GBA1 mutations reduce glucocerebrosidase expression and increase the risk of Parkinson’s disease. Murphy et al. assess glucocerebrosidase levels and consequent cellular changes in sporadic Parkinson’s disease. An early reduction in glucocerebrosidase precedes α-synuclein accumulation and relates to reduced chaperone-mediated autophagy, increased α-synuclein protein and decreased ceramide in sporadic Parkinson’s disease.
Keywords: Parkinson’s disease, glucocerebrosidase, α-synuclein, chaperone-mediated autophagy, ceramide
Abstract
Heterozygous mutations in GBA1, the gene encoding lysosomal glucocerebrosidase, are the most frequent known genetic risk factor for Parkinson’s disease. Reduced glucocerebrosidase and α-synuclein accumulation are directly related in cell models of Parkinson’s disease. We investigated relationships between Parkinson’s disease-specific glucocerebrosidase deficits, glucocerebrosidase-related pathways, and α-synuclein levels in brain tissue from subjects with sporadic Parkinson’s disease without GBA1 mutations. Brain regions with and without a Parkinson’s disease-related increase in α-synuclein levels were assessed in autopsy samples from subjects with sporadic Parkinson’s disease (n = 19) and age- and post-mortem delay-matched controls (n = 10). Levels of glucocerebrosidase, α-synuclein and related lysosomal and autophagic proteins were assessed by western blotting. Glucocerebrosidase enzyme activity was measured using a fluorimetric assay, and glucocerebrosidase and α-synuclein messenger RNA expression determined by quantitative polymerase chain reaction. Related sphingolipids were analysed by mass spectrometry. Multivariate statistical analyses were performed to identify differences between disease groups and regions, with non-parametric correlations used to identify relationships between variables. Glucocerebrosidase protein levels and enzyme activity were selectively reduced in the early stages of Parkinson’s disease in regions with increased α-synuclein levels although limited inclusion formation, whereas GBA1 messenger RNA expression was non-selectively reduced in Parkinson’s disease. The selective loss of lysosomal glucocerebrosidase was directly related to reduced lysosomal chaperone-mediated autophagy, increased α-synuclein and decreased ceramide. Glucocerebrosidase deficits in sporadic Parkinson’s disease are related to the abnormal accumulation of α-synuclein and are associated with substantial alterations in lysosomal chaperone-mediated autophagy pathways and lipid metabolism. Our data suggest that the early selective Parkinson’s disease changes are likely a result of the redistribution of cellular membrane proteins leading to a chronic reduction in lysosome function in brain regions vulnerable to Parkinson’s disease pathology.
Introduction
Homozygous mutations in the glucocerebrosidase gene (GBA1) cause Gaucher disease, a lysosomal storage disorder characterized by the accumulation of the lipid glucocerebroside in cells as a result of the functional loss of the lysosomal enzyme glucocerebrosidase (Hruska et al., 2008). Although considered non-neuropathic, patients with type 1, the mildest form of Gaucher disease, have a significantly increased risk for developing clinical Parkinson’s disease (Bultron et al., 2010), with pathological Lewy bodies at autopsy (DePaolo et al., 2009). Parkinson’s disease and associated Lewy body disorders are also observed in heterozygous GBA1 mutation carriers (Sidransky et al., 2009; Nalls et al., 2013), with similar levels of pathological Lewy bodies compared with sporadic Parkinson’s disease (Parkkinen et al., 2011). In large cohorts of patients with Parkinson’s disease, 4–7% were found to carry heterozygous GBA1 mutations (Neumann et al., 2009; Sidransky et al., 2009), rendering mutations in this gene the most common genetic risk factor for Parkinson’s disease (Leverenz et al., 2009).
All cases of sporadic Parkinson’s disease are characterized by the abnormal accumulation and aggregation of the neuronal protein α-synuclein in inclusions called Lewy bodies (Dickson et al., 2009). Normal α-synuclein is degraded primarily through lysosomal autophagic mechanisms (Cuervo et al., 2004; Vogiatzi et al., 2008), and the insoluble accumulation of this protein indicates defective lysosomal function in Parkinson’s disease (Boya and Kroemer, 2008; Martinez-Vicente et al., 2008; Alvarez-Erviti et al., 2010; Dehay et al., 2010). In cell and animal studies, decreased wild-type glucocerebrosidase leads to α-synuclein accumulation, and increased α-synuclein inhibits normal glucocerebrosidase function (Manning-Bog et al., 2009; Mazzulli et al., 2011; Yap et al., 2011). In post-mortem brain tissue from patients with Parkinson’s disease and GBA1 mutations, glucocerebrosidase and α-synuclein colocalize in Lewy bodies (Goker-Alpan et al., 2010) and aggregated forms of α-synuclein are present (Choi et al., 2011). Recent reports show reduced glucocerebrosidase protein levels and enzyme activity in a range of affected brain regions in patients with Parkinson’s disease (Gegg et al., 2012), although whether this reduction relates to the amount of accumulated α-synuclein has not been determined. The reason for reduced glucocerebrosidase in patients with Parkinson’s disease without GBA1 mutations remains unknown.
This study assessed Parkinson’s disease-specific changes in glucocerebrosidase expression and function in two brain regions, one with increased α-synuclein levels in Parkinson’s disease (anterior cingulate cortex) and one without (occipital cortex). Proteins and sphingolipids in related lysosomal, autophagic and sphingolipid pathways were assessed to identify the cellular mechanisms most disrupted. Our aim was to determine whether deficient glucocerebrosidase, changes in glucocerebrosidase-related pathways, and increased α-synuclein levels were related in patients with sporadic Parkinson’s disease without GBA1 mutations to identify potential therapeutic targets and early disease biomarkers.
Materials and methods
Cases
Brain samples from longitudinally followed, autopsy-confirmed subjects with Parkinson’s disease (n = 19) and age- and post-mortem delay-matched neurological and neuropathological controls (n = 10; Table 1) were obtained from the Sydney Brain Bank and New South Wales Tissue Resource Centre after study approval and with appropriate institutional ethics approval. All cases with Parkinson’s disease were levodopa-responsive, had no other neurodegenerative conditions, and met the UK Brain Bank Clinical Criteria for diagnosis of Parkinson’s disease (Gibb and Lees, 1988). Parkinson’s disease cases with few concurrent non-Parkinson’s disease-related pathologies were selected (Montine et al., 2012) as well as a group with later stages of Parkinson’s disease, many of whom had dementia based on assessments within 2 years of their death using the clinical dementia rating (Morris, 1997) (Table 1). All cases and controls were of non-Jewish ancestry.
Table 1.
Demographic details for early and later stage Parkinson’s disease and control cohorts
| Diagnosis | Sex (M:F)a | Age at death (years)b | Post-mortem delay (hours)c | Disease duration (years)d | Braak Lewy stage (/6) | CERAD plaque score (/3)e | Braak neuritic stage (/6)e | Clinical dementia rating (/3)f |
|---|---|---|---|---|---|---|---|---|
| Early PD (n = 7) | 6M:1F | 78.3 ± 2.4 (71–88) | 13.7 ± 3.0 (3–24) | 14.9 ± 1.6 (8–20) | 7IV | 0.6 ± 0.3 (0–2) | 1.1 ± 0.3 (0–2) | 0 |
| Late PD (n = 12) | 8M:4F | 77.8 ± 1.3 (69–85) | 16.2 ± 3.6 (3–42) | 15.2 ± 2.2 (7–36) | 7V:5VI | 1.0 ± 0.3 (0–3) | 1.2 ± 0.4 (0–4) | 1.9 ± 0.3 (1–3) |
| Control (n = 10) | 5M:5F | 74.7 ± 2.9 (60–88) | 18.0 ± 3.2 (7–35) | – | – | 0.3 ± 0.2 (0–2) | 0.6 ± 0.3 (0–2) | 0 |
aNot significantly different between groups (Pearson chi-square, P = 0.31).
bNot significantly different between groups (one-way ANOVA, P = 0.48).
cNot significantly different between groups (one-way ANOVA, P = 0.72).
dNot significantly different between groups (independent samples t-test, P = 0.91).
eParkinson’s disease cases and controls do not meet diagnostic criteria for Alzheimer’s disease (Montine et al., 2012), with CERAD scores and Braak neuritic stages not significantly different between groups (one-way ANOVA, P = 0.16 and P = 0.44, respectively).
fLater stage Parkinson’s disease cases were significantly demented compared to both early stage Parkinson’s disease cases and controls (one-way ANOVA with post hoc Bonferroni comparisons, P < 0.0001), with early stage Parkinson’s disease cases and controls not significantly different (P = 1.0).
CERAD = Consortium to Establish a Registry for Alzheimer's Disease; PD = Parkinson’s disease.
Values are given as mean ± standard error and range for age at death, post-mortem delay, disease duration, Parkinson’s disease severity (Braak Lewy stage, Braak et al., 2003), Alzheimer’s disease severity (CERAD plaque score and Braak neuritic stage), and clinical dementia rating.
DNA extraction
Genomic DNA was extracted from 50–100 mg of fresh-frozen occipital cortex tissue from each of the 19 cases with Parkinson’s disease using a proteinase K and phenol method for the isolation of high molecular weight mammalian DNA (Sambrook and Russell, 2006), modified for tissue samples. Briefly, minced tissue samples were suspended in lysis buffer (0.32 M sucrose, 10 mM Tris-HCl pH 7.5, 5 mM MgCl2, 1% Triton™ X-100), centrifuged at 3000 rpm for 10 min at 4°C, and the resulting pellet repeatedly washed in lysis buffer before overnight incubation at 55°C in digestion buffer (50 mM Tris-HCl pH 8.5, 1 mM EDTA, 0.16 M NaCl, 20 μg/ml proteinase K and 0.4% SDS). Repeated addition of phenol:chloroform:isoamyl alcohol (25:24:1; Sigma P2069) and centrifugation at 3000 rpm for 20 min at 4°C resulted in the isolation of DNA in the upper aqueous phase. DNA was precipitated by the addition of 100% ethanol and pelleted by centrifugation at 13 000rpm for 5 min at room temperature. The final DNA pellet was air-dried before resuspension in TE solution (10 mM Tris pH 8.0, 1 mM EDTA). DNA was extracted from 25 mg of frozen occipital cortex from each of the 10 control samples using the DNeasy® Blood and Tissue Kit (Qiagen) according to manufacturer instructions. DNA concentration was measured using a NanoDrop™ spectrophotometer.
GBA1 screening
GBA1 mutation status was assessed in the Parkinson’s disease cases by performing complete sequencing of the 11 exons and flanking intronic regions of GBA1, amplified from genomic DNA in three fragments encompassing exons 1–5, 5–7 and 8–11, as previously described (Koprivica et al., 2000; Stone et al., 2000). Amplified fragments were purified using a QIAquick® PCR Purification Kit (Qiagen) and cycle sequencing was performed using the Dye Terminator Cycle Sequencing kit (Applied Biosystems) with both forward and reverse primers (Stone et al., 2000), using an ABI Prism 3730XL DNA Analyzer. The sequencing data were analysed by SeqScape v2.6 using GeneBank NG_009783.1 as the reference sequence.
The control cases were screened for six common and two recombinant GBA1 mutations that account for 70% of causative alleles for type one Gaucher disease in non-Jewish populations (Beutler et al., 1991; Koprivica et al., 2000). Specifically, sequence analysis of exons 9 and 10 was used to test for N370S (c.1226A>G), L444P (c.1448T>C), R463C (c.1504C>T) and D409H (c.1342G>C) mutations, whereas testing for the IVS2+1 (c.115+1G>A) mutation used restriction enzyme analysis and the 84GG (c.84dupG) mutation used amplification-refractory mutation system analysis. The recombinant alleles RecNcil and RecTL were excluded based on the absence of the L444P mutation and other sequence variants in exon 10.
Protein extraction and lysosomal-enriched fraction preparation
Soluble and insoluble proteins were serially extracted from 250 mg of fresh-frozen brain from the anterior cingulate (with increased α-synuclein levels) and occipital (no increased α-synuclein) cortices, as previously described (Zhou et al., 2011). Briefly, tissue was homogenized in TBS homogenization buffer [50mM Tris, 125 mM NaCl, pH 7.4, 5 mM EDTA, 0.02% sodium azide containing protease inhibitor cocktail (Complete, EDTA-free; Roche 04693132001)], centrifuged at 120 000g for 2 h at 4°C and the supernatant collected as the TBS-soluble fraction containing cytosolic proteins. The pellet was resuspended in TBS homogenization buffer containing 5% SDS, centrifuged at 100 000g for 30 min at 25°C, and the supernatant collected as the SDS-soluble fraction containing membrane-associated proteins.
Lysosomal membrane-enriched fractions were isolated from 300 mg fresh-frozen tissue from each region of interest. Tissue was thawed on ice, minced with a scalpel blade and homogenized in 10× volume of homogenization medium [0.32 M sucrose, 1 mM EDTA, 10 mM Tris-HCl pH 7.4, containing protease inhibitor cocktail (Complete, EDTA-free; Roche)] using 20 strokes of a Potter homogenizer rotating at 600 rpm. A small aliquot of total homogenate (whole tissue extract) was reserved for later analysis. Total homogenate was centrifuged at 1000 g for 10 min at 4°C to sediment the nuclear pellet and cellular debris. The pellet was washed twice, and the resulting supernatant centrifuged at 17 000g for 15 min at 4°C to obtain the lysosome-enriched pellet. The supernatant was centrifuged at 100 000g to obtain a pure cytosolic fraction (supernatant) and a microsomal pellet. The lysosomal-enriched pellets were resuspended in homogenization medium. The three fractions of interest (whole tissue extract, lysosomal-enriched and cytosolic fractions) were assessed by western immunoblotting with appropriate cellular markers to confirm the enrichment of lysosomal membranes in the 17 000g pellet and their absence in the cytosolic 100 000g supernatant fraction (Supplementary Fig. 1).
Protein concentration of all fractions was measured using the BCA assay (Pierce BCA Protein Assay Kit; Thermo Scientific 23225), according to manufacturer instructions. Samples were stored at −80°C.
Western immunoblotting
TBS-soluble, SDS-soluble and lysosomal-enriched protein fractions were assessed by routine western immunoblotting for levels of glucocerebrosidase, α-synuclein, and lysosomal proteins. Briefly, 25 µg of protein samples were added to loading buffer (2% SDS, 20% glycerol, 2.5% bromophenol blue, 12.5 mM Tris-HCl, pH 6.8, 5% 2-mercaptoethanol), separated by reducing SDS-PAGE and transferred to nitrocellulose membranes (BioRad 162-0112). Membranes were blocked in 5% skimmed milk dissolved in TBS-T (0.87% NaCl, 0.01 M Tris, pH 7.4, 0.05% Triton™ X-100 or 0.1% Tween-20) and incubated overnight in primary antibodies, before detection with enhanced chemiluminescence (Amersham ECL Plus Western Blot Detection System; GE Healthcare RPN2133) using horseradish peroxidase-conjugated secondary antibodies. The relative levels of each protein of interest were analysed using ImageJ software (National Institutes of Health). The intensity of each protein band was quantified and expressed as arbitrary units standardized to β-actin protein levels.
Primary antibodies used for western immunoblotting were mouse monoclonal NeuN (Millipore MAB377), β-actin (Abcam ab6276), glucocerebrosidase (Abnova H00002629-M01), α-synuclein (BD Biosciences 610787), LAMP2 (Developmental Studies Hybridoma Bank H4B4) and LC3 (NanoTools 0231-100/LC3-5F10), rabbit polyclonal LAMP1 (Santa Cruz Biotechnology sc5570), LAMP3 (Aviva Biosystems ARP41598-P050), Beclin1 (Novus Biologicals NB500-249) and cathepsin K (Abcam ab19027), and goat polyclonal LIMP2 (Everest Biotech EB09295), cathepsin D (Santa Cruz Biotechnology sc6486) and cathepsin A (Santa Cruz Biotechnology sc26049).
Glucocerebrosidase enzyme activity assay
Glucocerebrosidase enzyme activity was assessed in lysosomal-enriched protein fractions using the standard fluorimetric assay (Peters et al., 1976). Thirty microlitres of the lysosomal-enriched protein fraction was incubated at 37°C for 40 min with 40 μl of 1 mM 4-methylumbellferyl β-glucopyranoside (4MUβGlu; Sigma M3633) in 0.2 M citrate buffer (pH 4.5) containing 1% bovine serum albumin, 0.25% sodium taurocholate and 0.25% Triton™ X-100. The reaction was stopped by addition of 70 μl of 1 M glycine-NaOH (pH 10.4), and 4-methylumbelliferone fluorescence measured at excitation 355 nm and emission 460 nm using a POLARstar plate reader (BMG 415-201). One unit of enzyme activity is the amount of enzyme that hydrolyses 1 nmol of substrate per minute at 37°C. Values are expressed as μmol normalized to the 4MUβGlu standard curve, with enzyme activity normalized to the total protein amount in each sample.
RNA extraction and quantitative polymerase chain reaction
Total RNA was extracted from 50 mg of fresh-frozen brain tissue from each region of interest using TRIzol® Reagent (Sigma 15596-026), according to manufacturer instructions. Five micrograms of RNA was reverse transcribed into complementary DNA using Moloney-murine leukaemia virus reverse transcriptase and random primers in a 20 μl reaction volume. Relative expression of GBA1, α-synuclein and β-actin messenger RNA was measured by quantitative PCR using a Mastercycler ep realplex S (Eppendorf) with the fluorescent dye SsoAdvanced™ SYBR® Green Supermix (BioRad 172-5264), according to manufacturer instructions. Briefly, each 20 μl reaction contained SYBR® Green Supermix, 5 pmol primers, and 1 μl complementary DNA template. Amplification consisted of 40 cycles of denaturation (94°C, 15 s), annealing and extension (60°C, 1 min). A complementary DNA template-free control was included for each PCR amplification. Primer sequences are provided in Supplementary Table 1. Gene expression was normalized to β-actin messenger RNA levels and relative gene expression calculated using the comparative threshold cycle (ΔCT) method.
Sphingolipid analyses
Sphingolipid species were extracted from 10 mg of fresh-frozen brain tissue from each region using a recently developed and validated mechanical bead homogenization (Precellys®24, Bertin Technologies) and methyl-tert-butyl ether lipid extraction protocol, as described (Abbott et al., 2013). The sphingolipid extracts were analysed using electrospray ionization mass spectrometry and sphingolipid species quantified in nmol/g of tissue wet weight using AB SCIEX Lipidview software as described (Abbott et al., 2013).
Statistical analyses
All statistical analyses were performed using SPSS Statistics software (IBM) and statistical significance set at P < 0.05. A series of analyses were performed to identify clinical and diagnostic structural changes between the cohort groups. Differences between group demographics (Table 1) were assessed using Pearson chi-square test (sex), ANOVA with post hoc Bonferroni comparisons (age at death, post-mortem delay, CERAD plaque score, Braak neuritic staging, and clinical dementia rating), or independent samples t-test (disease duration). A univariate ANOVA was performed to determine if there was any loss of neurons in the different cohort groups and regions, and a multivariate statistical analysis was performed to determine if there were any neuronal changes in the levels of α-synuclein protein and messenger RNA between cohort groups and brain regions. Two multivariate analyses were performed to determine any changes in glucocerebrosidase protein, enzyme, messenger RNA, lysosomal protein, and sphingolipid levels that related to Parkinson’s disease-specific increases in α-synuclein protein: one with control and early stage Parkinson’s disease cases to determine if there were early changes in these variables, and one with early and late stage Parkinson’s disease cases to determine if there were later changes in these variables in the anterior cingulate cortex when α-synuclein aggregates occur. Full details of these analyses are given in the relevant results sections. Non-parametric Spearman’s correlations and step-wise multiple linear regressions were used to identify related variables.
Results
Confirmation of changes typical of sporadic Parkinson’s disease
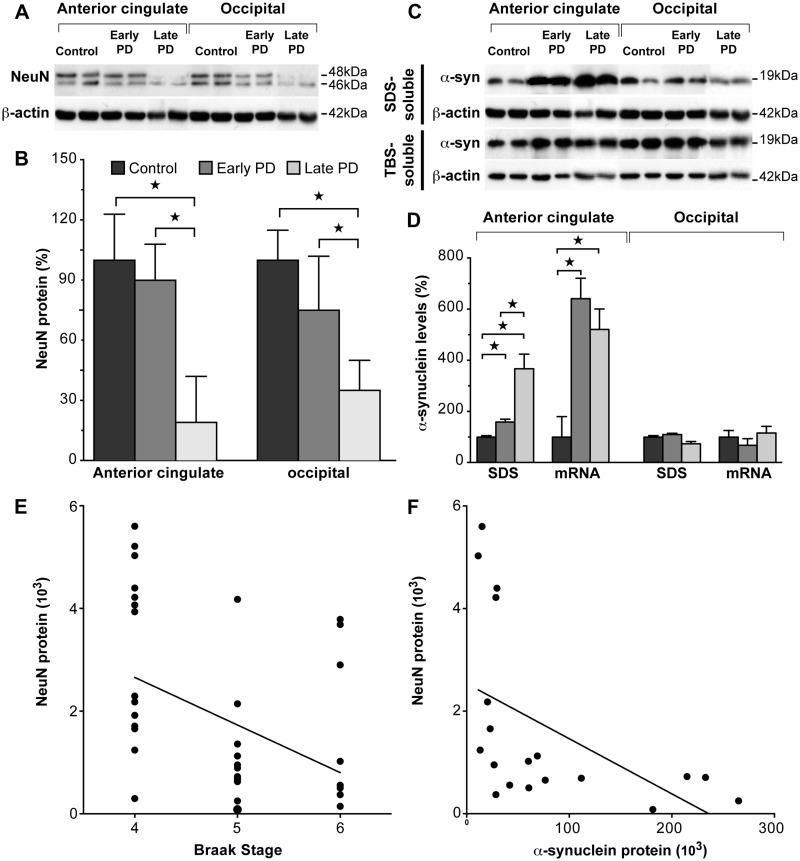
All Parkinson’s disease cases had typical pathological changes (Dickson et al., 2009) with limited other pathologies, seven reaching Braak Parkinson’s disease stage IV, seven reaching Braak Parkinson’s disease stage V and five reaching Braak Parkinson’s disease stage VI (Braak et al., 2003). For all analyses, the Parkinson’s disease cases were grouped into early stage Parkinson’s disease (Braak stage IV) and later stage Parkinson’s disease (Braak stages V and VI; Table 1). The absence of GBA1 mutations was confirmed in the 19 cases with sporadic Parkinson’s disease by sequencing all exons of GBA1, and in the 10 control cases by screening for eight common mutations (Beutler et al., 1991; Koprivica et al., 2000). To determine if there was any loss of neurons in the different cohort groups and regions, a two-way ANOVA was performed with the relative levels of NeuN protein as the dependent variable and cohort groups and brain regions as factors, covarying for the clinical dementia rating differences between the groups (other variables did not differ between groups). Among the early stage Parkinson’s disease cases, neither the anterior cingulate nor occipital cortex had substantial neuron loss using NeuN as a marker of neuron number in protein extracts (P > 0.92; Fig. 1A and B), as expected for early stage disease; however, NeuN was reduced in the cases with later stages of Parkinson’s disease (74 ± 15% reduction from control levels, P = 0.014, Fig. 1A and B).
Figure 1.
Characterization of neuron loss and α-synuclein changes in early and later stage sporadic Parkinson’s disease (PD). (A) Representative western blots of NeuN protein in control, early stage Parkinson’s disease and later stage Parkinson’s disease anterior cingulate and occipital cortices. (B) Quantitative western blot data of NeuN protein levels in control (n = 10), early stage Parkinson’s disease (n = 7) and later stage Parkinson’s disease (n = 12) cases. In early stage Parkinson’s disease anterior cingulate and occipital cortices, NeuN was not significantly different (P > 0.92), whereas NeuN was reduced in later stage Parkinson’s disease (P = 0.014). This confirms the lack of substantial cortical neuron loss in early stage Parkinson’s disease. ☆P < 0.05. (C) Representative western blots of SDS- and TBS-soluble α-synuclein protein in control, early stage Parkinson’s disease and later stage Parkinson’s disease anterior cingulate and occipital cortices. (D) Quantitative data of SDS-soluble α-synuclein protein and α-synuclein messenger RNA levels in control (n = 10), early stage Parkinson’s disease (n = 7) and later stage Parkinson’s disease (n = 12) cases. SDS-soluble α-synuclein was selectively increased in early stage Parkinson’s disease (P < 0.0001), and further increased in later stage Parkinson’s disease (P < 0.0001) anterior cingulate cortex, with unchanged levels in the occipital cortex. α-Synuclein messenger RNA was selectively increased to a similar extent in early stage Parkinson’s disease (P = 0.004) and later stage Parkinson’s disease (P = 0.004), with unchanged levels in the occipital cortex. These results confirm the regional selectivity of abnormal α-synuclein accumulation in early stage Parkinson’s disease. ☆P < 0.05. (E) Braak stage was significantly correlated with NeuN protein levels in all Parkinson’s disease samples (non-parametric Spearman’s rho = -0.47, P = 0.003), confirming the association of lower NeuN levels with the higher Braak stages of later stage Parkinson’s disease. (F) SDS-soluble α-synuclein protein levels were significantly correlated with NeuN levels (non-parametric Spearman’s rho = −0.69, P = 0.001) in Parkinson’s disease anterior cingulate cortex. This indicates that neuron loss associates with greater accumulation of membrane-associated α-synuclein in Parkinson’s disease anterior cingulate cortex.
Recent studies demonstrate that in regions vulnerable to Parkinson’s disease-specific aggregations of α-synuclein, the soluble form of α-synuclein is either unchanged or progressively reduced in sporadic Parkinson’s disease, whereas the SDS-soluble or membrane-associated fraction of α-synuclein increases significantly as pathological Lewy bodies form (Tong et al., 2010; Zhou et al., 2011; Lue et al., 2012; Murphy et al., 2013). To determine if there were any neuronal changes in the levels of α-synuclein protein and messenger RNA between cohort groups and brain regions, a multivariate statistical analysis was performed with the relative levels of α-synuclein protein and messenger RNA as the dependent variable and cohort groups and brain regions as factors, covarying for the clinical dementia rating and NeuN differences identified between groups. As expected (Zhou et al., 2011), we identified a significant increase in SDS-soluble α-synuclein in both early and late stage Parkinson’s disease anterior cingulate cortex (Fig. 1C and D, Table 2) but not in Parkinson’s disease occipital cortex at any disease stage. No change in TBS-soluble α-synuclein protein was observed (Fig. 1C and Table 2). This analysis was performed covarying for the NeuN identified neuronal loss in the later stages of Parkinson’s disease to ensure that the cellular comparisons were similar within the structurally changed anterior cingulate cortex, as there was a significant negative correlation between Braak stage and NeuN in all Parkinson’s disease samples (non-parametric Spearman’s rho = −0.47, P = 0.003; Fig. 1E). This confirms the association of lower NeuN levels with the higher Braak stages of late Parkinson’s disease. In addition, there was a significant negative correlation between NeuN and SDS-soluble α-synuclein levels in Parkinson’s disease anterior cingulate (non-parametric Spearman’s Rho = −0.69, P = 0.001; Fig. 1F), indicating that neuron loss is associated with greater accumulation of membrane-associated α-synuclein. The additional increase observed in SDS-soluble α-synuclein in late stage Parkinson’s disease anterior cingulate cortex (Table 2) was confirmed by the significant positive correlation between SDS-soluble α-synuclein and Braak staging in all Parkinson’s disease cases (non-parametric Spearman’s rho = 0.54, P = 0.018). The multivariate analysis also revealed α-synuclein messenger RNA to be significantly increased to a similar extent in both early and late stage Parkinson’s disease anterior cingulate cortex (Table 2), but not in Parkinson’s disease occipital cortex at any disease stage (Table 2 and Fig. 1D).
Table 2.
Results from the two main multivariate statistical analyses
| Variable | % control AC standard deviation | % change from control in early PD AC | % control OCC standard deviation | % change from control in early PD OCC | P-valuea | % early PD AC standard deviation | % change from early PD in late PD AC | P-valueb |
|---|---|---|---|---|---|---|---|---|
| SDS-soluble α-synuclein | 78 | 59 ± 11 ↑ | 45 | 10 | <0.0001 | 36 | 130 ± 36% ↑ | <0.0001 |
| TBS-soluble α-synuclein | 71 | 55 | 64 | 3 | 0.963 | 94 | 151 | 0.215 |
| α-Synuclein messenger RNA | 73 | 540 ± 80 ↑ | 101 | 32 | 0.004 | 52 | 19 | 0.215 |
| SDS-soluble glucocerebrosidase | 79 | 85 ± 15 ↓ | 53 | 39 | 0.006 | 40 | 73 | 0.215 |
| Lysosomal glucocerebrosidase | 46 | 38 ± 11 ↓ | 49 | 28 | 0.013 | 35 | 24 | 0.215 |
| Glucocerebrosidase enzyme activity | 9 | 27 ± 4 ↓ | 9 | 18 | 0.003 | 23 | 32 | 0.215 |
| GBA1 messenger RNAc | 88 | 70 | 81 | 27 | 0.252 | 122 | 72 | 0.215 |
| SDS-soluble LAMP1 | 44 | 26 | 47 | 36 | 0.123 | 32 | 33 | 0.215 |
| SDS-soluble LAMP2 | 72 | 71 ± 20 ↓ | 56 | 139 | 0.005 | 40 | 22 | 0.215 |
| SDS-soluble LAMP3 | 65 | 23 | 54 | 10 | 0.761 | 34 | 16 | 0.215 |
| SDS-soluble LIMP2 | 24 | 3 | 33 | 14 | 0.681 | 18 | 31 | 0.215 |
| TBS-soluble cathepsin A | 90 | 216 ± 61 ↑ | 60 | 59 | 0.004 | 88 | 217 | 0.215 |
| TBS-soluble cathepsin D | 12 | 312 ± 61 ↑ | 22 | 57 | 0.001 | 89 | 63 | 0.215 |
| TBS-soluble cathepsin K | 30 | 17 | 68 | 38 | 0.410 | 48 | 19 | 0.215 |
| SDS-soluble beclin 1 | 57 | 59 ± 13 ↓ | 13 | 113 | <0.0001 | 19 | 5 | 0.215 |
| SDS-soluble LC3-II | 62 | 2 | 80 | 5 | 0.909 | 58 | 0 | 0.215 |
| Ceramide | 17 | 59 ± 9% ↓ | 50 | 39 | 0.059 | 56 | 12 | 0.215 |
| Sphingomyelin | 23 | 48 | 58 | 40 | 0.142 | 35 | 22 | 0.215 |
AC = anterior cingulate cortex; OCC = occipital cortex.
aDependent variables were the relative levels of α-synuclein protein, α-synuclein messenger RNA, glucocerebrosidase protein, enzyme, messenger RNA, lysosomal proteins, and sphingolipid levels, factors were cohort groups and regions, covariates were NeuN protein levels.
bDependent variables were the relative levels of α-synuclein protein, α-synuclein messenger RNA, glucocerebrosidase protein, enzyme, messenger RNA, lysosomal proteins, and sphingolipid levels, factors were cohort groups and regions, covariates were Clinical Dementia Rating and NeuN protein levels.
cAs described in the results, GBA1 messenger RNA levels were not significantly altered in early stage PD anterior cingulate cortex (P = 0.252), but there was a strong trend for a non-selective reduction in both brain regions (54 ± 21% reduction from controls, P = 0.060), with no difference between early and later stage Parkinson’s disease anterior cingulate cortex (P > 0.215). The above data and Fig. 2D show that this reduction in GBA1 messenger RNA was greater in the anterior cingulate cortex than in the occipital cortex.
To determine any early changes in Parkinson’s disease, the firsta analysis compared early stage Parkinson’s disease cases with controls. To determine whether any early changes in Parkinson’s disease were enhanced with increasing disease stage, or if new changes occurred, the secondb analysis compared early stage Parkinson’s disease cases to later stage Parkinson’s disease cases. Data are given as a percentage of control values or early Parkinson’s disease values (i.e. percentage change ± SEM). Significant differences (italicized and direction of change arrowed), trends toward significance (bold and direction of change arrowed) or no change (plain text) are indicated.
Collectively, these data provide biochemical confirmation of the regional selectivity of increased α-synuclein levels in early stage Parkinson’s disease because of increased production of α-synuclein before substantial inclusion formation and cortical neuronal loss (Halliday and McCann, 2010). To identify the earliest cellular changes associated with increased α-synuclein before structural tissue changes, we first compared tissue changes between early stage cases with Parkinson’s disease and control subjects, then comparing the early and later stage Parkinson’s disease groups to identify any factors accompanying the later stage neuronal loss that might be associated with further increases in α-synuclein.
Decreased glucocerebrosidase protein levels and enzyme activity in regions with α-synuclein accumulation in early stages of Parkinson’s disease
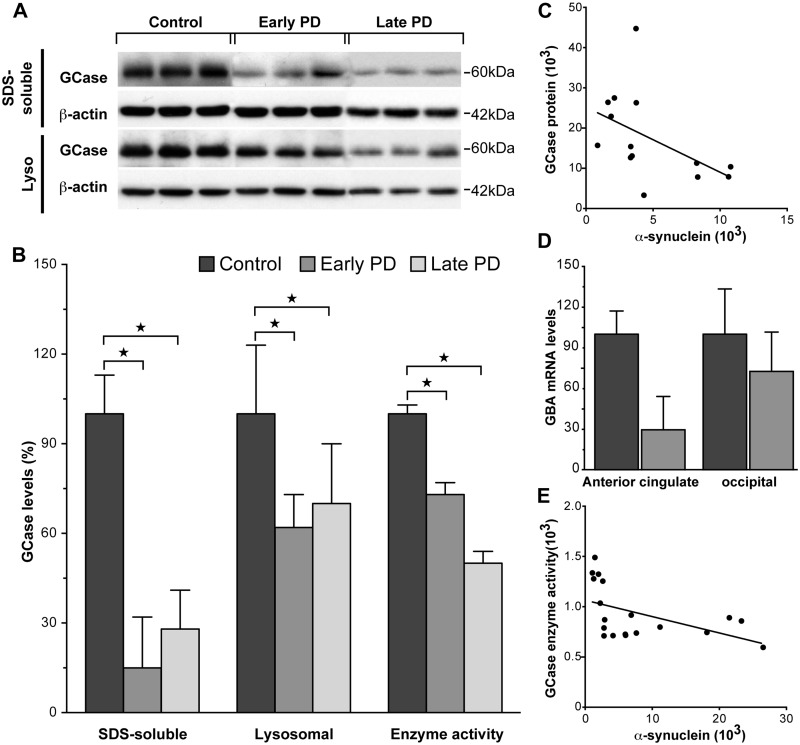
Glucocerebrosidase is predominantly a lysosomal membrane-associated protein under normal conditions (Qi and Grabowski, 1998). In controls, the majority of glucocerebrosidase protein was seen in the SDS-soluble fraction (99% is SDS-soluble in the anterior cingulate cortex). To determine if there were early changes in glucocerebrosidase protein, enzyme, messenger RNA, lysosomal protein and sphingolipid levels that related to Parkinson’s disease-specific increases in α-synuclein protein, a multivariate statistical analysis was performed with control and early stage Parkinson’s disease cases and the relative levels of α-synuclein protein and messenger RNA; glucocerebrosidase protein, enzyme activity and messenger RNA; lysosomal proteins; and sphingolipid levels as dependent variables and cohort groups and regions as factors, covarying for NeuN. SDS-soluble glucocerebrosidase was selectively reduced in anterior cingulate cortex samples from subjects with early stage Parkinson’s disease (Fig. 2A and B, and Table 2). The selective reduction of glucocerebrosidase protein in early stage Parkinson’s disease anterior cingulate, but not occipital cortex (Table 2), was confirmed in the lysosomal fraction using western blotting (Fig. 2A and B, Table 2). Glucocerebrosidase enzyme activity, assessed in the lysosomal-enriched protein fraction using standard methods (Peters et al., 1976), was also selectively reduced in early stage Parkinson’s disease anterior cingulate (Fig. 2B and Table 2) but not occipital cortex (Table 2). Non-parametric Spearman’s correlation analysis showed that the amount of increased α-synuclein observed in early stage Parkinson’s disease was related to the reduction in SDS-soluble glucocerebrosidase protein (Rho = −0.66, P = 0.01; Fig. 2C) and step-wise multiple regression analysis determined that the amount of increased α-synuclein observed in early stage Parkinson’s disease corresponded to the reduction in lysosomal glucocerebrosidase enzyme activity in the same cases (β = 0.3, P = 0.001). These data indicate that diminished glucocerebrosidase activity and protein levels occur selectively in brain regions with increased levels of α-synuclein in the early stages of Parkinson’s disease.
Figure 2.
Glucocerebrosidase protein levels and enzyme activity were selectively reduced in regions that accumulate abnormal α-synuclein in sporadic Parkinson’s disease. (A) Representative western blots of SDS-soluble and lysosomal glucocerebrosidase (GCase) protein in control, early stage Parkinson’s disease and later stage Parkinson’s disease anterior cingulate cortex. (B) Quantitative data of SDS-soluble and lysosomal glucocerebrosidase protein and glucocerebrosidase enzyme activity in control (n = 10), early stage Parkinson’s disease (n = 7) and later stage Parkinson’s disease (n = 12) cases. There were selective reductions in SDS-soluble glucocerebrosidase (P = 0.006), lysosomal glucocerebrosidase (P = 0.013), and glucocerebrosidase enzyme activity (P = 0.003) in early stage Parkinson’s disease anterior cingulate cortex. There was no difference between early and later stage Parkinson’s disease anterior cingulate cortex for SDS-soluble glucocerebrosidase (P > 0.21), lysosomal glucocerebrosidase (P > 0.21), or glucocerebrosidase enzyme activity (P > 0.21). *P < 0.05. (C) The amount of increased SDS-soluble α-synuclein protein observed in early stage Parkinson’s disease anterior cingulate cortex was related to the reduction in SDS-soluble glucocerebrosidase protein (non-parametric Spearman’s rho = −0.66, P = 0.01). (D) Although GBA1 messenger RNA expression was not significantly altered in early stage Parkinson’s disease anterior cingulate or occipital cortex (P > 0.25), the anterior cingulate cortex seemed to have less GBA1 messenger RNA. (E) The reduction in lysosomal glucocerebrosidase enzyme activity was significantly correlated with the increase in SDS-soluble α-synuclein protein in early and later stage Parkinson’s disease anterior cingulate cortex (non-parametric Spearman’s rho = −0.63, P = 0.005).
In contrast with the selective reduction in glucocerebrosidase in regions accumulating α-synuclein, GBA1 messenger RNA expression was not significantly altered in early stage Parkinson’s disease anterior cingulate cortex (Table 2), although there was a strong trend for a non-selective reduction in both brain regions (Fig. 2D and Table 2). This non-selective GBA1 messenger RNA reduction did not differ between early and later stage Parkinson’s disease (Table 2). This suggests that the reduction and redistribution of glucocerebrosidase in the anterior cingulate cortex is related to the increase and pathological redistribution of α-synuclein in this region.
To determine if there were later changes in glucocerebrosidase protein, enzyme, messenger RNA, lysosomal protein, and sphingolipid levels that related to Parkinson’s disease-specific increases in α-synuclein protein in the anterior cingulate cortex when α-synuclein aggregates occur, a multivariate statistical analysis was performed with early and late stage Parkinson’s disease cases and the relative levels of α-synuclein protein and messenger RNA; glucocerebrosidase protein, enzyme activity and messenger RNA; lysosomal protein; and sphingolipid levels as dependent variables and cohort group as the factor, covarying for the clinical dementia rating and NeuN differences identified between groups. Covarying for the neuronal loss in late stage Parkinson’s disease anterior cingulate cortex, the multivariate analysis showed that glucocerebrosidase protein levels and enzyme activity were not significantly different from the levels measured in early stage Parkinson’s disease (Fig. 2A and B, Table 2). NeuN protein levels had a significant negative correlation with lysosomal glucocerebrosidase protein levels (non-parametric Spearman’s rho = −0.56, P = 0.013) as well as glucocerebrosidase enzyme activity (non-parametric Spearman’s rho = 0.80, P < 0.0001). SDS-soluble α-synuclein levels in all Parkinson’s disease anterior cingulate cortex negatively correlated with glucocerebrosidase enzyme activity (non-parametric Spearman’s rho = −0.62, P = 0.005; Fig. 2E). This suggests that while glucocerebrosidase protein and enzyme activity deficits are an early pathological event associated with abnormal α-synuclein accumulation in Parkinson’s disease, glucocerebrosidase enzyme activity continues to decline in the absence of additional glucocerebrosidase protein deficits.
Glucocerebrosidase deficits in early stage Parkinson’s disease are not because of fewer lysosomes but are associated with lysosomal dysfunction and reduced ceramide levels
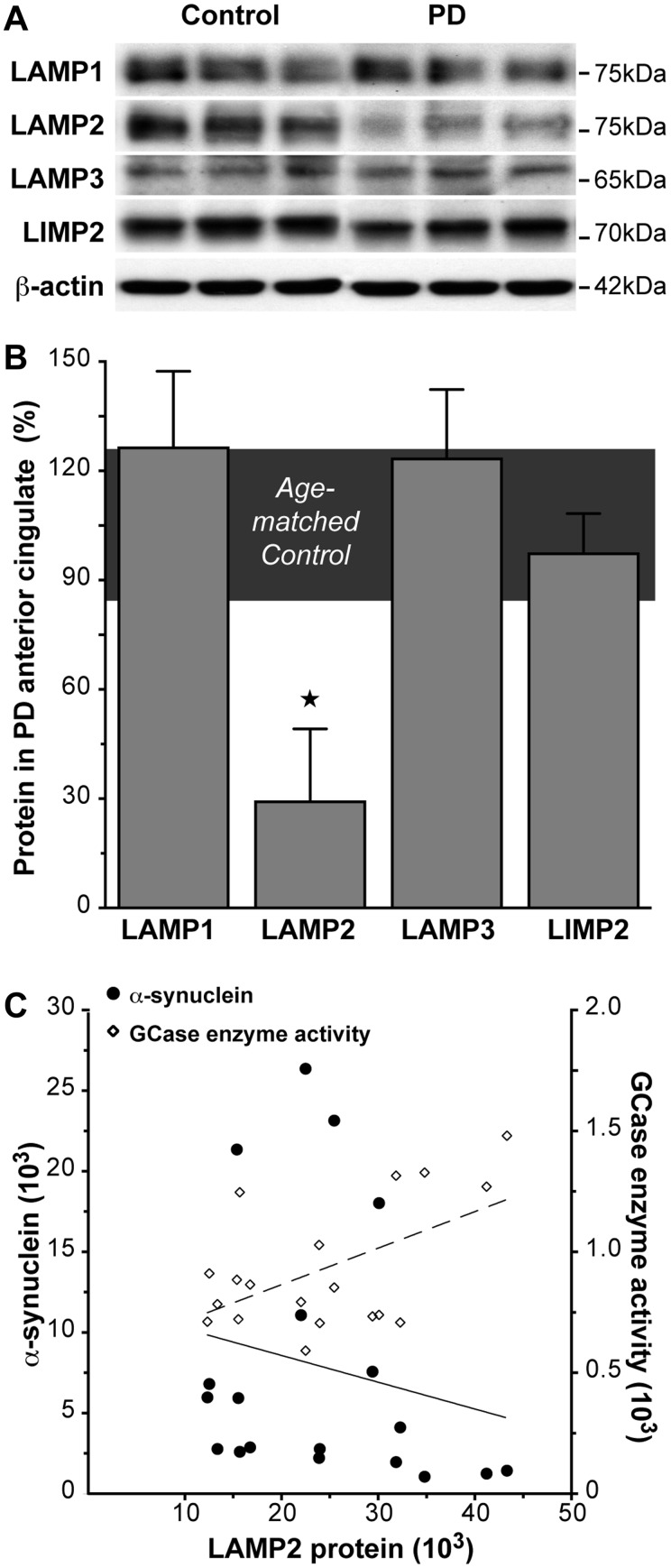
At the early Parkinson’s disease stage examined, many constituent lysosomal membrane proteins were not affected in regions accumulating α-synuclein, with no change in SDS-soluble levels of LAMP1, LAMP3 or LIMP2 observed in the anterior cingulate cortex (Fig. 3A and B, Table 2). Factoring in neuronal loss, these lysosomal membrane proteins were also unchanged in late stage Parkinson’s disease anterior cingulate cortex (Table 2). This suggests no overt loss of lysosomes with α-synuclein accumulation before neuronal loss in Parkinson’s disease. Therefore, a loss of lysosomes does not appear to be the underlying cause of the glucocerebrosidase reductions observed.
Figure 3.
SDS-soluble lysosomal membrane protein LAMP2 levels were selectively reduced in association with reduced glucocerebrosidase (GCase) in regions that accumulate abnormal α-synuclein in early stage Parkinson’s disease (PD). (A) Representative western blots of SDS-soluble lysosomal membrane proteins LAMP1, LAMP2, LAMP3 and LIMP2 in control and early stage Parkinson’s disease anterior cingulate cortex. (B) Quantitative data of SDS-soluble lysosomal membrane proteins in control (n = 10; horizontal bar at 100 ± 15%) and early stage Parkinson’s disease (n = 7) anterior cingulate cortex showed no significant changes in LAMP1 (P = 0.12), LAMP3 (P = 0.76) and LIMP2 (P = 0.68), but a selective reduction of LAMP2 in early stage Parkinson’s disease (P = 0.005). *P < 0.05. (C) The reduction in SDS-soluble LAMP2 protein was negatively correlated with increased SDS-soluble α-synuclein (circles with unbroken line; non-parametric Spearman’s rho = −0.82, P = 0.023), and positively correlated with decreased glucocerebrosidase enzyme activity (diamonds with dashed line; non-parametric Spearman’s rho = 0.89, P = 0.007) in early stage Parkinson’s disease.
Lysosome dysfunction in early stage Parkinson’s disease is indicated by changes in selected lysosomal proteins. In early stage Parkinson’s disease anterior cingulate cortex, there was a selective reduction in the lysosomal membrane protein LAMP2 (Fig. 3A and B, Table 2). LAMP2A, an isoform of LAMP2, is important for chaperone-mediated autophagic degradation of α-synuclein (Cuervo and Dice, 2000; Cuervo et al., 2004). Non-parametric Spearman’s correlation analysis showed that in early stage Parkinson’s disease the reduction in SDS-soluble LAMP2 was related to increased SDS-soluble α-synuclein protein (rho = −0.82, P = 0.023) and decreased glucocerebrosidase enzyme activity (rho = 0.89, P = 0.007) (Fig. 3C). Accounting for neuronal loss, LAMP2 protein levels in late stage Parkinson’s disease anterior cingulate cortex did not differ from early stage Parkinson’s disease anterior cingulate cortex (Table 2). These data implicate that chaperone-mediated autophagy pathways contribute to the glucocerebrosidase deficits observed in the early stages of Parkinson’s disease.
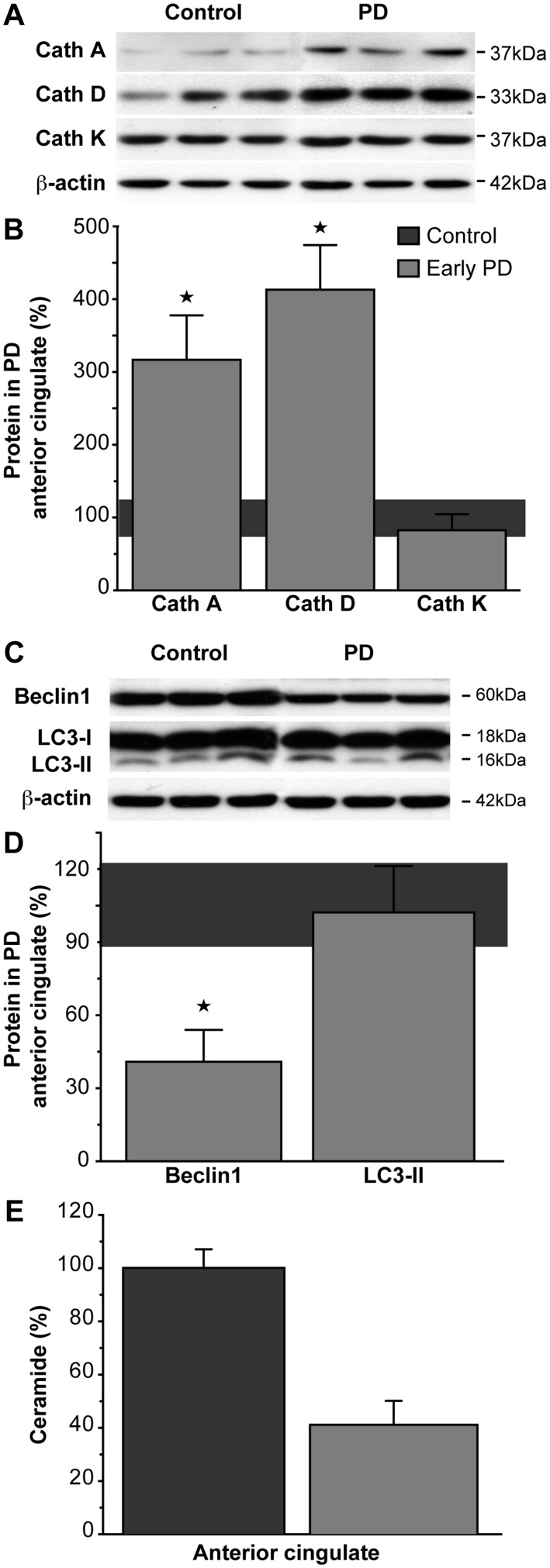
Lysosomes degrade chaperone-mediated autophagy-delivered cytosolic proteins using a range of soluble hydrolases called cathepsins. Two cathepsins associated with chaperone-mediated autophagic degradation of α-synuclein include cathepsin D, the primary lysosomal enzyme that degrades α-synuclein (Sevlever et al., 2008); and cathepsin A, involved in the regulation of the chaperone-mediated autophagy membrane receptor and rate-limiting factor LAMP2A (Cuervo et al., 2003). Cathepsin K is involved in bone matrix destruction and its gene expression is upregulated in Gaucher disease (Moran et al., 2000). In early stage Parkinson’s disease anterior cingulate cortex, there was a selective increase in the chaperone-mediated autophagy-related cathepsins A and D, but not the bone-related cathepsin (Fig. 4A and B, Table 2). However, there were no correlations between the levels of these affected chaperone-mediated autophagy-related cathepsins and the levels of glucocerebrosidase or α-synuclein in early stage Parkinson’s disease. Furthermore, the multivariate analysis showed that the levels of cathepsins A, D and K in the anterior cingulate cortex were similar in early and late stage Parkinson’s disease (Table 2). This indicates that although significant early dysfunction is likely in the lysosomal chaperone-mediated autophagy system, the glucocerebrosidase deficits observed in Parkinson’s disease may be more related to membrane interactions or factors external to the lysosome than to lysosomal hydrolase dysregulation.
Figure 4.
Chaperone-mediated autophagy- and macroautophagy-related proteins and total ceramide were selectively altered in regions that accumulate abnormal α-synuclein in early stage Parkinson’s disease. (A) Representative western blots of TBS-soluble lysosomal cathepsins (Cath) in control and early stage Parkinson’s disease anterior cingulate cortex. (B) Quantitative data of TBS-soluble lysosomal cathepsins in control (n = 10; horizontal bar at 100 ± 15%) and early stage Parkinson’s disease (n = 7) anterior cingulate cortex showed that the cathepsins related to the chaperone-mediated autophagic degradation of α-synuclein were significantly increased in early stage Parkinson’s disease (cathepsin A P = 0.004; cathepsin D P = 0.001), with no change to the GBA1-related cathepsin K levels (P = 0.41). *P < 0.05. (C) Representative western blots of SDS-soluble autophagosome marker proteins in control and early stage Parkinson’s disease anterior cingulate cortex. (D) Quantitative data of SDS-soluble autophagosome marker proteins in control (n = 10; horizontal bar at 100 ± 15%) and early stage Parkinson’s disease (n = 7) anterior cingulate cortex showed that beclin1 was significantly reduced in early stage Parkinson’s disease (P < 0.0001), while LC3-II levels remained within normal levels (P = 0.91). *P < 0.05. (E) Ceramide trended toward a selective reduction in early stage Parkinson’s disease anterior cingulate (P = 0.059). Data are presented as percentage changes in early stage Parkinson’s disease (n = 7) compared to controls (n = 10).
Chronic reductions in chaperone-mediated autophagy activity can result in constitutive activation of macroautophagy (Massey et al., 2006; Kaushik et al., 2008), where autophagosomes are delivered to the lysosome for degradation. To determine if changes in this pathway are relevant, we assessed two proteins involved in the induction of macroautophagy, LC3-II is required for autophagosome formation and beclin 1 is involved in the early formation and maturation of autophagosomes (Tanida, 2011). In early stage Parkinson’s disease anterior cingulate cortex, there was a selective reduction in levels of beclin 1 but not LC3-II (Fig. 4C and D, Table 2). The multivariate analysis revealed that the levels of beclin 1 and LC3-II in late stage Parkinson’s disease anterior cingulate cortex were not different from early stage Parkinson’s disease anterior cingulate cortex (Table 2). Step-wise multiple regression analysis showed that the reduction in SDS-soluble beclin 1 related to the reduction in SDS-soluble glucocerebrosidase (β = 0.32, P < 0.0001), but not to increasing levels of α-synuclein in early stage Parkinson’s disease. The LC3-II results indicate no change in steady-state levels of autophagosome number in Parkinson’s disease; however, they do not rule out an alteration in the rate of autophagosome formation (Mizushima and Yoshimori, 2007).
Lipid accumulation within the lysosome is the major defect observed in Gaucher disease (Schueler et al., 2004). We analysed the relevant sphingolipids using mass spectrometry. In early stage Parkinson’s disease anterior cingulate cortex, there was a strong trend for a selective reduction in total ceramide (Fig. 4E) but not sphingomyelin (Table 2). Total ceramide and sphingomyelin in late stage Parkinson’s disease anterior cingulate cortex were not different from early Parkinson’s disease anterior cingulate cortex in the multivariate analysis (Table 2). These data indicate that ceramide is selectively reduced in brain regions that accumulate α-synuclein and show glucocerebrosidase deficiency in sporadic Parkinson’s disease.
Discussion
The present study provides a comprehensive analysis of the relationship between wild-type glucocerebrosidase, α-synuclein, and the consequent lysosomal changes in a region with biochemical but not structural changes early in sporadic Parkinson’s disease. The loss of lysosomal glucocerebrosidase early in sporadic Parkinson’s disease corresponds to reduced lysosomal chaperone-mediated autophagy (but not macroautophagy), increased α-synuclein levels, and a decrease in ceramide. Although a deficit in glucocerebrosidase is expected in Parkinson’s disease cases harbouring GBA1 mutations, determining why glucocerebrosidase is deficient in sporadic Parkinson’s disease without GBA1 mutations is important. In cell and mouse models of Gaucher disease there are direct and reciprocal interactions between glucocerebrosidase and α-synuclein, where decreased glucocerebrosidase activity causes abnormal α-synuclein accumulation and elevated α-synuclein inhibits normal glucocerebrosidase function (Manning-Bog et al., 2009; Cullen et al., 2011; Mazzulli et al., 2011; Sardi et al., 2011, 2013; Xu et al., 2011; Yap et al., 2011, 2013). Reduced glucocerebrosidase protein levels and enzyme activity have been previously reported in sporadic Parkinson’s disease cases, with greatest reductions seen in the vulnerable substantia nigra, and no significant loss of glucocerebrosidase in frontal cortex (Gegg et al., 2012). This contrasts with the results of the present study and other reports of glucocerebrosidase reductions in frontal cortex in sporadic and GBA1-mutant Parkinson’s disease (Mazzulli et al., 2011; Kurzawa-Akanbi et al., 2012), although our data would suggest that such variation is most likely because of the degree of α-synuclein accumulation and neuronal loss in different frontal cortical regions at different stages of Parkinson’s disease (Braak et al., 2003). Overall, these studies confirm diminished glucocerebrosidase in Parkinson’s disease.
Although there was some evidence for reduced GBA1 messenger RNA expression in the same early stage Parkinson’s disease cases, this reduction did not reach significance, did not occur selectively in the region accumulating α-synuclein and was not associated with reduced glucocerebrosidase activity in the occipital cortex. These data are consistent with other studies showing relatively normal levels of GBA1 messenger RNA in sporadic Parkinson’s disease putamen, despite a 23–48% reduction in glucocerebrosidase protein and enzyme activity in this region (Gegg et al., 2012), and differs from data obtained in patients with Gaucher disease. In patients with Gaucher disease with different types of GBA1 mutations and a reduction in normal messenger RNA, greater residual glucocerebrosidase enzyme activity tends to be associated with milder Gaucher disease symptoms (Montfort et al., 2004; Goker-Alpan et al., 2005), and GBA1 mutation carriers remain asymptomatic despite a 50% reduction in glucocerebrosidase activity (Choy, 1988; Aerts et al., 2011). The reduced glucocerebrosidase activity levels in asymptomatic GBA1 mutation carriers are presumably sufficient for normal lysosomal function. It would be of interest to know the level to which normal GBA1 expression can be reduced before significant deficits in glucocerebrosidase levels and activity occur. It should be noted that therapeutic enzyme replacement therapies have significant efficacy for symptomatic patients with Gaucher disease (Aerts et al., 2011).
Importantly, there have been no previous studies assessing the relationships between glucocerebrosidase protein levels and enzyme activity and α-synuclein accumulation, or other relevant lysosomal proteins and lipids in Parkinson’s disease brains. Our data identifies important relationships between levels and activity of glucocerebrosidase and the early, selective increase in membrane-associated α-synuclein before substantial neuronal loss and Lewy body formation in sporadic Parkinson’s disease. Glucocerebrosidase is transported from the endoplasmic reticulum to the lysosome by the trafficking receptor LIMP2 (Reczek et al., 2007). Cells over-expressing α-synuclein show less glucocerebrosidase delivered to the lysosome as a result of less binding to LIMP2 (Gegg et al., 2012). We confirm that the glucocerebrosidase deficiency seen in early and late Parkinson’s disease is not because of reduced LIMP2 protein levels, consistent with previous studies (Gegg et al., 2012; Kurzawa-Akanbi et al., 2012), and although the deficit is associated with increased SDS-soluble α-synuclein, it is not associated with increased α-synuclein messenger RNA, suggesting that the glucocerebrosidase deficits in Parkinson’s disease may be associated with the cellular redistribution of both α-synuclein and glucocerebrosidase proteins. Some retention of glucocerebrosidase in the endoplasmic reticulum has been observed in Parkinson’s disease (Mazzulli et al., 2011). We also observe that lysosomes are not overtly lost in Parkinson’s disease, the formation of autophagosomes is not reduced, and that changes in the levels of GBA1 messenger RNA do not account for this relationship. We show that the reduction in glucocerebrosidase is not simply because of a loss of neurons in early stage Parkinson’s disease, consistent with known regional selectivity of neurodegeneration in Parkinson’s disease (Halliday and McCann, 2010). Finally, we show associated dysregulation in chaperone-mediated autophagy-associated proteins and enzymes, and a decrease in cellular ceramide in both early and later stages of Parkinson’s disease.
Notably, these abnormalities are not significantly different between the early and later stages of Parkinson’s disease, although our analysis included covarying to account for the degree of neuronal loss. The data suggest that the pathological event/s underlying such lysosomal dysfunction occur early within surviving neurons in Parkinson’s disease and may contribute to the increase in α-synuclein aggregation observed over time. Of interest, the degree of lysosomal dysfunction appears insufficient to immediately kill the neurons, but was of a substantial level and, in the neurons remaining, was not exacerbated with disease progression. Perhaps this indicates that any increasing level of deficit would have more detrimental consequences. Of course, ongoing and/or more substantial neuronal lysosomal dysfunction is likely to contribute to the loss of neurons we and others have observed in regions with α-synuclein pathology. The identification of such relationships enhances our understanding of the lysosomal pathways involved in Parkinson’s disease.
Glucocerebrosidase is a membrane-associated lysosomal enzyme, and a portion of α-synuclein is normally delivered to the lysosome via chaperone-mediated autophagy (Cuervo et al., 2004) and degraded primarily by the lysosomal enzyme cathepsin D resident in the lysosome lumen (Sevlever et al., 2008). Our data in early stage Parkinson’s disease show a selective increase in cathepsin D, consistent with some (Kurzawa-Akanbi et al., 2012) but not all (Chu et al., 2009; Gegg et al., 2012) previous studies in Parkinson’s disease and Alzheimer’s disease (Cataldo et al., 1991; Mackay et al., 1997). Again, the variation in Parkinson’s disease studies may relate to the amount of regional α-synuclein accumulation and neuronal loss at different disease stages. Although cathepsin D-deficient mice display abnormal α-synuclein accumulation that can be rescued by over-expression of cathepsin D (Qiao et al., 2008; Cullen et al., 2009), our data show the opposite effect in sporadic Parkinson’s disease, with an increase rather than a decrease in this enzyme. However, we also found a novel increase in the levels of cathepsin A in sporadic Parkinson’s disease, an enzyme that contributes to multienzyme complexes to protect glycosidases against proteolytic inactivation (Hiraiwa, 1999). The proteolytic activity of cathepsin A also triggers the degradation of LAMP2A, with cathepsin A-deficient cells displaying reduced rates of LAMP2A degradation, increased LAMP2A levels and chaperone-mediated autophagy rates (Cuervo et al., 2003). Our data do not support the elevation of cathepsin A levels in Parkinson’s disease brain tissue as a protective response to the reduction in glucocerebrosidase, as although reduced glucocerebrosidase protein levels stabilized in late Parkinson’s disease, glucocerebrosidase enzyme activity continued to decline, indicating that the remaining glucocerebrosidase was not protected against inactivation. Rather, our data suggest that increased cathepsin A would subsequently lead to increased LAMP2A degradation and reduced chaperone-mediated autophagy. This would result in the increased accumulation of chaperone-mediated autophagy substrates like α-synuclein.
Chaperone-mediated autophagy and macroautophagy exhibit crosstalk that results in the constitutive activation of one pathway when the other is impaired (Massey et al., 2006; Kaushik et al., 2008). In Parkinson’s disease, α-synuclein accumulates and neuronal dysfunction occurs over a long progressive disease course, and hence upregulated macroautophagy might be expected due to chronic impairment of chaperone-mediated autophagy (Massey et al., 2008). However, we saw no change in the autophagosome marker LC3-II, whereas others have found increased levels of this marker in Parkinson’s disease (Yu et al., 2009; Alvarez-Erviti et al., 2010; Gegg et al., 2012). Again, this may be because of the assessment of tissues at different disease stages. As stated in the results, LC3-II levels in post-mortem human tissue only represent autophagosome number, and do not identify whether an LC3-II increase is because of greater induction of autophagosome formation (upregulation of macroautophagy) or decreased fusion of autophagosomes with lysosomes. We also identified a decrease in beclin 1, also required for autophagosome formation in macroautophagy, which was significantly correlated with the reduced glucocerebrosidase in the same cases. This differs from previous studies showing no change (Crews et al., 2010) or an increase (Yu et al., 2009) in beclin 1 in Parkinson’s disease (variation could be related to disease stage), with our data being more consistent with cell and mouse models of synucleinopathy (representing early disease stages) where over-expression of beclin1 reduces abnormal α-synuclein accumulation by inducing autophagy (Spencer et al., 2009). Interestingly, beclin 1 has been implicated in the regulation of retrograde endosomal transport as well as macroautophagy (Ruck et al., 2011; Wirawan et al., 2012), suggesting that the beclin 1 deficits we observed may be related to endosomal trafficking. Recent work has identified dysfunction of endosome to Golgi trafficking by the retromer complex as important in Parkinson’s disease, with three Parkinson’s disease susceptibility genes involved in this pathway, LRRK2, PARK16/RAB7L1 and VPS35 (MacLeod et al., 2013). The association of beclin1 and glucocerebrosidase deficits in early stages of Parkinson’s disease may also link GBA1 to this Parkinson’s disease-related pathway rather than to deficits in macroautophagy.
We provide novel data on relevant sphingolipid changes that occur selectively in brain regions with glucocerebrosidase deficits and increased α-synuclein levels in early stages of sporadic Parkinson’s disease, identifying reductions in total ceramide. Ceramide may be generated through three main pathways: (i) de novo synthesis beginning with serine and palmitoyl-CoA; (ii) through the breakdown of sphingomyelin by sphingomyelinase; and (iii) by the breakdown of glucosylceramide by glucocerebrosidase (Kitatani et al., 2008). The latter route for ceramide production, also known as the salvage pathway, has been proposed as the most energy efficient mechanism for ceramide generation in non-dividing cells, and could account for 50% to 90% of sphingolipid production, depending on the lipid molecular species and the cell type (Gillard et al., 1998; Tettamanti et al., 2003). The decreased levels of ceramide detected in the Parkinson’s disease cases suggest that an impairment in the salvage pathway, which is critically dependent on lysosomal glucocerebrosidase, is not compensated for by an upregulation of de novo synthesis, and this may eventually have an impact on cellular membrane structure and signalling pathways (Soreghan et al., 2003; Fortin et al., 2004; Jana et al., 2009). This could suggest significant changes in neuronal membrane properties in patients with Parkinson’s disease (Fabelo et al., 2011). It should be noted that ceramide is not reduced in patients with Gaucher disease, even though they have significantly reduced glucocerebrosidase activity (Almeida Mdo, 2012), supporting the concept of alternate production pathways for this lipid, at least in the peripheral macrophages that are most abnormal in Gaucher disease. This suggests that neurons rely more on the ceramide-producing salvage pathway compared with other cell types, a concept that may increase their vulnerability to certain lysosomal perturbations. Interestingly, mutations affecting enzymes involved in alternate ceramide synthesis pathways (PANK2 in the de novo pathway and PLA2G6 in the sphingomyelin breakdown pathway) also present with parkinsonian symptoms and display α-synuclein neuronal pathology (Bras et al., 2008), supporting a relationship between the dysregulation of ceramide production and Parkinson’s disease.
This study assessed post-mortem Parkinson’s disease cases and cannot address early cellular changes occurring before, or at, symptom onset in Parkinson’s disease. However, the early stage Parkinson’s disease cases examined were the earliest disease stage with largely biochemical rather than structural α-synuclein changes in frontal cortical regions (Zhou et al., 2011; Lue et al., 2012; Murphy et al., 2013) and limited cortical pathology compared with later disease stages (Braak stages V/VI) (Braak et al., 2003). We analysed two cortical regions that do not undergo substantial neuron loss at this early Parkinson’s disease stage, to assess the earliest cellular changes associated with increases in α-synuclein. We then assessed later stage Parkinson’s disease cases (Braak stages V/VI) to determine what effect increased α-synuclein accumulation and aggregation have on these early cellular changes. We determined that there are reduced NeuN levels in the anterior cingulate cortex in these later disease stages indicative of neuron loss. The neuronal loss was associated with increased α-synuclein accumulation and continued, but not exacerbated, lysosomal dysregulation. Chronic lysosomal dysregulation in Parkinson’s disease is consistent with suggestions of lysosomal membrane destabilization and permeabilization as potential cell death mechanisms in neurodegenerative disorders (Ditaranto-Desimone et al., 2003; Boya and Kroemer, 2008).
This study suggests that glucocerebrosidase is reduced in association with the early abnormal accumulation of α-synuclein in sporadic Parkinson’s disease, leading to substantial alterations in lysosomal chaperone-mediated autophagy pathways and altered lipid metabolism. Although reduced glucocerebrosidase levels alone are not sufficient to cause Parkinson’s disease, as only a small percentage of GBA1 mutation carriers develop Parkinson’s disease (Westbroek et al., 2011), reduced glucocerebrosidase levels are present even in sporadic Parkinson’s disease. Our data show that the redistribution of cellular membrane proteins may be key to the selective Parkinson’s disease changes observed, and that such protein redistribution may occur through endosomal membrane trafficking. The reduced glucocerebrosidase is likely to contribute to lysosomal dysfunction by altering lysosomal contents and membrane properties, exacerbating any age-related diminished lysosomal capacity. Further elucidation of the nature and timing of dysregulation in these affected pathways in sporadic Parkinson’s disease will aid in identifying the catalytic event(s) that initiate the pathogenic process, as well as potential targets for disease-modifying therapies and early disease biomarkers (Balducci et al., 2007; Parnetti et al., 2009).
Supplementary Material
Acknowledgements
Tissues were received from the Sydney Brain Bank at Neuroscience Research Australia and the New South Wales Tissue Resource Centre at the University of Sydney which are supported by the National Health and Medical Research Council of Australia (NHMRC), University of New South Wales, Neuroscience Research Australia, Schizophrenia Research Institute and National Institute of Alcohol Abuse and Alcoholism (NIH (NIAAA) R24AA012725). We thank Heidi Cartwright for assistance with preparation of figures and Claudia Landazabal for assistance with DNA sequencing.
Funding
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) project grants (#1008307 to G.M.H. and B.G., and #1010839 to A.A.C.). K.E.M. is a NHMRC Postgraduate Biomedical Scholar (#630752) and a Hicksons Lawyer Neuroscience Research Australia Postgraduate Scholar. G.M.H. is a NHMRC Senior Principal Research Fellow (#630434). B.G. is an ARC Future Fellow (#FT0991986) and NHMRC Senior Research Fellow (Hon, #630445). Contributions of E.S. and N.T. were supported by the intramural research programs of the National Human Genome Research Institute and the National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
References
- Abbott SK, Jenner AM, Mitchell TW, Brown SH, Halliday GM, Garner B. An improved high-throughput lipid extraction method for the analysis of human brain lipids. Lipids. 2013;48:307–18. doi: 10.1007/s11745-013-3760-z. [DOI] [PubMed] [Google Scholar]
- Aerts JM, Kallemeijn WW, Wegdam W, Joao Ferraz M, van Breemen MJ, Dekker N, et al. Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J Inherit Metab Dis. 2011;34:605–19. doi: 10.1007/s10545-011-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida Mdo R. Glucocerebrosidase involvement in Parkinson disease and other synucleinopathies. Front Neurol. 2012;3:65. doi: 10.3389/fneur.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–72. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Balducci C, Pierguidi L, Persichetti E, Parnetti L, Sbaragli M, Tassi C, et al. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson's disease. Mov Disord. 2007;22:1481–4. doi: 10.1002/mds.21399. [DOI] [PubMed] [Google Scholar]
- Beutler E, Gelbart T, Kuhl W, Sorge J, West C. Identification of the second common Jewish Gaucher disease mutation makes possible population-based screening for the heterozygous state. Proc Natl Acad Sci USA. 1991;88:10544–7. doi: 10.1073/pnas.88.23.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–51. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: Potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–73. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultron G, Kacena K, Pearson D, Boxer M, Yang R, Sathe S, et al. The risk of Parkinson's disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33:167–73. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Paskevich PA, Kominami E, Nixon RA. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10998–1002. doi: 10.1073/pnas.88.24.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Stubblefield B, Cookson MR, Goldin E, Velayati A, Tayebi N, et al. Aggregation of alpha-synuclein in brain samples from subjects with glucocerebrosidase mutations. Mol Genet Metab. 2011;104:185–8. doi: 10.1016/j.ymgme.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy FY. Intrafamilial clinical variability of type 1 Gaucher disease in a French-Canadian family. J Med Genet. 1988;25:322–5. doi: 10.1136/jmg.25.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson's disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–98. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cuervo AM, Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 2000;1:570–83. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Mann L, Bonten EJ, d'Azzo A, Dice JF. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2003;22:47–59. doi: 10.1093/emboj/cdg002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. 2009;2:5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011;69:940–53. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–44. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo J, Goker-Alpan O, Samaddar T, Lopez G, Sidransky E. The association between mutations in the lysosomal protein glucocerebrosidase and parkinsonism. Mov Disord. 2009;24:1571–8. doi: 10.1002/mds.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Ditaranto-Desimone K, Saito M, Tekirian TL, Saito M, Berg M, Dubowchik G, et al. Neuronal endosomal/lysosomal membrane destabilization activates caspases and induces abnormal accumulation of the lipid secondary messenger ceramide. Brain Res Bull. 2003;59:523–31. doi: 10.1016/s0361-9230(02)00948-6. [DOI] [PubMed] [Google Scholar]
- Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, et al. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson's disease and incidental Parkinson's disease. Mol Med. 2011;17:1107–18. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–23. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72:455–63. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard BK, Clement RG, Marcus DM. Variations among cell lines in the synthesis of sphingolipids in de novo and recycling pathways. Glycobiology. 1998;8:885–90. doi: 10.1093/glycob/8.9.885. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Hruska KS, Orvisky E, Kishnani PS, Stubblefield BK, Schiffmann R, et al. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet. 2005;42:e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–9. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann N Y Acad Sci. 2010;1184:188–95. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M. Cathepsin A/protective protein: an unusual lysosomal multifunctional protein. Cell Mol Life Sci. 1999;56:894–907. doi: 10.1007/s000180050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–83. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- Jana A, Hogan EL, Pahan K. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J Neurol Sci. 2009;278:5–15. doi: 10.1016/j.jns.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179–92. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica V, Stone DL, Park JK, Callahan M, Frisch A, Cohen IJ, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–86. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzawa-Akanbi M, Hanson PS, Blain PG, Lett DJ, McKeith IG, Chinnery PF, et al. Glucocerebrosidase mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease. J Neurochem. 2012;123:298–309. doi: 10.1111/j.1471-4159.2012.07879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Lopez OL, Dekosky ST. The expanding role of genetics in the lewy body diseases: the glucocerebrosidase gene. Arch Neurol. 2009;66:555–6. doi: 10.1001/archneurol.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Adler CH, Shill H, Tran H, Akiyama H, et al. Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol. 2012;22:745–56. doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay EA, Ehrhard A, Moniatte M, Guenet C, Tardif C, Tarnus C, et al. A possible role for cathepsins D, E, and B in the processing of beta-amyloid precursor protein in Alzheimer's disease. Eur J Biochem. 1997;244:414–25. doi: 10.1111/j.1432-1033.1997.00414.x. [DOI] [PubMed] [Google Scholar]
- MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron. 2013;77:425–39. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–32. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey AC, Follenzi A, Kiffin R, Zhang C, Cuervo AM. Early cellular changes after blockage of chaperone-mediated autophagy. Autophagy. 2008;4:442–56. doi: 10.4161/auto.5654. [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–10. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Montfort M, Chabas A, Vilageliu L, Grinberg D. Functional analysis of 13 GBA mutant alleles identified in Gaucher disease patients: pathogenic changes and “modifier” polymorphisms. Hum Mutat. 2004;23:567–75. doi: 10.1002/humu.20043. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MT, Schofield JP, Hayman AR, Shi GP, Young E, Cox TM. Pathologic gene expression in Gaucher disease: up-regulation of cysteine proteinases including osteoclastic cathepsin K. Blood. 2000;96:1969–78. [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics. 1997;9(Suppl. 1):173–6. doi: 10.1017/s1041610297004870. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Cottle L, Gysbers AM, Cooper AA, Halliday GM. ATP13A2 (PARK9) protein levels are reduced in brain tissue of cases with Lewy bodies. Acta Neuropathol Commun. 2013;1:11. doi: 10.1186/2051-5960-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70:727–35. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132:1783–94. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen L, Neumann J, O'Sullivan SS, Holton JL, Revesz T, Hardy J, et al. Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson's disease. Mol Genet Metab. 2011;103:410–2. doi: 10.1016/j.ymgme.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Balducci C, Pierguidi L, De Carlo C, Peducci M, D'Amore C, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in Dementia with Lewy Bodies. Neurobiol Dis. 2009;34:484–6. doi: 10.1016/j.nbd.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Peters SP, Coyle P, Glew RH. Differentiation of beta-glucocerebrosidase from beta-glucosidase in human tissues using sodium taurocholate. Arch Biochem Biophys. 1976;175:569–82. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- Qi X, Grabowski GA. Acid beta-glucosidase: intrinsic fluorescence and conformational changes induced by phospholipids and saposin C. Biochemistry. 1998;37:11544–54. doi: 10.1021/bi980785+. [DOI] [PubMed] [Google Scholar]
- Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–83. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Ruck A, Attonito J, Garces KT, Nunez L, Palmisano NJ, Rubel Z, et al. The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic retrograde transport in addition to autophagy in C. elegans. Autophagy. 2011;7:386–400. doi: 10.4161/auto.7.4.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Isolation of High-molecular-weight DNA from Mammalian Cells Using Proteinase K and Phenol. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4036. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108:12101–6. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci USA. 2013;110:3537–42. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler UH, Kolter T, Kaneski CR, Zirzow GC, Sandhoff K, Brady RO. Correlation between enzyme activity and substrate storage in a cell culture model system for Gaucher disease. J Inherit Metab Dis. 2004;27:649–58. doi: 10.1023/b:boli.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–87. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreghan B, Thomas SN, Yang AJ. Aberrant sphingomyelin/ceramide metabolic-induced neuronal endosomal/lysosomal dysfunction: potential pathological consequences in age-related neurodegeneration. Adv Drug Deliv Rev. 2003;55:1515–24. doi: 10.1016/j.addr.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–8. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal. 2011;14:2201–14. doi: 10.1089/ars.2010.3482. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, Bassi R, Viani P, Riboni L. Salvage pathways in glycosphingolipid metabolism. Biochimie. 2003;85:423–37. doi: 10.1016/s0300-9084(03)00047-6. [DOI] [PubMed] [Google Scholar]
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–88. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–56. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17:485–93. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM, Piacentini M, et al. Beclin1: a role in membrane dynamics and beyond. Autophagy. 2012;8:6–17. doi: 10.4161/auto.8.1.16645. [DOI] [PubMed] [Google Scholar]
- Xu YH, Sun Y, Ran H, Quinn B, Witte D, Grabowski GA. Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab. 2011;102:436–47. doi: 10.1016/j.ymgme.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, et al. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem. 2011;286:28080–8. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound alpha-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab. 2013;108:56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Dorado B, Figueroa HY, Wang L, Planel E, Cookson MR, et al. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–47. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Broe M, Huang Y, Anderson JP, Gai WP, Milward EA, et al. Changes in the solubility and phosphorylation of alpha-synuclein over the course of Parkinson's disease. Acta Neuropathol. 2011;121:695–704. doi: 10.1007/s00401-011-0815-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.