Abstract
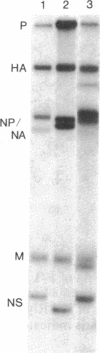
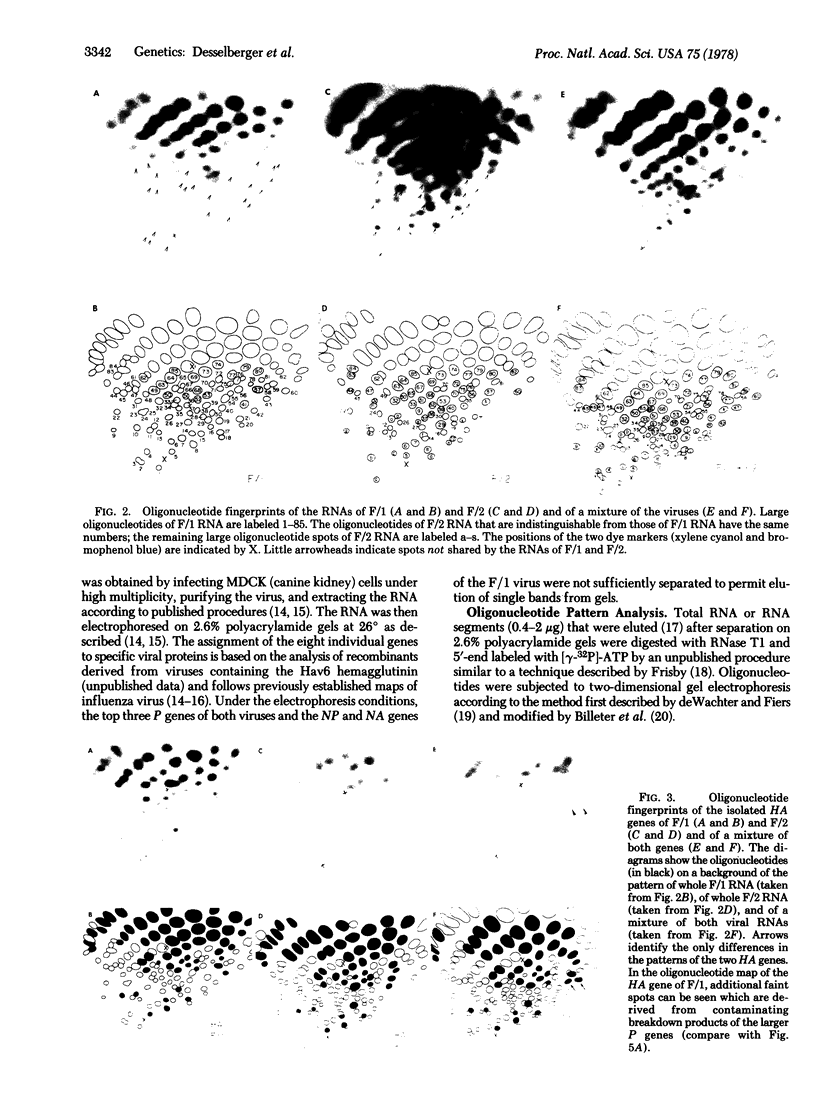
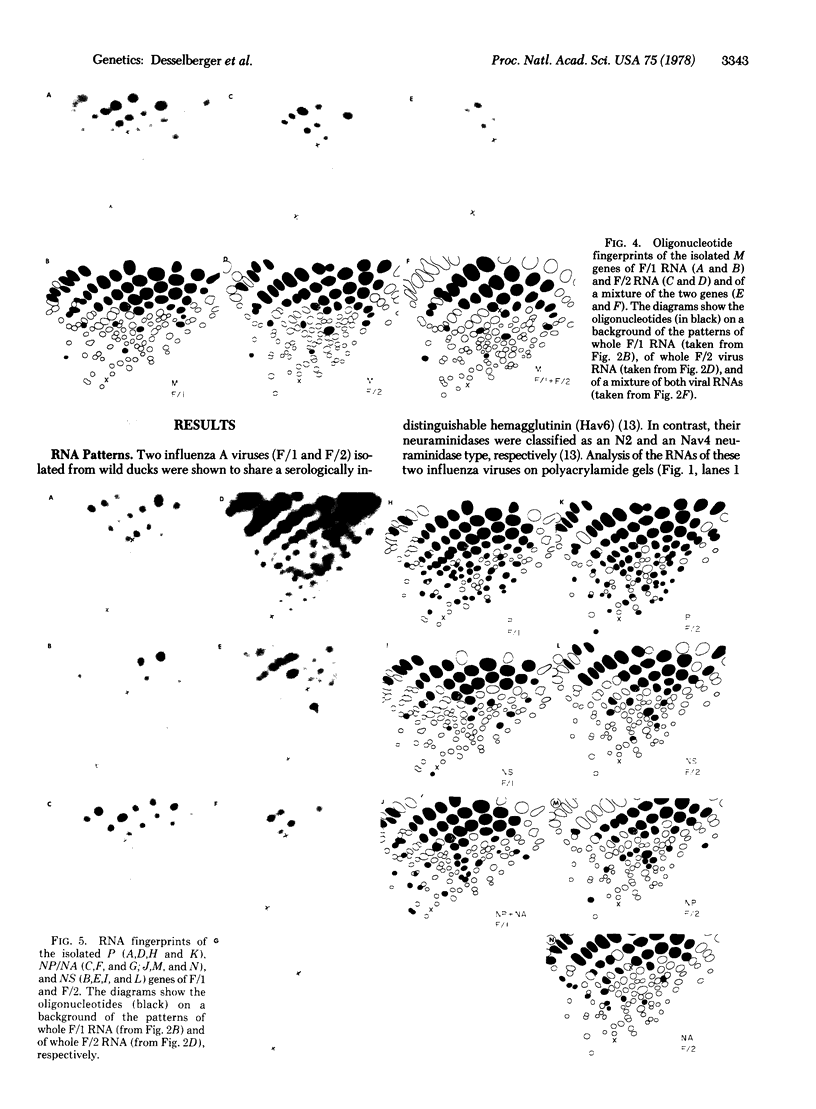
Oligonucleotide analysis of two avian influenza A viruses (Hav6N2 and Hav6Nav4) isolated in nature showed identical or almost identical patterns for the corresponding M and HA genes; 24 of 25 and 13 of 13 large oligonucleotides were indistinguishable by two-dimensional gel analysis. On the other hand, remarkable differences in the oligonucleotide patterns of the remaining genes were observed. Only 2 of 11 oligonucleotide spots of the NS gene, 10 of 27 spots of the NA/NP genes, and 22 of 49 spots of the P genes were indistinguishable between the two strains. On the basis of this observation that at least two genes of these viruses are virtually identical whereas others show easily detectable differences, we conclude that the two avian strains are related to each other by a recombinational event. In addition, it was found that animals in nature can be doubly infected with influenza viruses. Both lines of evidence strongly suggest that recombination is at least one mechanism by which "new" influenza virus strains emerge in nature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbourne E. D. Recombination of influenza A viruses of human and animal origin. Science. 1968 Apr 5;160(3823):74–76. doi: 10.1126/science.160.3823.74. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973 Feb;51(2):383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. II. Peptide maps of the light and heavy polypeptide chains from the hemagglutinin subunits of A 2 influenza viruses isolated before and after the appearance of Hong Kong influenza. Virology. 1972 May;48(2):445–455. doi: 10.1016/0042-6822(72)90055-4. [DOI] [PubMed] [Google Scholar]
- MULDER J., MASUREL N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet. 1958 Apr 19;1(7025):810–814. doi: 10.1016/s0140-6736(58)91738-0. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. Mapping of the influenza virus genome. II. Identification of the P1, P2, and P3 genes. Virology. 1977 Jan;76(1):114–121. doi: 10.1016/0042-6822(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge K. F., Butterfield W. K., Webster R. G., Campbell C. H. Isolation and characterization of influenza A viruses from avian species in Hong Kong. Bull World Health Organ. 1977;55(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- Tumova B., Pereira H. G. Genetic interaction between influenza A viruses of human and animal origin. Virology. 1965 Nov;27(3):253–261. doi: 10.1016/0042-6822(65)90104-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H., Granoff A. The "in vivo" production of "new" influenza viruses. 3. Isolation of recombinant influenza viruses under simulated conditions of natural transmission. Virology. 1973 Jan;51(1):149–162. doi: 10.1016/0042-6822(73)90375-9. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Campbell C. H. The in vivo production of "new" influenza A viruses. II. In vivo isolation of "new" viruses. Virology. 1972 May;48(2):528–536. doi: 10.1016/0042-6822(72)90063-3. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Tumová B., Hinshaw V. S., Lang G. Characterization of avian influenza viruses. Designation of a newly recognized haemagglutinin. Bull World Health Organ. 1976;54(5):555–560. [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]