Abstract
Relatively few data are available on the prevalence of hyperthyroidism (TSH concentrations of < 0.3 mIU/L, with normal or elevated concentrations of free T4) in individuals exposed to radioiodines at low levels. The accident at the Chornobyl (Chernobyl) nuclear plant in Ukraine on April 26, 1986 exposed large numbers of residents to radioactive fallout, principally to iodine-131 (I-131) (mean and median doses = 0.6 Gray (Gy) and 0.2 Gy). We investigated the relationship of I-131 and prevalent hyperthyroidism among 11,853 individuals exposed as children or adolescents in Ukraine who underwent an in-depth, standardized thyroid gland screening examination 12–14 years later. Radioactivity measurements taken shortly after the accident were available for all subjects and were used to estimate individual thyroid doses. We identified 76 cases of hyperthyroidism (11 overt, 65 subclinical). Using logistic regression, we tested a variety of continuous risk models and conducted categorical analyses for all subjects combined and for females (53 cases, n=5,767) and males (23 cases, n=6,086) separately, but found no convincing evidence of a dose response relationship between I-131 and hyperthyroidism. There was some suggestion of elevated risk among females in an analysis based on a dichotomous dose model with a threshold of 0.5 Gy chosen empirically (OR=1.86, P=0.06), but the statistical significance level was reduced (P=0.13) in a formal analysis with an estimated threshold. In summary, after a thorough exploration of the data, we found no statistically significant dose response relationship between individual I-131 thyroid doses and prevalent hyperthyroidism.
Introduction
Hyperthyroidism, a relatively infrequent form of thyroid functional disease, involves a decrease in serum thyroid stimulating hormone (TSH) that is accompanied by elevated serum free T4 (fT4) in the overt form of the disease or, in subclinical cases, by fT4 levels in the normal range (1). Estimates of prevalence range from 0.5% to 2.0% for overt hyperthyroidism and from 0.7% to 6.0% for subclinical hyperthyroidism, depending upon the criteria used as well as gender, age, race, ethnicity, and intake of stable iodine (1, 2). The rates are higher in females than in males, and increase at older ages (3). Few risk factors or co-morbidities for hyperthyroidism have thus far been identified but include thyroid adenomas and goiter (particularly multinodular) (3,4,5). Subclinical hyperthyroidism, typically an autoimmune disorder in younger patients (6), rarely leads to overt hyperthyroidism (2) but has been associated with increased risk for atrial fibrillation, reproductive problems such as miscarriage, as well as decreased bone density and possible osteoporosis (4).
The accident at the Chornobyl (Chernobyl) nuclear power plant in Ukraine in April of 1986 exposed large numbers of residents to radioactive iodines and iodine-131 (I-131) in particular. Previous studies by our group and others (7,8,9) have found a more than six-fold increase in thyroid cancer risk among persons exposed as children or adolescents to 1 Gy of radiation to the thyroid relative to those who were minimally exposed. The data from Chornobyl were virtually the first, and certainly the most convincing, to show increased risk of thyroid cancer following internal exposure to I-131 during childhood.
There have also been limited reports on risk of functional thyroid abnormalities related to Chornobyl fallout, although with mixed results and methodological limitations (10,11,12,13,14,15,16). We have been studying thyroid function in a cohort of ~13,000 persons exposed to I-131 in Ukraine under the age of 18 years, who had thyroid radioactivity measurements taken within eight weeks of the accident and who were screened by clinicians using a standardized protocol. We have found a linear dose-response relationship for prevalence of subclinical hypothyroidism (17) and a non-linear dose-response relationship for prevalence of elevated antibodies to thyroperoxidase (ATPO) (18). Results from the few studies evaluating the relationship between thyroid functional disorders and environmental exposure to I-131 in other circumstances, including fallout from nuclear weapons testing which exposed residents living downwind of the test sites (19,20) and releases from the Hanford nuclear facility (21,22) were largely negative and the data on hyperthyroidism in particular were extremely limited, presumably because the relatively small cohorts yielded few cases. To fill this gap, we evaluated I-131 thyroid dose estimates from Chornobyl fallout and prevalence of hyperthyroidism in a cohort of 11,853 exposed children and adolescents from affected areas of Ukraine.
Subjects and Methods
Study population
A detailed description of the study design and methods can be found in Stezhko et al. (23). In brief, in Ukraine a cohort was assembled of individuals who had direct measurements of thyroid radioactivity made in May or June 1986, who were under age 18 on the day of the accident (April 26, 1986), and who resided in adjacent Chernihiv, Zhytomyr or Kyiv Oblasts (similar in size to a state) or the city of Kyiv at the beginning of the study in the late-1990s. The study protocol called for detailed thyroid screening examinations of cohort members every two years. The current analysis is based on the data collected during the first screening examination conducted between 1998 and 2000 and therefore reflects only prevalent cases.
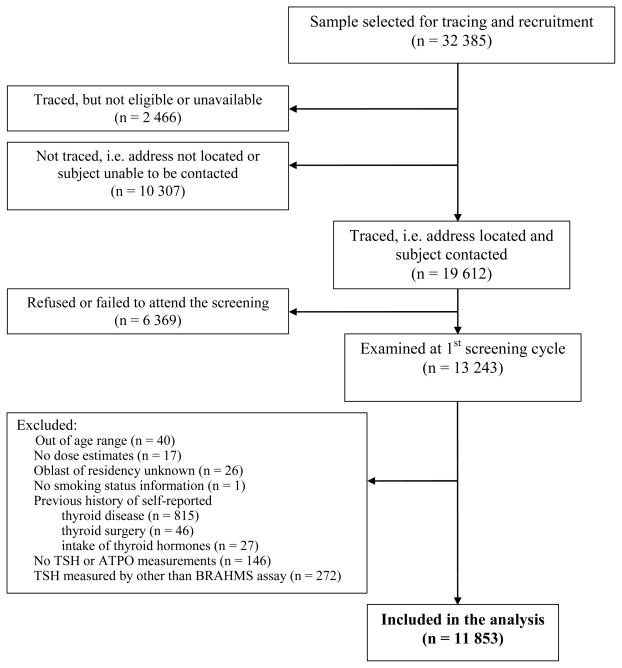
A summary of individuals who were originally selected, traced, screened, or excluded from the analysis is presented in Figure 1. Of the 32,385 individuals who were targeted, 19,612 could be traced and contacted. Approximately one-third had moved (n=10,307), were not at their residence (e.g., at university or in the military) (n=2,466), refused to participate or failed to attend the screening (n=6,369). To help insure that TSH measurements at first examination reflect spontaneous levels, individuals who reported thyroid disease (n=815, 90% with simple diffuse goiter), thyroid surgery (n=46) or intake of thyroid hormones (n=27) prior to the first screening examination were excluded from this analysis. Individuals without TSH or ATPO measurements (n=146) or those with TSH measurements performed by other than the BRAHMS assay (n=272) were also excluded. After these and other exclusions (see Figure 1), the cohort for analysis consisted of a total of 11,853 individuals.
Figure 1.
Study Flow Chart
The study was evaluated and approved by the institutional review boards in Ukraine and the United States National Cancer Institute, and all subjects signed an informed consent form.
Screening examination
Screening was carried out either by a mobile medical team visiting the local area or at the Institute of Endocrinology and Metabolism in Kyiv. Screening procedures included thyroid palpation and ultrasound examination by an ultrasonographer and an independent clinical examination and palpation by an endocrinologist. Any differences were resolved by joint examination by both doctors.
In addition, a serum sample and a spot urine sample were collected for measurement of thyroid hormones/antibodies and current iodine levels respectively. A series of structured questionnaires were also administered to elicit information on demographics, individual and family medical history, and items relevant to thyroid dose estimation, such as residential history, consumption of contaminated milk and leafy vegetables, and intake and timing of iodine prophylaxis (23).
Serum Assays
TSH, fT4, and ATPO were measured in serum samples with LUMitest immunochemiluminescense assays (BRAHMS Diagnostica GMBH, Heningsdorf, Germany) using a Bertoldt 953 luminometer (Pforzheim, Germany). While TSH and ATPO measurements were performed for everyone with a sufficient serum sample (99% of the cohort), fT4 measurements were performed only for those whose TSH result was outside the reference range. All assays were conducted according to the manufacturer’s instructions.
The analytical sensitivity of the TSH assay was 0.008 mIU/L. Intraassay coefficients of variation (CV) for the TSH assay at 0.03 and 2.0 mIU/L were 3.0 and 2.2%, respectively, and the interassay CVs were 10.9 and 2.8%, respectively. Intraassay CVs for the fT4 assay at 7.4 and 33.5 pmol/L were 5.6 and 2.8%, respectively, and the interassay CVs at 6.7 and 53.9 pmol/L were 9.0 and 7.3%, respectively. Intraassay CVs for the ATPO assay at 84 and 375 U/ml were 8.1 and 6.5% respectively, and the interassay CVs were 11.4 and 7.3% respectively.
Based on evaluation of the range of values in a sample from our cohort, reference limits for TSH were set between 0.3 and 4.0 mIU/L. Reference limits for fT4 were set between 10.0 – 25.0 pmol/L based on BRAHMS’s recommendation. An elevation of ATPO above 60 U/ml, consistent with BRAHMS, was considered positive or elevated.
Thyroid volume
The thyroid was examined using a 7.5-MHz linear transducer (EUB-405, Hitachi Medical Systems and Toshiba 240, Tokyo, Japan) with the subject supine and neck extended. The thyroid volume was calculated based on the volume of an ellipsoid (length x width x depth x 0.479) as described elsewhere (24). Because thyroid volume ≥ 20 ml is typically above the 97th percentile and considered large for populations whose age is comparable to our cohort (mean=21 years, range 12–32 years), we dichotomized the thyroid volume values as ≥ 20 ml/< 20 ml.
Iodine determination
Urinary iodine content in spot urine samples was measured using the Sandell-Kolthoff reaction and expressed in micrograms per liter (25). The analytic sensitivity of the assay was 6 μg/L.
Outcome definition
We defined hyperthyroidism as a serum TSH concentration < 0.3 mIU/L, the lower limit of the reference range. Overt hyperthyroidism was defined as a serum TSH concentration < 0.3 mIU/L with fT4 > 25.0 pmol/L, while subclinical hyperthyroidism was defined as a serum TSH concentration of < 0.3 mIU/L with normal concentrations of fT4 (10–25 pmol/L). Additional sensitivity analyses were carried out using two alternative definitions of hyperthyroidism: TSH < 0.25 mIU/L and TSH < 0.4 mIU/L.
Dosimetry
Details of dosimetric methods have been published elsewhere (26, 27). The unique feature of our study is that doses were primarily based on thyroid radioactivity measurements taken within eight weeks of the accident. Using these measurements, along with data on dietary and lifestyle habits, and environmental transfer models, we estimated individual I-131 thyroid doses and their uncertainties. The distributions of 1,000 individual thyroid dose estimates, obtained using a Monte Carlo procedure, were approximately lognormal (26). The arithmetic means of the 1,000 individual I-131 dose realizations were used for the current analyses after correction for the typical thyroid masses of the Ukrainian population (IIya Likhtarev, personal communication). The dose estimates are for I-131, which typically accounts for more than 90–95% of the total thyroid dose (28). The remaining portions of thyroid dose from external exposure and from internal exposure to cesium and other isotopes of iodine are minor contributors and are currently not taken into account. The mean and median for I-131 dose in the analysis cohort are 0.6 Gy and 0.2 Gy respectively, with a range of 0–40.8 Gy. Because of the small size of the gland in children and their high consumption of milk, much of which was contaminated, children generally received the highest thyroid doses (29). Age at exposure and age at examination are highly correlated in our cohort. For the groups examined at ages 12–14, 15–19, 20–24 and 25–32, respectively, the overall age-specific mean doses (Gy) were 1.58, 0.79, 0.46 and 0.39.
Statistical Analysis
Excess odds ratio (EOR) models based on logistic regression were employed to evaluate the association between hyperthyroidism prevalence and I-131 thyroid dose estimates among the study population. Let p(x,d) denote the hyperthyroidism prevalence of the group of subjects characterized by a set of covariate variables x and dose d. We assumed that the prevalence could be described as an excess odds ratio (OR) model of the form: Odds(x,d) = p(x,d)/{1-p(x,d)} = r(x){1+ f(d)}, where r is a function of background risk factors and f(d) is a dose response function such that f(0)=0. For example, if we assume the risk increases linearly with I-131 dose, we have f(d)=βd with the coefficient β interpreted as the excess relative odds ratio per unit dose (1 Gy). In addition to the linear dose response, we conducted analyses based on various types of functions including f(d)=βlog(d), f(d)=βdexp(-γd), categorical dose response models, and a dichotomous dose response model, f(d)=β if d≥θ and f(d)=0 otherwise. For each of these dose functions, the dose-response parameters were estimated by the maximum likelihood method based on the total group of subjects, as well as on males and females separately. In particular, the value of θ in the dichotomous dose response model was estimated by a profile likelihood method in which the θ that maximizes the likelihood was iteratively sought by fixing θ at each iteration to maximize the likelihood with respect to the other parameters. Confidence intervals (CIs) of model parameters were computed using likelihood ratio statistics. Significance of parameters and trends, and model comparisons were tested using the likelihood ratio test (LRT) with degrees of freedom (df) equal to the difference in number of parameters of compared models. We also measured Akaike Information Criteria or AIC (30) to compare non-nested models. All computations in this study were conducted using the generalized non-linear model package of R software (31) and the GMBO package of Epicure software (32).
To select a set of background risk factors x that explain hyperthyroidism prevalence in the absence of radiation, we conducted a selection procedure in which we started with a base model containing a gender-age (at examination) interaction and oblast of residency and then examined the inclusion effect of each of the following potential risk factors previously associated with functional thyroid outcomes in this cohort (17) or in other non-irradiated populations (1,3,4): smoking status (current/past/never), multivitamin consumption, thyroid disease history in relatives, year and season of screening examination (blood collection), ATPO level, urinary iodine concentration, thyroid volume, and presence of ultrasound-detected thyroid nodules. In addition, we retained those factors that were not significantly associated with the outcome, but affected the estimate of the dose-response. The final set of adjustment factors included age at examination by gender, oblast of residency, season of blood collection, ATPO level, thyroid volume and presence of thyroid nodules at ultrasound. Because of the inverse correlation between age at exposure and dose, adequate adjustment for attained age (strongly correlated in our cohort with age at exposure) was particularly important in analyzing the relationship of I-131 dose to risk of hyperthyroidism, a condition more prevalent at later ages (3).
For evaluation of factors that modify the effect of I-131 dose, we allowed the dose-response function to vary within categories of those factors such as gender, age at exposure, ATPO level, place of residency, etc. The significance of effect modification was examined in each instance by two likelihood ratio tests: one comparing a nested model with an interaction term (e.g., dose*gender) relative to a model with two main effects (dose and gender) and the other one comparing a model with an interaction term relative to a model which included one main effect (gender), but no parameter for the effect of I-131 dose.
Results
Case characteristics
There were 76 individuals out of 11,853 included in the analysis (0.6%) who met the definition of hyperthyroidism. Eleven of these satisfied criteria for overt (clinical) hyperthyroidism (TSH < 0.3 mIU/L and fT4 > 25.0 pmol/L) while the remaining 65 cases met criteria for subclinical hyperthyroidism (TSH < 0.3 mIU/L and fT4 10–25 pmol/L). The overall mean TSH level for the 11 overt hyperthyroid cases was 0.06 mIU/L, with a range from 0–0.20 mIU/L. One subject with overt hyperthyroidism had a TSH level below the limit of detection (< 0.008 mIU/L). The mean fT4 value among overt cases was 35.1 pmol/L (range 25.8–54.0 pmol/L) and 54.6% of the 11 cases had elevated ATPO levels (> 60 U/ml). The mean thyroid volume among the 11 cases of overt hyperthyroidism was 14.2 ml, with a range from 5.8–34.6 ml. Among the 65 cases of subclinical hyperthyroidism, the mean value for TSH was 0.12 mIU/L, with a range from 0–0.28 mIU/L. Five of the subclinical cases had a TSH level below the limit of detection. The mean fT4 value for subclinical cases was 15.9 pmol/L (range 10.0–23.4 pmol/L) and the percentage with elevated ATPO levels was 45.4%. The mean for thyroid volume in subjects with subclinical hyperthyroidism was 12.8 ml, with a range from 5.9–36.2 ml. The majority of the 76 hyperthyroid cases occurred in females (n=53, 69.7%).
Background risk factors
Table 1 presents estimated odds ratios (ORs) and 95% CIs for the effects of age at examination on hyperthyroidism prevalence. The overall adjusted OR estimates for the prevalence of hyperthyroidism in the absence of radiation exhibit an increasing trend with age at examination (P=0.05). The trend is more pronounced among females (P=0.03) than among males (P=0.80), although a formal test for interaction was not significant (P=0.33).
Table 1.
Odds ratio (OR) and 95% confidence intervals (CI) for prevalence of subclinical hyperthyroidism: effects of age at examination by gender.
| Gender Age at examination |
Case (76) | Noncase (11,777) | Rate per 103 | ORa | 95% CI | Test for
|
||
|---|---|---|---|---|---|---|---|---|
| heterogeneityb | trendc | |||||||
| All: | 12–14 | 3 | 1,243 | 2.41 | 0.43 | 0.10,1.28 | 0.07 | 0.05 |
| 15–19 | 18 | 3,493 | 5.13 | 1 | ||||
| 20–24 | 30 | 3,612 | 8.24 | 1.61 | 0.89,2.99 | |||
| 25–32 | 25 | 3,429 | 7.24 | 1.37 | 0.73,2.63 | |||
|
| ||||||||
| Male: | 12–14 | 1 | 624 | 1.60 | 0.39 | 0.02,2.20 | 0.73 | 0.80 |
| 15–19 | 8 | 1,758 | 4.53 | 1 | ||||
| 20–24 | 6 | 1,879 | 3.18 | 0.67 | 0.22,1.96 | |||
| 25–32 | 8 | 1,802 | 4.42 | 0.90 | 0.32,2.51 | |||
|
| ||||||||
| Total | 23 | 6,063 | 3.78 | |||||
|
| ||||||||
| Female: | 12–14 | 2 | 619 | 3.22 | 0.48 | 0.07,1.84 | 0.02 | 0.03 |
| 15–19 | 10 | 1,735 | 5.73 | 1 | ||||
| 20–24 | 24 | 1,733 | 13.66 | 2.42 | 1.17,5.39 | |||
| 25–32 | 17 | 1,627 | 10.34 | 1.76 | 0.79,4.13 | |||
|
| ||||||||
| Total | 53 | 5,714 | 9.19 | |||||
|
| ||||||||
| Test for interactiond | 0.33 | |||||||
All analyses performed with adjustment for gender, place of residency, period of examination, ATPO level, thyroid volume, ultrasound-detected nodules and I-131 dose with the 3-category non-parametric response.
Based on the LRT for categorical age effects vs no age effect (df=3).
Based on the LRT for logit-linear age effects vs no age effect (df=1).
Based on the LRT for the interaction between gender and categorical age effects (df=3).
Table 2 shows associations of other background risk factors with the prevalence of hyperthyroidism. Subjects with ATPO levels ≥ 60 U/ml compared to those with normal levels of ATPO (< 60 U/ml) had an OR of 2.63 (95% CI: 1.51–4.40, P<0.01). The OR was also elevated among subjects with ultrasound-detected thyroid nodules compared to those without such nodules (OR=2.63, 95% CI: 0.99–5.82, P=0.05). Individuals with thyroid volume ≥ 20 ml on ultrasound had about twice the risk of prevalent hyperthyroidism as those with thyroid volume below 20 ml (OR=2.07, 95% CI: 0.93–4.10, P=0.07), and risks were also higher among subjects examined between June and November compared with December through May (P=0.11). Associations with other background factors were close to null.
Table 2.
Odds ratio (OR) and 95% confidence intervals (CI) for prevalence of hyperthyroidism: effects of potential risk factors.
| Characteristic | Case (76) | Noncase (11,777) | Rate per 103 | ORa | 95% CI | Test for Heterogeneity, Pb |
|---|---|---|---|---|---|---|
| Place of residency | 0.60 | |||||
|
| ||||||
| Zhytomyr | 20 | 3221 | 6.17 | 1 | ||
| Kyiv | 17 | 2375 | 7.11 | 1.27 | 0.64,2.50 | |
| Chernihiv | 39 | 6181 | 6.27 | 1.38 | 0.74,2.64 | |
|
| ||||||
| Period of examination | 0.11 | |||||
|
| ||||||
| Dec-Feb | 12 | 2092 | 5.7 | 1 | ||
| Mar-May | 15 | 3412 | 4.38 | 0.67 | 0.30,1.56 | |
| Jun-Aug | 22 | 2850 | 7.66 | 1.44 | 0.70,3.07 | |
| Sep-Nov | 27 | 3423 | 7.83 | 1.33 | 0.67,2.79 | |
|
| ||||||
| ATPO level, U/mL | <0.01 | |||||
|
| ||||||
| < 60 | 56 | 10369 | 5.37 | 1 | ||
| ≥ 60 | 20 | 1408 | 14.01 | 2.63 | 1.51,4.40 | |
|
| ||||||
| Thyroid volume, ml | 0.07 | |||||
|
| ||||||
| < 20 | 67 | 11063 | 6.02 | 1 | ||
| ≥ 20 | 9 | 714 | 12.45 | 2.07 | 0.93,4.10 | |
|
| ||||||
| Ultrasound-detected nodules | 0.05 | |||||
|
| ||||||
| No | 70 | 11523 | 6.04 | 1 | ||
| Yes | 6 | 254 | 23.08 | 2.63 | 0.99,5.82 | |
|
| ||||||
| Year of examination | 0.50 | |||||
|
| ||||||
| 1998 | 16 | 2805 | 5.67 | 1 | ||
| 1999 | 11 | 1296 | 8.42 | 1.68 | 0.67,4.08 | |
| 2000 | 49 | 7676 | 6.34 | 1.05 | 0.59,1.97 | |
|
| ||||||
| Urinary iodine, μg/L | 0.29 | |||||
|
| ||||||
| < 20 | 5 | 1524 | 3.27 | 1 | ||
| 20–49 | 31 | 4574 | 6.73 | 2.16 | 0.91,6.38 | |
| 50–99 | 21 | 3263 | 6.39 | 2.06 | 0.83,6.23 | |
| ≥100 | 11 | 1324 | 8.24 | 2.55 | 0.91,8.21 | |
|
| ||||||
| Missing/Unknown | 8 | 1092 | 7.27 | 1.85 | 0.61,6.21 | |
|
| ||||||
| Current smoking | 0.84 | |||||
|
| ||||||
| No | 56 | 7823 | 7.11 | 1 | ||
| Yes | 20 | 3954 | 5.03 | 1.06 | 0.57,1.92 | |
|
| ||||||
| Vitamin consumption | 0.64 | |||||
|
| ||||||
| No | 71 | 11096 | 6.36 | 1 | ||
| Yes | 5 | 681 | 7.29 | 1.26 | 0.43,2.89 | |
|
| ||||||
| Thyroid disease history in relatives | 0.96 | |||||
|
| ||||||
| No | 52 | 7956 | 6.49 | 1 | ||
| Yes | 7 | 875 | 7.94 | 1.02 | 0.42,2.13 | |
|
| ||||||
| Unknown | 17 | 2946 | 5.74 | 0.92 | 0.51,1.57 | |
All analyses performed with adjustment for factors gender, age at examination by gender, place of residency, period of examination, ATPO level, thyroid volume, ultrasound-detected nodules and I-131 dose with the 3-category non-parametric response.
Based on the LRT for the categorical effects vs no effect.
Dose-response analysis
The overall mean (median) I-131 thyroid doses among cases and non-cases were 0.55 (0.21) Gy and 0.66 (0.20) Gy, respectively.
Results of analyses examining a linear dose-response between radioiodine exposure and hyperthyroidism are presented in Table 3, for all subjects combined and for males and females separately. In the sample overall, there was no evidence to support a statistically significant relationship; the linear EOR/Gy was 0.02 (P=0.90). The estimate for females was higher (EOR/Gy=0.22), although it was also not statistically different from zero (P=0.31). Due to the scarcity of male cases exposed at higher doses, the linear EOR model for males could not be successfully fitted, except by imposing a non-negative constraint on the dose response parameter (Table 3(b)).
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) for prevalence of hyperthyroidism: effects of I-131 thyroid dose.
| Model | Dose (Gy) | Case (76) | Noncase (11,777) | Rate Per03 | ORa | 95% CI | Test for trend / heterogeneityb P |
|---|---|---|---|---|---|---|---|
| (a) All subjects combined | |||||||
|
| |||||||
| Linear | EOR/Gy = 0.02 | NA,0.53 | 0.90 | ||||
|
| |||||||
| 7 categ. | < 0.1 | 24 | 3729 | 6.39 | 1 | 0.94 | |
| 0.1–0.25 | 18 | 2769 | 6.46 | 1.11 | 0.58,2.08 | ||
| 0.25–0.50 | 12 | 1987 | 6.00 | 1.15 | 0.54,2.36 | ||
| 0.50–1 | 11 | 1443 | 7.57 | 1.61 | 0.72,3.41 | ||
| 1–2.5 | 7 | 1232 | 5.65 | 1.39 | 0.51,3.38 | ||
| 2.5–5 | 3 | 403 | 7.39 | 1.65 | 0.37,5.33 | ||
| ≥ 5 | 1 | 214 | 4.65 | 1.38 | 0.07,7.48 | ||
|
| |||||||
| 3 categ. | <0.1 | 24 | 3729 | 6.39 | 1 | 0.45 | |
| 0.1–0.5 | 30 | 4756 | 6.27 | 1.13 | 0.64,2.00 | ||
| ≥ 0.5 | 22 | 3292 | 6.64 | 1.53 | 0.77,3.01 | ||
|
| |||||||
| dichotomous | <0.5 | 54 | 8485 | 6.32 | 1 | 0.23 | |
| ≥ 0.5 | 22 | 3292 | 6.64 | 1.41 | 0.79,2.45 | ||
|
| |||||||
| (b) Male | |||||||
|
| |||||||
| Linear | EOR/Gy = −0.025c | NA | 0.61 | ||||
|
| |||||||
| 7 categ. | < 0.1 | 6 | 1794 | 3.33 | 1 | 0.83 | |
| 0.1–0.25 | 7 | 1435 | 4.85 | 1.54 | 0.51,4.83 | ||
| 0.25–0.50 | 5 | 1060 | 4.69 | 1.54 | 0.43,5.28 | ||
| 0.50–1 | 3 | 786 | 3.80 | 1.25 | 0.25,4.94 | ||
| 1–2.5 | 2 | 669 | 2.98 | 1.10 | 0.15,5.08 | ||
| 2.5–5 | 0 | 205 | 0 | 0.00 | NA | ||
| ≥ 5 | 0 | 114 | 0 | 0.00 | NA | ||
|
| |||||||
| 3 categ. | < 0.1 | 6 | 1794 | 3.33 | 1 | 0.57 | |
| 0.1–0.5 | 12 | 2495 | 4.79 | 1.55 | 0.59,4.52 | ||
| ≥ 0.5 | 5 | 1774 | 2.81 | 1 | 0.27,3.54 | ||
|
| |||||||
| dichotomous | <0.5 | 18 | 4289 | 4.18 | 1 | 0.49 | |
| ≥ 0.5 | 5 | 1774 | 2.81 | 0.75 | 0.24,1.98 | ||
|
| |||||||
| (c) Female | |||||||
|
| |||||||
| Linear | EOR/Gy = 0.22 | −0.10,1.23 | 0.31 | ||||
|
| |||||||
| 7 categ. | < 0.1 | 18 | 1935 | 9.22 | 1 | 0.71 | |
| 0.1–0.25 | 11 | 1334 | 8.18 | 0.94 | 0.42,2.01 | ||
| 0.25–0.50 | 7 | 927 | 7.49 | 0.98 | 0.37,2.35 | ||
| 0.50–1 | 8 | 657 | 12.03 | 1.82 | 0.71,4.32 | ||
| 1–2.5 | 5 | 563 | 8.80 | 1.58 | 0.48,4.38 | ||
| 2.5–5 | 3 | 198 | 14.93 | 2.38 | 0.51,8.03 | ||
| ≥ 5 | 1 | 100 | 9.90 | 2.20 | 0.12,12.56 | ||
|
| |||||||
| 3 categ. | < 0.1 | 18 | 1935 | 9.22 | 1 | 0.17 | |
| 0.1–0.5 | 18 | 2261 | 7.90 | 0.96 | 0.48,1.91 | ||
| ≥ 0.5 | 17 | 1518 | 11.07 | 1.83 | 0.85,3.93 | ||
|
| |||||||
| dichotomous | <0.5 | 36 | 4196 | 8.51 | 1 | 0.06 | |
| ≥ 0.5 | 17 | 1518 | 11.07 | 1.89 | 0.97,3.54 | ||
All analyses performed with adjustment for gender, age at examination by gender, place of residency, period of examination, ATPO level, thyroid volume and ultrasound-detected nodules.
Based on the LRT for the dose response vs no dose effect.
Estimated with the parameter space constrained on >-max(dose)−1.
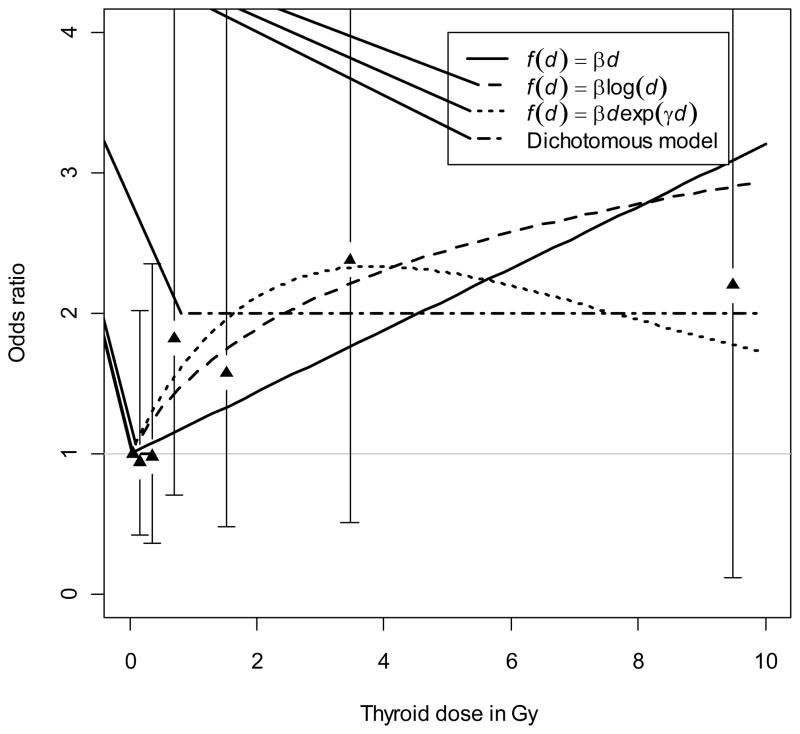
Categorical dose response analyses were also conducted. Based on analyses with seven dose categories, there was a suggestion of an increase in the estimated ORs at doses above 0.5 Gy overall and among females in particular, although the estimates were not statistically significant. (Table 3) Analyses based on alternative categorization of doses into three categories, chosen to assure approximately even distribution of cases with hyperthyroidism, were consistent with the previous categorization in that ORs for doses ≥ 0.5 Gy tended to be higher, but not significantly different from the ORs for lower dose categories. We pursued this finding further by fitting a dichotomous dose response model (with ≥ 0.5 Gy and < 0.5 Gy dose categories). The OR for individuals with dose ≥ 0.5 Gy compared to those with dose < 0.5 Gy was estimated as 1.41 (95% CI: 0.79–2.45, P=0.23) for all subjects combined, 0.75 (95% CI: 0.24–1.98, P=0.49) for males and 1.89 (95% CI: 0.97–3.54, P=0.06) for females. A more formal approach to the finding for females from the dichotomous dose response analysis was based on fitting a model in which the threshold was allowed to vary. The dichotomous model with an estimated threshold of 0.52 Gy (95%CI: 0–1.2) yielded an OR of 2.00 (95%CI: 0.6–3.7, P=0.13). In addition, we fitted other threshold models allowing for a trend before/after the threshold but did not find any improvement in goodness of fit compared to the dichotomous model without trend.
Table 4 summarizes the fit statistics of the dichotomous dose response model with estimated threshold as well as those of other models considered in this study. The fits of the models among females are also illustrated in Figure 2. Both the figure and the AIC data in Table 4 appear to favor the dichotomous dose response model, although we were not able to discriminate among the fitted models statistically. An analysis restricted to subclinical cases only produced results that were virtually the same as for the two types of hyperthyroidism combined, although the p-values were slightly reduced (data not shown).
Table 4.
Model fits of I-131 thyroid dose response analyses for all subjects and females only.
| Modela population | f(d) | df | test for significance change in deviance | P | AIC |
|---|---|---|---|---|---|
| Null | 902.63 | ||||
|
| |||||
| combined | βd | 1 | 0.49 | 0.48 | 904.13 |
| βlog(d) | 1 | 0.02 | 0.90 | 904.61 | |
| βd exp(−γd) | 2 | 1.40 | 0.50 | 905.22 | |
| Dichotomousb | 2 | 2.47 | 0.29 | 904.16 | |
|
| |||||
| female | βd | 1 | 1.02 | 0.31 | 903.61 |
| βlog(d) | 1 | 1.88 | 0.17 | 902.74 | |
| βd exp(−γd) | 2 | 2.21 | 0.33 | 904.41 | |
| Dichotomous | 2 | 4.08 | 0.13 | 902.55 | |
All analyses performed with adjustment for gender, age at examination by gender, place of residency, period of examination, ATPO level, thyroid volume and ultrasound-detected nodules.
With estimated threshold
Figure 2.
Association between hyperthyroidism prevalence and I-131 thyroid dose estimates for females. Continuous EOR dose response models as well as a threshold model were fitted and estimated functions plotted for the female-specific dose response analyses. 95% confidence intervals were plotted for odds ratio estimates from the categorical response model.
Additional dose-response analyses using alternative TSH cut-offs for the definition of hyperthyroidism produced results largely similar (data not shown) to the main definition, particularly for the lower cut-off of 0.25 mIU/L that is thought to be more relevant clinically for predicting risk of atrial fibrillation. Although there were too few overt cases to evaluate separately from subclinical cases, a re-analysis excluding the overt cases yielded similar results to those for the two types of hyperthyroidism combined (not shown).
Effect modification
Table 5 presents data on modifying effects of selected factors based on the dichotomous dose response model (≥0.5Gy vs. <0.5Gy). There was some suggestion that the effect of I-131 on risk of prevalent hyperthyroidism may vary according to age at exposure (strongly correlated in our cohort with age at examination), both overall (P=0.07/0.06 from the LRT comparing the model with interaction against the no-dose-effect model/the no interaction model) and among females (P=0.05/0.12). The highest I-131-related ORs were observed for those exposed at 15–18 years old.
Table 5.
Odds ratios (OR) and 95% confidence intervals (CI) for prevalence of hyperthyroidism: the estimated effects of I-131 thyroid dose (≥ 0.5 Gy vs. < 0.5 Gy) according to levels of other potential effect modifiers.
| Characteristic | Combined | Female | ||
|---|---|---|---|---|
|
| ||||
| ORa | 95%CI | ORa | 95%CI | |
| ATPO level, U/mL | ||||
|
| ||||
| < 60 | 1.48 | 0.76,2.76 | 1.98 | 0.92,4.04 |
| ≥ 60 | 1.26 | 0.43,3.28 | 1.70 | 0.52,4.68 |
|
| ||||
| test for heterogeneityb/interactionc | P=0.47 / P=0.78 | P=0.16 / P=0.80 | ||
|
| ||||
| Place of residency | ||||
|
| ||||
| Zhytomyr | 0.75 | 0.28,1.86 | 1.66 | 0.61,4.30 |
| Kyiv | 1.97 | 0.67,5.28 | 1.86 | 0.51,5.49 |
| Chernihiv | 1.97 | 0.82,4.24 | 2.22 | 0.73,5.61 |
|
| ||||
| test for heterogeneityb/interactionc | P=0.22 / P=0.23 | P=0.29 / P=0.91 | ||
|
| ||||
| Period of examination | ||||
|
| ||||
| Dec-Feb | 3.02 | 0.91,11.76 | 4.31 | 1.28,15.52 |
| Mar-May | 0.88 | 0.20,2.80 | 1.74 | 0.39,5.76 |
| Jun-Aug | 2.79 | 1.02,6.97 | 2.46 | 0.68,7.14 |
| Sep-Nov | 0.62 | 0.17,1.71 | 0.81 | 0.18,2.50 |
|
| ||||
| test for heterogeneityb/interactionc | P=0.09 / P=0.08 | P=0.09 / P=0.22 | ||
|
| ||||
| Ultrasound-detected nodules | ||||
|
| ||||
| No | 1.53 | 0.84,2.70 | 2.05 | 1.03,3.93 |
| Yes | 0.53 | 0.03,3.47 | 0.82 | 0.04,5.45 |
| test for heterogeneityb/interactionc | P=0.30 / P=0.31 | P=0.12 / P=0.57 | ||
|
| ||||
| Diffuse goiter | ||||
|
| ||||
| No | 1.45 | 0.77,2.63 | 2.04 | 1.00,3.96 |
| Yes | 1.31 | 0.43,3.20 | 1.42 | 0.33,4.19 |
| test for heterogeneityb/interactionc | P=0.49 / P=0.85 | P=0.14 / P=0.39 | ||
|
| ||||
| Current smoking status | ||||
|
| ||||
| No | 1.56 | 0.83,2.82 | 1.84 | 0.91,3.54 |
| Yes | 1.02 | 0.30,2.66 | 2.30 | 0.36,8.04 |
| test for heterogeneityb/interactionc | P=0.37 / P=0.45 | P=0.16 / P=0.78 | ||
|
| ||||
| Urinary iodine, μg/L | ||||
|
| ||||
| < 20 | 1.16 | 0.27,3.35 | 2.17 | 0.50,6.46 |
| ≥ 20 | 1.48 | 0.79,2.67 | 2.03 | 0.98,3.99 |
| Missing/Unknown | 1.30 | 0.21,4.36 | 0.86 | 0.05,4.12 |
|
| ||||
| test for heterogeneityb/interactionc | P=0.66 / P=0.92 | P=0.22 / P=0.63 | ||
|
| ||||
| Age at exposure, years | ||||
|
| ||||
| 0–4 | 0.54 | 0.15,1.57 | 0.47 | 0.07,1.91 |
| 5–9 | 1.43 | 0.56,3.17 | 1.88 | 0.68,4.50 |
| 10–14 | 1.82 | 0.71,4.09 | 3.03 | 1.15,7.11 |
| 15–18 | 5.08 | 1.40,14.65 | 4.88 | 0.73,19.42 |
|
| ||||
| test for heterogeneityb/interactionc | P=0.07 / P=0.06 | P=0.05 / P=0.12 | ||
All analyses performed with adjustment for gender, age at exposure by gender, place of residency, periodof examination, ATPO level, thyroid volume and ultrasound-detected nodules.
Based on the LRT for the no dose effect vs dichotomous effect with interaction.
Based on the LRT for the interaction with the dichotomous dose effect.
Discussion
In a screening study carried out in Ukraine in a cohort of 11,853 individuals under age 18 when they were exposed to fallout from the Chornobyl accident (principally to I-131), we found no solid evidence of a dose response relationship with prevalent hyperthyroidism, although statistical power to detect significant effects was limited by the relatively small number of cases.
We examined categorical as well as parametric dose response function models in both the overall study population and males and females separately. Among females there was an indication (P=0.13) of elevated risk (OR=2.00) in a dichotomous model with an estimated threshold of 0.52 Gy. Testing more conventional models of dose-dependence did not yield better fits to the data. The AIC results and figure plot also seem to favor the dichotomous dose response model with estimated threshold. However, this observation in females has to be interpreted cautiously for a number of reasons: it did not reach conventional levels of statistical significance; it was initially identified in an empirical analysis; and it could be a chance finding given the number of analyses carried out. In addition, the dose response form is unusual but if there is an interaction with age at examination and/or age at exposure as suggested in our analyses, this may have affected the shape of the model. We plan to investigate this further using data from future screenings that will add new cases of hyperthyroidism as the cohort ages and increase statistical power.
There has been very little research into hyperthyroidism following environmental exposure to I-131. Other studies of populations of children exposed to I-131 from Chornobyl fallout have focused on hypothyroidism, which is a far more common form of thyroid dysfunction, but few have reported positive results (13,14). Although the exposure in question was not internal I-131 radiation, a recent publication based on atomic bomb survivors in Hiroshima and Nagasaki who participated in a comprehensive thyroid screening study (33) reported a nonsignificant elevated EOR/Sv for hyperthyroidism in those exposed to external radiation at 10 years of age (EOR/Sv=0.49, 95% CI: −0.06–1.69, P=0.10). This estimate was based on 38 cases with Graves disease.
The study we report here did have certain limitations, including some related to case identification. For differential diagnosis of hyperthyroidism in a clinical setting, measurement of fT3 as well as fT4 is standard practice (4, 34) since in subclinical cases, levels of T3 as well as T4 should fall within laboratory reference ranges while in overt cases both T3 and T4 levels will be elevated (35). However, in a large, population-based screening program like ours, the number of assays had to be restricted due to cost constraints and T3 measurements were not included in the study protocol.
The absence of data on serum fT3 also prevented us from evaluating certain other diagnoses as alternative explanations for the observation of low TSH levels among adolescent females. A differential diagnosis of depression, for example, would be appropriate in some cases with low serum TSH accompanied by decreased levels of T3, but T3 levels are frequently normal in depressed patients, with reduced levels more typically found in cases of severe depression (36). Although misclassification of depressed young females as cases of hyperthyroidism would have attenuated any radiation dose-response in this gender group, the extent of such misclassification is probably small. Serum TSH levels also decrease during the late first trimester of pregnancy (2, 37). Females in our sample, many of child-bearing age, were not asked whether they were pregnant at the time of the screening examination when blood samples were drawn for measurement of TSH and other thyroid hormones. In the absence of such information, pregnancy could potentially be misdiagnosed as thyroid dysfunction. However, the dip in TSH levels during pregnancy is of brief duration, lasting for only a few weeks. Moreover, the prevalence of hyperthyroidism in our cohort is no greater than in studies of females of comparable age that excluded pregnant women (e.g., 37). It therefore seems likely that our case group included few, if any, women whose subnormal levels of serum TSH were a reflection of early pregnancy.
Statistical power is another consideration. Despite the larger sample size than in previous studies, our power to detect modest effects was relatively low based on the 76 cases identified at the initial screening. In addition, there were uncertainties in individual dose estimates, the magnitude of which can be described by geometric standard deviations (median of 1.7, range 1.6–5.0). Assuming that the contributions to these uncertainties were a mixture of Berkson and classical errors, it is possible they may have led to underestimation of a dose response (39).
We should point out that our study also had several notable strengths. In the context of a thyroid screening program, we evaluated hyperthyroidism in a standard manner using several alternative cut points to define subnormal TSH concentrations. Insofar as possible given our limited numbers, we assessed the impact of case heterogeneity. In addition, I-131 thyroid dose estimates were based on individual thyroid radioactivity measurements taken shortly after exposure, and data were available on a range of potential confounders collected at interview or medical examination using standardized forms. After controlling for the effect of I-131, we observed relationships with risk factors for hyperthyroidism that were similar to those reported for non-irradiated populations (1–4), indicating that there appear to be no unusual characteristics of our study group apart from their radioiodine exposure. As expected, the ORs for hyperthyroidism in cohort members tended to increase with age and to be higher in females and in those with large thyroids, ultrasound-detected nodules, and elevated levels of ATPO. Although we screened only 40% of the target population identified from a file of thyroid activity measurements, and 67.5% of those actually invited to participate, the measured thyroid radioactivity was similar for participants and non-participants (23). Selection bias is therefore unlikely.
In summary, the data presented here show that after evaluating a variety of continuous and categorical dose response models in our analytic cohort overall and in each gender group separately, we found no clear-cut evidence of a dose response relationship between I-131 and the prevalence of hyperthyroidism at the initial screening.
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Cancer Institute, NIH, DHHS, and the Department of Energy. The U.S. Nuclear Regulatory Commission and the French Institute of Radioprotection and Nuclear Safety provided the initial funds for purchase of equipment. We thank the Louise Hamilton Kyiv Data Management Center and its head, Oleksander Zvinchuk, for their excellent help with database management. The authors are also grateful to the late Drs. Ovisy Epshstein and Daniel Fink for their laboratory skills, insights and support, as well as to the late Dr. Jacob Robbins, who always encouraged the Chornobyl team to investigate functional disorders of the thyroid. Finally, we would like to thank the Journal’s Associate Editor and anonymous reviewers for their valuable comments.
References
- 1.Vanderpump M. The Epidemiology of Thyroid Diseases. In: Braverman L, Utiger RD, editors. Werner & Ingbar’s The Thyroid A Fundamental and Clinical Text. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 398–406. [Google Scholar]
- 2.Marqusee E, Haden ST, Utiger RD. Subclinical thyrotoxicosis. Endocrinol Metab Clin North Am. 1998;27:37–49. doi: 10.1016/s0889-8529(05)70296-6. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med. 1995;46:197–204. doi: 10.1016/0300-2977(94)00089-r. [DOI] [PubMed] [Google Scholar]
- 4.Biondi B, Palmieri EA, Klain M, Schlumberger M, Filetti S, Lombardi G. Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol. 2005;152:1–9. doi: 10.1530/eje.1.01809. [DOI] [PubMed] [Google Scholar]
- 5.Evans TC. Thyroid disease. Prim Care Clin Office Pract. 2003;30:625–640. doi: 10.1016/s0095-4543(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 6.Eheman CR, Garbe P, Tuttle RM. Autoimmune thyroid disease associated with environmental thyroidal irradiation. Thyroid. 2003;13:453–464. doi: 10.1089/105072503322021115. [DOI] [PubMed] [Google Scholar]
- 7.Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, Likhtarev IA, Fink DJ, Markov VV, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 8.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 9.Davis S, Kopecky KJ, Hamilton TE, Onstad L. Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford nuclear site. JAMA. 2004;292:2600–2613. doi: 10.1001/jama.292.21.2600. [DOI] [PubMed] [Google Scholar]
- 10.Pacini F, Vorontsova T, Molinaro E, Kuchinskaya E, Agate L, Shavrova E, Astachova L, Chiovato L, Pinchera A. Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet. 1998;352:763–766. doi: 10.1016/s0140-6736(97)11397-6. [DOI] [PubMed] [Google Scholar]
- 11.Agate L, Mariotti S, Elisei R, Mossa P, Pacini F, Molinaro E, Grasso L, Masserini L, Mokhort T, et al. Thyroid autoantibodies and thyroid function in subjects exposed to Chernobyl fallout during childhood: evidence for a transient radiation-induced elevation of serum thyroid antibodies without an increase in thyroid autoimmune disease. J Clin Endocrinol Metab. 2008;93:2729–2736. doi: 10.1210/jc.2008-0060. [DOI] [PubMed] [Google Scholar]
- 12.Vermiglio F, Castagna MG, Volnova E, Lo Presti VP, Moleti M, Violi MA, Artemisia A, Trimarchi F. Post-Chernobyl increased prevalence of humoral thyroid autoimmunity in children and adolescents from a moderately iodine-deficient area in Russia. Thyroid. 1999;9:781–786. doi: 10.1089/thy.1999.9.781. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith JR, Grossman CM, Morton WE, Nussbaum RH, Kordysh EA, Quastel MR, Sobel RB, Nussbaum FD. Juvenile hypothyroidism among two populations exposed to radioiodine. Environ Health Perspect. 1999;107:303–308. doi: 10.1289/ehp.99107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quastel MR, Goldsmith JR, Mirkin L, Poljak S, Barki Y, Levy J, Gorodischer R. Thyroid-stimulating hormone levels in children from Chernobyl. Environ Health Perspect. 1997;105(Suppl 6):1497–1498. doi: 10.1289/ehp.97105s61497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vykhovanets EV, Chernyshov VP, Slukvin, Antipkin YG, Vasyuk AN, Klimenko HF, Strauss KW. 131 I dose-dependent thyroid autoimmune disorders in children living around Chernobyl. Clin Immunol Immunopathol. 1997;84:251–259. doi: 10.1006/clin.1997.4379. [DOI] [PubMed] [Google Scholar]
- 16.Kasatkina EP, Shilin DE, Rosenbloom AL, Pykov MI, Ibragimova GV, Sokolovskaya VN, Matkovskaya AN, Volkova TN, Odoud EA, et al. Effects of low level radiation from the Chernobyl accident in a population with iodine deficiency. Eur J Pediatr. 1997;156:916–920. doi: 10.1007/s004310050742. [DOI] [PubMed] [Google Scholar]
- 17.Ostroumova E, Brenner A, Oliynyk VA, McConnell R, Robbins J, Terekhova GM, Zablotska L, Likhtarev I, Bouville A, et al. Prevalence of subclinical hypothyroidism following environmental radioiodine exposure: Results from the Ukrainian-American Cohort Study of Thyroid Cancer and other Thyroid Diseases after the Chornobyl Accident (1998–2000) Environ Health Perspect. 2009;117:745–750. doi: 10.1289/ehp.0800184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tronko MD, Brenner AV, Olijnyk VA, Robbins J, Epstein OV, McConnell RJ, Bogdanova TI, Fink DJ, Likhtarev IA, et al. Autoimmune thyroiditis and exposure to iodine 131 in the Ukrainian cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: results from the first screening cycle (1998–2000) J Clin Endocrinol Metab. 2006;91:4344–4351. doi: 10.1210/jc.2006-0498. [DOI] [PubMed] [Google Scholar]
- 19.Kerber RA, Till JE, Simon SL, Lyon JL, Thomas DC, Preston-Martin S, Rallison ML, Lloyd RD, Stevens W. A cohort study of thyroid disease in relation to fallout from nuclear weapons testing. J Amer Med Assoc. 1993;270:2076–2082. [PubMed] [Google Scholar]
- 20.Lyon JL, Alder SC, Stone MB, Scholl A, Reading JC, Holubkov R, Sheng X, White GL, Jr, Hegmann KT, et al. Thyroid disease associated with exposure to the Nevada nuclear weapons test site radiation: a reevaluation based on corrected dosimetry and examination data. Epidemiology. 2006;17:604–614. doi: 10.1097/01.ede.0000240540.79983.7f. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Kopecky KJ, Hamilton TE, Onstad L. Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford nuclear site. JAMA. 2004;292:2600–2613. doi: 10.1001/jama.292.21.2600. [DOI] [PubMed] [Google Scholar]
- 22.Davis S, Kopecky K, Hamilton TE, Onstad L, King B, Saporito MS, Callahan CR. Hanford Thyroid Disease Study Final Report. HTDS; 2007. [Google Scholar]
- 23.Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, Minenko VF, Ostapenko VA, Petrenko SV, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 24.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound (authors’ transl) Dtsch Med Wochenschr. 1981;106:1338–1340. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 25.Dunn FJT, Crutchfield HE, Gutekunst R, Dunn AD. Methods for measuring iodine in urine. International Council for Control of Iodine Deficiency Disorders. 1993:18–27. [Google Scholar]
- 26.Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voilleque P, Chepurny M. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]
- 27.Likhtarov I, Kovgan L, Vavilov S, Chepurny M, Ron E, Lubin J, Bouville A, Tronko N, Bogdanova T, et al. Post-Chernobyl thyroid cancers in Ukraine. Report 2: risk analysis. Radiat Res. 2006;166:375–386. doi: 10.1667/RR3593.1. [DOI] [PubMed] [Google Scholar]
- 28.UNSCEAR, SOURCES AND EFFECTS OF IONIZING RADIATION. UNSCEAR 2000 Report. United Nations; New York: pp. 453–487. [Google Scholar]
- 29.UNSCEAR, SOURCES AND EFFECTS OF IONIZING RADIATION. UNSCEAR 1998 Report. United Nations; New York: [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 32.Preston DL, Lubin JH, Pierce DA, McConney ME. EPICURE. Hirosoft Corporation; Seattle, WA: 1993. [Google Scholar]
- 33.Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, Ashizawa K, Hida A, Soda M, et al. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. JAMA. 2006;295:1011–1022. doi: 10.1001/jama.295.9.1011. [DOI] [PubMed] [Google Scholar]
- 34.Koutras DA. Subclinical hyperthyroidism. Thyroid. 1999;9:311–315. doi: 10.1089/thy.1999.9.311. [DOI] [PubMed] [Google Scholar]
- 35.Shrier DK, Burman KD. Subclinical hyperthyroidism: Controversies in management. Am Fam Physician. 2002;65:431–438. [PubMed] [Google Scholar]
- 36.Kirkegaard C, Faber J. The role of thyroid hormones in depression. Eur J Endocrinol. 1998;138:1–9. doi: 10.1530/eje.0.1380001. [DOI] [PubMed] [Google Scholar]
- 37.Vila L, Serra-Prat M, Palomera E, Casamitjana R, de Castro A, Legaz G, Barrionueva C, Garcia A-J, lal-Trehan S, et al. Reference values for thyroid function tests in pregnant women living in Catalonia, Spain. Thyroid. 2010;20:221–225. doi: 10.1089/thy.2008.0264. [DOI] [PubMed] [Google Scholar]
- 38.Hollowell JG, Staehling NM, Flanders WD, Hanson WH, Gunter EG, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Guolo A, Hoffman FO, Carroll RJ. Shared uncertainty in measurement error problems, with application to Nevada Test Site fallout data. Biometrics. 2007;63:1226–1236. doi: 10.1111/j.1541-0420.2007.00810.x. [DOI] [PubMed] [Google Scholar]