Mutations in the mitochondrial fission factor GDAP1 are associated with severe peripheral neuropathies, but why the CNS remains unaffected is unclear. Using a Gdap1−/− mouse, Niemann et al. demonstrate that a CNS-expressed Gdap1 paralogue changes its subcellular localisation under oxidative stress conditions to also act as a mitochondrial fission factor.
Keywords: animal models, Charcot-Marie-Tooth disease, mitochondria, axonal transport, demyelinating disease
Abstract
The ganglioside-induced differentiation-associated protein 1 (GDAP1) is a mitochondrial fission factor and mutations in GDAP1 cause Charcot–Marie–Tooth disease. We found that Gdap1 knockout mice (Gdap1−/−), mimicking genetic alterations of patients suffering from severe forms of Charcot–Marie–Tooth disease, develop an age-related, hypomyelinating peripheral neuropathy. Ablation of Gdap1 expression in Schwann cells recapitulates this phenotype. Additionally, intra-axonal mitochondria of peripheral neurons are larger in Gdap1−/− mice and mitochondrial transport is impaired in cultured sensory neurons of Gdap1−/− mice compared with controls. These changes in mitochondrial morphology and dynamics also influence mitochondrial biogenesis. We demonstrate that mitochondrial DNA biogenesis and content is increased in the peripheral nervous system but not in the central nervous system of Gdap1−/− mice compared with control littermates. In search for a molecular mechanism we turned to the paralogue of GDAP1, GDAP1L1, which is mainly expressed in the unaffected central nervous system. GDAP1L1 responds to elevated levels of oxidized glutathione by translocating from the cytosol to mitochondria, where it inserts into the mitochondrial outer membrane. This translocation is necessary to substitute for loss of GDAP1 expression. Accordingly, more GDAP1L1 was associated with mitochondria in the spinal cord of aged Gdap1−/− mice compared with controls. Our findings demonstrate that Charcot–Marie–Tooth disease caused by mutations in GDAP1 leads to mild, persistent oxidative stress in the peripheral nervous system, which can be compensated by GDAP1L1 in the unaffected central nervous system. We conclude that members of the GDAP1 family are responsive and protective against stress associated with increased levels of oxidized glutathione.
Introduction
Charcot–Marie–Tooth disease, also called hereditary motor and sensory neuropathies, is one of the most common inherited neurological diseases (Skre, 1974). The disease phenotype is usually associated with distal muscle weakness and atrophy, distal sensory loss and deformities of the limbs. Charcot–Marie–Tooth disease is caused by mutations in many different genes, with autosomal recessive, dominant or X-linked modes of inheritance (Suter and Scherer, 2003). Depending on the gene and the specific mutation, the disease manifests either by damaging neurons, Schwann cells, as the myelinating glia of the PNS, or both cell types. In axonal forms of Charcot–Marie–Tooth disease, loss of myelinated axons is the primary pathological feature of the disease, detectable by reduced compound muscle action potential amplitudes. In primary demyelinating forms, myelin formation or maintenance is impaired, leading to decreased nerve conduction velocities, invariably followed by axonal loss at later stages. In intermediate forms, a mild nerve conduction velocity reduction and reduced compound muscle action potential amplitudes are detectable simultaneously, indicative of a synchronous impairment of the myelin-axonal entity (Shy et al., 2001; Suter and Scherer, 2003). Only a few Charcot–Marie–Tooth disease forms are caused by mutations in genes that are exclusively or mainly expressed in peripheral nerves, raising the question of why mutations in Charcot–Marie–Tooth disease-linked genes specifically damage peripheral nerves (Berger et al., 2006; Niemann et al., 2006). Animal models for some Charcot–Marie–Tooth disease forms have been established and allowed the elucidation of the molecular disease mechanisms, leading to the development of new therapeutic strategies (Martini, 2000; Meyer Zu Horste and Nave, 2006).
In peripheral nerves, one of the essential pathways is the maintenance of mitochondrial dynamics. Charcot–Marie–Tooth disease-linked mutations interfere with mitochondrial transport by affecting motor protein complexes and the integrity of the cytoskeleton (Niemann et al., 2006; Baloh, 2008; Vital and Vital, 2012). Furthermore, mitochondria form an interconnected network by continuous fusion and fission processes. Fusion allows exchange of lipids, proteins and mitochondrial DNA. Fission leads to more mitochondrial entities by generating smaller units. Those can be transported, sequestered for autophagic degradation, or they fuse back with other mitochondrial units (Chan, 2012). The balance of fusion and fission processes determines the mitochondrial morphology and is influenced by stress conditions, altered energy demands, or apoptosis (Youle and van der Bliek, 2012). Impairment of mitochondrial dynamics interferes with mitochondrial transport, mitochondrial biogenesis, and leads to loss of mitochondrial DNA and increased redox stress (Bossy-Wetzel et al., 2003; Chan, 2012; Youle and van der Bliek, 2012).
Different fusion or fission factors of the outer and inner mitochondrial membrane have been identified (Chan, 2012), three of which are also mutated in Charcot–Marie–Tooth disease. First, mutations in the gene encoding the fusion factor of the mitochondrial outer membrane mitofusin 2 (MFN2) cause a dominantly inherited axonal form of Charcot–Marie–Tooth disease (CMT2A; Zuchner et al., 2004). Expression of mutant MFN2 alters the mitochondrial morphology and impairs mitochondrial transport and respiratory activity in neuronal cultures (Baloh et al., 2007). Transgenic mice expressing Charcot–Marie–Tooth disease-associated mutant forms of MFN2 develop motor deficits, associated with altered axonal mitochondrial density and a reduction of the axonal calibre size and number (Cartoni et al., 2010). Second, the inverted formin 2 protein (INF2) has been linked to mitochondrial fission in Charcot–Marie–Tooth disease with glomerulopathy (Boyer et al., 2011). INF2 induces the formation of actin filaments, which initiate mitochondrial constrictions that precede mitochondrial fission (Korobova et al., 2013). However, it remains to be determined whether the Charcot–Marie–Tooth disease-associated mutations in INF2 relate to mitochondrial dynamics and/or to impairments of the cytoskeleton (Ramabhadran et al., 2012). The third factor, ganglioside-induced differentiation associated protein 1 (GDAP1), is a mitochondrial fission factor (Niemann et al., 2005, 2009; Pedrola et al., 2005). In addition GDAP1 influences the peroxisomal morphology (Huber et al., 2013). Unlike any other Charcot–Marie–Tooth disease-linked gene, the various identified mutations in GDAP1 are associated with demyelinating, axonal, or mixed forms of Charcot–Marie–Tooth disease with recessive or dominant modes of inheritance, showing a wide range in the severity and onset of disease (Cassereau et al., 2011a, b). In general, dominantly inherited forms of GDAP1-associated Charcot–Marie–Tooth disease have a later onset than recessively inherited forms (Zimon et al., 2011). Mutations leading to a premature stop of translation or lying within the carboxy-terminal tail-anchor of GDAP1, the mitochondrial and peroxisomal targeting domain, cause the most severe forms with onset of disease within the first decade of life (Wagner et al., 2009; Cassereau et al., 2011a; Kabzinska et al., 2011; Huber et al., 2013). Mutated forms of GDAP1 impair mitochondrial dynamics in cultured cells. Recessively inherited GDAP1 mutants have reduced fission activity, whereas dominantly inherited mutants impair mitochondrial fusion (Niemann et al., 2005; Pedrola et al., 2005; Wagner et al., 2009). Based on sequence comparisons, GDAP1 and its paralogue GDAP1L1 have been characterized as founders of a new glutathione S-transferase family (Marco et al., 2004). Whether GDAP1 has glutathione S-transferase activity is unclear, as for recombinant GDAP1 no glutathione S-transferase activity was detected in vitro (Shield et al., 2006). However, the involvement of GDAP1 in glutathione-mediated processes has been indirectly demonstrated, as GDAP1 expression levels correlated with the survival of cultured cell lines under oxidative stress conditions by influencing the cellular glutathione content (Noack et al., 2012). Charcot–Marie–Tooth disease-associated GDAP1 mutants have lost this protective effect, thereby providing a hint that the disease might be linked to oxidative stress (Noack et al., 2012). Interestingly, MFN2 activity is increased by oxidized glutathione (Shutt et al., 2012) and CMT2F-causing mutations in HSP27 (now known as HSPB1) have also been associated with oxidative stress (Zuchner and Vance, 2006).
Here we introduce an animal model mimicking severe forms of Charcot–Marie–Tooth disease caused by GDAP1 mutations. The Gdap1 knockout mice develop a late-onset peripheral neuropathy with a reduced nerve conduction velocity and hypomyelination. The phenotype is dependent on loss of GDAP1 expression in Schwann cells, with mild impairment of the mitochondrial transport in axons. Further analysis reveals a novel mechanistic link between the GDAP1-family of proteins and oxidized glutathione-associated stress.
Materials and methods
Gdap1 mutant mice
Transgenic mice carrying loxP sites flanking exon 5 of the Gdap1 gene (Gdap1flox), were generated by the Mouse Clinical Institute (MCI, Strasbourg, France) using standard procedures of homologous recombination in murine embryonic stem cells. Gdap1 knockout (Gdap1−/−) mice were established by breeding Cre deleter mice with homozygous Gdap1flox/flox mice. Genotypes of mice were determined by PCR on genomic DNA derived from ear biopsies (Supplementary material). For conditional ablation the P0-cre and Hb9-cre lines have been described (Arber et al., 1999; Feltri et al., 1999, 2002). Nerve crush injury and electron microscopy of nerve tissues were as described (Somandin et al., 2012). Electrophysiological measurements and behavioural tests were analysed as described (Horn et al., 2012). All performed experiments with mice followed approved protocols (Veterinary Office of the Canton Zurich, Switzerland). Wild-type animals, cre-negative animals or cre-positive animals without floxed GDAP1 alleles served as controls.
Quantitative polymerase chain reaction
Reverse transcription was performed as described (Niemann et al., 2005). Mitochondrial DNA and genomic DNA from tissue were purified by using genomic DNA isolation Kit and G20 columns (Qiagen). DNA was resuspended in Tris-EDTA (TE) buffer, sonicated in a water bath for 10 min and dissolved overnight (Malik and Czajka, 2013). The quantitative PCR was performed in two different dilutions of DNA input (between 5 ng and 160 ng) using primers (330 nM) and 6-carboxyflurescein (FAM)-labelled tetramethylrhodamine (TAMRA)-quenched probes (20 nM; Microsynth). Gdap1l1 transcripts were detected by quantitative PCR using SYBR® Green PCR Master Mix (Applied Biosystems) in relation to 18S ribosomal RNA as published (Niemann et al., 2005), all other quantitative reverse transcribed PCRs were quantified in relation to β-actin on a Light Cycler 480 II (Roche), using LightCycler 480 SYBR® Green I Master Mix or Light Cycler 480 Probes Master (Roche). Primers and probes are listed in the Supplementary material.
Constructs
Constructs for transient transfections, lentiviral vectors and production of viruses have been described (Niemann et al., 2005, 2009; Wagner et al., 2009; Huber et al., 2013). GDAP1L1 was cloned from murine complementary DNA (Supplementary material). Mutations and fusion proteins were generated as described (Wagner et al., 2009).
Cell culture
Dorsal root ganglia of Gdap1−/− and control animals were isolated from embryonic Day 13.5 mice and infected with lentivirus (Supplementary material). HEK-293T and N1E-115 cells were maintained, transfected and infected as described previously (Niemann et al., 2009). N1E-115 cells were incubated for 2 h with 10 µM rotenone, 1 µM dithiothreitol (DTT), 0.1 mM hydrogen peroxide (H2O2), 10 µM cyanide m-chlorophenylhydrazone (cccp), 20 µM menadione, 100 µM tert-butylhydroquinone (TBHQ), 20 µM 2,3-dimethoxy-1,4-naphthoquinone (DNMQ), if not indicated differently. 1 mM N-acetyl-l-cystein (NAC) was applied 3 min before treatment with other drugs. All drugs were purchased from Sigma.
Live cell imaging and analysis
Dorsal root ganglion explant cultures were imaged with a ×40 Plan-Apo lens at the Olympus IX81 equipped with Cell-R software (Olympus) and Hamamatsu Orca ER (Hamamatsu). During imaging cultures were maintained at 37°C and 5% CO2. The proximal 100 to 200 µm of a neurite were imaged for 5 min with 30 images/min. The acquired movies were analysed by MetaMorph software 7.7.5 (Molecular Devices). Results were exported to Microsoft Excel.
Immunocytochemistry
Immunocytochemistry, immunohistochemistry and image processing was performed as described (Niemann et al., 2005). Antibodies: anti-GDAP1 (Niemann et al., 2005), rabbit anti-GDAP1L1 antiserum (Pineda; Supplementary material and Supplementary Fig. 4C), anti-cytochrome c (Pharmingen).
Biochemical methods
Fractionations experiments from spinal cord and cultured cells were performed as described (Niemann et al., 2009; Li et al., 2010). Western blotting, detection and quantification were performed as described (Niemann et al., 2005). Antibodies: anti-GDAP1 (Niemann et al., 2005), anti-GDAP1L1 (Pineda; Supplementary material and Supplementary Fig. 4A), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; HyTest), anti-Porin/voltage dependent anion channel (VDAC; Abcam) and anti-β-actin (Sigma).
Results
Challenging peripheral nerves reveals defects in Gdap1−/− mice
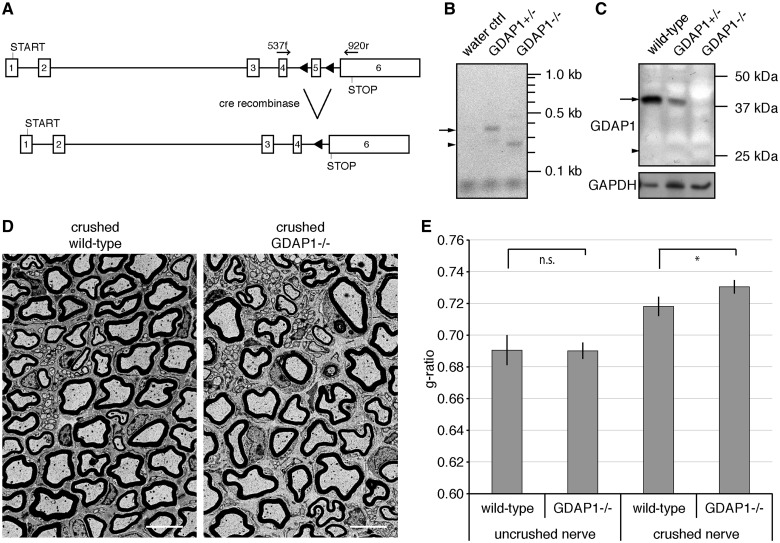
Mutations in GDAP1 cause various forms of Charcot–Marie–Tooth disease. The most severe forms with early onset and severe progression of disease are caused by recessively inherited mutations leading to a truncated protein or by a mutation within the C-terminal tail-anchor domain, which interferes with the targeting information (Cassereau et al., 2011b; Kabzinska et al., 2011). We generated a transgenic mouse line with exon 5 of Gdap1 flanked by loxP sites (Fig. 1A). Gdap1flox/flox mice were crossed with cre-deleter lines to ablate GDAP1 expression in the germline and bred to obtain wild-type, heterozygous and knockout animals (Gdap1 wt/wt, Gdap1 wt/- and Gdap1−/−). We confirmed by reverse transcriptase PCR that deletion of exon 5 resulted in a transsplicing from exon 4 to exon 6 (Fig. 1B). The resulting transcript lacking exon 5 harbours the start codon and the first 193 amino acids of GDAP1 plus 25 extra amino acids as a result of a frame-shift by the splicing from exon 4 to 6 (K193fsX219; Fig. 1A). Thus the Gdap1−/− mice resemble truncation mutations found in patients with Charcot–Marie–Tooth disease (S162fsX166, I186fsX205, R191X or S194X), which have been linked to axonal, demyelinating or intermediate forms of Charcot–Marie–Tooth disease with early onset (Cassereau et al., 2011a). Western blot analysis revealed loss of GDAP1 expression in Gdap1−/− animals. No truncated protein was detectable at the predicted size of 25 kDa, suggesting protein instability (Fig. 1C). In line with this finding, the GDAP1 Q163X protein is also unstable upon transient overexpression in COS-7 cells (Niemann et al., 2005). Gdap1+/+, Gdap1+/− and Gdap1−/− mice were born in normal Mendelian ratio (not shown), grew normally (Supplementary Fig. 1A), and showed no signs of peripheral neuropathies. In 13-month-old mice, motor and sensory studies revealed no significant differences between mutant and control animals (Supplementary Fig. 1B and C). To investigate changes in gene expression patterns of Gdap1−/− mice, we isolated motor neurons by laser dissection and took sciatic nerve lysates of 2-month-old mice, purified RNA, and compared expression profiles for Gdap1−/− and wild-type animals by Affymetrix GeneChip arrays. Cluster analysis revealed no significant changes, nor did we identify alterations in the expression of individual genes that could provide hints for compensatory adjustments in Gdap1−/− mice. The expression profile array data have been deposited in NCBI's Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE51650 (Supplementary material).
Figure 1.
Gdap1−/− animals display hypomyelination after nerve crush. (A) The ablation of exon 5 in the Gdap1 locus generates a premature stop codon in exon 6 as a result of a frame shift (triangles: introduced loxP sites; START: translation start codon; STOP: translational stop codon; arrows indicate primer pairs used for reverse transcriptase-PCR). (B) Reverse transcriptase-PCR demonstrates the trans-splicing from exon 4 to 6 in Gdap1−/− animals. The sizes of the predicted PCR products for the wild-type transcript (arrow; 383 bp) and the trans-spliced transcript (arrow head; 269 bp) are indicated. (C) Loss of GDAP1 protein in mutant mice (arrow: wild-type protein; arrowhead: size of the predicted GDAP1 K193fsX219 protein: note that the antiserum detects the amino-terminus of GDAP1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as loading control. (D) Representative ultrathin sections of unilaterally crush-lesioned sciatic nerves of 4-month-old wild-type and Gdap1−/− mice analysed by transmission electron microscopy. Two month post crush sections were obtained 3 mm distal to the site of injury. Scale bar = 5 µm. (E) Quantitative morphological analysis of sciatic nerve sections. One hundred randomly selected axons per crushed and uncrushed nerve were measured per animal. Values represent mean and standard error of three animals per genotype: n.s. = not significant, *P < 0.05.
As no overt phenotype was detectable up to 13 months, we challenged peripheral nerves by performing a sciatic nerve crush in 2-month-old mice (Gillespie et al., 2000; Ewaleifoh et al., 2012; Bogdanik et al., 2013). After a regeneration period of 2 months, sciatic nerves of wild-type and Gdap1−/− mice were analysed distal to the site of injury by transmission electron microscopy. The nerve fibres of both Gdap1−/− and wild-type animals were remyelinated (Fig. 1D), but remyelination was less effective in Gdap1−/− animals as indicated by a significantly higher g-ratio compared with remyelinated controls (Fig. 1E). This hypomyelination in injured Gdap1−/− mice was accentuated in large calibre axons (quantification not shown), even though axons of all myelination-competent calibres were remyelinated (Fig. 1D). Uncrushed, contralateral nerves revealed no difference in the g-ratios of Gdap1−/− and control animals (Fig. 1E). In summary, we did not detect pathological changes in Gdap1−/− mice within the first 13 months of age. Nevertheless, the alterations observed after challenging peripheral nerves of mutant mice by nerve injury prompted us to analyse later time points.
Late onset peripheral neuropathic changes in Gdap1−/− mice
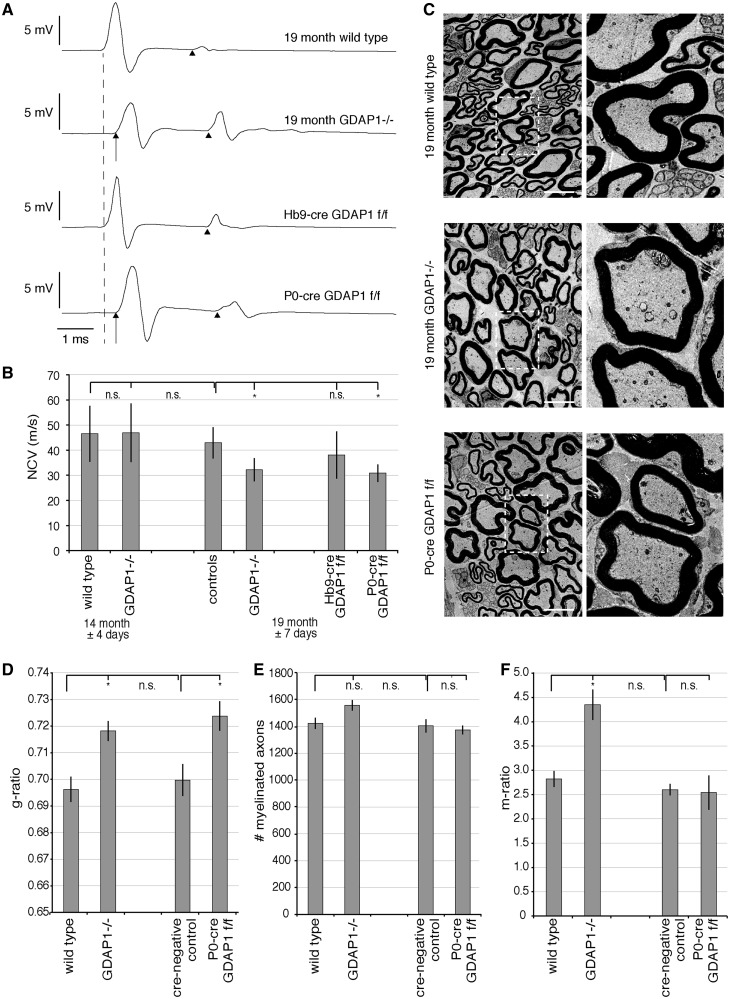
In line with our previous results, we found no changes in nerve conduction velocities or compound muscle action potential amplitudes by electrophysiological measurements in 14-month-old Gdap1−/− mice as compared with controls (Fig. 2B and Supplementary Table 1). In contrast, in 19-month-old Gdap1−/− animals, nerve conduction velocities were significantly reduced by 25% compared with age-matched controls (Fig. 2A and B), consistent with a late-onset demyelinating phenotype. After stimulation at the sciatic notch (proximal stimulation), the compound muscle action potential amplitudes were significantly reduced in 19-month-old Gdap1−/− mice when compared with controls, whereas significance was not reached using more distal stimulation at the tibial nerve (Supplementary Table 1). As the compound muscle action potentials were not clearly dispersed, the reduction in the compound muscle action potential amplitudes does not obviously attribute to a pure myelin disorder, but might also reflect an additional axonal phenotype. To investigate axonal pathology of 19-month-old animals, we carried out histological analysis of the plantar nerve, a distal peripheral nerve, as in Charcot–Marie–Tooth disease the longest nerves are the first and most severely affected. Only minor differences appeared in electron micrographs of knockout animals compared with age-matched controls (Fig. 2C). However, morphometric analyses revealed hypomyelination in 19-month-old Gdap1−/− mice compared with controls (Fig. 2D and Supplementary Fig. 2A) with no detectable axonal loss (Fig. 2E). Both, Schwann cells and neurons express GDAP1 in the myelinated peripheral nerves, and it has been proposed that GDAP1 is even mainly expressed by neurons (Niemann et al., 2005; Pedrola et al., 2005, 2008). Thus, to confirm the unexpected predominant myelin disorder phenotype, we disrupted Gdap1 by cre-mediated ablation specifically in Schwann cells (P0-cre Gdap1flox/flox) or in motor neurons (Hb9-cre Gdap1flox/flox). Ablation of GDAP1 in Schwann cells reduced nerve conduction velocities comparable to total knockout animals, whereas motor neuron-specific loss of GDAP1 caused no significant changes when compared with controls (Fig. 2A and B). Moreover, compound muscle action potentials were not decreased in Hb9-cre Gdap1flox/flox animals (3.78 ± 0.41 mV ms; n = 8; controls: 3.0 ± 0.35 mV ms n = 8), indicating that the compound muscle action potential reduction observed in Gdap1−/− mice was likely not due to a motor neuron/axon-autonomous pathology. To further support that ablation of GDAP1 expression in Schwann cells recapitulates the phenotype of Gdap1−/− mice, we analysed also the plantar nerve of 19-month-old P0-cre Gdap1flox/flox mice morphologically. We found significant hypomyelination and no axonal loss in 19-month-old P0-cre Gdap1flox/flox mice comparable to Gdap1−/− mice (Fig. 2D and E). Taken together, our results indicate that loss of GDAP1 expression in Schwann cells is sufficient to cause a hypomyelinating peripheral neuropathy phenotype in aged mice.
Figure 2.
Aged Gdap1−/− mice develop a hypomyelinating peripheral neuropathy. (A) Nerve conduction velocity (NCV) measurements of 19-month-old mice with different genotypes are shown as representative original recordings. The broken line represents onset (distal latency) of compound muscle action potentials in unaffected mice. Arrows denote a delayed compound muscle action potential onset (prolonged distal latency) in affected mice. Note that there is no relevant difference in shape and duration of compound muscle action potentials. F-waves are identified by arrowheads. (B) Nerve conduction velocities are represented as means and standard error, n = 6 to 11 animals per genotype and age group; variations of age are indicated as standard error; controls are a group of wild-type and cre-negative animals. n.s. = not significant, *P < 0.05. (C) Transverse ultrathin sections of plantar nerves of 19-month-old Gdap1−/− and P0-cre Gdap1flox/flox (f/f) animals reveal hypomyelination compared to age-matched controls. Scale bars = 5 µm, squares are shown at higher magnification. (D) Quantitative morphological analysis of ultrathin plantar nerve sections at 19 months showing hypomyelination in Gdap1−/− and P0-cre Gdap1flox/flox animals compared with wild-type and cre-negative controls. (E) Quantitative analysis of the total number of myelinated axons in the plantar nerve. (F) The m-ratio, defined as the percentage of the area covered by intra-axonal mitochondria divided by the total area of the axon, is significantly increased only in Gdap1−/− animals. (D and F) One hundred randomly selected myelinated axons were measured per animal. (D–F) represent the means and standard error of independent experiments; cre-negative controls and P0-cre Gdap1flox/flox: n = 3 animals each; wild-type and Gdap1−/− n = 4 animals each; n.s. = not significant, *P < 0.05.
Alterations of intra-axonal mitochondria in Gdap1−/− mice
GDAP1 influences mitochondrial morphology. Thus, mutations in GDAP1 might lead to impaired mitochondrial transport in line with that especially long peripheral axons might be afflicted (Pedrola et al., 2008). Initial studies in cell lines lead to inconsistent results (Niemann et al., 2009; Pla-Martin et al., 2013). Thus, to address this point, we analysed the distribution of mitochondria in axons of plantar nerves of wild-type, Gdap1−/−, P0-cre Gdap1flox/flox and cre-negative control animals. To quantify changes in the distribution of mitochondria, we determined the axonal area covered by mitochondria in percentage and defined this value as the m-ratio. This m-ratio was consistent for different mice of the same genotype, and was increased in Gdap1−/− mice compared with age-matched controls (Fig. 2C and F). In contrast, no change in the m-ratio was observed in P0-cre Gdap1flox/flox animals in comparison to age-matched controls (Fig. 2C and F), demonstrating that the mitochondrial changes in axons do not originate from the loss of GDAP1 expression in Schwann cells. As the m-ratio is influenced by the axonal calibre, and the number and sizes of mitochondria, we analysed these parameters in more detail. The axonal calibres were identical in Gdap1−/− mice and controls (Supplementary Fig. 2B), and the increase in the number of mitochondria in the axons of Gdap1−/− was minor, not reaching significance (Supplementary Fig. 2C). Yet, the size of the mitochondria in Gdap1−/− axons was significantly increased in comparison to control animals (Supplementary Fig. 2D). Taken together, the morphometric analysis of axonal mitochondria revealed that ubiquitous loss of GDAP1 affects mitochondria of the plantar nerve, whereas selective loss of GDAP1 expression in Schwann cells does not. Thus, our data are consistent with a cell-autonomous effect also in neurons. Furthermore, the increased m-ratio in Gdap1−/− nerves reflects changes in mitochondrial sizes and is not indicative of a major transport deficit.
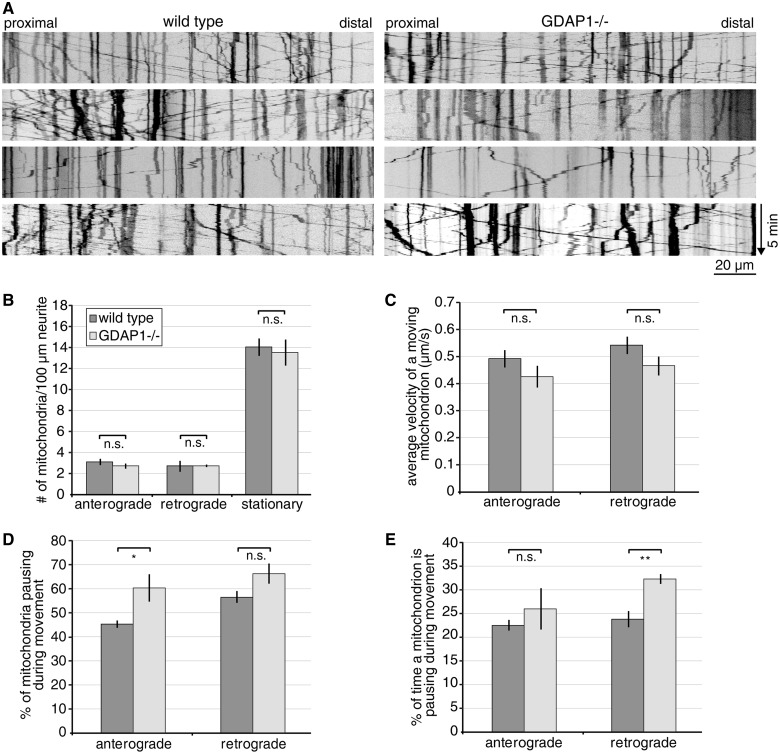
To pursue the transport issue directly, we investigated mitochondrial transport in dorsal root ganglion explant cultures of control and Gdap1−/− animals. Embryonic Day 13.5 embryos of wild-type or Gdap1−/− mice were dissected and dorsal root ganglion neurons were taken into culture and infected with lentiviral particles encoding DsRed with a mitochondrial leader sequence. When expression of mitochondrially targeted DsRed was detectable, cultures were analysed by live-cell imaging (Fig. 3A). As no evident difference was observed between wild-type and Gdap1−/− cultures, we quantified the transport events. Comparable numbers of mitochondria were stationary, or travelled in anterograde and retrograde direction in neurons of Gdap1−/− and wild-type mice (Fig. 3B). Also the velocities of the mitochondrial transport were not significantly reduced in Gdap1−/− cultures (Fig. 3C). However, the velocity calculations do not take into account that mitochondria often pause and then continue their movements. Indeed, when we analysed the pausing in more detail, we found that significantly more mitochondria in Gdap1−/− cultures pause during anterograde transport events (Fig. 3D), whereas the pausing lasted significantly longer for mitochondria transported in retrograde direction (Fig. 3E). In summary, we identified subtle changes in mitochondrial transport in cultured dorsal root ganglion neurons commensurate with the trend that we observed toward a slight increase in the number of mitochondria in the morphological analysis of peripheral nerve axons.
Figure 3.
Gdap1−/− dorsal root ganglia explant cultures reveal mild mitochondrial transport impairment. Five to nine dorsal root ganglion neurons expressing mitochondrially targeted DsRed were imaged per genotype and experiment, resulting in the analysis of 200 to 400 mitochondria per genotype and preparation. The movies were converted into kymographs (A) and quantified (B–E). (A) Representative kymographs of wild-type and Gdap1−/− neurons reveal no obvious impairment of mitochondrial transport. Vertical lines represent stationary mitochondria, lines crossing from left to right or right to left represent antero- or retrograde transport, respectively. (B) Quantification of the number of stationary and moving mitochondria (subdivided in anterograde or retrograde direction) per 100 µm of neurite length reveals no difference between Gdap1−/− and wild-type cultures. (C) The velocity of moving mitochondria is not altered in anterograde and retrograde direction in Gdap1−/− neurons. (D) During the acquisition period of 5 min, significantly more mitochondria moving in anterograde direction pause, (E) and the time a retrograde-moving mitochondrion spends pausing is significantly longer in Gdap1−/− cultures compared to wild-type controls. (B–E) Graphs represent means and standard error of n = 4 independent culture preparations per genotype, n.s. = not significant, *P < 0.05, **P < 0.01.
Increased mitochondrial DNA biogenesis in peripheral nerves of Gdap1−/− mice
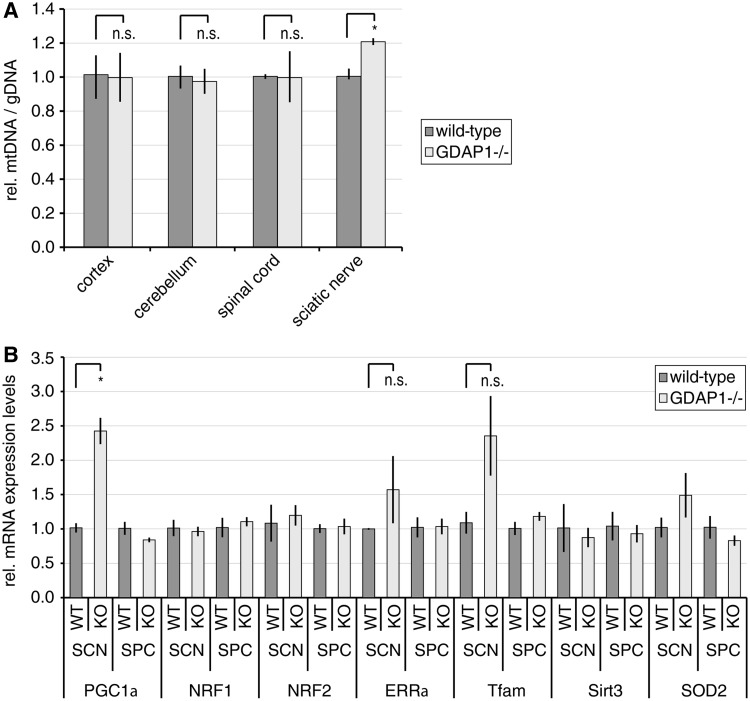
Impairing mitochondrial dynamics influences the mitochondrial DNA content. Blocking mitochondrial fusion leads to a reduction or loss of mitochondrial DNA, which can be quantified by the ratio between mitochondrial DNA and genomic DNA (Chen and Chan, 2010; Malik and Czajka, 2013). Expression knockdown of the mitochondrial fission factor dynamin-related protein 1 (DRP1, now known as DNM1L) also reduces the mitochondrial DNA content (Parone et al., 2008), whereas in Drp1−/− mouse embryonic fibroblasts the mitochondrial DNA content was not significantly altered (Ishihara et al., 2009). As GDAP1 is a mitochondrial fission factor and mitochondrial morphology was altered in the plantar nerve of Gdap1−/− animals (Supplementary Fig. 2D), we determined the mitochondrial DNA content in relation to the genomic DNA. Unexpectedly, the mitochondrial to genomic DNA-ratio was significantly increased in the sciatic nerve of Gdap1−/− animals compared with age-matched controls (Fig. 4A). Increased mitochondrial DNA contents are characteristic for mild, persistent oxidative stress, which is not overloading the intracellular antioxidant system (Lee and Wei, 2005; Scarpulla et al., 2012). Nuclear factors have been identified to regulate this protective response (Lee and Wei, 2005; Moreno-Loshuertos et al., 2006; Malik and Czajka, 2013). The peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α, now known as PPARGC1A) regulates various aspects of mitochondrial biogenesis, including the protective mitochondrial DNA increase. PGC1α influences the mitochondrial DNA replication in concert with other nuclear factors and coactivators, mainly nuclear respiratory factors 1 and 2 (NRF1, NRF2) and oestrogen-related receptor α (ERRα, now known as ESRRA). The transcription factor A, mitochondrial (TFAM) is one of the factors being induced by PGC1α and its coactivators. As mitochondrial factor, TFAM positively regulates the mitochondrial DNA copy number. Additionally, PGC1α and ERRα induce sirtuin 3 (SIRT3), which activates reactive oxygen species-protective pathways in mitochondria, i.e. the mitochondrial superoxide dismutase 2 (SOD2; Lee and Wei, 2005; Scarpulla et al., 2012). We found that the messenger RNA levels of Pgc1α are significantly increased in the sciatic nerves of Gdap1−/− mice compared with controls (Fig. 4B). In addition Errα, Tfam and Sod2 were elevated although significance was not reached due to variability in Gdap1−/− samples. No changes in Sirt3 levels were detectable (Fig. 4B). Collectively, our findings support that mild, persistent oxidative stress leads to an increase in the mitochondrial DNA content in sciatic nerves of 19-month-old Gdap1−/− mice. Strikingly, none of these changes were detected in highly GDAP1 expressing CNS tissues (Niemann et al., 2005; Pedrola et al., 2005) (Fig. 4A and B).
Figure 4.
Mitochondrial DNA biogenesis is increased in sciatic nerves of Gdpa1−/− mice. (A) DNA of the indicated tissues was isolated from 19-month-old Gdap1−/− and wild-type mice. The relative amount of mitochondrial DNA (mtDNA) in relation to genomic DNA (gDNA) was determined by quantitative PCR. (B) Total RNA was isolated from sciatic nerve (SCN) and spinal cord (SPC) lysates from 19-month-old Gdap1−/− and wild-type mice. The relative expression levels of nuclear factors involved in mitochondrial biogenesis and redox protection were determined by quantitative PCR in relation to β-actin levels. (A and B) Graphs represent means and standard error of tissues of n = 3 animals per genotype, n.s. = not significant, *P < 0.05.
To confirm further the tissue-specificity of the phenotype of Gdap1−/− mice, we analysed the cerebellum and the retina morphologically as these tissues are prevalently damaged by impaired mitochondrial dynamics (Chen and Chan, 2010). As hypomyelination was the major pathological change in the peripheral nerves, we also determined the g-ratio in the ventral funiculus of the spinal cord. We found neither neuronal loss nor hypomyelination in the analysed samples (Supplementary Fig. 3).
GDAP1L1 is responsive to changes in the cellular redox state
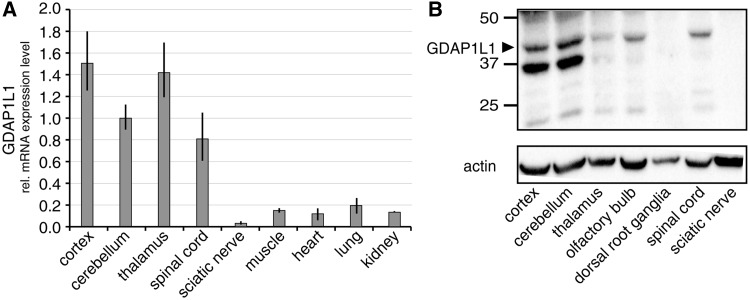
In search for a molecular mechanism explaining the lack of a CNS phenotype, we turned to Gdap1l1, the paralogue of Gdap1. We analysed the expression pattern of Gdap1l1 by quantitative reverse transcriptase PCR and western blots. Gdap1l1 is expressed in the CNS, but not in the PNS (Fig. 5). In immunostainings, the GDAP1L1 signal co-labels Purkinje cells in the cerebellum, and neuronal nuclei (NeuN)-positive neurons in cortex and hippocampus (Supplementary Fig. 4B and C), demonstrating that GDAP1L1 expression at least partially overlaps with GDAP1 (Pedrola et al., 2008).
Figure 5.
The paralogue of Gdap1, Gdap1l1, is expressed in the CNS. (A) Relative Gdap1l1 messenger RNA (mRNA) expression levels in mouse tissues were determined by quantitative reverse transcriptase PCR in relation to 18S ribosomal RNA. (B) GDAP1L1 protein expression was determined by western blot using 12 µg protein lysate of indicated murine tissues and anti-GDAP1L1 antiserum (Supplementary Fig. 4A). β-actin served as loading control. Arrowhead = predicted molecular weight of GDAP1L1.
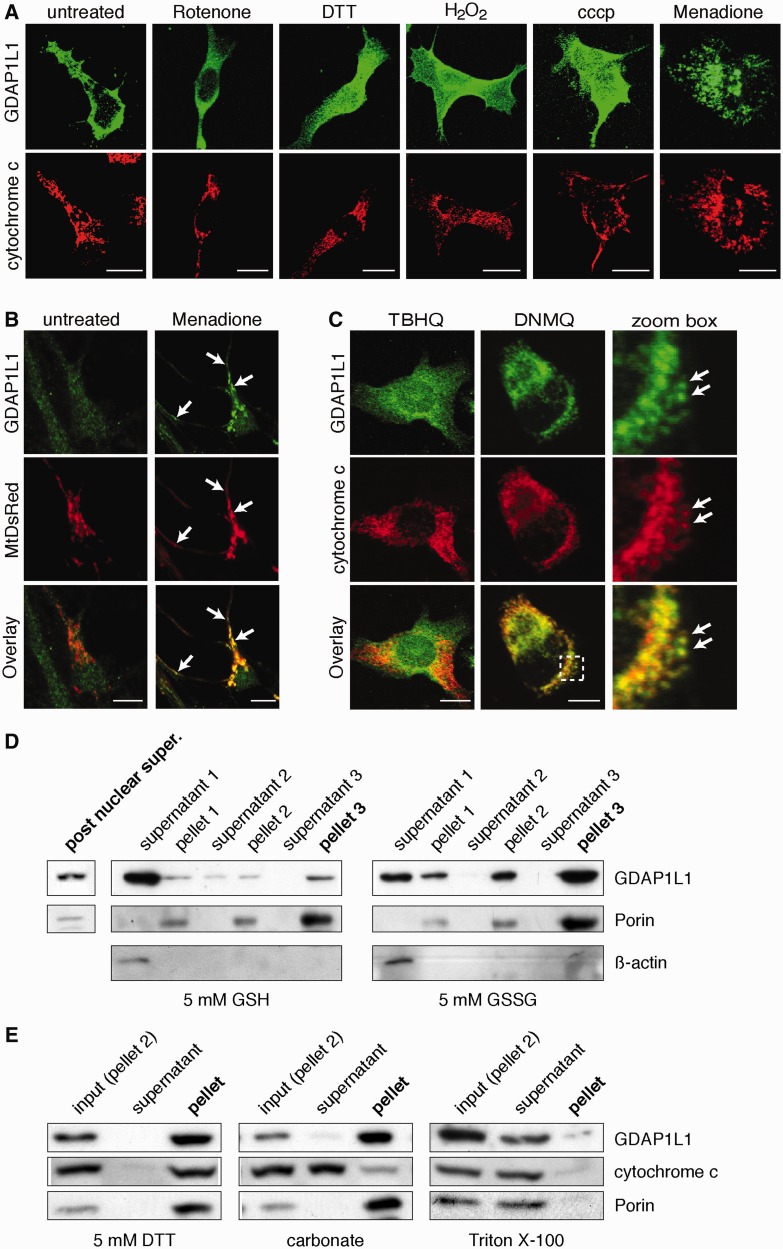
Surprisingly, we found that GDAP1L1 is not a mitochondrial protein but is mainly cytosolic (Fig. 6A). This was unexpected, as the targeting-domain of GDAP1, the C-terminal tail-anchor domain with surrounding basic amino acids (Wagner et al., 2009), is conserved in GDAP1L1 (Marco et al., 2004). Fusing the C-terminal domains of GDAP1L1 to the C-terminus of the green fluorescent protein (GFP) resulted in efficient targeting of the fusion proteins to mitochondria (Supplementary Fig. 5), demonstrating that the C-terminal domain of GDAP1L1 has the potential to efficiently target GDAP1L1 to mitochondria. As GDAP1L1 is mainly cytosolic, we reasoned that this targeting domain might be hidden under physiological conditions. Similarly, the tail-anchor domain containing protein BAX is cytosolic in healthy cells and needed for mitochondrial fusion. Upon induction of apoptosis, BAX translocates to mitochondria (Karbowski et al., 2006). In analogy, we transiently expressed GDAP1L1 or GDAP1 in N1E-115 cells and incubated the cells with different drugs and culture conditions (Fig. 6A and Supplementary Fig. 6A). In this initial screen GDAP1L1 is cytosolic under most conditions. Only the treatment with menadione induced a translocation of GDAP1L1 to mitochondria, which were identified by cytochrome c immunostaining (Fig. 6A). The translocation of GDAP1L1 because of menadione was concentration- and time-dependent and could be inhibited by N-acetyl-l-cysteine (Supplementary Fig. 6B–D). Menadione-treatment also induced the mitochondrial translocation of endogenous GDAP1L1 in primary hippocampal neurons (Fig. 6B).
Figure 6.
Translocation of GDAP1L1 to mitochondria. (A) N1E-115 cells were transiently transfected with GDAP1L1 expression constructs and treated for 2 h as indicated. Cells were fixed, stained for GDAP1L1 and cytochrome c and examined by immunofluorescence. Single plane confocal images are shown. GDAP1L1 has mainly a cytosolic localization, which is altered upon treatment with menadione (right). (B) Primary hippocampal neurons were infected with lentivirus encoding mitochondrially targeted DsRed (MtDsRed). Four days post-infection, cells were treated for 2 h with menadione or left untreated, fixed and stained for endogenously expressed GDAP1L1. Single plane confocal images are shown. (C) Twenty-four hours post-transfection with GDAP1L1 expression constructs and subsequent 2 h treatment with the indicated reagents, N1E-115 cells were analysed on single plane confocal pictures. 2,3-dimethoxy-1,4-naphthoquinoe (DNMQ, 20 µM) led to a redistribution of GDAP1L1 to mitochondria as visualized by the colocalization with the mitochondrial marker cytochrome c (arrows). In contrast, tert-butylhydroquinone (TBHQ, 100 µM) had no impact on the subcellular distribution of GDAP1L1. Broken line indicates area shown in higher magnification. (D) N1E-115 cells were transiently transfected with GDAP1L1 expression constructs and homogenized using a Dounce homogenizer on the next day. The post-nuclear supernatant of the lysate was divided in two and incubated with reduced glutathione (GSH) or oxidized glutathione (GSSG). In differential sedimentation steps, cytosolic proteins were removed as demonstrated by western blot of all purification steps against the cytosolic marker β-actin. In the oxidized glutathione (GSSG)-treated fraction of the post nuclear supernatant, more GDAP1L1 co-sediments with the mitochondrial marker Porin compared with the reduced glutathione (GSH)-treated fraction. (E) Pellet 2 of GDAP1L1 expressing cells treated with oxidized glutathione was resuspended in buffer plus 5 mM dithiothreitol (DTT), in 0.1 M carbonate (pH 11), or in buffer containing 0.1% Triton™ X-100 and sedimented to separate soluble protein supernatants from membranous pellets. GDAP1L1 and the known transmembrane protein Porin are only solubilized by detergent. (A–C) Scale bars = 10 µm.
Menadione is a quinone, which can be conjugated to glutathione by glutathione S-transferases. Alternatively, menadione can redox cycle, resulting in an increase in cellular oxidized glutathione at the expense of reduced glutathione (Watanabe et al., 2004). The naphthoquinone 2,3-dimethyoxy-1,4-naphthoquinone (DNMQ) can only redox cycle, whereas the quinone tert-butylhydroquinone (TBHQ) is detoxified by glutathione-conjugation. Only the treatment with DNMQ caused a translocation of transiently expressed GDAP1L1 to mitochondria in N1E-115 cells, suggesting that an increase in oxidized glutathione levels might induce the translocation (Fig. 6C). Measurements of reduced and oxidized glutathione concentrations in N1E-115 cells under the different drug-treatment conditions confirmed that increased oxidized glutathione levels correlate with GDAP1L1 translocation (Supplementary Table 2).
Next we reconstituted the translocation in vitro. We used post-nuclear supernatant of N1E-115 cells transiently expressing GDAP1L1. The post-nuclear supernatant was divided and incubated with either reduced or oxidized glutathione, before sedimentation of the heavy membrane fraction to enrich mitochondria (Fig. 6D). In untreated (not shown) and reduced glutathione-treated lysates, GDAP1L1 co-fractionated with the cytosolic marker protein ß-actin and only a minor fraction co-sedimented with the mitochondrial marker protein Porin [2.3 ± 0.8% of GDAP1L1 in the post-supernatant is recovered in pellet 3, n = 3; (mean ± standard error)]. Treating the same post-nuclear supernatant with oxidized glutathione caused a partial co-sedimentation of GDAP1L1 (10.7 ± 1.2%, n = 3). Thus, significantly more GDAP1L1 co-fractionated with Porin upon treatment with oxidized glutathione when compared with reduced glutathione (P < 0.005). Taking into consideration that the post-translational insertion of GDAP1L1 into the membrane occurs at 4°C, this represents efficient targeting (Wagner et al., 2009). As co-sedimentation does not prove that the protein inserted into the membrane, we subdivided Pellet 2 of the sedimentation and washed the pellets with dithiothreitol (DTT), carbonate or Triton™ X-100 (Fig. 6E). Like the mitochondrial membrane protein Porin, GDAP1L1 is solubilized only by Triton™ X-100. Surprisingly, treatment with DTT did not lead to the release of GDAP1L1 into the supernatant, suggesting that the protein, once inserted, will not leave the membrane in a reducing environment. Collectively, our results demonstrate that GDAP1L1 can be targeted to mitochondria under conditions associated with elevated oxidized glutathione concentrations.
GDAP1L1 induces fission upon mitochondrial translocation
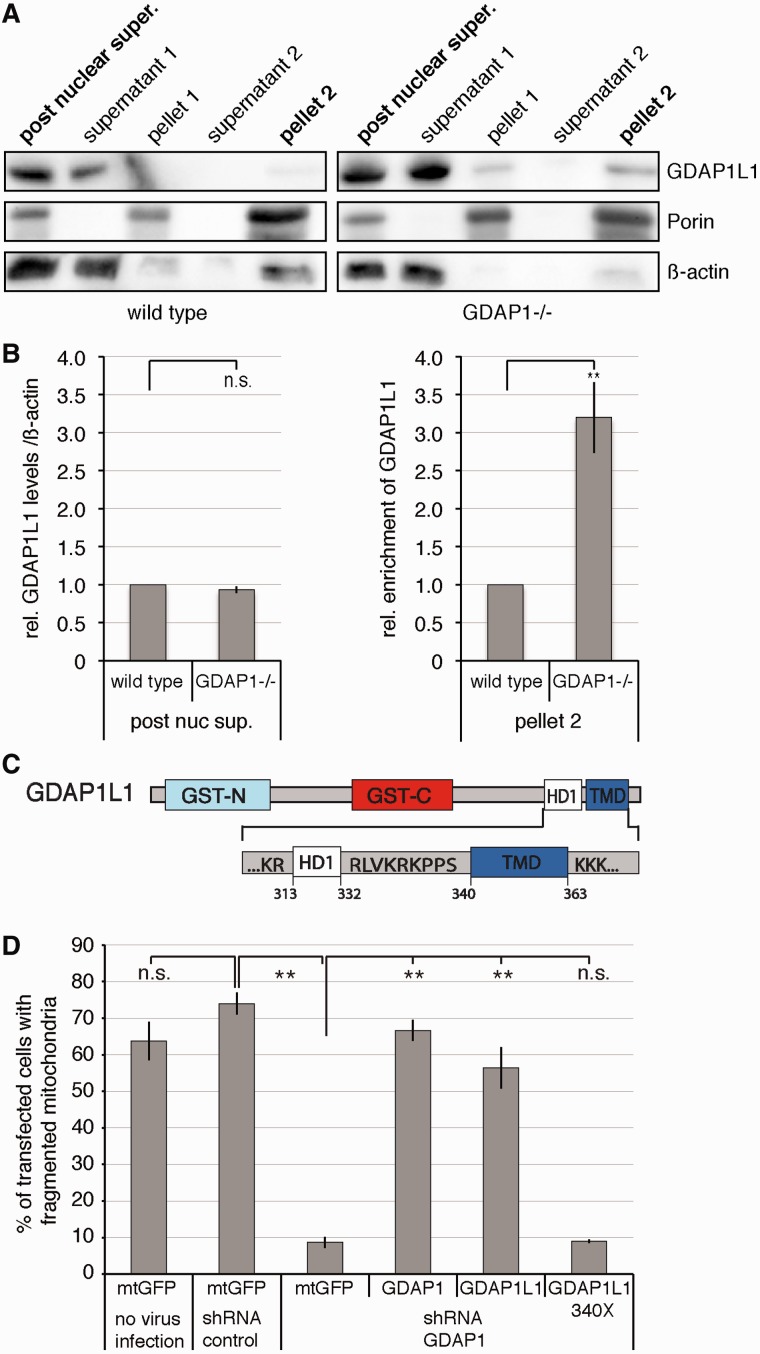
Long-term loss of GDAP1 expression causes mild oxidative stress conditions in peripheral nerves (Fig. 4A and B) and in cultured neuronal cell lines (Noack et al., 2012). Therefore, we carried out differential centrifugation to enrich mitochondria from the spinal cord to obtain evidence whether translocation of GDAP1L1 occurs also in vivo. In the spinal cord of 19-month-old Gdap1−/− mice, equal amounts of GDAP1L1 were expressed compared with age-matched controls (Fig. 7A and B). However, significantly more GDAP1L1 co-sedimented with Porin compared with the controls (Fig. 7A and B).
Figure 7.
Translocation of GDAP1L1 is needed to compensate for the loss of GDAP1 expression. (A) Spinal cords of 19-month-old wild-type or Gdap1−/− animals were fractionated by differential centrifugation. In homogenates from Gdap1−/− animals, more GDAP1L1 sediments with the mitochondrial marker Porin compared with age-matched wild-type controls. β-actin served as maker for the cytosolic fraction. (B) The relative amounts of GDAP1L1 were quantified by densitometry in relation to β-actin in the post-nuclear supernatant and the relative enrichment from GDAP1L1 was determined for pellet 2. The values obtained from wild-type animals were set to 1 in each of four independent experiments. Means and standard error of the mean, n = 4. n.s. = not significant, **P < 0.005. (C) The domain structure of GDAP1L1 is illustrated with focus on the carboxy-terminal domains harbouring the hydrophobic domain 1 (HD1) and the transmembrane domain (TMD; GST-N and GST-C: domains conserved in glutathione S-transferases). The tail-anchored domain is formed by the transmembrane domain and surrounding positively charged amino acids. (D) N1E-115 cells were infected with lentiviral particles encoding non-silencing short-hairpin RNA (shRNA control) or short-hairpin RNA against Gdap1 (shGDAP1) or were left uninfected. When the knockdown was detectable (Supplementary Fig. 7A and B), cells were transiently co-transfected with expression constructs encoding mitochondrially targeted DsRed together with mitochondrially targeted green fluorescent protein (mtGFP) as negative control, with GDAP1 as positive control, with GDAP1L1 or with GDAP1L1 340X, lacking the potential tail-anchor domain of GDAP1L1. The next day cells were fixed and stained. In blinded countings, the mitochondrial morphology was assessed by mitochondrially targeted DsRed fluorescence (Niemann et al., 2005). One hundred randomly selected cells were analysed per experiment and condition, means and standard error of the mean, n = 3, n.s. = not significant, **P < 0.01.
We reasoned that upon mitochondrial localization, GDAP1L1 and GDAP1 might fulfil similar functions. GDAP1 expression induces mitochondrial fission and the knockdown of GDAP1 in N1E-115 cells leads to mitochondrial elongation, which can be reverted by the re-expression of GDAP1 (Niemann et al., 2009). In the same experimental setup, GDAP1L1 expression also reverted the mitochondrial elongation in Gdap1-knockdown N1E-115 cells (Fig. 7D and Supplementary Fig. 7). In contrast, expression of GDAP1L1 without the tail-anchor domain (Fig. 7C; GDAP1L1 340X) was not able to revert the mitochondrial phenotype in Gdap1-knockdown cells (Fig. 7D and Supplementary Fig. 7C). We conclude that GDAP1L1 is responsive to oxidative stress conditions, which leads to mitochondrial translocation, where GDAP1L1 can compensate for the loss of GDAP1 expression.
Discussion
GDAP1 was characterized as founder of a new glutathione S-transferase family with unknown glutathione S-transferase activity and as fission factor (Marco et al., 2004; Niemann et al., 2005, 2009; Pedrola et al., 2005, 2008; Shield et al., 2006; Huber et al., 2013). In cellular assays, Charcot–Marie–Tooth disease-associated mutant forms of GDAP1 interfere with the capacity to influence mitochondrial dynamics (Niemann et al., 2005, 2009; Pedrola et al., 2005). It has been suggested that mutations in GDAP1 perturb transport, energy production and calcium buffering (Cassereau et al., 2011b; Pla-Martin et al., 2013). GDAP1 expression has pro-survival functions in neuronal cell death models associated with glutathione depletion (Noack et al., 2012). This protective survival effect was diminished in Charcot–Marie–Tooth disease-associated mutant forms of GDAP1 with reduced fission capacity (Noack et al., 2012). However, it remained unclear how members of the GDAP1-family are linked to oxidative stress and mitochondrial dynamics. The Gdap1−/− mice presented here carry a functional null allele and mimic mutations leading to Charcot–Marie–Tooth disease (Cassereau et al., 2011a), allowing us to investigate the underlying disease mechanisms.
Similar to patients with Charcot–Marie–Tooth disease, Gdap1−/− mice show a progressive phenotype, as young adult mice (up to 1 year of age) were indistinguishable from wild-type controls. First signs of a peripheral neuropathy were detected in aged, 19-month-old Gdap1−/− mice. The animals display mainly decreased nerve conduction velocities, accompanied by mild hypomyelination. These features were recapitulated in mice lacking GDAP1 specifically and exclusively in Schwann cells. Thus, GDAP1 function is Schwann cell-autonomously required for myelin regulation. In contrast, mice with motor neuron-specific loss of GDAP1 showed no detectable alterations in electrophysiological examinations. These findings are not surprising, albeit GDAP1-caused Charcot–Marie–Tooth disease is often associated with major loss of myelinated axons, as axonal neuropathies have been difficult to reproduce in mice for unknown reasons (Robertson et al., 2002; Bogdanik et al., 2013). As a consequence, in mouse models of intermediate forms of Charcot–Marie–Tooth disease, the myelin phenotype is likely to be favoured. Despite this discrepancy to human diseases, the analysis of these models is of major value for the understanding of the disease-underlying biochemical and cell biological mechanisms, including genetic interactions (Ewaleifoh et al., 2012; Bogdanik et al., 2013). In agreement with a less pronounced axonal phenotype we found alterations in the morphology of intra-axonal mitochondria in Gdap1−/− animals, but not in mice lacking GDAP1 exclusively in Schwann cells. Furthermore, we detected a mild transport deficit in primary dorsal root ganglion explant cultures of Gdap1−/− mice. The consequences of these changes might accumulate within the first decade of life in the longer axons of peripheral nerves in patients leading to the observed axonal deficits.
Mitochondrial fusion and fission, and mitochondrial transport are tightly linked (Baloh, 2008; Vital and Vital, 2012). The loss of MFN2 or expression of mutant forms of MFN2 found in patients with Charcot–Marie–Tooth disease impaired mitochondrial transport and increased mitochondrial pausing (Misko et al., 2010). We found that loss of GDAP1 expression did not impair transport, however, increased mitochondrial pausing in Gdap1−/− sensory neuron cultures was observed. The molecular trigger and function of pausing are still unclear. Recently, it has been proposed that the positioning of mitochondria is used to influence redox-dependent signalling pathways (Murphy, 2012). Along this line, changes in the redox microenvironment might cause the increasing pausing in MFN2 and GDAP1 mutant axons.
MFN2 activity has been linked to oxidized glutathione on a molecular level, and overlapping functions of MFN2 and GDAP1 in regulating glutathione levels were suggested (Ryan and Stojanovski, 2012; Shutt et al., 2012). Oxidized glutathione oxidizes MFN2 to form disulphide-mediated mitofusin oligomers, which increase mitochondrial fusion efficiency under redox stress conditions (Shutt et al., 2012). We found that GDAP1L1, the paralogue of GDAP1, translocates to mitochondria under stress conditions with increased reduced to oxidized glutathione ratio. In vitro, oxidized glutathione is sufficient to induce membrane insertion of GDAP1L1. In vivo, loss of GDAP1 expression increases the amount of GDAP1L1 in the mitochondrial fraction of the spinal cord of aged mice. As a change in redox conditions could not release GDAP1L1 from the membranous fraction in vitro, the accumulation of GDAP1L1 at mitochondria of the spinal cord is likely to be cumulative over time. Interestingly, GDAP1L1 also accumulates at the mitochondria of the spinal cord in the transgenic rats expressing mutated SOD1G93A compared with transgenic rats expressing wild-type SOD1 (Li et al., 2010), revealing that the translocation of GDAP1L1 to mitochondria is not a peculiar situation caused by loss of GDAP1 expression. Rather these data suggest broader relevance of our findings in that similar stress conditions might occur in the SOD1G93A model for amyotrophic lateral sclerosis and in the Gdap1−/− model for Charcot–Marie–Tooth disease. As the SOD1G93A mutation and GDAP1 expression levels were both linked to glutathione levels (Muyderman et al., 2009; Noack et al., 2012), we determined the reduced and oxidized glutathione concentrations in the peripheral nerves and in different regions of the CNS (spinal cord, cerebellum, cortex) in aged Gdap1−/− and age-matched controls. Our measurements did not reveal differences (not shown). This is not surprising, as drastic changes in glutathione-levels would be expected to lead to more dramatic damage, whereas minor changes might be missed in vivo (Townsend et al., 2003; Noack et al., 2012). Mild but persistent oxidative stress conditions in Gdap1−/− animals are supported by the increased mitochondrial DNA content and PGC1α expression levels that we found in sciatic nerves (Lee and Wei, 2005; Malik and Czajka, 2013), and by the increased translocation of GDAP1L1 in the spinal cord. Furthermore, as mitochondrial DNA content and PGC1α expression levels are not altered in the spinal cord of Gdap1−/− mice, and GDAP1L1 can induce mitochondrial fission in the absence of GDAP1, we propose that mitochondrial GDAP1L1 is able to compensate for GDAP1 loss in the CNS.
In summary, Gdap1−/− mice display reduced nerve conduction velocity and hypomyelination. Ablation of GDAP1 in Schwann cells is sufficient to cause this phenotype. Mild axonal impairments were also observed in Gdap1−/− mice, together with persistent oxidative stress. In the CNS, loss of GDAP1 is compensated by the mitochondrial translocation of GDAP1L1. We demonstrate that this recruitment is dependent on elevated oxidized glutathione levels, providing a novel protective mechanism for GDAP1-family members acting as sensors for altered cellular redox environment.
Supplementary Material
Acknowledgements
We acknowledge the generation of GDAP1-mutant mice by the Mouse Clinical Institute (Strasbourg, France). We thank Silvia Arber and Werner Kovacs for mouse strains and reagents; Stephen Helliwell for helpful suggestions; the Light Microscopy and Screening Centre and the Electron Microscopy Centre of the ETH Zurich for their valuable contributions.
Funding
This work was supported by the Swiss National Science Foundation and the National Centre for Competence in Research (NCCR) Neural Plasticity and Repair. K.V.T. is supported by a Senior Professorship Grant endowed by the University of Würzburg, Faculty of Medicine. Research by F.L.J. is supported by a Marie Curie International Incoming Fellowships (IIF) within the 7th European Community framework and an ETH fellowship.
Supplementary material
Supplementary material is available at Brain online.
References
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–74. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Baloh RH. Mitochondrial dynamics and peripheral neuropathy. Neuroscientist. 2008;14:12–8. doi: 10.1177/1073858407307354. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–30. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–57. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- Bogdanik LP, Sleigh JN, Tian C, Samuels ME, Bedard K, Seburn KL, et al. Loss of the E3 ubiquitin ligase LRSAM1 sensitizes peripheral axons to degeneration in a mouse model of Charcot-Marie-Tooth disease. Dis Model Mech. 2013;6:780–92. doi: 10.1242/dmm.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–16. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med. 2011;365:2377–88. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- Cartoni R, Arnaud E, Medard JJ, Poirot O, Courvoisier DS, Chrast R, et al. Expression of mitofusin 2(R94Q) in a transgenic mouse leads to Charcot-Marie-Tooth neuropathy type 2A. Brain. 2010;133(Pt 5):1460–9. doi: 10.1093/brain/awq082. [DOI] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Bonneau D, Verny C, Procaccio V, Reynier P, et al. A locus-specific database for mutations in GDAP1 allows analysis of genotype-phenotype correlations in Charcot-Marie-Tooth diseases type 4A and 2K. Orphanet J Rare Dis. 2011a;6:87. doi: 10.1186/1750-1172-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Gueguen N, Desquiret V, Verny C, Nicolas G, et al. Mitochondrial dysfunction and pathophysiology of Charcot-Marie-Tooth disease involving GDAP1 mutations. Exp Neurol. 2011b;227:31–41. doi: 10.1016/j.expneurol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–87. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Physiological functions of mitochondrial fusion. Ann N Y Acad Sci. 2010;1201:21–5. doi: 10.1111/j.1749-6632.2010.05615.x. [DOI] [PubMed] [Google Scholar]
- Ewaleifoh O, Trinh M, Griffin JW, Nguyen T. A novel system to accelerate the progression of nerve degeneration in transgenic mouse models of neuropathies. Exp Neurol. 2012;237:153–9. doi: 10.1016/j.expneurol.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, D'Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999;883:116–23. [PubMed] [Google Scholar]
- Feltri ML, D'Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;170:199–206. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Fleetwood-Walker SM, Cottrell DF, Tait S, Garry EM, et al. Peripheral demyelination and neuropathic pain behavior in periaxin- deficient mice. Neuron. 2000;26:523–31. doi: 10.1016/s0896-6273(00)81184-8. [DOI] [PubMed] [Google Scholar]
- Horn M, Baumann R, Pereira JA, Sidiropoulos PN, Somandin C, Welzl H, et al. Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells. Brain. 2012;135(Pt 12):3567–83. doi: 10.1093/brain/aws275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber N, Guimaraes S, Schrader M, Suter U, Niemann A. Charcot-Marie-Tooth disease-associated mutants of GDAP1 dissociate its roles in peroxisomal and mitochondrial fission. EMBO Rep. 2013;14:545–52. doi: 10.1038/embor.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–66. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Kabzinska D, Niemann A, Drac H, Huber N, Potulska-Chromik A, Hausmanowa-Petrusewicz I, et al. A new missense GDAP1 mutation disturbing targeting to the mitochondrial membrane causes a severe form of AR-CMT2C disease. Neurogenetics. 2011;12:145–53. doi: 10.1007/s10048-011-0276-7. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–62. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–7. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Li Q, Vande Velde C, Israelson A, Xie J, Bailey AO, Dong MQ, et al. ALS-linked mutant superoxide dismutase 1 (SOD1) alters mitochondrial protein composition and decreases protein import. Proc Natl Acad Sci USA. 2010;107:21146–51. doi: 10.1073/pnas.1014862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–92. doi: 10.1016/j.mito.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Marco A, Cuesta A, Pedrola L, Palau F, Marin I. Evolutionary and structural analyses of GDAP1, Involved in Charcot-Marie-Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol Biol Evol. 2004;21:176–87. doi: 10.1093/molbev/msh013. [DOI] [PubMed] [Google Scholar]
- Martini R. Animal models for inherited peripheral neuropathies: chances to find treatment strategies? J Neurosci Res. 2000;61:244–50. doi: 10.1002/1097-4547(20000801)61:3<244::AID-JNR2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Horste G, Nave KA. Animal models of inherited neuropathies. Curr Opin Neurol. 2006;19:464–73. doi: 10.1097/01.wco.0000245369.44199.27. [DOI] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–40. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, Rodriguez de Cordoba S, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet. 2006;38:1261–8. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- Murphy MP. Modulating mitochondrial intracellular location as a redox signal. Sci Signal. 2012;5:pe39. doi: 10.1126/scisignal.2003386. [DOI] [PubMed] [Google Scholar]
- Muyderman H, Hutson PG, Matusica D, Rogers ML, Rush RA. The human G93A-superoxide dismutase-1 mutation, mitochondrial glutathione and apoptotic cell death. Neurochem Res. 2009;34:1847–56. doi: 10.1007/s11064-009-9974-z. [DOI] [PubMed] [Google Scholar]
- Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–42. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–78. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A, Wagner KM, Ruegg M, Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis. 2009;36:509–20. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Noack R, Frede S, Albrecht P, Henke N, Pfeiffer A, Knoll K, et al. Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum Mol Genet. 2012;21:150–62. doi: 10.1093/hmg/ddr450. [DOI] [PubMed] [Google Scholar]
- Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrola L, Espert A, Valdes-Sanchez T, Sanchez-Piris M, Sirkowski EE, Scherer SS, et al. Cell expression of GDAP1 in the nervous system and pathogenesis of Charcot-Marie-Tooth type 4A disease. J Cell Mol Med. 2008;12:679–89. doi: 10.1111/j.1582-4934.2007.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrola L, Espert A, Wu X, Claramunt R, Shy ME, Palau F. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet. 2005;14:1087–94. doi: 10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- Pla-Martin D, Rueda CB, Estela A, Sanchez-Piris M, Gonzalez-Sanchez P, Traba J, et al. Silencing of the Charcot-Marie-Tooth disease-associated gene GDAP1 induces abnormal mitochondrial distribution and affects Ca homeostasis by reducing store-operated Ca entry. Neurobiol Dis. 2013;55:140–51. doi: 10.1016/j.nbd.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Ramabhadran V, Gurel PS, Higgs HN. Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J Biol Chem. 2012;287:34234–45. doi: 10.1074/jbc.M112.365122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J, Kriz J, Nguyen MD, Julien JP. Pathways to motor neuron degeneration in transgenic mouse models. Biochimie. 2002;84:1151–60. doi: 10.1016/s0300-9084(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Ryan MT, Stojanovski D. Mitofusins ‘bridge' the gap between oxidative stress and mitochondrial hyperfusion. EMBO Rep. 2012;13:870–1. doi: 10.1038/embor.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–66. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield AJ, Murray TP, Board PG. Functional characterisation of ganglioside-induced differentiation-associated protein 1 as a glutathione transferase. Biochem Biophys Res Commun. 2006;347:859–66. doi: 10.1016/j.bbrc.2006.06.189. [DOI] [PubMed] [Google Scholar]
- Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012;13:909–15. doi: 10.1038/embor.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Balsamo J, Lilien J, Kamholz J. A molecular basis for hereditary motor and sensory neuropathy disorders. Curr Neurol Neurosci Rep. 2001;1:77–88. doi: 10.1007/s11910-001-0079-6. [DOI] [PubMed] [Google Scholar]
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Somandin C, Gerber D, Pereira JA, Horn M, Suter U. LITAF (SIMPLE) regulates Wallerian degeneration after injury but is not essential for peripheral nerve development and maintenance: implications for Charcot-Marie-Tooth disease. Glia. 2012;60:1518–28. doi: 10.1002/glia.22371. [DOI] [PubMed] [Google Scholar]
- Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nature Rev Neurosci. 2003;4:714–29. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital A, Vital C. Mitochondria and peripheral neuropathies. J Neuropathol Exp Neurol. 2012;71:1036–46. doi: 10.1097/NEN.0b013e3182764d47. [DOI] [PubMed] [Google Scholar]
- Wagner KM, Ruegg M, Niemann A, Suter U. Targeting and function of the mitochondrial fission factor GDAP1 are dependent on its tail-anchor. PLoS One. 2009;4:e5160. doi: 10.1371/journal.pone.0005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Dickinson DA, Liu RM, Forman HJ. Quinones and glutathione metabolism. Methods Enzymol. 2004;378:319–40. doi: 10.1016/S0076-6879(04)78024-6. [DOI] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimon M, Baets J, Fabrizi GM, Jaakkola E, Kabzinska D, Pilch J, et al. Dominant GDAP1 mutations cause predominantly mild CMT phenotypes. Neurology. 2011;77:540–8. doi: 10.1212/WNL.0b013e318228fc70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–51. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Vance JM. Molecular genetics of autosomal-dominant axonal Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:63–74. doi: 10.1385/nmm:8:1-2:63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.