Figure 1a). Similar effects were observed in the HPV-16-positive SiHa and CaSki cells (Figure 1a), suggesting that the Star-PAP regulation of p53 and E6 expression is a broad mechanism for the high-risk HPV-infected cells. To verify these results, HeLa cells were selected as a model for further characterization. Immunofluorescence microscopy data demonstrated that the opposite changes in E6 and p53 levels after Star-PAP knockdown and VP-16 treatment resulted in enhanced nuclear accumulation of p53 with a loss of E6, and no detectable change of E6AP within nucleus (Figure 1b and Supplementary Figure 1b).
Figure 1.
Star-PAP controls p53 and E6 expression in high-risk HPV-positive cervical cancer cells downstream of DNA damage. (a) Star-PAP knockdown and VP-16 treatment (50 μM, 6 h) increased p53 and decreased E6 protein levels whereas E6AP levels remained unchanged in HeLa (ATCC), SiHa and CaSki (gifts from Dr Paul F Lambert’s Lab, UW-Madison) cells as analyzed by immunoblotting (IB). Star-PAP small interfering RNA (siRNA) 5′-GUGUGUUUGUCAGUGGCUU-3′; scrambled control siRNA 5′-AGGUAGUGUAAUCGCCUUG-3′. (b) Immunofluorescence (IF) staining demonstrated nuclear accumulation of p53 and depletion of E6 as well as decreased association of the two molecules after Star-PAP knockdown and VP-16 treatment. Scale bar = 10 μm. (c) Cellular protein fractionation and IB showed that the Star-PAP knockdown- and VP-16 treatment-induced increase in p53 levels were restricted in nucleus. Lamin B and β-tubulin were used as controls for nuclear and cytosolic protein fractions, respectively. IB and IF were performed as described.33 Antibodies used: rabbit polyclonal anti-Star-PAP (Anderson’s Lab homemade, Madison, WI, USA);13 mouse monoclonal anti-p53 (Santa Cruz Biotechnology, #sc-126, Santa Cruz, CA, USA); rabbit polyclonal anti-p53 (Santa Cruz Biotechnology, #sc-6243); mouse monoclonal anti-HPV-18 E6 (Santa Cruz Biotechnology, #sc-365089); mouse monoclonal anti-HPV-16 E6 (Santa Cruz Biotechnology, #sc-460); rabbit polyclonal anti-E6AP (Santa Cruz Biotechnology, #sc-25509); mouse monoclonal anti-β-tubulin (Upstate Biotechnology, #05-661, Lake Placid, NY, USA); mouse monoclonal anti-Lamin B (Santa Cruz Biotechnology, #sc-365962); and mouse monoclonal anti-actin (MP Biomedical, #691002, Solon, OH, USA).
Cellular protein fractionation analysis demonstrated that the increase in p53 protein levels induced by Star-PAP knockdown and VP-16 treatment was exclusively in the nucleus (Figure 1c), consistent with a role of the increased p53 in transcriptional activation of target genes and response to stress signals. Further, the complex of E6 with E6AP was diminished under the same experimental conditions potentially owing to the reduction in E6 expression (Supplementary Figure 1c), indicating that the proteosomal degradation pathway for p53 destruction was impaired. Interestingly, PIPKIα, PKCδ and CKIα/ε that mediate Star-PAP activities for the 3′-end processing and expression of certain target genes13–17 were not involved in the regulation of E6 expression (data not shown). This is possibly due to the chimeric property of the 3′-end sequence of the E6 pre-mRNA, which is a combinational derivative of the viral and the host genomes in HeLa cells. Although it still requires Star-PAP for transcript processing at the 3′-end, this chimeric sequence may combine to recruit different transactivating factors into the Star-PAP mRNA-processing complex.
Star-PAP regulates E6 mRNA levels by mediating the polyadenylation of the transcripts
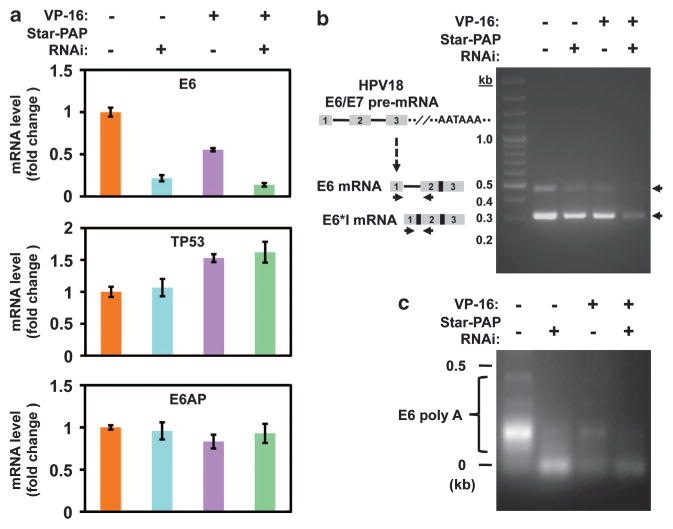
Star-PAP is a PAP.13 Thus, the decrease in E6 protein levels upon Star-PAP knockdown could be due to defective polyadenylation of the E6 mRNA and hence loss of expression. To assess this, the E6 mRNA levels were examined in cells treated with or without Star-PAP knockdown and in the presence or absence of VP-16. Quantitative real-time reverse transcriptase–PCR (qRT–PCR) data showed that loss of Star-PAP reduced E6 mRNA expression by 5-fold and VP-16 treatment by 2-fold, and the combination of Star-PAP loss and VP-16 treatment attenuated the expression by ~10-fold (Figure 2a). Similar results for E6 mRNA expression under the same experimental conditions were seen in HPV-16-positive SiHa and CaSki cells (Supplementary Figures 2a and 2b). This is further supported by reverse transcriptase–PCR for E6 products (with the inclusion of the first intron)4 using primers specific for the first and second exons of E6 pre-mRNA (Figure 2b). The E6 first intron spliced product E6*I20 and the E6 mRNA were detected using this method20,21 and were diminished by Star-PAP knockdown and/or VP-16 treatment (Figure 2b), demonstrating that the E6-host cell transcripts were lost. As the E6 and E6*I transcripts were generated using the same poly(A) signal,4,5,22 the loss of both transcripts after Star-PAP knockdown is consistent with the role of Star-PAP in mRNA 3′-end processing. This is directly modulated by Star-PAP as it interacts with the E6 mRNA as detected by RNA immunoprecipitation (data not shown). The E6*I mRNA encodes the E7 oncoprotein,20 and E7 binds and destabilizes the retinoblastoma tumor-suppressor pRb.3 Examination of E7 mRNA and protein levels by qRT–PCR and immunoblotting, respectively, showed that although the E7 mRNA contents were reduced in the Star-PAP knockdown cells, the E7 protein levels remained unchanged (Supplementary Figure 2c). VP-16 treatment also decreased E7 mRNA levels to some extent and slightly reduced E7 protein levels. The E7 target pRb levels, however, remained constant under the experimental conditions (Supplementary Figure 2c). The observed inconsistency in E7 mRNA expression and protein levels was reported before.23–25 It is well accepted that the cellular steady-state E7 protein levels are translationally or post-translationally regulated,25–27 and may not directly mirror the mRNA levels.23
Figure 2.
Star-PAP regulates E6 mRNA levels by mediating the polyadenylation of the transcripts. (a) qRT–PCR15 demonstrated reduced production of the E6 messages after knockdown of Star-PAP in the presence or absence of VP-16 (50 μM, 6 h), and treatment with VP-16 alone also to some extent decreased E6 expression. TP53 and E6AP mRNA levels were not significantly affected by the treatments. Target mRNA abundance was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression (internal non-Star-PAP target control), and the mRNA expression levels displayed on charts were normalized to mock-treated control. Error bars represent s.e.m. of three independent experiments with triplicates for each experimental condition. Primers used for the analysis: HPV-18 E6 forward 5′-GCGACCCTACAAGCTACC TG-3′, reverse 5′-GTTGGAGTCGTTCCTGTCGT-3′; TP53 forward 5′-CCCGGACGATATTGAACAATGG-3′, reverse 5′-CAGAATGCAAGAAGCCCAGAC-3′; E6AP forward 5′-CCCTGATGATGTGTCTGTGG-3′, reverse 5′-GGCAAAGCCATTTCCAGATA-3′; GAPDH forward 5′-GAAGGTCGGAGTCAACGGATTT-3′, reverse 5′-GAATTTGCCATGGGTGGAAT-3′. (b) Detection of the intron-containing E6 expression by RT–PCR using primers within the first and second exons of E6, which could also amplify the spliced E6*I expression. (c) Poly(A) test (PAT) analysis of the poly(A) tail length of E6 mRNA in cells treated with Star-PAP knockdown or/and VP-16 (50 μM, 6 h). The length of the extended poly(A) reflects the polyadenylation potential of the PAP. Total RNA prepared with the TRIzol Reagent (Life Technologies, #15596-026, Grand Island, NY, USA) was subjected to annealing with phosphorylated oligo(dT)12–18 and ligation with oligo(dT)-anchor primers,29 reverse transcribed and the E6 poly(A) mRNA generation assessed by PCR amplification of the corresponding complementary DNA using the gene-specific forward primer as described above and the oligo(dT)-anchor primer. The PCR products were then resolved on 1.5% agarose gel, stained with ethidium bromide and imaged.
Star-PAP knockdown did not alter TP53 and E6AP mRNA levels (Figure 2a) indicating that the dramatic increase in p53 protein levels after Star-PAP knockdown plus VP-16 treatment was not due to a transcriptional increase in TP53 or decrease in E6AP mRNAs. Addition of VP-16 slightly increased TP53 expression (Figure 2a). As an indicator of p53 transactivation, the effector of p53-mediated blockage of cell cycle S-phase entry, p21 mRNA expression was modestly increased after Star-PAP knockdown or VP-16 treatment but there was a large increase in p21 expression upon loss of Star-PAP and VP16 treatment (Supplementary Figure 3). The expression of Mdm2, which functions as an E3 ubiquitin ligase for p53 degradation in normal cells but is inactive in HPV-positive cervical cancer cells,18 and its modifying proteins SENP2 (for Mdm2 desumoylation) and p14ARF (for Mdm2 sequestration in nucleolus) were not significantly changed (Supplementary Figure 3).
To verify that E6 expression is controlled by Star-PAP-mediated polyadenylation of the mRNA, poly(A) test assays28,29 were performed to analyze the changes in intracellular polyadenylated target mRNA. Knockdown of Star-PAP diminished the production of the polyadenylated E6 mRNA in both VP-16-treated and non-treated cells (Figure 2c, compare lanes 2 and 4 with lane 1). VP-16 is able to stimulate Star-PAP activities toward the BCL2-interacting killer, BIK, mRNA polyadenylation and expression.15 In contrast, VP-16 treatment diminished E6 mRNA expression (Figure 2a), resulting in reduced E6 mRNA substrate for Star-PAP. Yet, the E6 mRNA was normally polyadenylated in the presence of VP-16 as demonstrated by the distinguishable bands below 0.5 kb (Figure 2c, compare lane 3 with lane 1). The combination of VP-16 treatment and Star-PAP loss resulted in a large decrease in the polyadenylated E6 mRNA (Figure 2c, lane 4). These data collectively demonstrate that Star-PAP mediates E6 mRNA 3′-end processing and expression, by which controls p53 protein levels.
Star-PAP-mediated p53 expression regulates cervical cancer cell proliferation and anchorage-independent growth
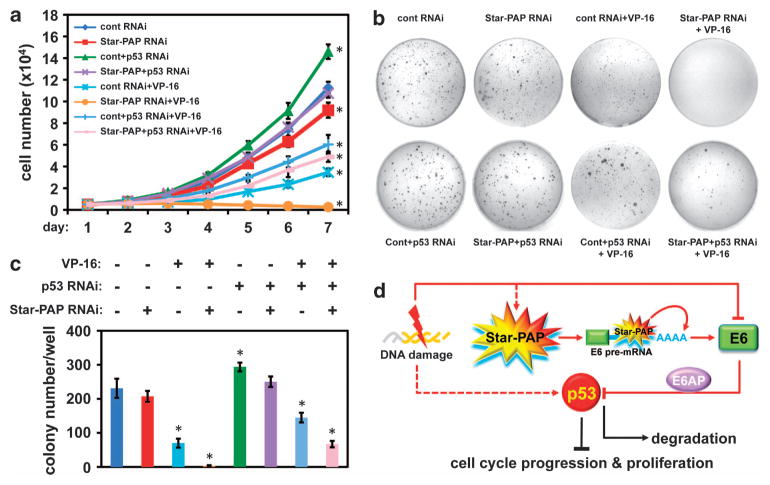
As E6 and p53 regulate HeLa cell proliferation and the combined treatment of Star-PAP knockdown and VP-16 resulted in a dramatic increase in p53 expression, we hypothesized that increased p53 and VP-16 treatment would synergistically regulate cell cycle progression, proliferation and anchorage-independent cell growth. To test this, cell cycle profiles were quantified by fluorescence-activated cell sorting analysis (FACS). The results showed that knockdown of Star-PAP reduced the cell numbers in S-phase and increased that of G1-phase while p53 knockdown resulted in an opposite trend (Supplementary Figure 4) in line with the role of p53 in cell cycle regulation.30 These modest changes in cell cycle profile could be due to the low basal level of p53 within the cell. VP-16-induced DNA damage arrested cells in G2-phase as reported31,32 and knockdown of p53 enhanced this arrest (Supplementary Figure 4b) because of a blockage of the cells progressing through S-phase. Interestingly, Star-PAP-knockdown cells appeared to progress into S-phase in the presence of VP-16 (Supplementary Figure 4b). As described before, the p53 levels were increased in Star-PAP knockdown cells and this was greatly enhanced in VP-16-treated cells. Therefore, the cells will eventually die in S-phase with sustained VP-16 treatment. This concept is supported by an independent observation, where expression of wild-type p53 could override the VP-16-induced G2 arrest and increase VP-16 cytotoxicity.31 Downregulation of p53 in addition to Star-PAP knockdown and VP-16 treatment slightly reduced the S-phase and increased the G2-phase cell numbers compared with that of the control RNA interference condition (Supplementary Figure 4b), a trend as seen under conditions of p53 knockdown and VP-16 treatment. The increase in S-phase entry after Star-PAP knockdown compared with the mock/control knockdown cells in the VP-16 treatment background suggests additional mechanisms in the control of S-phase entry might be involved.
To verify that the synergistic effect of Star-PAP knockdown and VP-16 treatment has an impact on cell survival, cell proliferation and colony assays were carried out in the presence or absence of a low dose of VP-16 (2 μM). Although p53 knockdown increased cell proliferation, Star-PAP knockdown attenuated this activity in both VP-16 non-treated and treated cells and is profound in the latter (Figure 3a and Supplementary Figure 4a). This is further confirmed by soft agar colony formation assays, which assess anchorage-independent growth that is fundamental for tumor progression. Star-PAP knockdown alone only slightly decreased the colony size and numbers compared with control. VP-16 treatment greatly reduced the colony numbers. Strikingly, the growth of HeLa cell colonies in soft agar was totally abolished under Star-PAP knockdown plus VP-16 treatment conditions (Figures 3b and c). To ensure that this phenotype is a synergistic effect of the enhanced p53 levels, p53 was knocked down and colony formation evaluated. Depletion of p53 augmented the colony size and numbers in the absence of VP-16, and substantially restored the colony formation attenuated by VP-16 treatment and Star-PAP knockdown (Figures 3b and c), indicating the growth-inhibiting effect of the combined treatment is through a p53-mediated pathway. Discrepancies between the FACS analysis and the colony formation experiments in terms of cell cycle blockage and growth arrest under Star-PAP/p53 knockdown and VP-16 treatment conditions are likely due to the use of VP-16 at different concentrations and treatment durations.
Figure 3.
Star-PAP-mediated p53 expression regulates cervical cancer cell proliferation. (a) Analysis of HeLa cell proliferation34 after Star-PAP or/and p53 knockdown in the presence or absence of contiguous low dose of VP-16 (2 μM) treatment for a period of time as indicated. Error bars represent s.e.m. of three individual experiments with triplicate for each experimental condition. For statistics, individual experimental conditions are compared with control group. *P≤0.05 (t-test). p53 small interfering RNA (siRNA) 5′-CCGUCCCAAGCAAUGGAUGAUUUGA-3′. (b) Soft agar colony formation assay using HeLa cells treated under the same conditions as described above. A representative batch of experiment is displayed, and the colony numbers of each experimental condition were quantified and the means of the colony numbers from three independent experimental runs were used for plotting (c). Error bars represent s.e.m. of three individual experiments. For statistics, individual experimental conditions are compared with control group. *P≤0.05 (t-test). This assay was carried out in 35 mm cell culture dishes, where three layers of soft agar (Fisher Scientific, #BP1423, Pittsburgh, PA, USA; bottom 0.6%, middle 0.4% and top 0.6%) were plated individually (0.5 ml liquefied agar at 40 °C for each layer) with the middle layer containing 104 cells per dish. After plating the sandwich agar and the agar solidified, 1 × Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and penicillin/streptomycin (50 U/ml) was overlaid on top and the cultures were grown at 37 °C in 5% CO2 for 4 weeks. The colonies were stained with Giemsa Stain (Sigma-Aldrich, #GS500, St Louis, MO, USA) and visible colonies were counted and quantified. (d) Model of the Star-PAP-regulated p53 expression in the control of cell proliferation in response to DNA damage signal.
We have identified E6 as a Star-PAP target in high-risk HPV-positive cervical cancer cells. Star-PAP-controlled E6 expression regulates p53 levels by interfering with the fine-tuned nuclear E6/E6AP/p53 complex formation required for p53 degradation, cell cycle progression and oncogenic growth downstream of DNA damage (Figure 3d). Therefore, this study implicates a role for Star-PAP as a nuclear sensor of the genotoxic stress pathway that controls p53 degradation, which is critical for the cell cycle surveillance system and cell survival. Our data support Star-PAP as a therapeutic target in cancers with downregulated wild-type p53 levels. Pharmacological compounds or short oligonucleotides directed to target Star-PAP during cancer treatment with DNA damage agents such as chemotherapeutic drugs or gamma irradiation may increase the sensitivities of the therapeutics and therefore could be clinically beneficial.
Supplementary Material
Acknowledgments
We thank members of the Anderson laboratory for discussion and comments, and thank Dr Paul F Lambert’s laboratory at UW-Madison for SiHa and CaSki cell lines. This work was supported by a grant (GM051968) from the US National Institutes of Health and a Scientist Development Grant (12SDG12100035) from the American Heart Association.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Wang Y, Suh YA, Fuller MY, Jackson JG, Xiong S, Terzian T, et al. Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation. J Clin Invest. 2011;121:893–904. doi: 10.1172/JCI44504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 3.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 4.Schneider-Gadicke A, Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986;5:2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinther J, Rosenstierne MW, Kristiansen K, Norrild B. The 3′ region of human papillomavirus type 16 early mRNAs decrease expression. BMC Infect Dis. 2005;5:83. doi: 10.1186/1471-2334-5-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90:3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, McKalip A, Herman B. Human papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human keratinocytes to apoptosis induced by chemotherapeutic agents: roles of p53 and caspase activation. J Cell Biochem. 2000;78:334–349. [PubMed] [Google Scholar]
- 8.Wesierska-Gadek J, Schloffer D, Kotala V, Horky M. Escape of p53 protein from E6-mediated degradation in HeLa cells after cisplatin therapy. Int J Cancer. 2002;101:128–136. doi: 10.1002/ijc.10580. [DOI] [PubMed] [Google Scholar]
- 9.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 12.Sauter ER, Takemoto R, Litwin S, Herlyn M. p53 alone or in combination with antisense cyclin D1 induces apoptosis and reduces tumor size in human melanoma. Cancer Gene Ther. 2002;9:807–812. doi: 10.1038/sj.cgt.7700492. [DOI] [PubMed] [Google Scholar]
- 13.Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, et al. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451:1013–1017. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- 14.Laishram RS, Anderson RA. The poly A polymerase Star-PAP controls 3′-end cleavage by promoting CPSF interaction and specificity toward the pre-mRNA. EMBO J. 2010;29:4132–4145. doi: 10.1038/emboj.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Laishram RS, Ji Z, Barlow CA, Tian B, Anderson RA. Star-PAP control of BIK expression and apoptosis is regulated by nuclear PIPKIalpha and PKCdelta signaling. Mol Cell. 2012;45:25–37. doi: 10.1016/j.molcel.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laishram RS, Barlow CA, Anderson RA. CKI isoforms alpha and epsilon regulate Star-PAP target messages by controlling Star-PAP poly(A) polymerase activity and phosphoinositide stimulation. Nucleic Acids Res. 2011;39:7961–7973. doi: 10.1093/nar/gkr549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales ML, Mellman DL, Anderson RA. CKIalpha is associated with and phosphorylates star-PAP and is also required for expression of select star-PAP target messenger RNAs. J Biol Chem. 2008;283:12665–12673. doi: 10.1074/jbc.M800656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci USA. 2001;98:1218–1223. doi: 10.1073/pnas.031470698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koivusalo R, Krausz E, Helenius H, Hietanen S. Chemotherapy compounds in cervical cancer cells primed by reconstitution of p53 function after short interfering RNA-mediated degradation of human papillomavirus 18 E6 mRNA: opposite effect of siRNA in combination with different drugs. Mol Pharmacol. 2005;68:372–382. doi: 10.1124/mol.105.011189. [DOI] [PubMed] [Google Scholar]
- 20.Tang S, Tao M, McCoy JP, Jr, Zheng ZM. The E7 oncoprotein is translated from spliced E6*I transcripts in high-risk human papillomavirus type 16- or type 18-positive cervical cancer cell lines via translation reinitiation. J Virol. 2006;80:4249–4263. doi: 10.1128/JVI.80.9.4249-4263.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotlar K, Stubner A, Diemer D, Menton S, Menton M, Dietz K, et al. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J Med Virol. 2004;74:107–116. doi: 10.1002/jmv.20153. [DOI] [PubMed] [Google Scholar]
- 22.Zheng ZM, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006;11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler M, Muller-Holzner E, Viertler HP, Widschwendter A, Laich A, Pfister G, et al. High level HPV-16 E7 oncoprotein expression correlates with reduced pRb-levels in cervical biopsies. FASEB J. 2004;18:1120–1122. doi: 10.1096/fj.03-1332fje. [DOI] [PubMed] [Google Scholar]
- 24.Lappalainen K, Pirila L, Jaaskelainen I, Syrjanen K, Syrjanen S. Effects of liposomal antisense oligonucleotides on mRNA and protein levels of the HPV 16 E7 oncogene. Anticancer Res. 1996;16:2485–2492. [PubMed] [Google Scholar]
- 25.Oh KJ, Kalinina A, Park NH, Bagchi S. Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein. J Virol. 2006;80:7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinstein E, Scheffner M, Oren M, Ciechanover A, Schwartz A. Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene. 2000;19:5944–5950. doi: 10.1038/sj.onc.1203989. [DOI] [PubMed] [Google Scholar]
- 27.Kanduc D. Translational regulation of human papillomavirus type 16 E7 mRNA by the peptide SEQIKA, shared by rabbit alpha(1)-globin and human cytokeratin 7. J Virol. 2002;76:7040–7048. doi: 10.1128/JVI.76.14.7040-7048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salles FJ, Strickland S. Rapid and sensitive analysis of mRNA polyadenylation states by PCR. PCR Methods Appl. 1995;4:317–321. doi: 10.1101/gr.4.6.317. [DOI] [PubMed] [Google Scholar]
- 29.Murray EL, Schoenberg DR. Assays for determining poly(A) tail length and the polarity of mRNA decay in mammalian cells. Methods Enzymol. 2008;448:483–504. doi: 10.1016/S0076-6879(08)02624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 31.Skladanowski A, Larsen AK. Expression of wild-type p53 increases etoposide cytotoxicity in M1 myeloid leukemia cells by facilitated G2 to M transition: implications for gene therapy. Cancer Res. 1997;57:818–823. [PubMed] [Google Scholar]
- 32.Clifford B, Beljin M, Stark GR, Taylor WR. G2 arrest in response to topoisomerase II inhibitors: the role of p53. Cancer Res. 2003;63:4074–4081. [PubMed] [Google Scholar]
- 33.Li W, Kotoshiba S, Berthet C, Hilton MB, Kaldis P. Rb/Cdk2/Cdk4 triple mutant mice elicit an alternative mechanism for regulation of the G1/S transition. Proc Natl Acad Sci USA. 2009;106:486–491. doi: 10.1073/pnas.0804177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Petrimpol M, Molle KD, Hall MN, Battegay EJ, Humar R. Hypoxia-induced endothelial proliferation requires both mTORC1 and mTORC2. Circ Res. 2007;100:79–87. doi: 10.1161/01.RES.0000253094.03023.3f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.