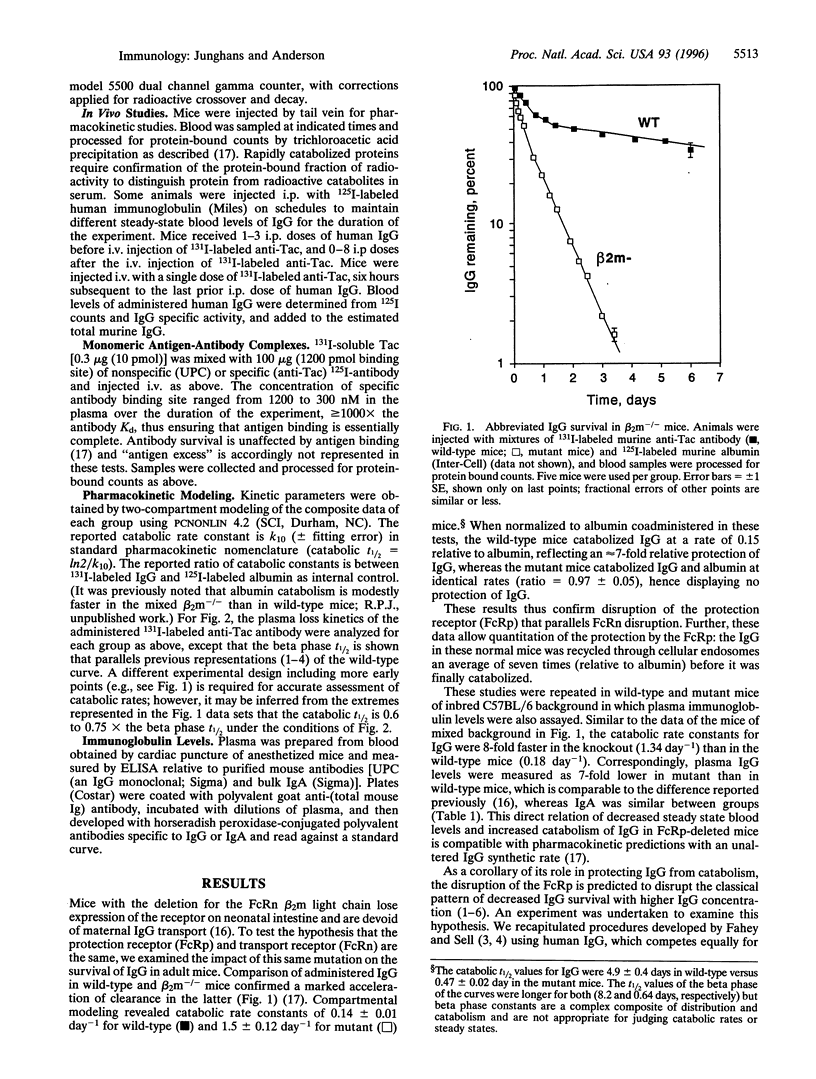
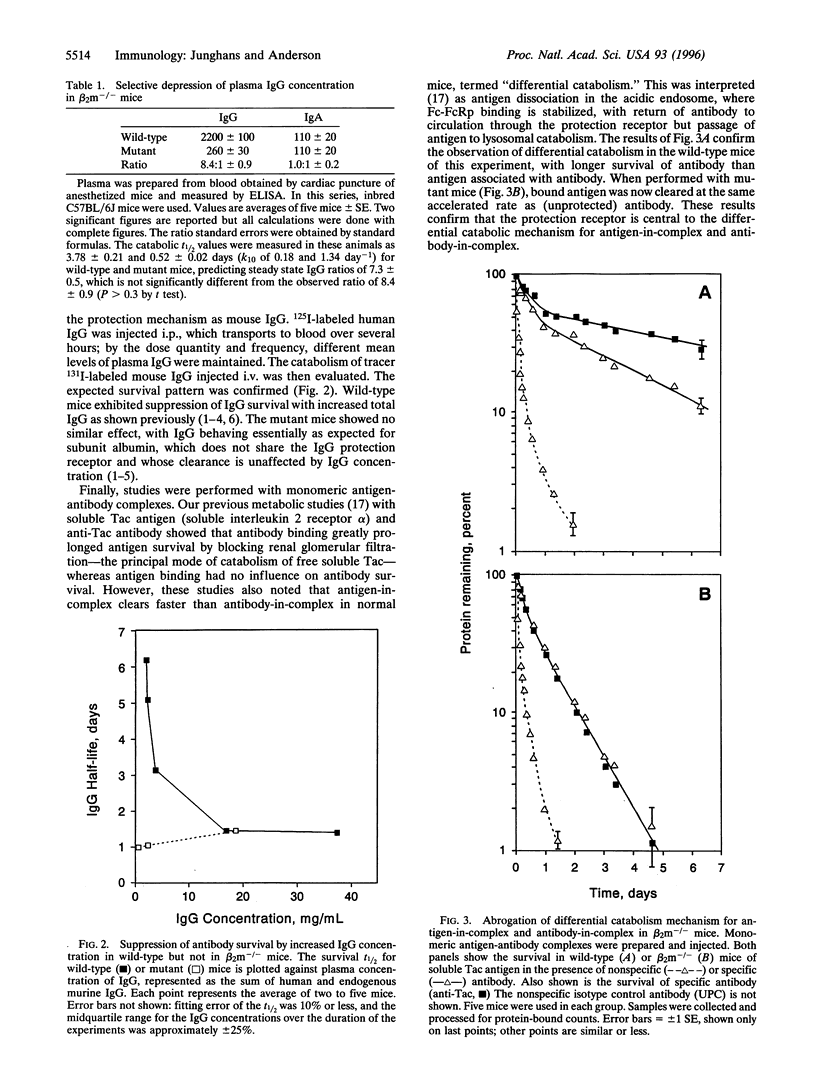
Abstract
More than 30 years ago, Brambell published the hypothesis bearing his name [Brambell, F. W. R., Hemmings, W. A. & Morris, 1. C. (1964) Nature (London) 203, 1352-1355] that remains as the cornerstone for thinking on IgG catabolism. To explain the long survival of IgG relative to other plasma proteins and its pattern of increased fractional catabolism with high concentrations of IgG, Brambell postulated specific IgG "protection receptors" (FcRp) that would bind IgG in pinocytic vacuoles and redirect its transport to the circulation; when the FcRp was saturated, the excess unbound IgG then would pass to unrestricted lysosomal catabolism. Brambell subsequently postulated the neonatal gut transport receptor (FcRn) and showed its similar saturable character. FcRn was recently cloned but FcRp has not been identified. Using a genetic knockout that disrupts the FcRn and intestinal IgG transport, we show that this lesion also disrupts the IgG protection receptor, supporting the identity of these two receptors. IgG catabolism was 10-fold faster and IgG levels were correspondingly lower in mutant than in wild-type mice, whereas IgA was the same between groups, demonstrating the specific effects on the IgG system. Disruption of the FcRp in the mutant mice was also shown to abrogate the classical pattern of decreased IgG survival with higher IgC concentration. Finally, studies in normal mice with monomeric antigen-antibody complexes showed differential catabolism in which antigen dissociates in the endosome and passes to the lysosome, whereas the associated antibody is returned to circulation; in mutant mice, differential catabolism was lost and the whole complex cleared at the same accelerated rate as albumin, showing the central role of the FcRp to the differential catabolism mechanism. Thus, the same receptor protein that mediates the function of the FcRn transiently in the neonate is shown to have its functionally dominant expression as the FcRp throughout life, resolving a longstanding mystery of the identity of the receptor for the protection of IgG. This result also identifies an important new member of the class of recycling surface receptors and enables the design of protein adaptations to exploit this mechanism to improve survivals of other therapeutic proteins in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAMBELL F. W., HEMMINGS W. A., MORRIS I. G. A THEORETICAL MODEL OF GAMMA-GLOBULIN CATABOLISM. Nature. 1964 Sep 26;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- Brambell F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966 Nov 19;2(7473):1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P., Huber A. H., Bjorkman P. J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994 Nov 24;372(6504):379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Nolan J. A., Conrad P. J., Vasavada H. A., Vasavada H. H., Ploegh H. L., Ganguly S., Janeway C. A., Jr, Weissman S. M. Tissue-specific and cell surface expression of human major histocompatibility complex class I heavy (HLA-B7) and light (beta 2-microglobulin) chain genes in transgenic mice. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7690–7694. doi: 10.1073/pnas.85.20.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetie V., Hubbard J. G., Kim J. K., Tsen M. F., Lee Y., Ward E. S. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996 Mar;26(3):690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- Henderson L. A., Baynes J. W., Thorpe S. R. Identification of the sites of IgG catabolism in the rat. Arch Biochem Biophys. 1982 Apr 15;215(1):1–11. doi: 10.1016/0003-9861(82)90272-7. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Fahey J. L. THE METABOLISM OF NORMAL PLASMA PROTEINS AND GAMMA-MYELOMA PROTEIN IN MICE BEARING PLASMA-CELL TUMORS. J Clin Invest. 1961 Sep;40(9):1696–1705. doi: 10.1172/JCI104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel E. J., Patel V. K., Taylor S. F., Marshak-Rothstein A., Simister N. E. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol. 1995 Jun 15;154(12):6246–6251. [PubMed] [Google Scholar]
- Jones E. A., Waldmann T. A. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972 Nov;51(11):2916–2927. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Dobbs D., Brechbiel M. W., Mirzadeh S., Raubitschek A. A., Gansow O. A., Waldmann T. A. Pharmacokinetics and bioactivity of 1,4,7,10-tetra-azacylododecane off',N'',N'''-tetraacetic acid (DOTA)-bismuth-conjugated anti-Tac antibody for alpha-emitter (212Bi) therapy. Cancer Res. 1993 Dec 1;53(23):5683–5689. [PubMed] [Google Scholar]
- Junghans R. P., Waldmann T. A. Metabolism of Tac (IL2Ralpha): physiology of cell surface shedding and renal catabolism, and suppression of catabolism by antibody binding. J Exp Med. 1996 Apr 1;183(4):1587–1602. doi: 10.1084/jem.183.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. K., Tsen M. F., Ghetie V., Ward E. S. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994 Oct;24(10):2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Janeway C. A., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993 Apr;14(4):151–154. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J Cell Biol. 1984 Apr;98(4):1163–1169. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V. Fc receptors: rubor redux. Cell. 1994 Aug 26;78(4):553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S. EVIDENCE FOR SPECIES' DIFFERENCES IN THE EFFECT OF SERUM GAMMA-GLOBULIN CONCENTRATION ON GAMMA-GLOBULIN CATABOLISM. J Exp Med. 1964 Nov 1;120:967–986. doi: 10.1084/jem.120.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S., FAHEY J. L. RELATIONSHIP BETWEEN GAMMA-GLOBULIN METABOLISM AND LOW SERUM GAMMA-GLOBULIN IN GERMFREE MICE. J Immunol. 1964 Jul;93:81–87. [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Story C. M., Mikulska J. E., Simister N. E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994 Dec 1;180(6):2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wawrzynczak E. J., Denham S., Parnell G. D., Cumber A. J., Jones P. T., Winter G. Recombinant mouse monoclonal antibodies with single amino acid substitutions affecting Clq and high affinity Fc receptor binding have identical serum half-lives in the BALB/c mouse. Mol Immunol. 1992 Feb;29(2):221–227. doi: 10.1016/0161-5890(92)90103-5. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]



