Abstract
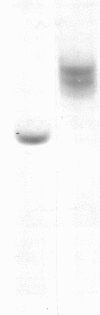
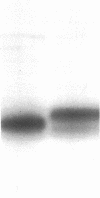
Papain solubilized H-2a histocompatibility antigens (H-2Kk plus H-2Dd) have been purified by a large-scale procedure that can routinely provide 2-3 mg of heavy chain from 1 kg of mouse liver. The heavy chains were homogeneous by sodium dodecyl sulfate electrophoresis. Disc gel electrophoresis resolved two protein bands that were identified as H-2Dd and H-2Kd by immune complex formation and autoradiography. Comparative amino acid composition and NH2-terminal sequence analyses of unfractionated H-2a, H-2Kk, and HLA suggested close structural relationships. However, the following observations suggest that papain cleaves these membrane bound antigens at different positions with respect to the COOH terminus: the molecular weight of the peptide portion of papain solubilized HLA is smaller than that of H-2 (30,000 versus 33,700); the COOH-terminal sequences are different; and, finally, papain-solubilized H-2 contains a free cysteine residue in addition to the two disulfide bridges that are present in both H-2 and HLA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Henriksen O., Natori T., Tanigaki N., Law L. W., Pressman D. Basic structure of mouse histocompatibility antigens. Transplant Proc. 1975 Jun;7(2):191–194. [PubMed] [Google Scholar]
- Appella E., Tanigaki N., Fairwell T., Pressman D. Partial amino acid sequences of the heavy chains of human HLS histocompatibility antigens. Biochem Biophys Res Commun. 1976 Jul 12;71(1):286–292. doi: 10.1016/0006-291x(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Appella E., Tanigaki N., Natori T., Pressman D. Partial amino acid sequence of mouse beta2-microglobulin. Biochem Biophys Res Commun. 1976 May 17;70(2):425–430. doi: 10.1016/0006-291x(76)91063-9. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Gillett D., Hill D. F., Petersen G. B. Automated sequencing of insoluble peptides using detergent. Bacteriophage fl coat protein. J Biol Chem. 1977 Apr 10;252(7):2218–2225. [PubMed] [Google Scholar]
- Ballou B., McKean D. J., Freedlender E. F., Smithies O. HLA membrane antigens: sequencing by intrinsic radioactivity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4487–4491. doi: 10.1073/pnas.73.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunitzer G., Schrank B., Ruhfus A. Zum vollständigen und autoatischen Abbau von Peptiden nach der Quadrolmethode. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1589–1590. [PubMed] [Google Scholar]
- Capra J. D., Vitetta E. S., Klapper D. G., Uhr J. W., Klein J. Structural studies on protein products of murine chromosome 17: partial amino acid sequence of an H-2Kb molecule. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3661–3665. doi: 10.1073/pnas.73.10.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Freed J. H., Mole L. E., Nathenson S. G. Localization of the papain cleavage site of H-2 glycoproteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):915–918. doi: 10.1073/pnas.73.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. H., Nathenson S. G. Similarity of the carbohydrate structures of H-2 and Ia glycoproteins. J Immunol. 1977 Aug;119(2):477–482. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen O., Appella E., Smith D. F., Tanigaki N., Pressman D. Comparative chemical analyses of the alloantigenic fragments of HL-A antigens. J Biol Chem. 1976 Jul 25;251(14):4214–4219. [PubMed] [Google Scholar]
- Hess M., Davies D. A. Basic structure of mouse histocompatibility antigens. Eur J Biochem. 1974 Jan 3;41(1):1–13. doi: 10.1111/j.1432-1033.1974.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Klein J., Shreffler D. C. The H-2 model for the major histocompatibility systems. Transplant Rev. 1971;6:3–29. doi: 10.1111/j.1600-065x.1971.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Kvist S., Sandberg-Trägårdh L., Ostberg L., Peterson P. A. Isolation and partial characterization of papain-solubilized murine H-2 antigens. Biochemistry. 1977 Oct 4;16(20):4415–4420. doi: 10.1021/bi00639a014. [DOI] [PubMed] [Google Scholar]
- Natori T., Katagiri M., Tanigaki N., Pressman D. The 11,000-dalton component of mouse H-2. Isolation and identification. Transplantation. 1974 Dec;18(6):550–555. doi: 10.1097/00007890-197412000-00015. [DOI] [PubMed] [Google Scholar]
- Natori T., Katagiri M., Tanigaki N., Pressman D. The 11,000-dalton component of mouse H-2. Isolation and identification. Transplantation. 1974 Dec;18(6):550–555. doi: 10.1097/00007890-197412000-00015. [DOI] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Appella E., Pressman D. Amino acid composition and physicochemical properties of mouse beta2-microglobulin. Biochem Biophys Res Commun. 1975 Jul 22;65(2):611–617. doi: 10.1016/s0006-291x(75)80190-2. [DOI] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Pressman D., Henriksen O., Appella E., Law L. W. The component fragments obtained by acid dissociation of papain-solubilized H-2 molecules. J Immunogenet. 1976 Feb;3(1):35–47. doi: 10.1111/j.1744-313x.1976.tb00554.x. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Rask L., Ostberg L., Lindblom B., Fernstedt Y., Peterson P. A. The subunit structure of transplantation antigens. Transplant Rev. 1974;21(0):85–105. doi: 10.1111/j.1600-065x.1974.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Shimada A., Nathenson S. G. Murine histocompatibility-2 (H-2) alloantigens. Purification and some chemical properties of soluble products from H-2b and H-2d genotypes released by papain digestion of membrane fractions. Biochemistry. 1969 Oct;8(10):4048–4062. doi: 10.1021/bi00838a023. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Detergent-solubilised H-2 alloantigen is associated with a small molecular weight polypeptide. Nature. 1974 Jun 21;249(459):764–765. doi: 10.1038/249764a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Structure and evolution of transplantation antigens: partial amino-acid sequences of H-2K and H-2D alloantigens. Proc Natl Acad Sci U S A. 1976 Feb;73(2):599–603. doi: 10.1073/pnas.73.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober S., Appella E., Law L. W. Serological and immunogenic activity of soluble mouse transplantation antigens controlled by the H-2 locus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):765–772. doi: 10.1073/pnas.67.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Klein J., Pazderka F., Moticka E. J., Ruth R. F., Capra J. D. Homology of (murine) H-2 and (human) HLA with a chicken histocompatibility antigen. Nature. 1977 Dec 8;270(5637):535–536. doi: 10.1038/270535a0. [DOI] [PubMed] [Google Scholar]