Abstract
The Dietary Guidelines for Americans (DGA) are the cornerstone of US government efforts to promote health and prevent disease through diet and nutrition. The DGA currently provides guidelines for ages ≥2 y. In an effort to determine the strength of the evidence to support the inclusion of infants and children from birth to age 24 mo, the partner agencies led by the Department of Health and Human Services Office of Disease Prevention and Health Promotion and the USDA Center for Nutrition Program and Policy initiated the project entitled “Evaluating the evidence base to support the inclusion of infants and children from birth to 24 months of age in the Dietary Guidelines for Americans—the B-24 Project.” This project represents the first step in the process of applying systematic reviews to the process of deciding whether the evidence is sufficient to include this age group in future editions of the DGA. This supplement includes the B-24 Executive Summary, which describes the B-24 Project and the deliberations of the 4 working groups during the process of developing priority topics for the systematic review, and a research agenda to address the critical gaps. Also included in this supplement issue is an article on the Nutrition Evidence Library methodology for developing systematic review questions and articles from the invited content presenters at the B-24 Prime meeting.
INTRODUCTION
The Dietary Guidelines for Americans (DGA)4 are generated every 5 y via a partnership between the USDA and the US Department of Health and Human Services (DHHS). The DGA is the national nutrition policy that has served as the foundation for the US government guidance about the important role of diet in health promotion and disease risk prevention for over 40 y. The history of the DGA is shown in Supplemental Table 1 (under “Supplemental data” in the online issue).
The development of the DGA was legislatively mandated with the passage of the National Nutrition Monitoring and Related Research Act of 1990 (1), which codified the goal as to, “At least every 5 years the Secretaries shall publish a report entitled ‘Dietary Guidelines for Americans’. Each such report shall contain nutritional and dietary information and guidelines for the general public, and shall be promoted by each Federal agency in carrying out any Federal food, nutrition, or health program.”
Beginning with the first edition in 1980, the DGA focused on “healthy Americans 2 years and older.” While providing essential guidance for consumers, health professionals, nutrition educators, researchers, and policymakers alike, the omission of guidance specifically for infants and children from birth to 24 mo of age has been a topic of discussion throughout the history of the DGA. Although not specifically addressed, this critical developmental period has been referred to repeatedly in numerous editions of the DGA (Table 1).
TABLE 1.
References to children <2 y in DGA and related documents1
| 2nd edition DGA (1985): guidance relevant to infants2 |
| • “Infants also have special nutritional needs. Infants should be breast-fed unless there are special problems. The nutrients in human breast milk tend to be more available than those in cow's milk. In addition, breast milk serves to transfer immunity to some diseases from the mother to the infant.” (p 4). |
| • “Normally most babies are not given solid foods until they are 3 to 6 months old. At that time, other foods can be introduced gradually. Prolonged breast- or bottle-feeding—without solid foods or supplemental iron—can result in iron deficiency.” (p 4). |
| • “You should not add salt or sugar to the baby's foods. Infants do not need these inducements if they are really hungry. The foods are nourishing and extra flavoring with salt and sugar is not necessary.” (p 4). |
| • “To ensure your infant receives an adequate diet: |
| ○ Breastfeed unless there are special problems |
| ○ Delay other foods until the infant is 3–6 mo old |
| ○ Do not add salt or sugar to the infant's food” (p 4). |
| 3rd edition DGA (1990)3 |
| • “The guidelines in this bulletin are not intended for infants. Food and nutrient needs of infants are different from those of older children and adults.” (p 7) |
| • “Mother's milk is the best food for nearly all infants. It contains the ideal balance of nutrients and other substances to promote growth. It also transfers immunity to some diseases from the mother to the infant.” (p 7). |
| • “To help prevent tooth decay in newly growing teeth, infants should not use as pacifiers nursing bottles containing any beverage other than water.” |
| • “Babies are generally not given solid foods until they are 4–6 months old. The foods are introduced gradually—no more than one new food each week. The doctor should advise on how to get adequate iron into the baby's diet.” (p 7). |
| • “Salt and sugar should not be added to an infant's food; they are not needed as inducements to eat.” (p 7). |
| • The DGAC recognized some specific needs to be addressed by the DGA including the need to “give attention to special guidance for groups within the population such as infants, children, and elderly persons” (p 15). |
| • With regard to the audience for the DGA: “Children under 2 years of age are specifically excluded as users of these guidelines because their nutritional needs and dietary patterns differ from those of older children and adults” (p 20). |
| • “Infants. The Committee specifically excluded children under 2 years of age in developing these guidelines because their nutritional needs and eating patterns differ from those of older children and adults. However, the Committee wanted to include some dietary advice for this important part of the population. The text for the variety guideline seemed the best place for the advice. It appears near the front of the bulletin and earlier editions included some advice about diets of infants here. Also, the concepts in this guideline—meeting nutritional needs and consuming a variety of foods—are the main factors that distinguish the infant's diet from diets of older people” (p 22). |
| • “The brief advice on infants’ diets included is consistent with advice from the AAP and the AAPD.” (p 22). |
| • “A caution about using bottles as pacifiers was added because of the increased risk for tooth decay. Infants who use nursing bottles containing milk, formula, or juice as pacifiers and who breast feed on demand at times other than normal feedings and throughout the evening often develop early, multiple caries lesions. The AAPD believes that a meaningful portion of the caries observed in young children (12–24 mo of age) is traceable to such practices. The infant's need for adequate iron from foods and possibly a supplement as advised by a physician is emphasized.” (p 22). |
| • With regard to the use of dietary guideline regarding fat: “The Committee notes that this guideline is not for children under 2 years, recognizing that a few over-zealous parents have severely limited their infants’ intakes of fat, thus causing them to fail to thrive. The AAP Committee on Nutrition recommends against fat reduction for children under 2 years of age” (p 27). |
| 4th edition DGA (1995)4 |
| • “Although limiting fat intake may help to prevent excess weight gain in children, fat should not be restricted for children younger than 2 years of age.” (p 27). |
| 7th edition DGA (2010)5: questions and answers |
| • “Why are the Dietary Guidelines only for ages 2 years and older? The DGA has always focused on adults and children 2 years of age and older. Children under 2 years of age are not included because their nutritional needs and eating patterns vary by their developmental stage and differ substantially from those of older children and adults. A separate committee for reviewing nutrition and physical activity needs of pregnant women and children from birth to 2 years old could be beneficial as it would be made up of scientists and nutrition professionals who are experts in those very specialized topic areas of infant development and infant feeding practices.” |
AAP, American Academy of Pediatrics; AAPD, American Academy of Pediatric Dentistry; DGA, Dietary Guidelines for Americans; DGAC, Dietary Guidelines Advisory Committee.
US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 1985. Washington DC: US Department of Health and Human Services, US Department of Agriculture, 1985.
US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 1990. Washington DC: US Department of Health and Human Services, US Department of Agriculture, 1990.
US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 1995. Washington DC: US Department of Health and Human Services, US Department of Agriculture, 1995.
US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington DC: US Government Printing Office, 2010.
Because of the importance of nutrition to infant and young child growth, development, and health, this developmental period has emerged as a focal point for both domestic and international attention via programs such as Feed the Future (2) and other efforts to address the “1000 days” covering pregnancy through the first 2 y of life (3). The priority placed on this period was exemplified by the following statement by former Secretary of State Hillary R Clinton (4), who noted that the nutritional interventions “have the biggest impact when they occur during the first 1000 days of a child's existence. That begins with pregnancy and continues through a child's second birthday. Interventions after that second birthday make a difference, but often cannot undo the damage that was done because of the under-nutrition during the first 1000 days. So we can be very targeted with our investments to save and improve the greatest number of lives.” Despite the prominence and significance of the 1000 d in the overall development, a conundrum is presented by the lack of national guidance for infants and children from birth to 24 mo. Thus, it becomes a challenge to develop and implement US-driven, evidence-based health and nutrition promotion programs focused on this group in other settings.
Aside from the integral and inextricable role of nutrition in all aspects of human growth, development, and health, an additional driver for much of this attention has been the emerging evidence attesting to the importance of early-life exposures to long-term health and disease prevention. Stimulated to a great extent by the early work of David Barker (5), this theory has now given birth to a major area of research focus, referred to as Developmental Origins of Health and Disease (DOHaD), and has been expanded to include a range of conditions from obesity (6) to cardiovascular risk (7) and cancer. Clearly, the role of diet and nutrition in the prevention of these conditions is at the core of the DGA mission. Thus, identifying the evidence base and strategies to improve health and reduce the risk of these diseases as early in life as feasible is increasingly important.
As noted above, the past DGA have alluded to the birth-to–24-mo age group (Table 1). Although most of those references were limited to the exclusion of this group, limited advice was offered in some editions. For example, the fourth edition (1995) noted that guidance to limit total fat intake did not apply to children under the age of 2 y. Throughout its history, there was always recognition of the special needs of this group. A major impediment to inclusion in a comprehensive manner was the limited evidence to support specific guidance. As a result of the burgeoning body of new evidence about diet and health in this age group, the time may be right to consider including infants and children <2 y in the DGA. The challenge is to identify questions for which this new evidence would be sufficient to support a rigorous evaluation.
The confluence of increased public/global health attention and the emerging evidence has created an opportunity to more fully explore what we know and do not know about the importance of diet and nutrition during the first 24 mo of life. The DGA process is ideally suited to exploit such an opportunity. As a result of the efforts within the partner agencies to explore how best to proceed, the DHHS Office of Disease Prevention and Health Promotion and the USDA Center for Nutrition Program and Policy initiated the project entitled “Evaluating the evidence base to support the inclusion of infants and children from birth to 24 months of age in the Dietary Guidelines for Americans—the B-24 Project” that is the focus of this article. The intent is to use this project as a scientific foundation to support the inclusion of infants and children from birth to 24 mo in future iterations of the DGA beginning with the 2020 edition.
PROJECT DESCRIPTION
The goal of the B-24 Project is to inform the process that will eventually lead to the integration of infants and children from birth to 24 mo into future iterations of the DGA. This phase of the process is intended to identify the key topics and questions that will eventually be the focus of systematic reviews conducted by the USDA Nutrition Evidence Library (NEL). As highlighted in Supplemental Table 1 (under “Supplemental data” in the online issue), the application of the systematic review process by the NEL is still relatively new. The topics and systematic review questions identified as part of this project will serve as the basis for future phases of the NEL's DGA efforts, including the conduct of systematic reviews to inform the development of federal dietary guidance for infants and children from birth to 24 mo. The B-24 Project represents the first time the NEL has used a topic nomination and refinement process involving the input from technical working groups (WGs). The description of the NEL process is included in this supplement issue (8).
This first phase of the project was designed to better understand the nature of evidence available to inform eventual birth-to–24-mo dietary guidance. Concerns have been raised both in terms of the quantity [ie, number of well-designed randomized controlled trials (RCTs)] and quality (eg, inclusion of relevant variables) of data that can be evaluated systematically (9, 10). Consequently, part of the B-24 process included an effort to capture an appreciation of all the relevant types of data that might be of use to the NEL as well as to itemize data needs, both in terms of the ability to exploit more fruitfully existing data sources and the identification of new types of data not currently available.
An additional and critical element of the B-24 Project is the development of a targeted research agenda to address outstanding gaps in our understanding of diet and health relations as they pertain to this population group. Because the DGA are targeted public health messages, they are focused at a population level. Clearly, there is a range of important issues relevant to the nutritional care and management of individual infants and children. Although the DGA is not intended to address such issues, the B-24 Project was designed to capture those issues both in terms of the research needs and those for which there is sufficient evidence to support systematic reviews by agencies/organizations with a more clinical focus. Such agencies might include the Academy of Nutrition and Dietetics and the American Academy of Pediatrics (AAP). Moreover, because of the universal need for evidence-based guidelines, a need exists to explore many of these same issues in support of the development of guidelines for global health. As the lead agency for development of global health guidance, the WHO was also included as part of this first phase of the B-24 process.
STRUCTURE
The B-24 Project was organized to maximize input from key stakeholders. To accomplish this goal, a 3-tiered committee structure was developed. A conceptualization of the working relations between these 3 entities is provided in Figure 1.
FIGURE 1.
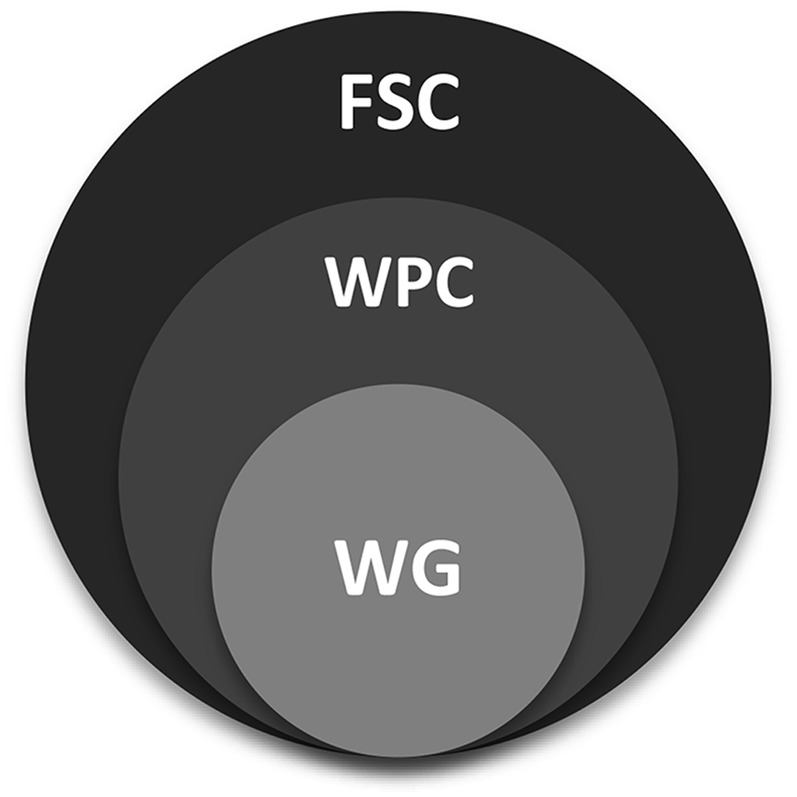
B-24 committee structure. FSC, Federal Steering Committee; WG, working group; WPC, Workshop Planning Committee.
PROCESS
The steps involved in the implementation of the B-24 process and the committee descriptions are outlined in Table 2. In addition to the recruitment of the 3 core groups, the process included a series of meetings and conference calls with a core Federal Steering Committee (FSC) to ensure that the process stayed on track and met the needs of the partner agencies. To support the work of the thematic WG, 2 technical workshops were held. The first, the “All Hands Meeting,” was intended to bring all of the B-24 participants together and to ensure clarity of the mission and to establish a time frame for actualizing the project plan. After a series of conference calls with each WG, a second content-focused “B-24 Prime” workshop was held.
TABLE 2.
The B-24 process1
| • FSC: Identify members from relevant agencies within the DHHS/USDA. This included a “core FSC” with representation from the DHHS/USDA responsible for actual implementation of the B-24 plan. |
| • WPC: The FSC identified candidates for the chair and membership to represent the relevant content areas and agency needs regarding DGA development. |
| • WG: The WPC in consultation with the FSC identified candidates for WG chairs. |
| • WG chairs: Identified candidates for their respective WGs. |
| • NICHD Secretariat in consultation with FSC, WPC, and WG chairs recruited WG members. |
| • October 2012: “All Hands Meeting” to ensure all participants were clear about the goals of the B-24 and the NEL methodologies. |
| • WG conference calls: Designed to coalesce the list of topics that could be supported by the NEL systematic review process, data needs, and research gaps for each WG. |
| • February 2013: “B-24 Prime” workshop to |
| ○ Allow each WG the opportunity to hear the results of the deliberations of the other WGs to identify “cross-cutting” issues and avoid overlap |
| ○ Bring in additional expertise to address specific content areas identified by each WG needing additional coverage and critical cross-cutting issues affecting more than one group (eg, food allergies) |
DGA, Dietary Guidelines for Americans; DHHS, Department of Health and Human Services; FSC, Federal Steering Committee; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; NEL, Nutrition Evidence Library; WG, working group; WPC, Workshop Planning Committee.
NEXT STEPS
The process described above constitutes the first phase of a larger project designed to develop dietary guidance for the birth−24-mo population. The ultimate goal is for the 2020 edition of the DGA to provide recommendations for the entire American population, including the birth-to–24-mo age group. The next steps for this project include the following (Figure 2):
FIGURE 2.

Process toward developing dietary guidelines for birth to 24 mo. DGA, Dietary Guidelines for Americans; DGAC, Dietary Guidelines Advisory Committee.
A 2-y interim period: Phase I will be followed by a period of time for USDA's NEL to further refine questions and plans for systematic reviews. It is anticipated that there will be approximately a 2-y break, during which time the 2015 Dietary Guidelines will be developed for the population aged ≥2 y. This 2-y gap will allow researchers time to identify high-priority topics for rapid publication, particularly if they have ongoing projects relevant to the priority research needs for the 0–24-mo age group. The hiatus will also allow time to collect new data or analyze the preexistent data to inform the impending dietary guidelines. This would expand the research base to inform future systematic reviews and guidance development.
Phase II is expected to begin in 2014 and will include conducting systematic reviews by the NEL with the use of the existing and new data identified with the support of an expert federal scientific panel. These systematic reviews and data analyses will be derived from the topic briefs developed during phase I, taking into consideration evolution in the science and policy needs.
Phase III will be the development of unified dietary guidance for the B-24 population, with the goal of releasing the guidance in 2018 for consideration by the experts convened to develop the 2020 DGA in early 2018. Federal agencies would be able to use the final policy document for their programs, once it has been approved by the USDA and DHHS and is publicly available.
Phase IV, the policy document will be provided to the 2020 Dietary Guidelines Advisory Committee for their use in incorporating the B-24 population into the 2020 Dietary Guidelines Advisory Committee report. The process will be transparent, and public input will be collected and considered throughout this phase of the process.
SUMMARY OF B-24 PRIME
The goals of the B-24 Prime meeting were to provide additional input from outside experts identified by each WG, provide additional expertise to all WGs with regard to specific “cross-cutting” issues, provide an opportunity for the WGs to interact to ensure full coverage and avoid overlap, and to finalize the process for completing the WG report. The intent was to be as interactive as possible and included several opportunities for the WGs to share their work and interact to ensure the fullest coverage of the priority areas.
The B-24 Prime meeting opened with a series of presentations by representatives from all participating agencies on agency-specific needs with regard to the DGA. The agenda moved to sessions focused on both the content-specific needs of each WG interspersed with presentations on the following 5 critical cross-cutting issues:
The state of the science and policy with regard to prevention and treatment of food allergies in infants and young children (11)
An overview of the current evidence with regard to the impact of infant feeding on the development of the human microbiome and the implications of these relations for growth and development (12)
Factors influencing the ontogeny of taste preferences, both in terms of the biology and implications for health (13)
The current thinking about diet and developmental origins of obesity and long-term health outcomes
Factors affecting the development of eating behavior (14)
The content-specific subjects selected by the individual WG included the following:
WG 1 (0–6 mo): Infant formula and infant nutrition, including coverage of what is known about bioactive components of human milk and implications for composition of infant formulas (15).
WG 2 (6–12 mo): Current knowledge about nutrition, metabolism, and growth, particularly with regard to protein needs and long-term health (16).
WG 3 (12–24 mo): Developmental aspects and measurement of physical activity (17). An overview of knowledge gained from the Feeding Infants and Toddlers Study (FITS) was provided by Anna-Maria Siega-Riz. This presentation included a summary of several published reports on the analysis of the FITS data (18–21).
WG 4 (caregivers: mothers and others): The impact of maternal diet on human milk composition and neurologic development of infants (22). Kelley Scanlon provided a summary of the Food and Drug Administration/CDC Infant Feeding Practices Study (IFPS) (23).
In addition to the WG-specific topic session, a session was included to address issues pertaining to the currently available data/analyses regarding infant and toddler diet, including the contribution of the national nutrition monitoring system (eg, NHANES) (24) and a summary of recent analyses of the USDA programs addressing infant and young child feeding (25).
Finally, the participants were provided with a summary of the approaches used by the Institute of Medicine (IOM)/Food and Nutrition Board to develop Dietary Reference Intake (DRI) recommendations in the face of limited data specific to the B-24 target populations. Linda Meyers, former Director of the Food and Nutrition Board, provided an overview of the DRI process, including a brief discussion of some of the challenges specific to infants and children <2 y of age.
Throughout the meeting, participants were provided with opportunities to interact and discuss the content presentations and identify issues of common concern. Some of the overarching priority data needs identified during this meeting are outlined in Table 3.
TABLE 3.
Data and research needs for supporting guidelines for infants from birth to 24 mo
| • Human milk composition: need for up-to-date analyses of human milk across populations including |
| ○ Nutrients |
| ○ Bioactive components of human milk |
| • Nutrient specification for infant formulas: the need to update is driven by new information about human milk composition |
| • Factors affecting the ontogeny of the gut miocrobiome: need for expanded understanding of its impact on nutrition and role in human health and development |
| • Dietary patterns of infants >6 mo: the need for expanded understanding of |
| ○ Optimal duration of exclusive breastfeeding (is the 6-mo “line in the sand” justified?) |
| ○ Duration of breastfeeding (is there benefit of extended breastfeeding in the United States?) |
| ○ Timing and composition of complementary foods |
| ○ Timing of introduction of allergens (is earlier better?) |
| • Role of maternal nutrition and health on successful lactation initiation and performance |
| ○ Dietary factors influencing human milk composition |
| ○ Impact of body composition on breastfeeding initiation, duration |
| • Social/behavioral context influencing infant feeding choice |
These proceedings include the summary reports from each of the 4 thematic WGs and manuscripts of papers presented at the B-24 Prime meeting and included in this supplement.
WG REPORTS
Each WG was asked to develop a set of topics, specific questions that might be the focus of NEL systematic reviews, priority data needs (that could be derived either from existing data sets, eg, NHANES, or met with new data/surveillance), and research priorities. The charge to the WGs and their products was developed based on instructions from the NEL (8). The WGs used the template found in Figure 3 to summarize their work relative to each identified topic.
FIGURE 3.
Template for working group topics. PICO, Population, Intervention/Exposure, Comparator Outcomes.
The full reports from the WGs are available at the NEL website (26). WG, FSC, and Workshop Planning Committee rosters are found in Supplemental Table 2 (under “Supplemental data” in the online issue). Selected references identified by the WG for each topic are listed by WG in Supplemental Table 3 (under “Supplemental data” in the online issue).
The WG summaries include priority topics with a brief rationale, associated questions that might be addressed via systematic reviews, and research priorities.
In terms of the presentation of these summaries it should be noted that 1) the topics are not listed in any particular order of priority and 2) the WGs recognized that the line of demarcation between these groups was somewhat arbitrarily based on age and current guidance with regard to diet (eg, 6 mo of “exclusive” human milk/formula feeding, mixed feeding through 12 mo, and the transition from human milk/formula by 24 mo). In general, the WGs all identified topics that fall in the categories of biology and context (including social/behavioral factors).
The WGs also acknowledge a number of “cross-cutting issues” particularly regarding the role of the caregivers across the 3 age groups. Consequently, there are several of these cross-cutting issues that are addressed both within specific groups and across groups. These summaries reflect the WGs best efforts to capture the most important issues in support of the NEL and the DGA process.
WG 1: INFANCY—PERIOD OF SOLE NUTRIENT SOURCE FEEDING (0–6 mo)
Chair
Susan Baker, University of Buffalo
WG members
Leila Beker, Food and Drug Administration
Teresa Davis, Baylor College of Medicine, USDA
Kirsi Jarvinen Seppo, Albany Medical Center
Shannon Kelleher, Pennsylvania State University
Rafael Pérez-Escamilla, Yale University
Topics
1) Duration of exclusive breastfeeding
2) Relation between breast-milk composition and infant health outcomes
3) Delivery mechanism for human milk
4) Micronutrient supplements for breastfed infants (iron, zinc, vitamin D, fluoride)
5) Maternal diet and allergy risk
6) Introduction of complementary and transitional foods into the diets of infants/toddlers in those at high risk of allergic disease
7) Infant formula: which type of formula or formulas are comparable to human milk in terms of health outcomes in healthy infants
8) Health outcomes in formula-fed compared with breastfed infants
9) Type (eg, suckled, fresh, stored, banked) of human milk consumed
10) Factors influencing infant appetite
11) Infant microbiome
1. Duration of exclusive breastfeeding
Rationale
Current US and global recommendations are based largely on the WHO's 2002 systematic review “The optimal duration of exclusive breastfeeding” (27). However, studies published since the WHO review suggest that this recommendation should be reevaluated in light of concerns regarding nutritional status, food allergies, iron deficiency, and celiac disease related to delayed introduction of complementary foods.
Suggested systematic review questions
What is the optimal duration of exclusive breastfeeding for promoting appropriate nutritional status?
What is the optimal duration of exclusive breastfeeding for promoting appropriate growth and development?
What is the optimal duration of exclusive breastfeeding for promoting appropriate cognitive, behavioral, and neuromotor development?
What is the optimal duration of exclusive breastfeeding for preventing food allergies and asthma?
What is the optimal duration of exclusive breastfeeding for promoting long-term health outcomes [eg, cardiovascular disease (CVD), hypertension, diabetes—types 1 and 2, obesity, inflammatory bowel disease]?
Data and research priorities
Data on nutrient and bioactive components of human milk, particularly as these reflect changes in dietary patterns in the United States and globally
Definition and prevalence of “exclusive breastfeeding.” Some research makes very clear what definition was used, and others do not. In addition, “exclusive” did not always mean that the infant received only human milk from his/her mother
Prevalence of different modes of infant feeding, ie, who is feeding “at the breast” compared with via bottle (pumped fresh expressed human milk, stored mother's human milk, banked human milk, or any combination thereof)
Relative differences between growth standards for assessing the impact of feeding practices on infants
Impact of timing of blood sampling on nutrient concentrations relative to feeding, eg, the timing of the blood draw may matter, or the last feeding may be what is reflected in the concentrations found
Impact of stage of development on nutrient assessment methodologies
Factors that affect the definition, prevalence, and effect of food “allergies” compared with “sensitivities”
Best methods for defining/confirming “allergy”
Prevalence of infants/toddlers with confirmed allergy (ie, by appropriate testing) compared with self-reported allergy, and comparisons based on method of feeding
2. Relation between breast-milk composition and infant health outcomes
Rationale
Human milk is a complex, biologically active fluid that changes throughout the course of a feeding and over the course of lactation. Therefore, it is important to understand the specific factors in human milk that either positively or negatively affect infant health outcomes.
Suggested systematic review questions
What factors in human milk affect an infant's
growth, body composition, and physical development;
cognitive, behavioral, and neuromotor development;
intake self-regulation; and
immune function, morbidity/mortality?
Data and research priorities
Best methods (biomarkers, intake estimation, etc) for assessing the impact of maternal diet on nutrient/bioactive components in human milk
Data to estimate dietary intake patterns of lactating women in the United States and globally including dietary supplement/herbal/botanical use
Confirmation of the role of confounders affecting analysis/composition of human milk including time of day of milk collection, time since last (mother's) meal, method of milk collection (one or both breasts, full or partial collection, pump or hand expression), stage of lactation, extent of breastfeeding (exclusive or predominant), and milk storage
Impact of storage method on human milk composition, hedonics
Differences in composition of banked human milk from single (mother) or multiple sources
Impact of infant on milk composition (eg, infant sex, parity, number of infants/children nursing)
3. Delivery mechanism for human milk
Rationale
Many infants fed human milk in the United States consume expressed human milk by bottle. Numerous studies have shown protective effects of human milk when compared with formula feeding, but these have not distinguished the potential effects of mode of milk delivery on outcomes. For example, not only may differences in the composition of the milk (breast compared with formula) influence outcomes such as growth but the potential for caregiver to influence volume of consumption (bottle compared with breast) may also have an effect. Moreover, bottle feeding, regardless of type of milk, may have an effect on an infant's ability to self-regulate milk intake. A recent small study by Bartok (28) suggested that the delivery method for human milk may not affect growth for the first 4 mo of life but may increase weight velocity from 4 to 6 mo. With the use of data from the IFPS II, Li et al (29) reported that weight gain during infancy was greater in infants who received a larger proportion of human milk from a bottle than from the breast. Further analysis from IFPS II showed that bottle feeding early in life, regardless of milk type, was associated with increased intake in late infancy compared with those fed directly at the breast.
Suggested systematic review questions
The WG concluded that insufficient evidence is currently available to support any systematic reviews at this time.
Data and research priorities
The relative impact of human milk consumed via the breast compared with a bottle on an infant's
growth and development;
cognitive, behavioral, and neuromotor development;
oral health; and
long-term outcomes (eg, CVD, hypertension, diabetes—types 1 and 2, obesity, inflammatory bowel disease).
4. Micronutrient supplements for breastfed infants (iron, zinc, vitamin D, fluoride)
Rationale
Micronutrient supplements are routinely prescribed for breastfed infants. Are they necessary, and if so, when, and what is the effective dose, duration, and timing? What are the determinants of the need for supplementation and what are the criteria for efficacy?
Suggested systematic review questions
What is the impact of specific micronutrient supplements (iron, zinc, vitamin D, and fluoride) for breastfed infants on
physical growth;
cognitive, behavioral, and neuromotor development; and
relevant health outcomes (eg, iron and anemia)?
Data and research priorities
Prevalence of maternal supplement use in the United States and globally and impact on human milk composition
Impact of maternal iron supplementation on infant status including potential interactions with other micronutrients (eg, zinc, vitamin A, and folate)
Prevalence of dietary supplement use in infants 0–6 mo, including relative use in breastfed compared with formula-fed infants
Prevalence of micronutrient malnutrition (over-/or undernutrition) in 0- to 6-mo-old infants in the United States
-
Specific priority nutrients include
○ relative impact of iron form, dose, duration, and compliance on infant iron status
○ prevalence of zinc supplement use in mothers and potential impact on maternal status
○ better biomarkers for assessing zinc status in this age group
○ prevalence of vitamin D use and impact on maternal and infant health
○ better data on primary vitamin D exposure scenarios (diet, sunlight) and impact on maternal and infant health, including impact of dose, and timing
○ data on intake of “dietary” fluoride (water, toothpaste) and potential health implications
5. Maternal diet and allergy risk
Rationale
Maternal diet during breastfeeding has been implicated in potential increased risk of infant allergy, celiac disease, milk “sensitivities” (ie, not true allergies), and infant colic. Infant exposure to potential allergens through human milk has been the focus of a number of recent studies (30–32). Sufficient concern has been raised to warrant a closer evaluation of these potential relations.
Suggested systematic review questions
-
What is the relation between maternal diet during pregnancy and risk of infant
○ allergy and asthma,
○ celiac disease,
○ milk “sensitivities,” and
○ colic?
-
What is the relation between maternal diet during lactation and risk of infant
○ allergy and asthma,
○ celiac disease,
○ milk “sensitivities,” and
○ colic?
Data and research priorities
Relative risk of maternal diet in nonatopic compared with high-risk atopic families
Prevalence of food allergy compared with food “sensitivity”
Best methods for distinguishing between allergic response and sensitivity; the former is a clinical diagnosis based on a double-blind placebo-controlled food challenge, whereas food sensitivity is assessed by skin-prick testing or serum-specific IgE concentrations
Relative impact of maternal diet on infant asthma or atopic dermatitis (atopic eczema)
6. Introduction of complementary and transitional foods into the diets of infants/toddlers in those at high risk of allergic disease
Rationale
The issue of timing of exposure to food allergens is a core component of questions regarding guidance about infant and young child feeding. The area has been the subject of new studies and reviews (33–35) that reflect a changing approach to this question, eg, in most patients no delay in introduction is necessary for many of these putative food allergens, and early introduction may prevent food allergy. Consequently, the WG deemed this an important area for targeted systematic reviews.
Suggested systematic review questions
-
What is the relation between timing of introduction of complementary foods and the development of
○ food allergies and asthma;
○ immune system function, infection, or inflammation;
○ atopic dermatitis;
○ obesity;
○ types 1 and 2 diabetes; and
○ celiac disease among infants and toddlers?
-
What is the relation between consuming highly allergenic complementary foods and the development of
○ food allergies and asthma;
○ immune system function, infection, or inflammation;
○ atopic dermatitis;
○ obesity;
○ types 1 and 2 diabetes; and
○ celiac disease among infants and toddlers?
Data and research priorities
Criteria for definition of high risk (most commonly having a first-degree relative with an atopic disease)
Better understanding of the role of confounders in both experimental design and evaluation of outcomes: for example, reverse causation in which atopic families delay introduction of complementary foods, therefore creating the appearance that delaying introduction causes allergy
Need well-designed RCTs
Data are needed on the prevalence of relevant practices regarding timing of introduction of suspect foods
7. Infant formula: which types of formula or formulas are comparable to human milk in terms of health outcomes in healthy infants?
Rationale
Notwithstanding the universal recognition of human milk as the “gold standard” for infant feeding, infant formulas continue to play a large role in infant feeding in the United States. Commercially available infant formulas have a remarkable record of safely providing adequate nutrition for normal growth and development. The ongoing effort to create a food that mimics the composition of human milk has resulted in periodic additions of new components. These changes demand ongoing vigilance to ensure continued safety and efficacy. Areas of continued interest include the nature and effect of available protein sources, eg, cow-milk (casein/whey) or soy based, and addition of ingredients such as specific long-chain PUFAs (LC-PUFAs) and other bioactive components, including pre- and probiotics. Further knowledge is needed about the best formulation to inform recommendations on what to feed non- or partially breastfed infants.
Suggested systematic review questions
Which type of infant formula results in similar risk of food allergies and asthma between formula-fed infants and breastfed infants?
Which type of infant formula results in similar risk of diabetes mellitus between formula-fed infants and breastfed infants?
Which type of infant formula results in similar diet quality between formula-fed infants and breastfed infants?
Data and research priorities
Improved data on composition of currently available commercial formulas
Specific data are needed to define “extensively” or “partially” hydrolyzed formula
Better data on physicochemical properties of components of infant formula are needed to better evaluate the biological impact and to address issues such as potential allergenicity
Impact of other components in human milk on protein metabolism to provide better context for evaluating this issue with regard to infant formula matrix
Estimates of the exposure of infants to the range of new ingredients that have been added to infant formulas in recent years, eg, LC-PUFAs, and comparison to intakes in breastfed infants
8. Health outcomes in formula-fed compared with breastfed infants
Rationale
Short-, medium-, and long-term health outcomes are likely linked to different infant feeding practices (exclusive breastfeeding, exclusive formula feeding, mixed feeding, etc). It is crucial to understand the relation between infant feeding practices and obesity and related chronic diseases. Focal points include the influence of infant feeding mode or modes on infant body composition and the immune system (eg, inflammation). Other key health outcomes might include the impact of infant feeding mode on oral health.
Suggested systematic review questions
What is the relation between infant feeding practices (ie, exclusive breastfeeding, exclusive formula feeding) and cognitive, behavioral, or neuromotor development?
What is the relation between infant feeding practices (ie, exclusive breastfeeding, exclusive formula feeding) and immune system development and function, infection, or inflammation?
What is the relation between infant feeding practices (ie, exclusive breastfeeding, exclusive formula feeding) and overweight/obesity?
Data and research priorities (in addition to the priorities highlighted in no. 8 above)
Need to standardize definitions of different infant feeding modes
Prevalence and type of mixed feeding during ages 0–6 mo in the United States
Impact of mixed feeding during ages 0–6 mo (solids, water, and either human milk or infant formula) on health outcomes
Need for good prospective cohort studies, because RCTs are unethical in this area
Need for data on household socioeconomic status and maternal education, because they are critical distal confounders
Need to have a better understanding of the role of maternal lifestyles and quality of caregiving as intermediate confounders
Need for better data on type of formula, quality, and amount of complementary foods and drinks, as well as an infant's physical activity patterns because each is likely to be a key proximal determinant or effect modifier
9. Type of human milk consumed
Rationale
Numerous modes exist for feeding human milk including directly suckled from the breast, freshly expressed via a bottle, stored mother's milk, banked human milk, or any combination thereof. It is therefore important to assess the impact of changes in composition (ie, nutritional and immunologic content) (36) and hedonics (taste and/or smell) (37) over time (eg, during a feeding, over a day or longer, consequent to these different modes of feeding/storage).
Suggested systematic review questions
The WG concluded that insufficient data exist to support any systematic reviews at this time.
Data and research priorities
Do different human milk storage methods result in differences in the composition and/or taste of different types of human milk (ie, milk suckled directly from the mother's breasts, freshly expressed human milk, stored mother's human milk, banked human milk, or any combination thereof)?
Are there differences in the composition and/or taste of different types of human milk (ie, milk suckled directly from the mother's breasts, freshly expressed human milk, stored mother's human milk, banked human milk, or any combination thereof)?
Are there differences in changes over time in the composition and/or taste of different types of human milk (ie, milk suckled directly from the mother's breasts, freshly expressed human milk, stored mother's human milk, banked human milk, or any combination thereof)?
Are there differences in infant health outcomes when different types of human milk are consumed, including 1) immune system development and function, infection, or inflammation; 2) growth and physical development; 3) cognitive, behavioral, and neuromotor development; and 4) weight status (overweight/ obesity)?
10. Factors influencing infant appetite
Rationale
Infant appetite is likely affected by the method of feeding, the nutrient content of the food (eg, tryptophan may make an infant sleepy and thus lessen interest in feeding) (38); free glutamate may be associated with early satiety (39), timing of feedings, and the hormone profile of the infant (40). Therefore, an understanding of appetite regulation and factors that can alter that regulation are important because they affect the immediate health of the infant and may affect long-term health outcomes.
Suggested systematic review questions
How is infant appetite regulated?
What infant cues of developmental readiness for complementary foods should be used to determine timing of introduction of complementary foods?
What factors in the infant diet and environment affect appetite regulation in infants?
What is the impact of appetite regulation on infant health, including growth and physical development, and cognitive, behavioral, and neuromotor development?
Data and research priorities
Biomarkers of relevant neurological function
Methodologies to distinguish between biological and environmental factors
Better appreciation of critical periods in development specific to appetite (hedonics and mechanics of eating)
11. Infant microbiome
Rationale
Study of the infant microbiome, considered to be the collective genomes and gene products of bacteria resident within and on the infant, is a new field in which information that may directly affect health is accruing rapidly (41, 42). The infant gastrointestinal tract microbiome is highly variable and can be influenced by mode of delivery, type of feeding, timing, and infectious events the infant experiences. The gut microbiome may directly affect infant health by playing a role in the type of infections and in infant nutritional status. It may also have long-term effects on allergy, obesity, and chronic inflammatory diseases, among others. The key issue is to understand if or how the infant microbiome affects health outcomes in infancy and later in life and how infant feedings can be manipulated to provide desirable outcomes.
Suggested systematic review questions
The WG concluded that insufficient data exist to support any systematic reviews at this time.
Data and research priorities
-
Characterization of the nature and ontology of the gut/oral/dermal microbiome, including the following:
○ Characterization of the gut microbiome across different populations and racial-ethnic groups
○ Relative impact of genetics compared with environment
-
Characterization of the bidirectional relations between diet/specific nutrients and the gut microbiome, such as the following:
○ What is the role of diet/specific nutrients in the ontogeny of the gut microbiome?
○ What is the impact of the gut microbiome on nutrient absorption and metabolism?
How does the infant gut microbiome affect the development of food allergies and asthma?
What is the contribution of the gut microbiome to the metabolome, and what is the potential for this relation in terms of biomarkers of nutrition exposure, status, and function?
How does the infant gut microbiome affect the development of the immune system, infection, or inflammation?
How does the infant gut microbiome affect the development of overweight and/or obesity and other chronic noncommunicable diseases?
How can infant feeding practices be manipulated to affect the infant gut microbiome in such a way as to improve health outcomes?
WG 2: INFANCY—PERIOD OF COMPLEMENTARY FEEDING (FOCUS: 6–12 mo)
Chair
Frank Greer, University of Wisconsin
WG members
Ronette Briefel, Mathematica Policy Research
Jatinder Bhatia, Georgia Health Sciences University
Kay Dewey, University of California, Davis
Nancy Krebs, University of Colorado
Julie Mennella, Monell Center
Kelley Scanlon, Centers for Disease Control and Prevention
Topics
-
1) The impact of differences in protein intake in infants in the first 12 mo of life, including
○ total amount and source (human milk compared with protein sources in available infant formula options) of protein consumed
○ the timing and duration of exposure to various protein sources during the first year of life
2) The role of beverages (including fruit juices and sugar-sweetened beverages) in complementary feeding between 6 and 12 mo
3) Can fluid cow milk be introduced before 12 mo of age?
4) Micronutrients of concern: iron, zinc, vitamin D, LC-PUFAs, fluoride, and vitamin B-12
5) Appropriate complementary food choices for human milk–, formula-, or mixed-fed infants from a macro- and micronutrient standpoint
6) Early dietary influences on food and flavor preferences, especially for nutrient-dense foods (fruit, vegetables, meat, dairy, etc)—What are the evidence-based strategies to enhance acceptance of nutrient-dense foods such as fruit and vegetables?
7) Development of taste preferences for salt and sweet in infants and the impact on dietary intake and long-term health outcomes—How do preferences for foods with added salt and sugars develop?
8) The role, timing, and value of snacking (ie, food consumed between meals)
9) Method or methods of complementary feeding
10) Physical activity in prevention of childhood obesity
11) Impact of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) food package on infant and child diet
1. The impact of differences in protein intake in infants in the first 12 mo of life
The scope and focus of this topic might include the following:
Total amount and source (human milk compared with protein sources in available infant formula options) of protein consumed
The timing and duration of exposure to various protein sources during the first year of life
Rationale
Available data indicate that formula-fed infants have a higher intake of protein compared with infants fed mature human milk (ie, past the period of colostrum consumption) (43). In addition to the consideration of total protein, the source of protein (human milk, cow milk, soy, hydrolyzed proteins), particularly as it pertains to the amino acid profiles and relative amounts of free amino acids (FAAs) of available protein sources, is critical in evaluating the needs of infants through the first 12 mo of life. Differences in total protein, FAAs, and other factors such as peptides associated with hydrolyzed protein formulas, as well as the standard used to evaluate protein quality (the so-called protein efficiency ratio or PER), have been comprehensively reviewed (44). However, the impact of increased FAA intake on satiety and growth is still poorly understood. Higher protein intake has been shown to result in increased weight gain in formula-fed compared with human milk–fed infants (45). The relative impact of differences in protein intake in formula-fed infants compared with human milk–fed infants continues to be an open question particularly with regard to the potential differences in physical growth including risk of overweight and obesity, neurologic development, and renal solute load.
Suggested systematic review questions
-
What is the association between relatively high intakes of protein in infant formula compared with lower protein intakes of human milk on
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development;
○ overweight/obesity;
○ renal function; and
○ serum insulin-like growth factor I (IGF-I) and insulin concentrations?
-
What is the association between protein intake and
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development;
○ overweight/obesity;
○ renal function and solute load; and
○ serum IGF-I and insulin concentrations?
Data and research priorities
What is the impact of hydrolyzed formulas on cognitive, behavioral, or neuromotor development?
What is the impact of hydrolyzed formulas on long-term health outcomes including overweight and obesity?
How do concentrations of IGF-I or insulin in human milk–fed infants compare with those in infant formulas including those that are extensively hydrolyzed?
2. The role of beverages (including fruit juices and sugar-sweetened beverages) in complementary feeding between 6 and 12 mo
Rationale
The current WIC food packages do not contain any fruit juices for infants. The AAP recommends no juice before 6 mo of age, introduction into the diet only when the infant is drinking from a cup, and limited to 4–6 ounces/d. Fruit juice is not the equivalent to fresh fruit and should not take its place in the diet (46). Type of juice may also be important; eg, apple juice is low in folate (47). Sugary drinks such as soda should be avoided because they are nutrient poor and decrease the appetite for other, more nutrient-dense, foods. Despite these admonitions, the limited available data indicate that infants and toddlers in the United States are consuming these beverages at rates that could be of potential concern (48). In addition, the AAP (Pediatric Nutrition Handbook) notes that there is little indication for water except to mix with infant formula; thus, any additional water requirement for infants in this age group is a subject for research.
Suggested systematic review questions
-
What is the relation between beverage consumption (including fruit juices and sugar-sweetened beverages) between 6 and 12 mo of age and
○ growth and physical development,
○ oral health,
○ overweight/obesity, and
○ impact on achieving recommended dietary intake and/or diet quality?
Data and research priorities
What is the range of water intake seen in infants in the United States?
What is the prevalence of intakes of different beverages in infants aged 6–12 mo in the United States?
How much water intake is needed between 6 and 12 mo of age?
-
What is the impact of water intake on outcomes, such as
○ growth and physical development,
○ oral health,
○ hyponatremia,
○ overweight/obesity, and
○ achieving recommended dietary intake and/or diet quality?
3. Can fluid cow milk be introduced before 12 mo of age?
Rationale
Current guidance recommends against introducing fluid cow milk before 12 mo of age (49), and the current WIC food package does not allow the introduction of whole milk before 12 mo of age. Despite these policies, the use of cow milk as a source of nutrition for infants <12 mo continues to be prevalent (50). Factors such as economics and cultural attitude contribute to this use. Moreover, historical concerns about the safety of cow milk vis-à-vis the potential to increase gastrointestinal bleeding have been obviated by recent studies indicating that occult blood losses consequent to cow-milk consumption are minor in infants older than 6 mo of age (51). Nevertheless, questions remain about the relative safety and efficacy of cow milk, particularly in the period beyond exclusive breastfeeding and weaning (ie, between 6 mo and in cases in which infants have stopped breastfeeding before 12 mo of age).
Suggested systematic review questions
What is the relation between consumption of fluid cow milk between 6 and 12 mo of age and risk of iron deficiency or anemia?
What is the relation between consumption of fluid cow milk by infants 6 and 12 mo of age and growth and physical development?
Data and research priorities
What is the prevalence of liquid cow milk (whole milk, low-fat, or skim) consumption (as sole source of liquid or in combination with human milk) in infants aged 6–12 mo in the United States?
Do differences exist based on demographic characteristics or race-ethnicity?
-
What is the relation between consumption of liquid cow milk and
○ immune function/allergenicity,
○ gastrointestinal problems, and
○ risk of other conditions including diabetes and obesity?
Are there other components of concern in liquid cow milk for infants 6–12 mo of age?
What is the ideal time for introduction of liquid cow milk?
4. Micronutrients of concern: iron, zinc, vitamin D, LC-PUFAs, fluoride, and vitamin B-12
Rationale
The human milk concentrations of these nutrients are limited in later stages of lactation, and the quality of the other food sources assume greater significance. Currently available infant formulas serve as a good source of all of the nutrients of concern, with the exception of fluoride, which is provided primarily via the fluoride content of the water source used to prepare the formula. For infants who have been formula-fed from birth and who are continued on infant formula along with complementary food through 12 mo of age, deficiencies of these nutrients are rare. On the other hand, because human milk is a poor source of vitamin D and cannot meet the needs for iron and zinc by ∼6 mo, the infant's needs for these nutrients are a concern (52–55). Intake of complementary foods rich in iron and zinc is important for breastfed infants and even for infants fed a mixture of formula and human milk after 6 mo. A number of authoritative organizations have addressed the issue of vitamin D supplementation for infants including the American Academy of Pediatrics (56) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) (57). For infants maintained on a mixed vegetarian and human milk diet, intakes of iron, zinc, vitamin B-12, and vitamin D remain a concern (58). The question of the need for supplemental LC-PUFAs for term infants and infants older than 6 mo remains controversial (59). Finally, because the fluoride content of human milk is relatively low and is not responsive to maternal intake, breastfed infants are dependent on other sources of fluoride after 6 mo (60). On the basis of these considerations, these nutrients will require further attention in the context of dietary guidance for this age group.
Suggested systematic review questions
-
What is the relation between iron intake and/or status and infant and toddler
○ cognitive, behavioral, or neuromotor development;
○ anemia;
○ growth and physical development; and
○ immune function?
-
What is the relation between zinc intake and/or status and infant and toddler
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development; and
○ immune function?
-
What is the relation between vitamin D intake and/or status and infant and toddler
○ growth and physical development;
○ immune function; and
○ bone development?
-
What is the relation between LC-PUFA intake and/or status and infant and toddler
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development and visual acuity; and
○ immune function?
-
What is the relation between fluoride intake and/or status and infant and toddler
○ growth and physical development (bones, teeth)?
-
What is the relation between vitamin B-12 intake and/or status and infant and toddler
○ cognitive/behavioral and neuromotor development; and
○ risk of anemia?
What strategies can be used to improve dietary quality and micronutrient intake in infants 6–12 mo of age, and would different nutrients (eg, iron, fluoride) require different strategies?
Data and research priorities
-
Data are needed on the prevalence of single/multiple micronutrient deficiencies in the United States and how these prevalences may differ by
○ demographic characteristics and
○ method of feeding (human milk plus complementary foods, human milk plus other liquids plus complementary foods, formula plus complementary feeding).
Research is needed to determine how micronutrient needs can be met with foods from 6 to 12 mo of age, or whether supplements are needed.
Sensitive, specific, and noninvasive biomarkers of micronutrient status, function, and effect are needed for this age group.
Functional biomarkers are needed that reflect high-priority health outcomes and potential mechanisms of effect, including biomarkers of growth (beyond anthropometric measurements), immunocompetence, and neurologic function.
More data from RCTs are needed for this age group.
Data are needed on the relative contribution of complementary foods introduced between 4 and 6 mo on the nutrient status of exclusively breastfed infants (to 6 mo).
5. Appropriate complementary food choices for human milk–, formula-, or mixed-fed infants from a macro- and micronutrient standpoint
Rationale
The prevalence of obesity in the United States and globally is increasing at an alarming rate. The factors that contribute to this are many, but clearly, early dietary exposures and habits make up critical aspects of this scenario and affect numerous other aspects of short- and long-term health. Timing of introduction (61) and quality/type of complimentary foods introduced (62) have been scrutinized for their contributions to the obesity epidemic. The ideal distribution of daily energy intake from human milk or formula (or a combination) and complementary foods is uncertain. The appropriate amounts and choices of complementary foods are dependent on the percentage of the infant's required caloric intake and the DRIs they provide. To meet micronutrient needs, the breastfed infant is dependent on the choices of complementary foods (or supplements), whereas if a formula-fed infant continues to receive a substantial intake of formula, micronutrient inadequacy is unlikely.
Suggested systematic review questions
-
What is the optimal distribution of daily energy and macronutrient intake from complementary foods, human milk, and/or infant formula (or a combination of the 2) that promotes favorable health outcomes, such as
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development;
○ prevention of food allergies and asthma; and
○ prevention of overweight/obesity?
-
What types and amounts of complementary foods are necessary for infants fed human milk, formula, or mixed feedings to promote favorable health outcomes, such as
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development;
○ prevention of food allergies and asthma; and
○ prevention of overweight/obesity?
Data and research priorities
Improved content data are needed for common commercially available foods marketed for infants/toddlers
Prevalence data on specific dietary intake patterns are needed for infants 6–12 mo of age
Data are needed on the distribution of daily energy and nutrient intake from complementary foods, human milk, and/or infant formula (or a combination of the 2) that ensures meeting the nutrient requirements established by the DRIs
6. Early dietary influences on food and flavor preferences, especially for nutrient-dense foods (fruit, vegetables, meat, dairy, etc)—What are the evidence-based strategies to enhance acceptance of nutrient-dense foods such as fruit and vegetables?
Rationale
Before the transition to a mixed diet, a striking contrast exists between the sensory experience of the breastfed and formula-fed infant (63, 64). Breastfed infants have a more diversified exposure to the volatile elements of the maternal diet via mother's milk, whereas the formula-fed infant's dietary exposure is more “monotonous.” The myriad of factors that might contribute to the development of taste preferences in human milk–fed compared with formula-fed infants and their implications for health have been recently reviewed (65). Once weaned to complementary foods, both breastfed and formula-fed infants learn through repeated exposure to a particular food as well as through exposure to a variety of foods (in both flavor and texture), which, in turn, promotes the willingness to eat these complementary foods as well as novel foods. Thus, concerns about “how to” (eg, order of introduction of foods, types of foods) represent one of the primary matters discussed by mothers/caregivers with their child's pediatrician. Ultimately, the goal is to accustom children gradually to a varied diet that meets nutritional needs for growth and development with appropriate nutrient-dense foods, leading to a preference for nutrient-dense foods.
Suggested systematic review questions
(Note: The WG emphasized that the following suggested systematic review questions as well as the data and research priorities address the following: 1) the biology underlying the senses that provide acceptance/preference of foods, 2) the relation of this biology to dietary intake and long-term consequences on health, and 3) evidence-based strategies, based on the biology, to increase the acceptance of nutrient-dense foods.)
Is there a relation between appropriate/increased intake (quantity, timing, frequency) of nutrient-dense foods (meats, dairy, fruit, vegetables, etc) by the mother during pregnancy and acceptance/preference of nutrient-dense foods in infants?
Is there a relation between appropriate/increased intake (quantity, timing, frequency) of nutrient-dense foods (meats, dairy, fruit, vegetables, etc) by mothers after infants’ birth on the acceptance/preference of nutrient-dense foods by their children?
Are there differences in the acceptance/preference of nutrient-dense foods between breastfed and formula-fed infants?
Does exposure (timing, quantity, frequency) to nutrient-dense foods in weaned infants increase acceptance of nutrient-dense foods?
Are there differences in pattern or duration of acceptance between infants who were formula-fed or breastfed or both during first few months of life, and if so, how does this relate to maternal diet and feeding practices? Are there differences between formula-fed infants depending on the type of formula used and whether the infant was breastfed or formula-fed in early life?
Does increased acceptance/preference for nutrient-dense foods in the first year of life persist? Does it improve dietary intake of nutrient-dense foods at 12–24 mo? Does this affect growth variables during infancy and childhood?
Data and research priorities
Improved data on complementary food (type and amount) patterns in the United States, particularly with regard to potential differences between breastfed, formula-fed, and mixed-fed infants
Improved data/analyses to document impact of maternal dietary patterns during pregnancy and lactation
What is the biology (taste, aroma, textures) underlying the preference of foods by infants?
Using the biology, what are the best strategies to increase the acceptance of nutrient-dense foods and decrease the preference for nutrient-poor, energy-dense foods?
How does the preference for nutrient-dense foods compared with the preference for nutrient-poor foods in the first 24 mo of life affect long-term health?
What is the relative contribution of protein sources to the development of taste preferences in formula-fed infants?
7. Development of taste preferences for salt and sweet in infants and the impact on dietary intake and long-term health outcomes
Rationale
Excessive intakes of foods that contain high amounts of salt (NaCl) and refined sugars (and consequently, taste salty and sweet) cause or exacerbate a number of illnesses, including hypertension, diabetes, and obesity. The DGA as well as other authoritative documents make clear public health concerns about avoiding foods with added salt and sugar. Despite this concern, people consume sugar and salt in amounts that most health professionals consider to be unhealthy starting at a young age. Not only do processed foods, which make up a large part of modern diets, have high quantities of salt and added sugars, but foods that taste sweet or salty and beverages that taste sweet have powerful hedonic appeal, especially to children. The elevated preferences for sugars and salt during childhood reflect basic biology: children are programmed to like mother's milk and foods containing energy (signaled by sweet taste) and minerals (signaled by salt taste) during periods of growth (66, 67). Other biological influences might include maternal status, eg, hyperemesis gravidarum, which has been suggested as a potential precursor to salt preference (68). In addition, a body of evidence suggests a role for socioeconomic status and race-ethnicity in the development of preferences for salt and sweet (69). Our ability to develop evidence-based dietary guidance aimed at health promotion and disease prevention is contingent on a better understanding of the factors that contribute to the ontogeny of salt and sweet preferences during critical periods of development.
Suggested systematic review questions
(Note: The WG suggested that the following questions as well as the data and research priorities address the following: 1) the biology of preferences for salt and sweet; 2) the relation between preferences for salt and sweet and dietary intake of foods with added salt and sugar later in childhood and adulthood, as well as long-term consequences on health; and 3) evidence-based strategies to reduce intake of foods with added salt and sugar.)
Do infants and children differ in their preferences for sugar and salt?
If there are differences, what are the mechanisms underlying these age-related changes?
-
Are there differences based on
○ race,
○ genetics,
○ sex, or
○ emotional state of the child (eg, depression)?
Is there a relation between the health of the mother prenatally (eg, hyperemesis gravidarum) and preference for salt and sweet?
Does the intake of foods with added salt and sugar in infancy influence the preference and analgesic appeal of dietary salt and sweet in infants, young children, and adults?
Does repeated exposure to foods with added sugar lead to an “addiction” later in life (physiologic endorphin response)? How much of the addiction is predisposed and inborn compared with conditioned?
-
Is there a relation between intake of foods with added salt and sugar during infancy (timing of introduction, quantity, frequency) and preference for salt and sweet later in childhood and adulthood? Does this relation affect
○ growth variables at 12–24 mo and
○ body composition/health later in life?
Data and research priorities
Additional data are needed on what foods children are eating and the sources of added sugars and salt in their diet (48, 70). When are these foods first introduced into infants’ diets and why?
Is there a relation between an increased or decreased intake (quantity, timing frequency) of foods with added salt and sugar in pregnant women and intake/preference for foods with added salt and sugar in their infants?
Is there a relation between an increased or decreased intake (quantity, timing frequency) of foods with added salt and sugar in postpartum women whether breast- or bottle-feeding) and intake/preference for foods with added salt and sugar in their infants?
Is there a difference in intake/preference for added salt and sugar between infants who are predominantly breastfed or formula-fed during the first 6 mo after birth?
How does the amount of salt or sugar vary among formulas, and how does it compare with breast milk? If the amounts are variable, does the mode of differential exposure to salts and sugars affect salt/sugar preference and dietary habits among infants and children?
What evidence-based strategies are associated with reduced 1) preferences and 2) intake of salt and sugar among children?
8. The role, timing, and value of snacking (ie, food consumed between meals)
Rationale
Snacking continues to be common in the 6–12-mo age group (20). However, our understanding about its prevalence, type, effect on growth and development, the development or establishment of eating patterns, and caloric intake needs further systematic exploration to help inform dietary guidance for these infants/toddlers.
Suggested systematic review questions
-
What is the association between scheduled and self-regulated snacking on infant and toddler
○ growth and physical development,
○ overweight/obesity, and
○ dietary intake (quality)?
Are there differences between breastfed and formula-fed infants?
-
What is the association between frequency of snacks and infant/toddler
○ growth and physical development,
○ overweight/obesity, and
○ dietary intake?
What snacks optimize nutrient intakes in infants and toddlers, and are there differences between breastfed and formula-fed infants?
Data and research priorities
What are infants/toddlers currently eating for snacks?
How frequently are infants/toddlers eating snacks and/or meals?
What is the definition of snacking? Sometimes it is defined by researchers as the number of foods or calories consumed “between meals” or by respondents as to whether they called it a meal or a snack. In infants, the name does not mean much because infants eat frequently, so snacks and meals may be interchangeable for this population.
Are there longitudinal data that provide measured health outcomes needed to supplement existing cross-sectional data?
9. Method of complementary feeding
Rationale
Aside from the potential health impact of the timing of introduction of complementary foods (61, 71, 72), the methods of introducing both liquids/beverages and solid complementary foods may also have important implications for infant growth and development. The ease of accepting complementary foods is dependent on developmental readiness, and this should be shown before complementary foods are introduced. Because strategies for introducing complementary foods are varied and can occur before children are developmentally ready, parents need guidance on the best methods for introducing complementary foods. Another area in need of guidance is the timing of use of complementary foods within a given feeding episode, ie, before or after breast- or bottle-feeding. These practices may result in overfeeding, the displacement of nutrients or calories from human milk or infant formula, or have other health consequences.
Suggested systematic review questions
-
What is the relation between adding solid foods into bottles and infant/toddler
○ oral health,
○ overweight/obesity, and
○ dietary intake (eg macronutrient, including calories, and micronutrient intake)?
-
What is the relation between putting an infant to bed with a bottle and infant/toddler
○ oral health,
○ overweight/obesity, and
○ risk of otitis media?
-
What methods of introducing solid foods results in optimal infant/toddler
○ oral health,
○ overweight/obesity, and
○ dietary (quality and quantity) intake?
Data and research priorities
Improved data on the prevalence of different methods for introduction and use of complementary foods.
Does it make a difference whether or not complementary foods are introduced before breastfeeding or bottle-feeding at a given meal time?
10. Physical activity in prevention of childhood obesity
Rationale
A need exists for consistent messaging related to structured and unstructured activity, including play for infants and toddlers. The type and amount of activity will affect energy expenditure/needs, the development of healthful activity habits at an early age, physical and social development, and overall well-being. The National Association of Sports and Physical Education has developed specific guidelines for the physical activity of children from birth to age 5 y that address the developing child's unique characteristics (73). These guidelines reflect the current evidence with regard to motor development, movement, and exercise and the physical activity needs of young children during the first years of life. Including activity guidelines for infants from birth to 24 mo is consistent with the DGA goals of health promotion and disease prevention. Of specific interest is the relation between “tummy time” (74), other developmentally appropriate activities, and inactivity (“containerization”) on short- and long-term health. Specific physical activities to consider at 6–12 mo include locomotor activities (crawling, walking, etc), nonmanipulative activity (tummy time, ie, pushing up on extended arms and pivoting on stomach, unrestrained sitting time), and manipulative activity (encouraging infants to manipulate objects). In addition, there are concerns about “containment” of infants and “back to sleep practices” that do not promote the physical activities listed above, and it is recommended that caregivers allow periods for tummy time and unrestrained activity.
Suggested systematic review questions
The WG concluded that there are insufficient data at this time to support systematic reviews on this topic for this age group.
Data and research priorities
-
What is the relation between “tummy time” or other unrestrained activity on
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development; and
○ overweight/obesity?
-
What is the relation between participation in developmentally appropriate physical activity and
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development; and
○ overweight/obesity?
-
What is the relation of physical inactivity due to restrained/contained time (including television/video exposure) and
○ growth and physical development;
○ cognitive, behavioral, or neuromotor development; and
○ overweight?
11. Impact of the WIC food package on infant and child diet
Rationale
Nearly one-half of the infants born in the United States participate in the WIC program. WIC is designed to help families with limited resources meet the nutrition needs of pregnant, breastfeeding, and nonbreastfeeding postpartum women, as well as infants and children to 5 y of age who are at nutritional risk. WIC provides supplemental food and nutrition education to program participants to improve dietary quality and practices. For infants, the contents of the WIC food package are dependent on whether the infant is formula-fed, breastfed, or fed both formula and breast milk (75). By October 2009, all WIC clinics introduced a new WIC food package to reflect the DGA as well as the infant feeding practice guidelines of the AAP. The new food package was designed in response to concerns raised by the IOM and others regarding breastfeeding duration, juice consumption, age at introduction to solid foods and cow milk, consumption of fruit and vegetables, and frequency of exposure to new foods among WIC participants. The impact of the new food packages on the dietary intake and behavior of participating infant and toddlers is of importance. Another priority is the comparison of the diets of participating infants and young children with diets of those not participating in WIC.
Suggested systematic review questions
(Note: The WG suggested that each of the following study questions be repeated, comparing new and old WIC food packages.)
Is participation in WIC associated with improved breastfeeding initiation, duration, and exclusivity?
-
With regard to complementary feeding, is participation in WIC associated with
○ age at which infants are introduced to solid foods,
○ the types of first food introduced, and
○ a specific pattern of complementary feeding during infancy (dietary pattern, types of foods consumed, added sugar and salt content of complementary foods, age in which whole milk is introduced)?
How do associations between WIC participation and infant and young child feeding practices differ for breastfed compared with formula-fed infants?
How do associations between WIC participation and infant and young child feeding differ for children of different race and ethnic groups?
Is participation in WIC associated with childhood obesity or other diet-related adverse health outcomes?
Is participation in WIC associated with improved food security (compared with non-WIC, not WIC eligible and non-WIC, WIC eligible)?
Data and research priorities
The WG was aware of ongoing data analyses of the WIC programs, and the review is included as part of this supplement issue (25).
WG 3: PERIOD OF TRANSITIONAL FEEDING (12–24 mo)
Chairs
Stephanie Atkinson, McMaster University
Leann Birch, Pennsylvania State University
WG members
Maureen Black, University of Maryland
Kristen Copeland, Cincinnati Children's Hospital Center
William Dietz, Centers for Disease Control and Prevention (formerly)
David Fleischer, National Jewish Health
Mary Kay Fox, Mathematica Policy Research
Madeleine Sigman-Grant, University of Nevada Reno
Topics
1) What are the specific energy needs of infants and children aged 12–24 mo to promote optimal growth, health, and physical development?
2) What is the optimal type and amount of physical activity to promote health of infants and toddlers 12–24 mo of age?
3) Micronutrients and other dietary constituents that may be under-/overconsumed by infants/toddlers 12–24 mo of age
4) Impact of different approaches to weaning off the breast or bottle on infant/toddler health
5) Implication of consumption of different types of postweaning beverages in infant/toddler growth, development, and health
6) Implications of sleep patterns for infant/toddler nutrition, growth, and health
7) “Microenvironmental” effects on the transition to the adult diet. How do inborn responses to basic tastes, learning and experience with food and eating, and exposure to caregiver practices influence the transition to the adult diet during the period of 12–24 mo of age?
8) Factors affecting exposure to and impact of media (eg, television, handheld computers, etc) in infants/toddlers
9) Impact of food insecurity on infant/toddler health and development
10) Specific food safety concerns for this population, such as exposure to lead and mercury and potential choking risks associated with food (texture, shape, etc)
11) General food preparation, handling, and storage issues related to this population
1. What are the specific energy needs of infants and children aged 12–24 mo to promote health and prevent disease?
Rationale
Alarming patterns of overweight and obesity occurring in early life (<4 y of age) continue in the United States (76). Although many factors may contribute to this pattern (77), it is clear that energy requirements of toddlers are critical components to be considered. Of particular interest is the relation of energy intake to growth velocity, which decelerates after 1 y of age (78, 79), and to physical activity levels of toddlers in today's society. The key question is how should dietary guidance reflect these changes?
Suggested systematic review question
What are the energy requirements for toddlers aged 12–24 mo to promote optimal growth and physical development?
Data and research priorities
-
Data on food consumption patterns in infants/toddlers in the US including
○ total calories and contributions of specific food groups
○ demographic/cultural/ethnic determinants
○ impact of foods consumed in home compared with those consumed out of home including in child care facilities
-
Factors contributing to “unhealthy” body composition, eg,
○ differences between parental attitudes, beliefs, and habits and those of other caregivers
Determination of the optimal mathematical model for Estimated Energy Expenditure in toddlers from knowledge of energy expenditure, growth, and physical activity level
2. What is the optimal type and amount of physical activity to promote health of infants and toddlers 12–24 mo of age?
Rationale
As noted by WG 2 topic 10 above, the National Association of Sports and Physical Education has developed specific guidelines for the physical activity of children from birth to age 5 y that address the developing child's unique characteristics (73). These guidelines reflect the current evidence with regard to motor development, movement, and exercise and the physical activity needs of young children during the first years of life. Because of the importance of physical activity for growth and physical development, energy balance, and bone development in infants and toddlers, its measurement is critical for the evaluation of its impact on energy needs and ultimately the ability to develop evidence-based guidance for infants and toddlers. Limited information is available to determine what types and amounts of physical activity optimize the health and development of infants and toddlers. In addition, a need exists to better understand what types of opportunities for physical activity, including indoor and outdoor activities, should be recommended.
Suggested systematic review questions
-
What types of physical activity during ages 6–24 mo are associated with
○ optimal growth and physical development,
○ optimal bone development, and
○ reduced risk of overweight/obesity?
-
What amount (ie, duration, frequency, number of daily occasions) of physical activity from age 6 to 24 mo is associated with
○ optimal growth and physical development,
○ optimal bone development, and
○ reduced risk of overweight/obesity?
Data and research priorities
-
Prevalence data are needed for US children with regard to
○ types and
○ amounts of physical activity
-
The most common and available settings to be physically active and of relative value to the child's health and development:
○ home
○ outside the home
3. Micronutrients and other dietary constituents that may be under-/overconsumed by infants/toddlers 12–24 mo of age
Rationale
Limited information is available on the consumption of specific nutrients in infants 12–24 mo old (80) and their adequacy relative to existing recommendations (19). New data are needed with regard to their adequacy in the diet in relation to current DRIs [Estimated Average Requirements (EARs) and Tolerable Upper Intake Levels (ULs)] and the association of the individual nutrients with specific health outcomes. Nutrients noted to be of special concern include the following: suboptimal intakes of iron, zinc, vitamin E, vitamin D, fiber, and potassium; and excessive intakes of energy, protein, sodium, vitamin A, synthetic folate, saturated fat, water, and fluoride (19). A particular need is further guidance with regard to folate, fiber, vitamins, and percentage milk fat (eg, skim, 2%, or whole milk) recommended for 12–24-mo-olds to ensure proper neurodevelopment while minimizing obesity and cardiovascular risk.
Suggested systematic review questions
What is the relation between observed intakes of fiber, vitamin A, and folate and the EARs and ULs for toddlers 12–24 mo of age?
What is the relation between fiber, vitamin A, and folate and 1) markers of growth in relation to WHO reference growth standards or 2) biomarkers of adequacy?
Data and research priorities
What is the average consumption of food intake by food groups in infants 12–24 mo of age in relation to BMI and overweight at 2 y?
What are the average nutrient intakes, including the contribution of dietary supplements (eg, iron, zinc, vitamin E, vitamin D, fiber, potassium, energy, protein, sodium, vitamin A, synthetic folate, saturated fat, water, and fluoride), of toddlers, and how do intakes compare with the current DRIs (EARs and ULs)?
Improved understanding of the relation between vitamin D, fluoride, and calcium intake in pregnancy and in infants up to 2 y of age and bone health outcomes in young children.
Improved understanding of the relation between intake of fiber in toddlers and functional outcomes such as bowel motility/function.
Improved understanding of the relation between sodium and potassium intake in toddlers and outcomes of blood pressure.
Improved understanding of the relation between total and saturated fat intake in toddlers and outcomes of blood triglyceride, HDL-cholesterol, and LDL-cholesterol concentrations.
Knowledge of the optimal adequate macronutrient distribution range for infants 12–24 mo of age including sugars and starches to optimize growth, body composition, and functional outcomes such as bowel motility cognition (related to sugars), food behavior, hyperactivity, and glucose tolerance.
4. Impact of different approaches to weaning off the breast or bottle on infant/toddler health
Rationale
Delayed weaning from (and/or inappropriate use of) bottle feeding may lead to dental caries (81) and/or anemia. Limited evidence implicates early weaning with increased calorie intake and overweight/obesity (82, 83), but available literature is scant, and the results of extant studies are inconsistent (84). Because of changes in nutrient composition of mature human milk over time and increased caloric/nutrient needs, delayed weaning from the breast may affect growth and development. Thus, a need exists to better understand how the timing of weaning affects consumption of other foods and how this may influence diet quality, the development of eating behaviors, and growth and development, including the prevalence of overweight and obesity. It is important to understand whether there are differences between breastfeeding and bottle feeding of expressed human milk.
Suggested systematic review questions
-
What is the relation between delayed weaning from the breast on toddler
○ dietary intake (including energy and iron intake);
○ eating behaviors;
○ growth and development, including risk of overweight/obesity;
○ oral health; and
○ anemia?
-
What is the relation between delayed weaning from the bottle and/or inappropriate use (including formula, human milk, and juice) of a bottle on a toddler? Does the example of “inappropriate” use include either use that is inconsistent with current guidance (eg, off the bottle by 18 mo) or use of food to manage behavior: soothe, quiet, redirect, or control a nonhungry infant/toddler? Information is also needed on
○ dietary intake (quantity and quality), particularly energy and iron intake;
○ eating behaviors;
○ growth and development, including risk of overweight/obesity;
○ oral health; and
○ anemia.
Data and research priorities
-
Are there differences between delayed weaning from human milk in a bottle and formula in a bottle? Potential differences of interest between human milk– and formula-fed infants might include
○ nutrient intake;
○ health outcomes, including body composition;
○ how weaning is accomplished; and
○ caregiver behavior/demographic characteristics.
5. Implication of consumption of different types of postweaning beverages in infant/toddler growth, development, and health
Rationale
In addition to complementary foods, the period from 6 to 24 mo involves the introduction of several complementary beverages, including fruit juice, sweetened beverages, water, and milk (cow milk or other alternative milks such as soy, rice, almond, and coconut) (85). Because the 12–24-mo period also usually involves the discontinuation of human milk and/or infant formulas, the replacement fluids provide an important source of energy and other nutrients but also involve trade-offs and, often, diminishing sources of essential nutrients. Consumption of many of these types of beverages may have adverse health effects, including increased risk of obesity (86), CVD (87), and anemia (88), among others. Excess quantities of these fluids can also displace the appetite for healthful solid foods, and certain beverages may be associated with adverse outcomes such as irritable bowel syndrome and diarrhea. Consequently, guidance is needed to specify a maximum recommended intake of these beverages. Guidance is also needed about the percentage of milk fat recommended for 12–24-mo-olds to ensure proper neurodevelopment while minimizing obesity and cardiovascular risk. Recent guidance from the AAP (49) suggests reduced-fat milk for 12–24-mo-olds who are believed to be at risk of obesity and/or cardiovascular risk. Similarly, with an increase in the prevalence of allergies, the past 20 y has seen an increase in the types of milk-alternative beverages offered (soy, rice, almond, coconut). The effect of these types of alternative milks compared with human or cow milk on young children's nutritional status, nutrient intake, risk of allergies, and cardiovascular risk is unclear.
Suggested systematic review questions
-
Is there a level (minimum and maximum) of cow-milk consumption that is ideal for infants/toddlers aged 12–24 mo
○ to support physical growth and development,
○ to support cognitive/behavioral development, and
○ to prevent increased risk of anemia and noncommunicable conditions such as obesity and CVD?
-
What is the effect of different percentages of milk fat (eg, whole, reduced, skim) on 12–24-mo-olds with regard to
○ energy intake,
○ neurologic development,
○ obesity, and
○ CVD risk?
-
What are the effects of milk-alternative beverages (eg, soy, rice, almond, and coconut beverages) compared with human or cow milk on children's
○ nutritional status,
○ nutrient intake (eg, effect on vitamin D and calcium status),
○ risk of allergy, and
○ CVD risk?
-
What is the effect of juice consumption in this age group on
○ risk of diarrhea,
○ physical growth and development (decreased risk of obesity or poor weight gain), and
○ diet quality (ie, displacement of other foods in diet).
What amount of free water is recommended for children aged 12–24 mo to promote optimal growth and development and adequate hydration and to limit displacement of other foods in the diet?
Data and research priorities
-
What are the consequences of the provision of human milk to toddlers over 12 mo of age for
○ physical growth and development,
○ cognitive/behavioral and emotional development, and
○ nutritional status (micronutrients, essential fatty acids, etc)?
What compositional changes (nutrients and/or bioactive components) occur in human milk during the period of extended breastfeeding (>12 mo) that might affect the nutritional status of these infants?
What is the impact of the introduction of cow milk, alternative milks, and/or juice on allergies in infants/toddlers aged 12–24 mo?
What are the current consumption patterns of juice, alternative milks, and water in infants and toddlers in the US?
What are the predictors of consumption of cow milk, alternative milks, and/or juice?
-
Has the use of fortified/supplemented beverages changed in infants/toddlers in the US in recent years and what is the impact on
○ physical growth and development and
○ nutrient status?
Is there a critical period when the amount of milk fat or total fat in the diet needs to be controlled to avoid increased risk of CVD?
6. Implications of sleep patterns for infant/toddler nutrition, growth, and health
Rationale
Short sleep duration and its relation with obesity risk, appetite, and dietary quality is an emerging area of inquiry (89). Most studies have been performed in adults and older children (90), but there are also some cohort studies in infants and toddlers that have shown a link between shortened sleep duration (<12 h in a 24-h period) and increased BMI z score, subscapular and triceps skinfold thicknesses, and odds of overweight among infants aged 0–24 mo (91). Moreover, short sleep duration is highly prevalent, particularly among minority ethnic groups (92, 93), and tends to persist into later childhood (94). Understanding the mechanisms for the observed relation between sleep and obesity comes primarily from the adult literature, where sleep restriction has been associated with reduced leptin concentrations, increased ghrelin concentrations, and increased appetite. Through these studies, it is apparent that sleep plays an important role in appetite, dietary quality, and subsequent obesity risk.
Suggested systematic review questions
What is the effect of short sleep duration on obesity risk among infants aged 0–24 mo?
What is the effect of short sleep duration on appetite and dietary quality among children consuming complementary foods?
Data and research priorities
If ideal duration of sleep is 12 h of the 24 h, is there an ideal duration of sleeping episodes, ie, is 12 continuous hours better than 3 sessions of 4 h or some other combination?
What is the impact of sleep periodicity on dietary intake patterns?
What are the predictors of documented differences in sleep patterns/practices among different racial-ethnic groups?
How does composition (quantity/quality) of the diet affect sleep patterns?
How do eating patterns (regular meals, on-demand feeding, etc) affect sleep patterns?
7. “Microenvironmental” effects on the transition to the adult diet—How do inborn responses to basic tastes, learning and experience with food and eating influence the transition to the adult diet during the period of 12–24 mo of age?
Rationale
From birth to 24 mo, feeding occasions include the interaction of the child, the caregiver, and the food. Each has a set of unique characteristics that affect the nature and impact of this interaction (95, 96). For example, child factors might include inborn taste preferences, ability to learn, developmental level, and temperament; and the caregiver factors might include eating habits and eating style. The interactions between child and caregiver include feeding practices and parenting style. Finally, the food contributes via flavors, portion size, and energy/nutrient content. How these myriad of factors affect feeding from 12 to 24 mo need to be better understood.
Suggested systematic review questions
-
How do children's 1) inborn ability to learn food likes and dislikes (ie, the ability to learn to like or dislike foods and other things via familiarization and associative learning processes), 2) predispositions for basic tastes (ie, unlearned liking to sweet, salty, or umami; rejection of sour and bitter tastes), and 3) individual differences (ie, individual differences in 1 and 2 due to genetic differences) contribute to
○ making the transition to consumption of the adult diet (food preferences and dietary intake),
○ growth and development, and
○ risk of overweight/obesity?
-
How do parent/caregiver feeding practices affect
○ the transition to the adult diet (food preferences and dietary intake, development of reactivity, self-regulation of food intake),
○ growth and development, and
○ risk of overweight/obesity?
What food characteristics (eg, taste/flavor characteristics; portion size; energy and nutrient density; novel or familiar) affect the development of food preferences and dietary intake?
What factors (either from the child or the parent/caregiver) precipitate or exacerbate “picky eating”?
-
What is the relation between picky eating and
○ dietary intake (quality and quantity),
○ long-term eating behaviors, and
○ health outcomes (eg, growth and development and risk of overweight/obesity)?
What factors contribute to the ability to self-regulate energy intake?
Can changes in infant/toddler feeding patterns and/or environmental characteristics affect the ability to self-regulate energy intake?
Data and research priorities
Evidence for how children learn food likes and dislikes (through familiarization, observational learning, and associative learning)
How do children's predispositions for basic tastes and individual differences (eg, temperament, previous experience with food in the first year of life) contribute to making the transition to consuming the foods of the adult diet?
Evidence for food (energy) intake regulation in toddlers
Examine the age at which the ability to self-regulate energy intake begins to deteriorate
What factors (eg, feeding practices, palatability, schedule/routine, and social context) contribute to this deterioration?
Can changes in infant/toddler feeding patterns and/or environmental characteristics prevent or reduce the deterioration?
-
Evidence for parent/caregiver effects on the transition to the adult diet (food preferences, intake patterns, and feeding practices)
○ Are new foods introduced systematically to promote acceptance?
○ Is feeding responsive to child cues?
○ Are there differences in general parenting style (eg, authoritative, authoritarian, permissive), parents’/caregivers’ own eating habits, and other characteristics that affect a child's developing food likes or dislikes and developing reactivity and regulatory ability that affect what foods are consumed, and how much is consumed?
Evidence for the effects of food characteristics on liking and intake (taste/flavor characteristics), portion size, and energy and nutrient density? Novel or familiar?
Etiology of “picky eating” (including food refusal, food jags) and consequences on dietary intake and eating behaviors. What factors precipitate or exacerbate picky eating and how does picky eating affect dietary intake, long-term eating behaviors, and other outcomes?
8. Factors affecting exposure to and impact of media (eg, television, handheld computers, etc) in infants/toddlers
Rationale
Television viewing has been associated with increased risk of adverse dietary outcomes including obesity risk (97). Advertising and other marketing strategies to promote foods contribute to obesity (98), and exposure to television may represent the earliest exposure to a screen in 0–24-mo-old infants. Efforts to control exposure to advertisements that promote food may contribute to obesity prevention. In addition, foods marketed to children are often high in sources of fat and added sugars (99), so reduced consumption of foods advertised on television may help reduce the risk of later CVD. Parents and other caretakers model and control television time.
Suggested systematic review questions
What are the impacts of exposure to advertising on food choice? On food intake and weight status?
-
What is the relation between screen time and
○ growth and physical development,
○ overweight/obesity, and
○ cognitive, behavioral, or neuromuscular development in infants and toddlers?
Data and research priorities
What types and amounts of advertising are infants and toddlers exposed to?
What are the impacts of exposure to advertising on food intake and weight status?
-
What is the relation between screen time and
○ growth and physical development,
○ overweight/obesity, and
○ cognitive, behavioral, or neuromuscular development in infants and toddlers?
Are there critical periods/milestones in cognitive development that play a role in “receptivity” to the influence of specific types of media?
What impacts do caregivers and child care providers have on infant and toddler screen time?
9. Impact of food insecurity on infant/toddler health and development
Rationale
Household food insecurity (lack of access to an available and nutritious diet) remains a major public health problem, affecting an estimated 21.8% of US households with children under age 6 y. Rates may be as high as 49.9% for low-income, female-headed households with children (100). Toddlers in food-insecure households are at risk of numerous adverse health outcomes, including iron deficiency, increased hospitalizations, poor perceived health, and developmental delays (101–104). National nutritional programs [eg, WIC, the Supplemental Nutrition Assistance Program (SNAP)] reduce food insecurity. Further guidance is needed with regard to the impact of food insecurity and programs designed to address it on infant and toddler diet and health relations.
Suggested systematic review questions
-
What is the relation between food insecurity in infants/toddlers and
○ cognitive, behavioral, or neuromotor development;
○ dietary intake/diet quality;
○ poor perceived health;
○ risk of iron deficiency/anemia;
○ weight status?
Data and research priorities
Prevalence of food insecurity in the US by demographic characteristics/race-ethnicity
Quality of dietary intakes of infants living in food-insecure environments
Primary sources of food for families living in food-insecure environments
Role of the household compared with other child care settings in food-insecure settings
-
Impact of the community on dietary quantity and quality for food-insecure infants/toddlers including the following:
○ National food assistance programs
○ Community-based/charitable organizations
○ Impact of episodic (eg, monthly or seasonal) compared with chronic food insecurity on infant/toddler growth, health, development
○ Implications of the reduction of WIC benefits after the first year of life on WIC participation and its impact on infant/toddler food security, nutrition, and health
10. Specific food safety concerns for this population, such as exposure to lead and mercury and potential choking risks associated with food (texture, shape, etc)
Rationale
Because infants and toddlers increase their exposure to things within their environment as a result of increasing mobility and independent movement, they are at increasing risk of choking (105, 106) and exposure to potential toxic materials/substances in their food and environment (107). Guidance is needed to limit exposure to toxic/unsafe materials in the environment of infants and toddlers.
Suggested systematic review questions
What dietary behaviors increase the risk of lead poisoning among infants and toddlers?
What dietary behaviors increase a toddler's risk of exposure to mercury among infants and toddlers?
What food characteristics (ie, texture, shape) are associated with increased risk of choking among infants and toddlers?
Data and research priorities
What is the role of nutritional status (eg, general nutrition, calcium/vitamin D, thiamine, etc) both as a predictor and an outcome of heavy metal exposure?
What is the role of body composition in heavy metal exposure toxicity?
What is the role of the caregiver? Are there parenting “style” issues that might contribute to increased exposure to either toxic material or choking risk?
Are there differences in incidence of choking at home compared with at daycare?
11. General food preparation, handling, and storage issues related to this population
Rationale
Aside from general concerns about the safety of the infant food supply (108), specific concerns related to toddler feeding include issues related to food preparation, “the 5 second rule,” and the parental habit of carrying food for children for long periods without refrigeration (109).
Suggested systematic review questions
What is the risk of foodborne illness associated with consuming food that has been mishandled?
What is the risk of foodborne illness associated with consuming food that has not been refrigerated properly?
Data and research priorities
Who is the source of greatest risk—child/caregiver characteristics?
Is race or sex or type of caregiver (mother vs father vs other givers) a predictor for this behavior?
Are there nutritional risks/consequences?
WG 4: CAREGIVERS (MOTHERS, OTHER)—FACTORS INFLUENCING NUTRIENT NEEDS, INFANT FEEDING CHOICE, DIETARY QUALITY, AND FOOD HABITS
Chairs
Kathleen Rasmussen, Cornell University
Emily Oken, Harvard Medical School
WG members
Sara Benjamin-Neelon, Duke Global Health Institute
Sharon Donovan, University of Illinois
Laura Hubbs-Tait, Oklahoma State University
Cheryl Lovelady, University of North Carolina at Greensboro
Shelley McGuire, Washington State University
Topics
(Note: WG 4 developed a conceptual model to help identify core themes and linkages to their topics. The model can be found in Supplemental Figure 1 under “Supplemental data” in the online issue.)
1) What maternal factors (other than dietary intake and/or nutritional status) have been documented to affect lactation initiation and success?
-
2) What predicts maternal feeding choices
○ to feed human milk at the breast or with a bottle and
○ to feed on demand or on schedule?
3) What are the relations of maternal feeding practices and styles (including control, pressure, restriction, responsive/intrusive/indulgent, and responsiveness to satiety and hunger cues) to infant growth and physical development, overweight/obesity, and eating behaviors?
4) What is the evidence for any benefit or harm of maternal fish consumption during lactation?
5) What is the relation between lactating mothers’ energy balance (energy intake, energy expenditure, and postpartum weight loss) on breast-milk volume and composition, as well as on infant growth and body composition?
6) What are the maternal and caregiver predictors of infant dietary quality (ie, what do we know about maternal and caregiver adherence to dietary guidelines for infants and how can we increase the number of infants and toddlers who would benefit, especially low-income/high-risk infants/toddlers)?
7) What are the effects of dietary patterns (eg, vegan, vegetarian, macrobiotic diets) on breast-milk composition?
8) What is the influence of maternal dietary intake on micronutrients (including fat- and water-soluble vitamins) and macronutrients (including total fat, n–3 PUFAs, n–6 PUFAs, and trans fats) on human milk composition?
9) What are infant/children aged 0–24 mo being fed and what are they consuming in nonparental child care settings?
10) What are the effects (or lack thereof) of maternal alcohol consumption during lactation on milk production, milk composition, and infant outcomes?
11) What are the effects (or lack thereof) of maternal caffeine consumption during lactation on milk production, milk composition, and infant outcomes?
12) How can we assist women across the BMI spectrum to reach national goals for breastfeeding duration?
13) What are the effects of maternal probiotic consumption on human milk composition or infant outcomes?
1. What maternal factors (other than dietary intake and/or nutritional status) have been documented to affect lactation initiation and success (incorporating both duration and intensity)?
Rationale
A number of factors may directly or indirectly affect the initiation and success of lactation (110, 111). Recent studies have emphasized factors such as delivery mode (cesarean section compared with vaginal, preterm compared with term) (112, 113) and maternal inflammatory conditions such as obesity (114, 115), impaired glucose tolerance (116), and mastitis (117, 118). Other factors could include the influence of the postnatal hospital environment (eg, providing formula, rooming-in) and pharmaceutical drug use during the peripartum period (including antiinflammatory drugs). Any of these factors can positively or negatively affect initiation or success of lactation, leading to a child not being fed human milk as recommended; thus, a better understanding of the various factors that potentially affect breastfeeding performance is needed to inform future guidance targeting this age group and their mothers/caregivers. A systematic assessment of these myriad of factors will help women and their caregivers in making informed decisions about best feeding choices for them and their infants.
Suggested systematic review questions
What impact does delivery mode (cesarean section compared with vaginal delivery) have on lactation initiation and sustainability?
What impact do maternal inflammatory conditions (eg, eclampsia, diabetes, mastitis) have on lactation initiation and sustainability?
What impact do breast irregularities (eg, inverted nipples) have on lactation initiation and sustainability?
What impact do “baby-friendly” hospital practices (eg, not providing formula, having access to a lactation specialist, making rooming-in available) have on lactation initiation and sustainability?
What impact does pharmaceutical drug use during the peripartum period (including antiinflammatory drugs) have on lactation initiation and sustainability?
What impact do maternal factors such as 1) delivery mode (cesarean section compared with vaginal delivery), 2) maternal inflammatory conditions (eg, eclampsia, diabetes, mastitis), and 3) “baby-friendly” hospital practices (eg, not providing formula, making rooming-in available) have on infant growth and physical development?
Data and research priorities
-
What impact do the following maternal factors have on infant growth and physical development?
○ Breast irregularities (eg, inverted nipples)
○ Pharmaceutical drug use during the peripartum period (including antiinflammatory drugs)
-
What impact do the following maternal factors have on infant immune system development, infection, or inflammation?
○ Delivery mode (cesarean section compared with vaginal delivery)
○ Maternal inflammatory conditions (eg, eclampsia, diabetes, mastitis)
○ Breast irregularities (eg, inverted nipples)
○ “Baby-friendly” hospital practices (eg, providing formula, rooming-in)
○ Pharmaceutical drug use during the peripartum period (including antiinflammatory drugs)
2. What predicts the following maternal feeding choices 1) to feed human milk at the breast or with a bottle and 2) to feed on demand or on schedule?
Rationale
These maternal feeding choices are linked with infant health outcomes (reviewed by WG 1) and knowledge of maternal characteristics associated with these choices will allow targeting of education/intervention to mothers who are least likely to adhere to best feeding practices.
Suggested systematic review questions
The WG concluded that insufficient evidence/data exist to support systematic reviews at this time.
Data and research priorities
What predicts the maternal feeding choice to feed human milk at the breast or with a bottle?
What predicts the maternal feeding choice to feed on demand or on schedule?
What is the prevalence of feeding human milk in a bottle?
3. What are the relations of maternal feeding practices and styles (including control, pressure, restriction, responsive/intrusive/indulgent, and responsiveness to satiety and hunger cues) to infant growth and physical development, overweight/obesity, and eating behaviors?
Rationale
Feeding styles and approaches differ greatly between women and across cultures (119–121). The impact of different feeding styles assumes greater importance in light of findings that 9.7% of infants and toddlers in 2009–2010 NHANES have weight-for-recumbent length >95th percentile (76). Furthermore, numerous reports have documented that excessive infant weight gain predicts childhood obesity (122–124). Finally, childhood overweight and obesity predict multiple health and social outcomes in adolescence and adulthood.
Suggested systematic review questions
What is the relation between maternal feeding style (including control, pressure, restriction, responsive/pressuring/indulgent styles, and responsiveness to satiety and hunger cues) and infant/toddler growth and physical development (including rapid weight gain, weight-for-length, BMI, or BMI z score)?
What is the relation between maternal feeding style (including control, pressure, restriction, responsive/pressuring/indulgent styles, and responsiveness to satiety and hunger cues) and infant, toddler, and childhood overweight/obesity?
What is the relation between maternal feeding style (including control, pressure, restriction, responsive/pressuring/indulgent styles, and responsiveness to satiety and hunger cues) and infant/toddler feeding/eating behaviors (eg, speed of eating; Baby Eating Questionnaire subscales)?
Data and research priorities
Research on impacts of other caregivers’ feeding styles on infant/toddler outcomes
Longitudinal studies and RCTs of interventions to increase parenting that is authoritative (ie, high in responsivity and appropriate limit setting)
4. What is the evidence for any benefit or harm of maternal fish consumption during lactation?
Rationale
Awareness has been raised about the potential risks and benefits of seafood consumption in the context of maternal and child health (125). Consequent to the concerns raised, a systematic review of the extant evidence with regard to risk/benefit of maternal seafood consumption is needed to further refine existing recommendations. A specific need is the review of the evidence related to lactating mothers and breast-milk content and infant health outcomes.
Suggested systematic review questions
-
What are the effects of maternal fish consumption on
○ human milk composition and
○ infant growth, immune function, and neurologic development?
Data and research priorities
Randomized trials of interventions to change fish consumption (amount/composition) among pregnant/lactating women.
-
Observational studies including information on all 3 factors of interest and their impact on human milk composition and infant outcomes. The 3 factors are as follows:
○ fish intake,
○ mercury intake/concentrations, and
○ n–3 LC-PUFA intake/concentrations in mothers, their milk, and their infants.
5. What is the relation between lactating mothers’ energy balance (energy intake, energy expenditure, and postpartum weight loss) on breast-milk volume and composition, as well as on infant growth and body composition?
Rationale
More than two-thirds of women in their childbearing years are overweight or obese. In addition, more than one-half of women gain more weight during pregnancy than recommended by the IOM. A bidirectional relation exists between body composition and lactating performance (ie, body composition affects lactation and vice versa) (126–128). Approaches to addressing these relations during pregnancy (129) (eg, exercise, weight loss, diet, etc) and lactation (130, 131) have been explored. Concerns about these relations may lead to practices that might be counterproductive in terms of both maternal needs and lactation success. Thus, women need guidance on how to lose excess weight during lactation, without compromising breast-milk volume and/or composition and, ultimately, infant growth.
Suggested systematic review questions
-
What is the relation between lactating mothers’ energy intake (including “dieting”), energy expenditure (including exercise), and postpartum weight loss (independently and in combination) with
○ human milk volume,
○ human milk composition, and
○ infant growth and/or body composition?
Data and research priorities
Prevalence of “dieting” in lactating women in the United States including potential difference by race-ethnicity
-
Nature of diets commonly used, such as
○ caloric restriction,
○ changes in macronutrient intake (“low carb,” high-protein, etc), and
○ use of dietary supplements as weight-loss aids (nutrient-based supplements and herbals/botanicals)
Is there an ideal timing for interventions to affect body composition postpartum?
Is there a difference between reducing total calories compared with reducing specific macronutrient components (protein, fat, carbohydrate) on weight loss, milk composition/volume, or infant health outcomes?
6. Maternal and caregiver predictors of infant dietary quality (ie, what do we know about maternal and caregiver adherence to dietary recommendations for infants and how can we increase the number of infants and toddlers who would benefit from improved adherence to dietary recommendations, especially low-income/high-risk infants/toddlers?)
Rationale
Disparities have been reported, irrespective of setting, between national dietary recommendations and actual dietary intakes of women, infants, and children (132, 133). Evidence exists to explain how and why mothers/caregivers might be able to implement and adhere to dietary recommendations effectively (134). There is also a growing appreciation of the potential impact of child care providers (beyond parents) on infant and young child diet and nutrition (135, 136). A need exists for analyses to inform policymakers, practitioners, and educators about which mothers/caregivers are most at risk of not adhering to dietary guidelines or best practices, and how to intervene to increase adherence. This topic is a needed research agenda topic after the 2020 DGA is implemented.
Suggested systematic review questions
Because no current guidelines exist that address infants/children aged 0–24 mo, no data/evidence exist that are specifically relevant to this age group. Consequently, no evidence is available to support systematic reviews.
Data and research priorities
(The WG suggested that the following questions be investigated in the future, after guidelines are developed and data can be generated in response to that guidance in relevant target groups.)
What are the maternal and caregiver predictors of infant diet quality and/or adherence to the DGA?
-
Does maternal/caregiver/child care provider adherence to dietary guidelines/best practices affect the infant's or toddler's
○ diet quality or dietary intake and
○ growth and physical development?
-
What are the modifiable mediators (ie, potential intervention targets) in addition to
○ parental socioeconomic status and education,
○ nutrition knowledge, and
○ self-efficacy and motivational variables?
7. What is the influence of maternal dietary intake on micronutrients (including fat- and water-soluble vitamins) and macronutrients (including total fat, n–3 PUFAs, n–6 PUFAs, and trans fats) on human milk composition?
Rationale
A basic understanding exists on the nutrient and bioactive component composition of human milk (137) and the impact of maternal diet and nutritional status on infant nutrient intake (138). Of the dietary essential nutrients, human milk content of lipids (total and fatty acid composition) (139) and specific water-soluble and fat-soluble vitamins (140, 141) are most affected by maternal diet. Hence, breastfeeding mothers will need guidance to ensure optimal human milk composition.
Suggested systematic review questions
What is the relation between maternal dietary water-soluble vitamin intake and human milk water-soluble vitamin composition?
What is the relation between maternal dietary fat-soluble (vitamin) intake and human milk fat-soluble composition?
What is the relation between maternal dietary fat intake and human milk fat composition?
Data and research priorities
Data on specific conditions that might affect milk composition via
changes in maternal intake patterns (eg, consequent to illness and/or treatment)
impact of acute and/or chronic inflammation on milk composition (including amounts of nutrients or immune components)
impact of xenobiotics including bioactive components of food or drugs on nutrient composition with particular emphasis on lipid soluble drugs
8. What are the effects of dietary patterns (eg, vegan, vegetarian, macrobiotic diets) on breast-milk composition?
Rationale
Although a basic knowledge of the nutrient and bioactive components of human milk exists (137), our understanding of the factors that influence that composition is still evolving.
It is clear that maternal intake can affect the concentrations of some nutrients in human milk (eg, lipids and some vitamins), whereas other nutrients are unaffected (eg, protein, lactose, most minerals). Certain restricted or “unconventional” dietary patterns (eg, vegetarianism, vegan diet, macrobiotic diets, etc) may affect these relations (142, 143). Because of the intimate relation between maternal diet/practices and human milk composition, guidance is needed with regard to the impact of restricted dietary patterns on maternal and infant nutrition during lactation.
Suggested systematic review questions
What is the relation between a maternal vegan dietary pattern during lactation and human milk composition?
What is the relation between a maternal vegetarian dietary pattern during lactation and human milk composition?
What is the relation between a maternal macrobiotic diet during lactation and human milk composition?
Data and research priorities
Prevalence of specific dietary patterns in the general population of women of reproductive age
Behavioral/ethnic/cultural predictors
Impact of special dietary patterns on maternal body composition and health during pregnancy/lactation
Impact of maternal special dietary patterns on nutritional status of infants (not just dietary quality)
Impact on infant body composition and neurologic development
9. What are infants/children aged 0–24 mo being fed and what are they consuming in nonparental child care settings?
Rationale
A large number of children in the United States are cared for outside of the home and receive one or more meals and snacks in nonparental child care settings. Today, ∼40% of infants and toddlers are cared for outside of the home for ∼30 h/wk (144). A body of evidence is emerging that documents the impact of differing child care settings on infant/toddler diet and nutrition (145–147). Efforts have been made to provide guidance for dietary/nutritional care in child care settings (148). A need exists for a systematic review of the evidence to provide evidence-based guidance to parents and caregivers and to provide a better understanding of the impact of this important trend.
Suggested systematic review questions
What is the relation between out-of-home feeding and infant/toddler dietary intake?
What is the relation between out-of-home feeding and infant/toddler diet quality?
What is the relation between out-of-home feeding and infant/toddler dietary behaviors?
Data and research priorities
Description of what children are fed in out-of-home care settings [existing data sets can provide some information (NHANES), but additional sources of data are also needed]
Research on more informal types of child care is limited
Research on dietary intake of infants in nonparental child care settings is extremely limited
Are there data to assess potential differences in health outcomes in infant/children in out-of-home child care settings?
10. Effects of maternal alcohol consumption during lactation on milk production, milk composition, and infant outcomes
Rationale
Many perceptions exist about the impact of alcohol on various aspects of lactation (149). A need exists to amalgamate the extant evidence to address the validity of these perceptions.
Suggested systematic review questions
What are the effects of (or associations between) maternal alcohol consumption during lactation on human milk caffeine concentration?
What are the effects of (or associations between) maternal alcohol consumption during lactation on human milk volume (or infant milk consumption)?
What are the effects of (or associations between) maternal alcohol consumption during lactation on infant (ages 0–6 mo) behavior (specifically suckling and sleep)?
Data and research priorities
What data exist with regard to the use of alcohol by lactating women in the United States?
-
Are there groups at greater risk of alcohol use?
○ Behavioral characteristics?
○ Ethnic/cultural influences?
○ Use of other recreational drugs?
Are there common perceptions about the use of alcohol and what are their prevalence?
-
What are the effects of maternal alcohol consumption during lactation on
○ human milk composition (other than alcohol),
○ infant and toddler (ages 0–24 mo) growth and physical development, and
○ infant and toddler (0–24 mo) cognitive, behavioral, and neuromotor development?
Is there a dose-response, safe dose, or upper limit of alcohol that imparts any benefit?
11. Effects (or lack thereof) of maternal caffeine consumption during lactation on milk production, milk composition, and infant outcomes
Rationale
Caffeine exposure continues to increase in the general population. The potential impact of maternal caffeine consumption on infant health has not been clearly delineated (150, 151). A need exists to assess the impact of caffeine exposure on various aspects of lactation as well as on relevant health outcomes in infants.
Suggested systematic review questions
What are the effects of (or associations between) maternal caffeine consumption during lactation on human milk caffeine concentration?
What are the effects of (or associations between) maternal caffeine consumption during lactation on human milk volume (or infant milk consumption)?
What are the effects of (or associations between) maternal caffeine consumption during lactation on infant (ages 0–6 mo) behavior (specifically suckling and sleep) as well as on growth?
Data and research priorities
What data exist with regard to the use of caffeine by lactating women in the United States?
-
Are there groups at greater risk of caffeine use?
○ Behavioral characteristics?
○ Ethnic/cultural influences?
○ Use of other recreational drugs?
Are there common perceptions about the use of caffeine and what are their prevalence?
-
What are the effects of maternal caffeine consumption during lactation on
○ human milk composition,
○ infant caffeine concentrations (ie, does caffeine reach the infant via human milk),
○ infant and toddler (ages 0–24 mo) growth and physical development, and
○ infant and toddler (0–24 mo) cognitive, behavioral, and neuromotor development?
Is there a safe dose of caffeine or an upper limit for its intake that imparts any benefit?
12. How can we assist women across the BMI spectrum to reach national goals for breastfeeding duration?
Rationale
A substantial literature exists that documents an association between higher maternal BMI and difficulty initiating and sustaining breastfeeding in most—but not all—populations that have been studied (111, 115, 127, 152, 153). An evaluation of this literature is needed to provide guidance to mothers who are dealing with this issue.
Suggested systematic review question
What are the effects of maternal body composition/weight status on initiation (ever breastfeeding) and duration of breastfeeding?
Data and research priorities
-
What is the relation between maternal body composition/weight status and
○ human milk volume and
○ human milk composition [macronutrients (fat, protein), micronutrients (vitamins/minerals), and bioactive components (eg, oligosaccharides, lactoferrin, etc)]?
What are the obstacles to achieve current breastfeeding goals (eg, initiation, exclusive breastfeeding for up to 6 mo, etc) and how can women (particularly overweight and obese women) achieve them?
What are the effects of maternal body composition/weight status on growth and development of infants and toddlers from age 0 to 24 mo?
-
What are the plausible biological mechanisms that might explain difficulties in initiating/sustaining breastfeeding in women with a high BMI?
○ What is the role of inflammation?
○ How might such information be used to identify biomarkers of risk for poor breastfeeding performance?
13. What are the effects of maternal probiotic consumption on human milk composition or infant outcomes?
Rationale
The importance of the various human “microbiomes” (gastrointestinal, vaginal, oral, and milk) has emerged as a major focal point of activity, both clinically and from a research perspective. As these microbial populations are better characterized, much information is learned about factors that might affect their growth, maintenance, and effect on host health. The role of maternal probiotic consumption during pregnancy/lactation and its influences on maternal and infant microbiomes remains undetermined. [Note: because this is an emerging area of scientific inquiry, WG 4 (as did WG 1) has designated this as being part of a perceived “research agenda,” rather than a high priority for a systematic literature review at this time. There is very little research on this topic; and with the recent description of the human milk microbiome (154), and its potential as both a pro-/prebiotic (155), this topic will likely assume great importance to infant health.]
Suggested systematic review questions
The WG concluded that insufficient evidence/data exist to support systematic reviews at this time.
Data and research priorities
What is the prevalence of probiotic use in lactating women in the United States (ie, use of any probiotics compared with use of specific probiotics, and if so, which ones)?
-
What is the evidence to support the use of probiotics to
○ improve maternal gastrointestinal function,
○ improve maternal overall health including immune function,
○ improve lactation (performance/milk composition), and
○ improve infant health (growth, immune function)?
Acknowledgments
We thank Richard Olson, Office of Disease Prevention and Health Promotion, DHHS, and Robert Post, Center for Nutrition Policy and Promotion, USDA, for their partnership. We acknowledge the contributions of the NEL team for this project. Special thanks to the FSC, Workshop Planning Committee (WPC), and WG chairs and members for their effort toward this project and to Ronald Kleinman, chair of the WPC, for his support.
The authors’ responsibilities were as follows—DJR, RR, and AP: represent the B-24 Project Secretariat and were responsible for the content of this manuscript; and JEO and JMS: were responsible for the development of the topic briefs, which served as the basis of the WG reports. All of the authors read and approved the final version of the manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: AAP, American Academy of Pediatrics; CVD, cardiovascular disease; DGA, Dietary Guidelines for Americans; DHHS, US Department of Health and Human Services; DRI, Dietary Reference Intake; FAA, free amino acid; FSC, Federal Steering Committee; IFPS, Infant Feeding Practices Study; IGF-I, insulin-like growth factor I; IOM, Institute of Medicine; LC-PUFA, long-chain PUFA; NEL, Nutrition Evidence Library; RCT, randomized controlled trial; WG, working group; WIC, Women, Infants, and Children.
REFERENCES
- 1.National Nutrition Monitoring and Related Research Act of 1990. Public Law 101–445. 1990. Available from: http://www.health.gov/dietaryguidelines/2015-binder/2015/dietaryGuidelinesLegislation.aspx (cited 13 January 2014).
- 2.Feed the Future. Homepage. Available from: http://www.feedthefuture.gov/ (cited 27 June 2013).
- 3.World Bank. Nutrition and early child development. 2011. Available from: http://web.worldbank.org/wbsite/external/topics/extcy/extecd/0,contentmdk:20207804~menupk:528430~pagepk:148956~pipk:216618~thesitepk:344939,00.html (cited 11 July 2013).
- 4.Clinton HR. 1,000 Days: change a life, change the future. US Department of State; September 21, 2010. Available from: http://www.state.gov/secretary/rm/2010/09/147512.htm (cited 11 July 2013).
- 5.Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–7. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr 2013;9(suppl 1):105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ. Human growth and cardiovascular disease. Nestle Nutr Workshop Ser Pediatr Program 2008;61:21–38. [DOI] [PubMed] [Google Scholar]
- 8.Obbagy JE, Blum-Kemelor DM, Essery EV, Lyon JMG, Spahn JM. USDA Nutrition Evidence Library: methodology used to identify topics and develop systematic review questions for the birth-to–24-mo population. Am J Clin Nutr 2014;99(suppl):692S–6S. [DOI] [PubMed] [Google Scholar]
- 9.Chung M, Balk EM, Ip S, Raman G, Yu WW, Trikalinos TA, Lichtenstein AH, Yetley EA, Lau J. Reporting of systematic reviews of micronutrients and health: a critical appraisal. Am J Clin Nutr 2009;89:1099–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SM. Should meta-analyses trump observational studies? Am J Clin Nutr 2013;97:237–8. [DOI] [PubMed] [Google Scholar]
- 11.Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol 2013;1:29–36. [DOI] [PubMed] [Google Scholar]
- 12.Sela DA, Mills DA. The marriage of nutrigenomics with the microbiome: the case of infant-associated bifidobacteria and milk. Am J Clin Nutr 2014;99(suppl):697S–703S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am J Clin Nutr 2014;99(suppl):704S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr 2014;99(suppl):723S–8S. [DOI] [PubMed] [Google Scholar]
- 15.Lönnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr 2014;99(suppl):712S–7S. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsen KF, Greer FR. Protein needs early in life and long-term health. Am J Clin Nutr 2014;99(suppl):718S–22S. [DOI] [PubMed] [Google Scholar]
- 17.Worobey J. Physical activity in infancy: developmental aspects, measurement, and importance. Am J Clin Nutr 2014;99(suppl):729S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briefel RR, Kalb LM, Condon E, Deming DM, Clusen NA, Fox MK, Harnack L, Gemmill E, Stevens M, Reidy KC. The Feeding Infants and Toddlers Study 2008: study design and methods. J Am Diet Assoc 2010;110(suppl):S16–26. [DOI] [PubMed] [Google Scholar]
- 19.Butte NF, Fox MK, Briefel RR, Siega-Riz AM, Dwyer JT, Deming DM, Reidy KC. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J Am Diet Assoc 2010;110(suppl):S27–37. [DOI] [PubMed] [Google Scholar]
- 20.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc 2010;110(suppl):S38–51. [DOI] [PubMed] [Google Scholar]
- 21.Dwyer JT, Butte NF, Deming DM, Siega-Riz AM, Reidy KC. Feeding Infants and Toddlers Study 2008: progress, continuing concerns, and implications. J Am Diet Assoc 2010;110(suppl):S60–7. [DOI] [PubMed] [Google Scholar]
- 22.Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 2014;99(suppl):734S–41S. [DOI] [PubMed] [Google Scholar]
- 23.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics 2008;122(suppl 2):S28–35. [DOI] [PubMed] [Google Scholar]
- 24.Ahluwalia N, Herrick K, Paulose-Ram R, Johnson C. Data needs for B-24 and beyond: NHANES data relevant for nutrition surveillance of infants and young children. Am J Clin Nutr 2014;99(suppl):747S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison GG, Hirschman JD, Owens TA, McNutt SW, Sallack LE. WIC Infant and Toddler Feeding Practices Study: protocol design and implementation. Am J Clin Nutr 2014;99(suppl):742S–6S. [DOI] [PubMed] [Google Scholar]
- 26.Nutrition Evidence Library. USDA's Nutrition Evidence Library. Available from: http://www.cnpp.usda.gov/NEL.htm (cited 21 October 2013).
- 27.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2002;1:CD003517. [DOI] [PubMed] [Google Scholar]
- 28.Bartok CJ. Babies fed breastmilk by breast versus by bottle: a pilot study evaluating early growth patterns. Breastfeed Med 2011;6:117–24. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med 2012;166:431–6. [DOI] [PubMed] [Google Scholar]
- 30.Gdalevich M, Mimouni D, David M, Mimouni M. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol 2001;45:520–7. [DOI] [PubMed] [Google Scholar]
- 31.Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol 2009;161:373–83. [DOI] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Mostrom M, Ludvigsson J, Duchen K. Exclusive breastfeeding and risk of atopic dermatitis in some 8300 infants. Pediatr Allergy Immunol 2005;16:201–8. [DOI] [PubMed] [Google Scholar]
- 33.Sansotta N, Piacentini GL, Mazzei F, Minniti F, Boner AL, Peroni DG. Timing of introduction of solid food and risk of allergic disease development: understanding the evidence. Allergol Immunopathol (Madr) 2012;41:337–45. [DOI] [PubMed] [Google Scholar]
- 34.Nwaru BI, Takkinen HM, Niemela O, Kaila M, Erkkola M, Ahonen S, Tuomi H, Haapala AM, Kenward MG, Pekkanen J, et al. Introduction of complementary foods in infancy and atopic sensitization at the age of 5 years: timing and food diversity in a Finnish birth cohort. Allergy 2013;68:507–16. [DOI] [PubMed] [Google Scholar]
- 35.Järvinen KM, Fleischer DM. Can we prevent food allergy by manipulating the timing of food exposure? Immunol Allergy Clin North Am 2012;32:51–65. [DOI] [PubMed] [Google Scholar]
- 36.Bertino E, Giribaldi M, Baro C, Giancotti V, Pazzi M, Peila C, Tonetto P, Arslanoglu S, Moro GE, Cavallarin L, et al. Effect of prolonged refrigeration on the lipid profile, lipase activity, and oxidative status of human milk. J Pediatr Gastroenterol Nutr 2013;56:390–6. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer J, Klos K, Buettner A. Monitoring aroma changes during human milk storage at +4 degrees C by sensory and quantification experiments. Clin Nutr 2013;32:1036–42. [DOI] [PubMed] [Google Scholar]
- 38.Arslanoglu S, Bertino E, Nicocia M, Moro GE. WAPM Working Group on Nutrition: potential chronobiotic role of human milk in sleep regulation. J Perinat Med 2011;40:1–8. [DOI] [PubMed] [Google Scholar]
- 39.Ventura AK, Beauchamp GK, Mennella JA. Infant regulation of intake: the effect of free glutamate content in infant formulas. Am J Clin Nutr 2012;95:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savino F, Grassino EC, Fissore MF, Guidi C, Liguori SA, Silvestro L, Oggero R, Miniero R. Ghrelin, motilin, insulin concentration in healthy infants in the first months of life: relation to fasting time and anthropometry. Clin Endocrinol (Oxf) 2006;65:158–62. [DOI] [PubMed] [Google Scholar]
- 41.Kelly D, Mulder IE. Microbiome and immunological interactions. Nutr Rev 2012;70(suppl 1):S18–30. [DOI] [PubMed] [Google Scholar]
- 42.Donovan SM, Wang M, Li M, Friedberg I, Schwartz SL, Chapkin RS. Host-microbe interactions in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr 2012;3(suppl):450S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hester SN, Hustead DS, Mackey AD, Singhal A, Marriage BJ. Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab 2012;2012:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raiten DJ, John M, Waters JH. eds. Assessment of nutrient requirements for infant formulas. J Nutr 1998; 128(11 suppl): i–iv, 2059S–293S. [PubMed] [Google Scholar]
- 45.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 46.American Academy of Pediatrics. The use and misuse of fruit juice in pediatrics. Pediatrics 2001;107:1210–3. [DOI] [PubMed] [Google Scholar]
- 47.Skinner JD, Ziegler P, Ponza M. Transitions in infants’ and toddlers’ beverage patterns. J Am Diet Assoc 2004;104(suppl 1):s45–50. [DOI] [PubMed] [Google Scholar]
- 48.Fox MK, Pac S, Devaney B, Jankowski L. Feeding infants and toddlers study: what foods are infants and toddlers eating? J Am Diet Assoc 2004;104(suppl 1):s22–30. [DOI] [PubMed] [Google Scholar]
- 49.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics 2008;122:198–208. [DOI] [PubMed] [Google Scholar]
- 50.Siega-Riz AM, Kinlaw A, Deming DM, Reidy KC. New findings from the Feeding Infants and Toddlers Study 2008. Nestle Nutr Workshop Ser Pediatr Program 2011;68:83–100; discussion 100–5. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. Guiding principles for feeding non-breastfed children 6-24 months of age. Geneva, Switzerland: World Health Organization, 2005.
- 52.Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am J Clin Nutr 2012;96:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and risk for iron deficiency in U.S. infants. Breastfeed Med 2007;2:63–73. [DOI] [PubMed] [Google Scholar]
- 54.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 55.Savino F, Viola S, Tarasco V, Lupica MM, Castagno E, Oggero R, Miniero R. Bone mineral status in breast-fed infants: influence of vitamin D supplementation. Eur J Clin Nutr 2011;65:335–9. [DOI] [PubMed] [Google Scholar]
- 56.Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics 2010;125:627–32. [DOI] [PubMed] [Google Scholar]
- 57.Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, et al. Vitamin D in the healthy paediatric population: a position paper by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2013;56:692–701. [DOI] [PubMed] [Google Scholar]
- 58.Mangels R, Driggers J. The youngest vegetarians: vegetarian infants and toddlers. ICAN 2012;4:8–20. [Google Scholar]
- 59.Simmer K, Patole SK, Rao SC. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 2011;12:CD000376. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. Using fluoride to prevent and control tooth decay in the United States. Atlanta, GA: Centers for Disease Control and Prevention, 2011.
- 61.Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond) 2013;37:1295–1306. [DOI] [PubMed] [Google Scholar]
- 62.Pearce J, Langley-Evans SC. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond) 2013;37:477–85. [DOI] [PubMed] [Google Scholar]
- 63.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001;107:E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mennella JA, Beauchamp GK. Mothers’ milk enhances the acceptance of cereal during weaning. Pediatr Res 1997;41:188–92. [DOI] [PubMed] [Google Scholar]
- 65.Trabulsi JC, Mennella JA. Diet, sensitive periods in flavour learning, and growth. Int Rev Psychiatry 2012;24:219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drewnowski A, Mennella JA, Johnson SL, Bellisle F. Sweetness and food preference. J Nutr 2012;142(suppl):1142S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein LJ, Cowart BJ, Beauchamp GK. The development of salty taste acceptance is related to dietary experience in human infants: a prospective study. Am J Clin Nutr 2012;95:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crystal SR, Bernstein IL. Infant salt preference and mother's morning sickness. Appetite 1998;30:297–307. [DOI] [PubMed] [Google Scholar]
- 69.Mennella JA. The sweet taste of childhood. In:Firestein S, Beauchamp G. eds. Olfaction and taste. San Diego, CA: Academic Press, 2008:183–8. [Google Scholar]
- 70.Elliott CD. Sweet and salty: nutritional content and analysis of baby and toddler foods. J Public Health (Oxf) 2011;33:63–70. [DOI] [PubMed] [Google Scholar]
- 71.Woo JG, Guerrero ML, Ruiz-Palacios GM, Peng YM, Herbers PM, Yao W, Ortega H, Davidson BS, McMahon RJ, Morrow AL. Specific infant feeding practices do not consistently explain variation in anthropometry at age 1 year in urban United States, Mexico, and China cohorts. J Nutr 2013;143:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jonsdottir OH, Thorsdottir I, Hibberd PL, Fewtrell MS, Wells JC, Palsson GI, Lucas A, Gunnlaugsson G, Kleinman RE. Timing of the introduction of complementary foods in infancy: a randomized controlled trial. Pediatrics 2012;130:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.The National Association of Sports and Physical Education. Active start: a statement of physical activity guidelines for children from birth to age 5. 2nd ed. 2013. Available from: http://www.aahperd.org/naspe/standards/nationalGuidelines/ActiveStart.cfm (cited 11 July 2013).
- 74.Kadey HJ, Roane HS. Effects of access to a stimulating object on infant behavior during tummy time. J Appl Behav Anal 2012;45:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Food and Nutrition Service. WIC food packages. 2012. Available from: http://www.fns.usda.gov/wic/benefitsandservices/foodpkg.htm (cited 11 July 2013).
- 76.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flores G, Lin H. Factors predicting overweight in US kindergartners. Am J Clin Nutr 2013;97:1178–87. [DOI] [PubMed] [Google Scholar]
- 78.Jordan PN, Hall KD. Dynamic coordination of macronutrient balance during infant growth: insights from a mathematical model. Am J Clin Nutr 2008;87:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butte NF, Wong WW, Hopkinson JM, Heinz CJ, Mehta NR, Smith EO. Energy requirements derived from total energy expenditure and energy deposition during the first 2 y of life. Am J Clin Nutr 2000;72:1558–69. [DOI] [PubMed] [Google Scholar]
- 80.Briefel R, Hanson C, Fox MK, Novak T, Ziegler P. Feeding Infants and Toddlers Study: do vitamin and mineral supplements contribute to nutrient adequacy or excess among US infants and toddlers? J Am Diet Assoc 2006;106(suppl 1):S52–65. [DOI] [PubMed] [Google Scholar]
- 81.Smith PJ, Moffatt ME. Baby-bottle tooth decay: are we on the right track? Int J Circumpolar Health 1998;57(suppl 1):155–62. [PubMed] [Google Scholar]
- 82.Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011;127:e544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sloan S, Gildea A, Stewart M, Sneddon H, Iwaniec D. Early weaning is related to weight and rate of weight gain in infancy. Child Care Health Dev 2008;34:59–64. [DOI] [PubMed] [Google Scholar]
- 84.Moorcroft KE, Marshall JL, McCormick FM. Association between timing of introducing solid foods and obesity in infancy and childhood: a systematic review. Matern Child Nutr 2011;7:3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fox MK, Condon E, Briefel RR, Reidy KC, Deming DM. Food consumption patterns of young preschoolers: are they starting off on the right path? J Am Diet Assoc 2010;110(suppl):S52–9. [DOI] [PubMed] [Google Scholar]
- 86.O'Connor TM, Yang SJ, Nicklas TA. Beverage intake among preschool children and its effect on weight status. Pediatrics 2006;118:e1010–8. [DOI] [PubMed] [Google Scholar]
- 87.Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet 2013;113:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maguire JL, Lebovic G, Kandasamy S, Khovratovich M, Mamdani M, Birken CS, Parkin PC. The relationship between cow's milk and stores of vitamin D and iron in early childhood. Pediatrics 2013;131:e144–51. [DOI] [PubMed] [Google Scholar]
- 89.Taylor BJ, Heath AL, Galland BC, Gray AR, Lawrence JA, Sayers RM, Dale K, Coppell KJ, Taylor RW. Prevention of Overweight in Infancy (POI.nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health 2011;11:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dattilo AM, Birch L, Krebs NF, Lake A, Taveras EM, Saavedra JM. Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. J Obes 2012;2012:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med 2008;162:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nevarez MD, Rifas-Shiman SL, Kleinman KP, Gillman MW, Taveras EM. Associations of early life risk factors with infant sleep duration. Acad Pediatr 2010;10:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics 2005;115(suppl):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kataria S, Swanson MS, Trevathan GE. Persistence of sleep disturbances in preschool children. J Pediatr 1987;110:642–6. [DOI] [PubMed] [Google Scholar]
- 95.Fisher J, Birch LL. Early experience with food and eating: influencing risk for the development of disordered eating and problems of energy balance In: Thompson K, Smolak L. eds. Body image, eating disorders, and obesity in youth: assessment, prevention, and treatment. 2nd ed. Washington, DC: American Psychological Association, 2009. [Google Scholar]
- 96.Ventura A, Johnson SL, Birch LL. Children's eating: the development of food-acceptance patterns In: Essa E, Burnham M. eds. Informing our practice: useful research on young children's development. Washington, DC: National Association for the Education of Young Children, 2009. [Google Scholar]
- 97.Ford C, Ward D, White M. Television viewing associated with adverse dietary outcomes in children ages 2-6. Obes Rev 2012;13:1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zimmerman FJ. Using marketing muscle to sell fat: the rise of obesity in the modern economy. Annu Rev Public Health 2011;32:285–306. [DOI] [PubMed] [Google Scholar]
- 99.Slining MM, Popkin BM. Trends in intakes and sources of solid fats and added sugars among U.S. children and adolescents: 1994-2010. Pediatr Obes 2013;8:307–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coleman-Jensen A, Nord M, Andrews M, Carlson S. Household food security in the United States in 2010. Washington, DC: USDA, 2011. Available from: http://www.ers.usda.gov/Publications/err125/ (cited 23 February 2013).
- 101.Skalicky A, Meyers AF, Adams WG, Yang Z, Cook JT, Frank DA. Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern Child Health J 2006;10:177–85. [DOI] [PubMed] [Google Scholar]
- 102.Cook JT, Frank DA, Berkowitz C, Black MM, Casey PH, Cutts DB, Meyers AF, Zaldivar N, Skalicky A, Levenson S, et al. Food insecurity is associated with adverse health outcomes among human infants and toddlers. J Nutr 2004;134:1432–8. [DOI] [PubMed] [Google Scholar]
- 103.Rose-Jacobs R, Black MM, Casey PH, Cook JT, Cutts DB, Chilton M, Heeren T, Levenson SM, Meyers AF, Frank DA. Household food insecurity: associations with at-risk infant and toddler development. Pediatrics 2008;121:65–72. [DOI] [PubMed] [Google Scholar]
- 104.Zaslow M, Bronte-Tinkew J, Capps R, Horowitz A, Moore KA, Weinstein D. Food security during infancy: implications for attachment and mental proficiency in toddlerhood. Matern Child Health J 2009;13:66–80. [DOI] [PubMed] [Google Scholar]
- 105.Cyr C. Preventing choking and suffocation in children. Paediatr Child Health 2012;17:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Altkorn R, Chen X, Milkovich S, Stool D, Rider G, Bailey CM, Haas A, Riding KH, Pransky SM, Reilly JS. Fatal and non-fatal food injuries among children (aged 0-14 years). Int J Pediatr Otorhinolaryngol 2008;72:1041–6. [DOI] [PubMed] [Google Scholar]
- 107.Piccinelli R, Pandelova M, Le Donne C, Ferrari M, Schramm KW, Leclercq C. Design and preparation of market baskets of European Union commercial baby foods for the assessment of infant exposure to food chemicals and to their effects. Food Addit Contam Part A 2010;27:1337–51. [DOI] [PubMed] [Google Scholar]
- 108.Koletzko B, Shamir R, Ashwell M. Quality and safety aspects of infant nutrition. Ann Nutr Metab 2012;60:179–84. [DOI] [PubMed] [Google Scholar]
- 109.Turck D. Safety aspects in preparation and handling of infant food. Ann Nutr Metab 2012;60:211–4. [DOI] [PubMed] [Google Scholar]
- 110.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003;112:607–19. [DOI] [PubMed] [Google Scholar]
- 111.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr 2010;92:574–84. [DOI] [PubMed] [Google Scholar]
- 112.Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr 2012;95:1113–35. [DOI] [PubMed] [Google Scholar]
- 113.Castellote C, Casillas R, Ramirez-Santana C, Perez-Cano FJ, Castell M, Moretones MG, Lopez-Sabater MC, Franch A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr 2011;141:1181–7. [DOI] [PubMed] [Google Scholar]
- 114.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr 2007;27:103–21. [DOI] [PubMed] [Google Scholar]
- 115.Lovelady CA. Is maternal obesity a cause of poor lactation performance. Nutr Rev 2005;63:352–5. [DOI] [PubMed] [Google Scholar]
- 116.Nommsen-Rivers LA, Dolan LM, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med 2012;7:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crepinsek MA, Crowe L, Michener K, Smart NA. Interventions for preventing mastitis after childbirth. Cochrane Database Syst Rev 2012;10:CD007239. [DOI] [PubMed] [Google Scholar]
- 118.Kvist LJ. Toward a clarification of the concept of mastitis as used in empirical studies of breast inflammation during lactation. J Hum Lact 2010;26:53–9. [DOI] [PubMed] [Google Scholar]
- 119.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics 1998;101:539–49. [PubMed] [Google Scholar]
- 120.Lumeng JC, Ozbeki TN, Appugliese DP, Kaciroti N, Corwyn RF, Bradley RH. Observed assertive and intrusive maternal feeding behaviors increase child adiposity. Am J Clin Nutr 2012;95:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thompson AL, Adair LS, Bentley ME. Pressuring and restrictive feeding styles influence infant feeding and size among a low-income African-American sample. Obesity (Silver Spring) 2013;21:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, Ekelund U, Levy-Marchal C, Jarvelin MR, Kuh D, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol 2012;26:19–26. [DOI] [PubMed] [Google Scholar]
- 123.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med 2011;165:993–8. [DOI] [PubMed] [Google Scholar]
- 124.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009;123:1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lando AM, Fein SB, Choiniere CJ. Awareness of methylmercury in fish and fish consumption among pregnant and postpartum women and women of childbearing age in the United States. Environ Res 2012;116:85–92. [DOI] [PubMed] [Google Scholar]
- 126.Winkvist A, Rasmussen KM. Impact of lactation on maternal body weight and body composition. J Mammary Gland Biol Neoplasia 1999;4:309–18. [DOI] [PubMed] [Google Scholar]
- 127.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wojcicki JM. Maternal prepregnancy body mass index and initiation and duration of breastfeeding: a review of the literature. J Womens Health (Larchmt) 2011;20:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen TR, Koski KG. Limiting excess weight gain in healthy pregnant women: importance of energy intakes, physical activity, and adherence to gestational weight gain guidelines. J Pregnancy 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCrory MA, Nommsen-Rivers LA, Mole PA, Lonnerdal B, Dewey KG. Randomized trial of the short-term effects of dieting compared with dieting plus aerobic exercise on lactation performance. Am J Clin Nutr 1999;69:959–67. [DOI] [PubMed] [Google Scholar]
- 131.Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J Med 2000;342:449–53. [DOI] [PubMed] [Google Scholar]
- 132.Thorisdottir AV, Gunnarsdottir I, Thorsdottir I. Revised infant dietary recommendations: the impact of maternal education and other parental factors on adherence rates in Iceland. Acta Paediatr 2013;102:143–8. [DOI] [PubMed] [Google Scholar]
- 133.Dubois L, Farmer A, Girard M, Burnier D, Porcherie M. Demographic and socio-economic factors related to food intake and adherence to nutritional recommendations in a cohort of pre-school children. Public Health Nutr 2011;14:1096–104. [DOI] [PubMed] [Google Scholar]
- 134.Wojcicki JM, Gugig R, Kathiravan S, Holbrook K, Heyman MB. Maternal knowledge of infant feeding guidelines and label reading behaviours in a population of new mothers in San Francisco, California. Matern Child Nutr 2009;5:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pearce A, Li L, Abbas J, Ferguson B, Graham H, Law C. Is childcare associated with the risk of overweight and obesity in the early years? Findings from the UK Millennium Cohort Study. Int J Obes (Lond) 2010;34:1160–8. [DOI] [PubMed] [Google Scholar]
- 136.Kim J, Peterson KE. Association of infant child care with infant feeding practices and weight gain among US infants. Arch Pediatr Adolesc Med 2008;162:627–33. [DOI] [PubMed] [Google Scholar]
- 137.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chapman DJ, Nommsen-Rivers L. Impact of maternal nutritional status on human milk quality and infant outcomes: an update on key nutrients. Adv Nutr 2012;3:351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rudolph MC, Neville MC, Anderson SM. Lipid synthesis in lactation: diet and the fatty acid switch. J Mammary Gland Biol Neoplasia 2007;12:269–81. [DOI] [PubMed] [Google Scholar]
- 140.Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr 2012;3:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dawodu A, Tsang RC. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr 2012;3:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baatenburg de Jong R, Bekhof J, Roorda R, Zwart P. Severe nutritional vitamin deficiency in a breast-fed infant of a vegan mother. Eur J Pediatr 2005;164:259–60. [DOI] [PubMed] [Google Scholar]
- 143.Dagnelie PC, van Staveren WA, Roos AH, Tuinstra LG, Burema J. Nutrients and contaminants in human milk from mothers on macrobiotic and omnivorous diets. Eur J Clin Nutr 1992;46:355–66. [PubMed] [Google Scholar]
- 144.United States Department of Education; National Center for Education Statistics. Child care and early education arrangements of infants, toddlers, and preschoolers: 2001. Available from: http://nces.ed.gov/pubs2006/2006039.pdf (cited 13 January 2014).
- 145.Nicklas TA, Baranowski T, Baranowski JC, Cullen K, Rittenberry L, Olvera N. Family and child-care provider influences on preschool children's fruit, juice, and vegetable consumption. Nutr Rev 2001;59:224–35. [DOI] [PubMed] [Google Scholar]
- 146.Fleischhacker S, Cason KL, Achterberg C. “You had peas today?”: a pilot study comparing a Head Start child-care center's menu with the actual food served. J Am Diet Assoc 2006;106:277–80. [DOI] [PubMed] [Google Scholar]
- 147.Erinosho T, Dixon LB, Young C, Brotman LM, Hayman LL. Nutrition practices and children's dietary intakes at 40 child-care centers in New York City. J Am Diet Assoc 2011;111:1391–7. [DOI] [PubMed] [Google Scholar]
- 148.Benjamin Neelon SE, Briley ME. Position of the American Dietetic Association: benchmarks for nutrition in child care. J Am Diet Assoc 2011;111:607–15. [DOI] [PubMed] [Google Scholar]
- 149.Mennella J. Alcohol's effect on lactation. Alcohol Res Health 2001;25:230–4. [PMC free article] [PubMed] [Google Scholar]
- 150.Nehlig A, Debry G. Consequences on the newborn of chronic maternal consumption of coffee during gestation and lactation: a review. J Am Coll Nutr 1994;13:6–21. [DOI] [PubMed] [Google Scholar]
- 151.Santos IS, Matijasevich A, Domingues MR. Maternal caffeine consumption and infant nighttime waking: prospective cohort study. Pediatrics 2012;129:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lepe M, Bacardi Gascon M, Castaneda-Gonzalez LM, Perez Morales ME, Jimenez Cruz A. Effect of maternal obesity on lactation: systematic review. Nutr Hosp 2011;26:1266–9. [DOI] [PubMed] [Google Scholar]
- 153.Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–51. [DOI] [PubMed] [Google Scholar]
- 155.Barile D, Rastall RA. Human milk and related oligosaccharides as prebiotics. Curr Opin Biotechnol 2013;24:214–9. [DOI] [PubMed] [Google Scholar]