Abstract
Context
Urothelial tumors are rare in young patients. Because of its rarity, the natural history of the disease in young patients remains poorly understood.
Objective
To understand the pathologic and clinical features of urothelial tumors of the urinary bladder in young patients.
Design
We identified 59 young patients with urothelial tumors of the urinary bladder treated at our institution and analyzed the tumors’ pathologic features and the patients’ clinical outcomes.
Results
All patients were 30 years old or younger, with a mean age of 23.5 years (range, 4 to 30). Thirty-eight patients were male, and 21 were female. Most tumors were noninvasive papillary urothelial tumors (n = 49), including papillary urothelial neoplasms of low malignant potential (n = 7), low-grade papillary urothelial carcinomas (n = 38), and high-grade papillary urothelial carcinomas (n = 4). Only a minority of urothelial tumors were invasive, invading the lamina propria (n = 5), muscularis propria (n = 4), or perivesical soft tissue (n = 1). Clinical follow-up information was available for 41 patients, with a mean follow-up time of 77 months. Of 31 patients with noninvasive papillary urothelial tumors, only 1 patient later developed an invasive urothelial carcinoma and died of the disease, and 30 of these patients were alive at the end of follow-up, although 10 had local tumor recurrences. In the 10 patients with invasive urothelial carcinomas, 3 patients died of the disease and 5 others were alive with metastases.
Conclusion
Urothelial tumors in young patients are mostly noninvasive papillary carcinomas and have an excellent prognosis; however, a small subset of patients may present with high-grade invasive urothelial carcinomas that result in poor clinical outcomes.
Keywords: Papillary urothelial carcinoma, Urinary bladder, Renal pelvis, Young patients
INTRODUCTION
Bladder cancer is the fourth most common cancer in men and the ninth most common cancer in women in the United States [1]. Its incidence has been steadily increasing during the past years. It is estimated that 73,510 patients are newly diagnosed with bladder cancer and 14,880 patients die of this disease in the United States every year [1]. The majority of bladder cancers consist of urothelial carcinoma, which can be classified into different categories with distinct clinical behavior [2]. Low-grade papillary urothelial carcinomas usually do not invade the bladder wall but often recur locally, necessitating a long-term surveillance. In contrast, high-grade nonpapillary urothelial carcinomas are more likely to be deeply invasive into the bladder wall and metastasize, resulting in a significantly higher mortality. Recent studies have indicated that papillary and nonpapillary urothelial carcinomas may utilize different molecular pathways, which may contribute to their distinct biological behaviors [3].
Urothelial tumors are typically a disease of elderly people with a male predominance. The median ages of patients at the time of the initial diagnosis are 69 years in men and 71 years in women [4]. Urothelial tumors are rare in young patients. Previous studies of patients with this disease have used different definitions of young age, ranging from 20 years to 40 years [5–18]. These differences have led to inconsistent results regarding the clinicopathologic features of this disease in young patients. Some studies have reported that younger patients with urothelial tumors had a more favorable prognosis than older patients [6,10–12], but other studies have shown that the clinical course of the disease in younger patients is similar to that in older patients [5,8,13,14]. Furthermore, because urothelial tumors are rare in young patients, most studies of urothelial tumors in young patients have been small series with the number of reported cases ranging from 12 to 50 [5–18]. To better characterize the clinicopathologic features of urothelial tumor in young patients, we retrospectively evaluated a large series of patients initially diagnosed at 30 years of age or younger at a single institution.
PATIENTS AND METHODS
After obtaining approval for our study from the Institutional Review Board, we searched the surgical pathology files at The University of Texas MD Anderson Cancer Center (Houston, Texas) from 1985 to 2011 and found 59 patients who were initially diagnosed with urothelial tumors in the urinary bladder at 30 years of age or younger. We reviewed the patients’ archived slides for histologic features, including tumor type, grade, stage, and any unusual features. We determined the tumor grade using the 2004 World Health Organization classification system (Table 1) [2] and the pathologic stage using the 2010 American Joint Committee on Cancer staging system [19]. We also reviewed the patients’ records to collect data on demographics, smoking history, medical history, tumor recurrence, metastasis, treatment, and clinical outcome.
Table 1.
Simplified WHO/ISUP 2004 histological classification of urothelial tumors
|
RESULTS
The mean age of the 59 patients at initial diagnosis was 23.5 years (range, 4 to 30 years). Forty-two patients were 21 to 30 years old, 14 patients were 11 to 20 years old, and only 3 patients were 10 years old or younger (Table 2). Thirty-eight patients were male, and 21 were female. Clinical histories were available for 36 patients, of whom 21 had a history of smoking tobacco and 2 had a family history of bladder cancer. The most common presenting symptom was gross hematuria (n = 31), followed by abdominal pain (n = 8). Six patients had a history of urinary tract infections, and 2 of them had recurrent urinary tract infections, which had required bilateral ureteral implantations.
Table 2.
Distribution of patients with urothelial tumors by age and tumor type
| Age (years)a | PUNLMP | LGPUC | HGPUC | INVUC | Total |
|---|---|---|---|---|---|
| ≤10 | 1 | 2 | 0 | 0 | 3 |
| 11–20 | 2 | 11 | 0 | 1 | 14 |
| 21–30 | 4 | 25 | 4 | 9 | 42 |
| Total | 7 | 38 | 4 | 10 | 59 |
Patient’s age at the initial diagnosis.
Abbreviations: PUNLMP, papillary urothelial neoplasm of low malignant potential; LGPUC, low-grade papillary urothelial carcinoma; HGPUC, high-grade papillary urothelial carcinoma, noninvasive; INVUC, high-grade invasive urothelial carcinoma.
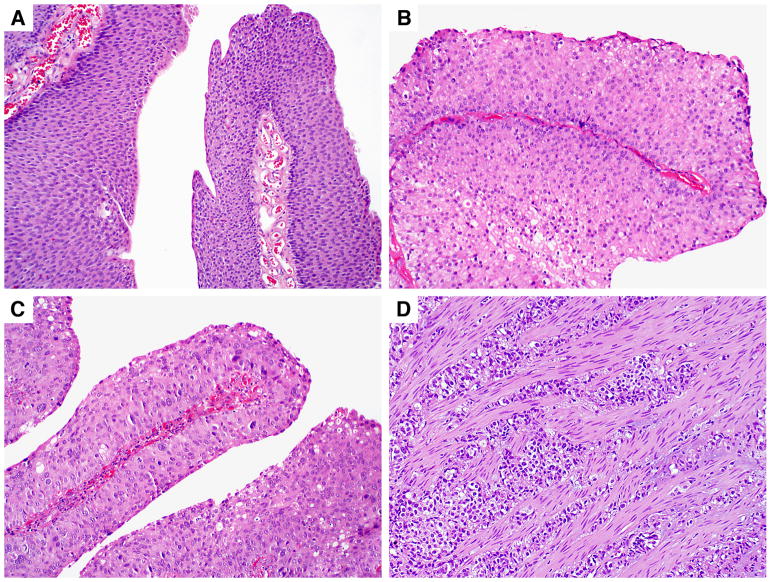
The urothelial tumors were noninvasive in 49 cases and invasive in 10 cases. The noninvasive urothelial tumors included papillary urothelial neoplasms of low malignant potential (PUNLMPs) (n = 7; Figure 1A), low-grade papillary urothelial carcinomas (LGPUCs) (n = 38; Figure 1B), and high-grade papillary urothelial carcinomas (HGPUCs) (n = 4; Figure 1C). The invasive urothelial carcinomas invaded the lamina propria (n = 5), muscularis propria (n = 4; Figure 1D), and perivesical soft tissue (n = 1). In addition, 1 invasive urothelial carcinoma showed focal nested variant, and another showed focal small cell carcinoma features.
Figure 1.
Urothelial tumors in young patients. A, Papillary urothelial neoplasm of unknown malignant potential (hematoxylin-eosin, X 100). B, Low-grade papillary urothelial carcinoma(hematoxylin-eosin, X 100). C, High-grade papillary urothelial carcinoma(hematoxylin-eosin, X 100). D, Urothelial carcinoma invading the muscularis propria(hematoxylin-eosin, X 100).
Follow-up information was available for 41 patients, with a mean follow-up time of 77 months (range, 3 to 250 months; Table 3). Seven patients with invasive urothelial carcinomas underwent radical cystoprostatectomy or cystectomy. Two patients with PUNLMPs who had follow-up data available were alive with no evidence of disease after 13 and 37 months. Of 26 patients with LGPUCs, 25 were alive after a mean of 71 months (range, 3 to 225 months), but 9 had local tumor recurrences. Only 1 patient later developed high-grade, invasive urothelial carcinoma and died of the disease after 160 months. All 3 patients with HGPUCs were alive after a mean of 129 months (range, 37 to 238 months); 1 had local tumor recurrence and another developed bone metastasis. All 5 patients with tumors invading the lamina propria in the bladder were alive after a mean of 85 months (range, 16 to 250 months), but 3 of them developed metastases in lymph nodes. Two of 4 patients with tumors invading the muscularis propria died of the disease after 15 and 102 months, and the other 2 were alive with metastases to the lungs and lymph nodes after 12 and 166 months, respectively. One patient with a tumor invading the perivesical soft tissue died after 10 months despite cystoprostatectomy and chemotherapy.
Table 3.
Outcomes for young patients with urothelial tumorsa
| Noninvasive (n=31) | Invasive (n=10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| PUNLMP (n = 2) | LGPUC (n = 26) | HGPUC (n = 3) | INVLP (n = 5) | INVMP (n = 4) | INVPS (n = 1) | |
| Mean follow-up time (range), months | 25 (13–37) | 71 (3–225) | 129 (37–238) | 85 (16–250) | 74 (15–166) | 10 |
| Alive with no recurrence | 2 | 16 | 1 | 2 | 0 | 0 |
| Alive with local recurrence | 0 | 9 | 1 | 0 | 0 | 0 |
| Alive with metastasis | 0 | 0 | 1 | 3 | 2 | 0 |
| Died of disease | 0 | 1b | 0 | 0 | 2 | 1 |
Follow-up information was available for 41 patients.
This patient later developed invasive high-grade urothelial carcinoma with metastasis and died after 160 months.
Abbreviations: PUNLMP, papillary urothelial neoplasm of low malignant potential; LGPUC, low-grade papillary urothelial carcinoma; HGPUC, high-grade papillary urothelial carcinoma, noninvasive; INVLP, urothelial carcinoma invading the lamina propria; INVMP, urothelial carcinoma invading the muscularis propria; INVPS, urothelial carcinoma invading the perivesical soft tissue.
COMMENT
Urothelial tumors are rare in young patients, but the exact incidence depends on the criteria defining the young age group. While 1.0% to 2.4% of urothelial tumors are found in patients younger than 40 years, only 0.1% to 0.4% of urothelial tumors are found in patients younger than 20 years [5–10]. The average ages of young patients with urothelial tumors in most previous reports were more than 30 but less than 40 years [6,8,14,18]. In the current study, all patients were 30 years old or younger when they were first diagnosed, with a mean age of 23.5 years. The majority of these patients (71%) were 21 to 30 years old, followed by the group of patients who were 11 to 20 years old (24%). Only 5% of our patients were younger than 10 years at diagnosis. This age distribution suggests that the incidence of urothelial tumors in young patients increases with age. In addition, the patients in our series had a male-to-female ratio of 1.8:1, which was lower than the ratio of 4:1 reported in older patients [1, 2].
Urothelial tumors in younger patients often have a lower grade and stage than those in older patients [5–18]. The majority of urothelial tumors in young patients are noninvasive, low-grade papillary urothelial neoplasms. As patient age increases, the incidence of low-grade urothelial tumors decreases while the incidence of high-grade urothelial tumors increases. Over 95% of patients younger than 30 years with urothelial tumors have low-grade (grade 1 or 2) papillary tumors [20]. In patients 30 to 40 years old, however, 80% of urothelial carcinomas were low-grade while 20% were high-grade (grade 2 or 3), a tumor grade distribution more like that of older patients than that of younger patients. In the current study, all 17 patients 20 years old or younger had low-grade papillary urothelial tumors, including LGPUCs and PUNLMPs, whereas 13 of 42 patients 21 to 30 years old had high-grade urothelial carcinoma, resulting in a higher rate (31%) than that previously reported in this age group. Furthermore, we did not find any urothelial papillomas in the patients we studied, although urothelial papillomas were relatively common in previous reports, accounting for 3% to 9% of urothelial tumors in young patients [20]. The differences are likely due to the referral nature of our institution, which tends to treat patients with aggressive oncologic diseases.
Studies have suggested that younger patients with urothelial tumors are likely to have more favorable outcomes than older patients [9–12,17,20]. Fine et al reported that 23 patients younger than 20 years with urothelial tumors were all alive with no evidence of disease after a mean follow-up of 4.5 years [16]. However, Yossepowitch et al found that younger (median age, 34.6 years) and older (median age, 65.3 years) patients with superficial urothelial tumors had similar outcomes [13], although we note that the latter series included a substantial number of patients who were 30 to 40 years old, an age group that tends to have urothelial tumors that are more similar to those of older patients than those of younger patients with respect to grade and stage. In the current study, 16 of the 17 patients diagnosed at 20 years old or younger had low-grade, noninvasive urothelial tumors, and only 1 had a high-grade, invasive urothelial carcinoma. No patients in this age group died of the disease, although local tumor recurrences were not uncommon; all 4 patients who died of the disease were in the age group of 21 to 30 years. Our results suggest that the prognosis for urothelial carcinoma is excellent in young patients, particularly those younger than 20 years, but becomes less favorable with increasing age.
Although the oncogenesis of urothelial tumors in young patients is not clear, multiple environmental and genetic factors may contribute to the etiology. Tobacco smoking is a major known risk factor for bladder cancer in old patients, and the risk is increased with duration of smoking. The incidence of tobacco smoking is also high in young patients with bladder cancer. An early study reported that 67% of patients under 30 years had a history of tobacco smoking and the rate was increased to 96% of patients between 30 and 40 years old [14]. In the current study, 58% of patients had a history of smoking, suggesting that tobacco smoking is likely to contribute to the development of urothelial tumors in young patients. Occupational exposure is another known risk factor for urothelial tumors in old patients, but its role is uncertain in young patients. Although urothelial tumors in young patients may lack some genetic alterations that are frequently observed in old patients [21,22], genetic factors are likely to play an important role in the early onset of urothelial tumors in young patients.
The pathologic diagnosis of urothelial tumors is not difficult, but diagnosis is often delayed in young patients because clinical suspicion of urothelial tumors is generally low for this age group [23]. As urine cytology is generally not effective for detecting low-grade papillary urothelial tumors, it has limited value for young patients, in whom these tumors predominate [24]. Several benign papillary lesions in the urinary bladder, such as polypoid cystitis and nephrogenic adenomas, should be differentiated from papillary urothelial carcinomas. Polypoid cystitis usually forms broad-based papillary or polypoid structures with edema and inflammation in the stroma. Nephrogenic adenomas may show papillary structures, but the lining cells are usually a single layer of cuboidal, flat, or “hobnailed” cells with no conspicuous cellular atypia. Another pitfall in diagnosis is overestimating the tumor grade. In the cohort studied by Fine et al, 10 patients (44% of the cohort) who were diagnosed with PUNLMPs according to the 2004 World Health Organization classification system would have been diagnosed with papillary urothelial carcinomas using older classification systems [17]. Thus, the use of the diagnostic category of PUNLMP can avoid labeling a significant number of young patients with carcinoma.
In summary, we analyzed the clinical and pathologic features of urothelial tumors in a large series of young patients from a single institution. The majority of urothelial tumors in this age group were noninvasive, low-grade papillary urothelial neoplasms, and only a minority of the tumors were invasive high-grade urothelial carcinomas. Patients with noninvasive papillary urothelial tumors have an excellent prognosis, although tumor recurrences are not uncommon. Patients with invasive high grade urothelial carcinomas often develop metastases and may die of disease, indicating an aggressive behavior in this age group. Thus, our results suggest that urothelial tumors with comparable pathologic features may have similar clinical courses in younger and older patients. The excellent clinical outcomes in young patients are likely due to the predominance of noninvasive, low-grade papillary urothelial neoplasms in this age group.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization (WHO) Classification of Tumours - Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Albany, NY: WHO Publication Center; 2004. pp. 90–157. [Google Scholar]
- 3.Czerniak B. Molecular pathology and biomarkers of bladder cancer. Cancer Biomark. 2010;9(1–6):159–176. doi: 10.3233/CBM-2011-0175. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, Bladder Section, 1975–2008. Bethesda, MD: National Cancer Institute; [Accessed November 7, 2011]. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 5.Kutarski PW, Padwell A. Transitional cell carcinoma of the bladder in young adults. Br J Urol. 1993;72(5 Pt 2):749–755. doi: 10.1111/j.1464-410x.1993.tb16261.x. [DOI] [PubMed] [Google Scholar]
- 6.Witjes JA, Debruyne FM. Bladder carcinoma in patients less than 40 years of age. Urol Int. 1989;44(2):81–83. doi: 10.1159/000281475. [DOI] [PubMed] [Google Scholar]
- 7.Migaldi M, Rossi G, Maiorana G, et al. Superficial papillary urothelial carcinomas in young and elderly patients: a comparative study. BJU Int. 2004;94(3):311–316. doi: 10.1111/j.1464-410X.2004.04929.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DE, Hillis S. Carcinoma of the bladder in patients less than 40 years old. J Urol. 1978;120(2):172–173. doi: 10.1016/s0022-5347(17)57090-1. [DOI] [PubMed] [Google Scholar]
- 9.Javadpour N, Mostofi FK. Primary epithelial tumors of the bladder in the first two decades of life. J Urol. 1969;101(5):706–710. doi: 10.1016/s0022-5347(17)62407-8. [DOI] [PubMed] [Google Scholar]
- 10.Benson RC, Jr, Tomera KM, Kelalis PP. Transitional cell carcinoma of the bladder in children and adolescents. J Urol. 1983;130(1):54–55. doi: 10.1016/s0022-5347(17)50950-7. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick JM, Reda M. Bladder carcinoma in patients 40 years old or less. J Urol. 1986;135(1):53–54. doi: 10.1016/s0022-5347(17)45513-3. [DOI] [PubMed] [Google Scholar]
- 12.Madgar I, Goldwasser B, Nativ O, et al. Long-term followup of patients less than 30 years old with transitional cell carcinoma of bladder. J Urol. 1988;139(5):933–934. doi: 10.1016/s0022-5347(17)42721-2. [DOI] [PubMed] [Google Scholar]
- 13.Yossepowitch O, Dalbagni G. Transitional cell carcinoma of the bladder in young adults: presentation, natural history and outcome. J Urol. 2002;168(1):61–66. [PubMed] [Google Scholar]
- 14.Wan J, Grossman HB. Bladder carcinoma in patients age 40 years or younger. Cancer. 1989;64(1):178–181. doi: 10.1002/1097-0142(19890701)64:1<178::aid-cncr2820640130>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.McGuire EJ, Weiss RM, Baskin AM. Neoplasms of transitional cell origin in first twenty years of life. Urology. 1973;1(1):57–59. doi: 10.1016/0090-4295(73)90114-3. [DOI] [PubMed] [Google Scholar]
- 16.Chang SY, Ma CP. Transitional cell carcinoma of the urinary bladder in patients under 40 years of age. Br J Urol. 1987;60(4):343–344. doi: 10.1111/j.1464-410x.1987.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 17.Fine SW, Humphrey PA, Dehner LP, et al. Urothelial neoplasms in patients 20 years or younger: a clinicopathological analysis using the World Health Organization 2004 Bladder Consensus Classification. J Urol. 2005;174(5):1976–1980. doi: 10.1097/01.ju.0000176801.16827.82. [DOI] [PubMed] [Google Scholar]
- 18.Ozbey I, Aksoy Y, Biçgi O, et al. Transitional cell carcinoma of the bladder in patients under 40 years of age. Int Urol Nephrol. 1999;31(5):655–659. doi: 10.1023/a:1007160522033. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7. New York, NY: Springer-Verlag; 2010. pp. 497–505. [Google Scholar]
- 20.Paner GP, Zehnder P, Amin AM, et al. Urothelial neoplasms of the urinary bladder occurring in young adult and pediatric patients: a comprehensive review of literature with implications for patient management. Adv Anat Pathol. 2011;18(1):79–89. doi: 10.1097/PAP.0b013e318204c0cf. [DOI] [PubMed] [Google Scholar]
- 21.Wild PJ, Giedl J, Stoehr R, et al. Genomic aberrations are rare in urothelial neoplasms of patients 19 years or younger. J Pathol. 2007;211(1):18–25. doi: 10.1002/path.2075. [DOI] [PubMed] [Google Scholar]
- 22.Owen HC, Giedl J, Wild PJ, et al. Low frequency of epigenetic events in urothelial tumors in young patients. J Urol. 2010;184(2):459–46. doi: 10.1016/j.juro.2010.03.131. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield SP, Williot P, Kaplan D. Gross hematuria in children: a ten year review. Urology. 2007;69(1):166–169. doi: 10.1016/j.urology.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Kurz KR, Pitts WR, Vaughan ED., Jr The natural history of patients less than 40 years old with bladder tumors. J Urol. 1987;137(3):395–397. doi: 10.1016/s0022-5347(17)44046-8. [DOI] [PubMed] [Google Scholar]