Abstract
In nonhuman animals, the transitive inference (TI) task typically involves training a series of four simultaneous discriminations involving, for example, arbitrary colors in which choice of one stimulus in each pair is reinforced [+] and choice of the other color is nonreinforced [−]. This can be represented as A+B−, B+C−, C+D−, D+E− and can be conceptualized as a series of linear relationships: A>B>C>D>E. After training, animals are tested on the untrained non endpoint pair, BD. Preference for B over D is taken as evidence of TI and occurs because B is greater than D in the implied series. In the present study we trained pigeons using a novel training procedure—a hybrid of successive pair training (training one pair at a time) and mixed pair training (training all pairs at once)—designed to overcome some of the limitations of earlier procedures. Using this hybrid procedure, we trained five premise pairs (A+B−, B+C−, C+D−, D+E−, and E+F−) which allowed us to test three untrained non endpoint pairs (BD, CE, and BE). A significant TI effect was found for most subjects on at least two out of three test pairs. Different theories of TI are discussed. The results suggest that this hybrid training is an efficient procedure for establishing mixed pair acquisition and a TI effect.
Keywords: transitive inference, value transfer, simultaneous discriminations, pigeons
Piaget (1928, 1955) described a transitive inference (TI) task in which a child might be given two propositions—for example, Alice is taller than Betty, and Betty is taller than Carol— and then be asked “Who is taller, Alice or Carol?” Piaget believed that giving the correct answer would provide evidence for the ability of the child to make an inference because the child would presumably use Betty as a mediator to infer that Alice must be taller than Carol. The problem can by symbolized as A>B>C in which A is greater than B and B is greater than C. However, solution of this problem does not require one to make an inference because A is always greater (than something) but C is never greater (than anything). Such a solution can be described as an end point effect because the end points are unique and thus allow for a nontransitive solution based on the absolute value of A and C.
Bryant and Trabasso (1971) reasoned that this end-point problem could be avoided by expanding the series to four propositions or premise pairs; thus, A>B, B>C, C>D, and D>E. By using four premise pairs one can avoid the end points and still ask a subject about the relation between two untrained terms, B and D, that are presumed to have ambiguous absolute value—B is sometimes greater (in the BC pair) and sometimes not greater (in the AB pair). Similarly, D is sometimes greater (in the DE pair) and sometimes not greater (in the CD pair).
With this procedure in mind, McGonigle and Chalmers (1977) were the first to examine TI performance in nonhumans. They presented successive pairs of stimuli of different colors to squirrel monkeys in the form of simple simultaneous discriminations. In their procedure, Stimulus A and Stimulus B were presented simultaneously and the choice of Stimulus A was reinforced, whereas the choice of B was not (i.e., A+B−). Then, Stimulus B and Stimulus C were presented, and the choice of B was reinforced (B+C−). Stimulus Pairs C+D− and D+E− were presented in a similar manner. Such training can be depicted as a five term A–E series, with Stimuli A and E as endpoints, each with a unique reinforcement history (always reinforced and never reinforced, respectively) and stimuli B, C, and D with mixed reinforcement history (both reinforced and nonreinforced). When McGonigle and Chalmers presented their subjects with the BD test pair, Stimulus B was consistently selected.
Although successful performance of the TI task has been found in a variety of species (e.g., Chalmers & McGonigle, 1984, with humans; Davis, 1992, with rats; Fersen, Wynne, Delius, & Staddon, 1991, with pigeons; Gillan, 1981, with chimpanzees; Grosenick, Clement, & Fernald, 2007, with fish; McGonigle & Chalmers, 1977, with monkeys), little consensus exists as to the mechanisms governing this behavior. Trabasso and Riley (1975) suggested that when children learn this task, they form a mental representation or linear mapping of the stimuli that preserves and organizes all the essential information required for subjects to correctly choose B over D, on a BD test. However, two noncognitive accounts of TI performance on the five term task also have been proposed (Couvillon & Bitterman, 1992; Fersen et al., 1991).
One of these noncognitive accounts of TI is based on the direct history of reinforcement associated with each of the test stimuli. Although this theory has been presented in the context of various stochastic linear operator models (Couvillon & Bitterman, 1992; Delius and Siemann, 1998; Wynne, Fersen, & Staddon, 1992), it can be somewhat simplified as follows: Although the non endpoints of the A–E series have each been associated with reinforced as well as nonreinforced responding, reinforcement histories with those central stimuli are not likely to be the same. In fact, experience with (i.e., responding to) B−, presented in the context of A+ (which is never associated with nonreinforced responding) is likely to be somewhat less than experience with D−, presented in the context of C+ (which is also associated with nonreinforced responding in the context of B+). Thus, less experience with B− than with D− could account for a preference for B− in the absence of relational learning. In addition, any differences in reinforced responding between B and D would also be relevant.
Reinforcement history has been empirically tested in a variety of experiments (Lazareva & Wasserman, 2006, 2010, 2012; Lazareva et al., 2004; Steirn, Weaver, & Zentall, 1995; Weaver, Steirn, & Zentall, 1997; Wright & Howells, 2008) typically resulting in the conclusion that it does not adequately account for many of the conditions in which TI occurs. The most direct test of this theory was conducted by Lazareva and Wasserman (2012). First, they trained pigeons on a five term task and found a TI effect. Then the pigeons were given massed presentations of D+E− until D had a greater history of reinforcement than B and thus, should have been preferred over B. Contrary to the predictions of reinforcement history, B was still preferred over D. These findings suggest that some other mechanism is responsible for TI.
The second noncognitive account of TI involves the transfer of associative value from one member of a stimulus pair to the other (Fersen et al., 1991). Value transfer is actually a form of generalization that is a function of the inherent spatial–temporal relation between the two stimuli. The mechanism underlying this transfer of value is likely to involve higher order Pavlovian conditioning in which the S+ serves as a mediator by which the S− becomes associated with reinforcement (Zentall, Sherburne, Roper, & Kraemer, 1996). According to value transfer theory (VTT), the member of each stimulus pair associated with nonreinforced responding (S−) acquires secondary positive value from the positive member of the pair (S+). It has been suggested that the amount of value transferred from the S+ to the S− is proportional to the amount of value accrued to the S+ (Fersen et al., 1991). Thus, in the case of B− presented in the context of A+ (a stimulus never associated with nonreinforced responding), the value transferred should be relatively high. However, in the case of D−, presented in the context of C+ (a stimulus also associated with nonreinforced responding in the context of B+), the value transferred should be somewhat less. According to Fersen et al., this discrepancy in value transferred is responsible for preference of B over D on test trials.
Evidence for VTT has been found independently of the TI paradigm (Steirn et al., 1995; Zentall & Sherburne, 1994). However, VTT has not been supported empirically when tested in the context of TI experiments. For example, Weaver et al. (1997) equated the value transferred from A to B with the value transferred from C to D by training pigeons on four simultaneous discriminations for which choice of A, C, or E was always reinforced 50% of the time, A±B−, B+C±, C±D−, and D+E±. Surprisingly, Weaver et al. still found a significant TI effect (a preference for B over D). This finding is consistent with the results of studies that have found that adding value transfer to mathematical models of TI does not improve the model's ability to predict TI (Lazareva et al., 2004).
Although it is possible that reinforcement history and to a lesser degree value transfer play a role in TI, neither can adequately account for the many conditions under which TI occurs. Comparison of the various studies that have investigated the conditions under which TI has been found is made difficult by the variety of training procedures used (Davis, 1992; Fersen et al., 1991; Higa & Staddon, 1993; Lazareva & Wasserman, 2012; Steirn et al. 1995). For example, the most direct training procedure that has been used is the mixed pair procedure, which involves presenting all four premise pairs during each training session (see e.g., Fersen et al.). Although simultaneous training of the premise pairs ensures that the subjects will have equal experience with the discriminations, acquisition can be very slow. For example, Fersen et al. trained for 125 sessions before four of their six pigeons reached a criterion of 80% correct with four premise pairs.
Alternatively, Steirn et al. (1995) trained pigeons with the successive pair procedure in which each premise pair was trained individually in a separate training phase (also see Davis, 1992). This procedure results in very rapid acquisition. Generally it takes only three 96 trial sessions to reach a criterion of 90% or better for two consecutive sessions for each stimulus pair (Daniels, Laude, & Zentall, 2013; Steirn et al., 1995; Weaver et al., 1997) and surprisingly, this procedure results in a significant TI effect. The great advantage of this procedure is the small number of training sessions (only about 12) needed before testing. However, as noted by Daniels et al. a potential problem with this procedure is the differential time between training and testing for the different premise pairs. For example, for the DE pair testing follows training immediately whereas for the AB pair about 9 days intervenes between training and testing. One might expect that this procedure would result in a preference for D over B because of very recent training with the D+E− pair but a transitive inference effect was still found. Furthermore, Steirn et al. found a similar TI effect when the D+E− pair was trained first and the A+B− pair was trained last.
Lazareva and Wasserman (2006) used a procedure, which we refer to as the stepped mixed pair procedure, in which the premise pairs were introduced successively but with each addition of a premise pair, they continued training with the previous pairs. Although this procedure required an average of only about 56 sessions of training, it resulted in far more training on the earlier stimulus pairs than on the later pairs which would have likely introduced a bias to choose Stimulus B over Stimulus D when they were presented as the test pair. For example, Lazareva and Wasserman (2012) found that on average there were 2.56 times as many reinforced responses to B than to D using this procedure. Although they also had some pigeons acquire the discriminations starting with D+E−, this reverse order is not the mirror image of the A+B− because, in the D+E− first condition, when each new pair is introduced, a former S+ is presented with a novel stimulus, whereas for the A+B− first condition, when each new pair is introduced, a former S− is presented with a novel stimulus. In fact, there is evidence that the second pair is acquired faster in the A+B− condition than in the D+E− condition (Steirn, et al., 1995). Furthermore, Lazareva and Wasserman found that although for both groups, whichever stimulus, B or D, that was trained as an S+ first, received more reinforced responses than the stimulus that was trained as an S+ second, the B/D reinforcement ratio for the A+B− group (2.56) was considerably higher than the D/B reinforcement ratio for the D+E− group (1.66). Thus, when this procedure is used, adding stimulus pairs to already discriminated pairs involves an inherent bias that may be difficult to overcome.
Weaknesses identified in the training procedures described suggest that none of them is ideal for testing mechanisms of TI. Furthermore, it would be beneficial to use a procedure that would allow for testing with multiple untrained, non endpoint pairs. Thus, the main purpose of the present experiment was to use a novel training procedure (a hybrid of the procedures used by Fersen et al., 1991 and Steirn et al., 1995) to train pigeons on a six term task that would allow for a TI test with three novel non endpoint (interior) pairs (BD, BE, and CE). To our knowledge there has been only one study that has trained pigeons with a task involving more than five terms (Fersen et al., 1991) and in that experiment following 125 sessions of training with the original four pairs they trained the pigeons on two added pairs (X+A− and E+F−) for 190 sessions of mixed pair training prior to testing. Thus, their pigeons received a total of 315 sessions of training.
As our pigeons were first trained on a successive pair procedure and then transferred to a mixed pair procedure, the second purpose of the present experiment was to evaluate whether the learning acquired under the successive pair procedure (e.g. Weaver et al., 1997) would result in animals transfer to the mixed pair procedure used by Fersen et al., (1991). Given that both training procedures have been shown to result in a TI effect, there should be positive transfer from successive pair training to mixed pair training. That is, animals trained on the successive pair procedure first should perform above chance levels on training pairs during the first session of the mixed pair procedure.
The third purpose of the experiment was to ask if the degree of TI accuracy depends on the level of accurate performance on the premise pairs prior to testing. To this end, we trained with the premise pairs for a total of only 70 sessions before testing the pigeons with the three untrained test pairs (BD, BE, and CE) that did not involve endpoints (A or F). Finally, we asked if the proportion of reinforced and nonreinforced responses to each stimulus in the test pairs could account for the results of the test.
Method
Subjects
Subjects were four unsexed white Carneau pigeons (Columba livia) (numbers 10742, 19306, 19276, and 19831) purchased from the Palmetto Pigeon Plant (Sumter, SC). The pigeons ranged from 3 to 5 years of age. All pigeons had prior experience with learning successive color discriminations; however they were naïve to simultaneous discriminations as well as to the TI procedure. All subjects were maintained at 85% of their free feeding weight and given ad lib access to grit and water. When not in an experimental session they were housed individually in wire cages in a colony room on a 12h/12h light/dark cycle. All pigeons were cared for in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
The experiment was conducted in a BRS/LVE sound attenuated standard operant test chamber (Laurel, MD) that measured 34 cm high, 30 cm from the response panel to the back wall, and 35 cm across the response panel. On the response panel were three circular response keys (2.5 cm diameter) that were horizontally aligned and separated from each other by 6.0 cm. Only two of the circular response keys (left and right) were active during the experiment. The bottom edge of the response keys was 24 cm from the wire mesh floor of the operant chamber. Mounted behind each circular response key was a 12 stimulus in line projector (Industrial Electronics Engineering, Van Nuys, CA) with a 28 V, 0.1 A lamps (GE 1820) that could project red (R), purple (P), yellow (Y), white (W), blue (B), and green (G) hues. For reinforcement, mixed grains (Purina Pro Grains) were provided for 1.5 s from a raised and illuminated hopper located behind an aperture (5.1 × 5.7 cm) horizontally centered and vertically located at the midpoint between the response keys and the floor of the chamber. A microcomputer located in the adjacent room controlled the daily sessions and recorded data.
Pretraining
In order to reduce possible side biases all pigeons were given 5 to 10 pretraining sessions in which they were required to peck once for 1.5 s of mixed grain to each of the six hues presented on either the left or right pecking key. Each trial was separated by a 10 s intertrial interval (ITI). There were four presentations of each colored stimulus (two on each key) and thus 24 trials per session.
Successive-Pair Training
Training began with single pair training. There were five phases of single pair training, one pair per phase (i.e., A+B−, B+C−, C+D−, D+E−, E+F−). For pigeons 10742 and 19306, stimuli A, B, C, D, E, and F were represented by R, P, Y, W, B, and G and for pigeons 19831 and 19276 the stimuli were represented by G, B, W, Y, P, and R. Pairs were trained sequentially from A+B− to E+F−. In each phase of training, one peck to either stimulus presented in the pair being trained constituted a choice. If the choice was incorrect then it resulted in termination of the pair and the onset of the 10 s ITI. If the choice was correct then it resulted in 1.5 s of reinforcement and the start of the 10 s ITI. Each session was composed of 96 trials in 24 four trial blocks. In each block, the location of the stimuli in the pair was counterbalanced between the left and right pecking keys. Each phase of training continued for a minimum of three sessions and until a correct choice was made on 90% of the trials (86 out of 96 trials) for two consecutive sessions. Sessions occurred once a day, 6 days a week.
Mixed-Pair Training
After completing all five phases of successive pair training, pigeons were transferred to mixed pair training. During mixed pair training, all of the training pairs were presented in each session. One peck to either stimulus presented constituted a choice. Again, if the choice was incorrect then it resulted in termination of the pair and the onset of the 10 s ITI. If the choice was correct then it resulted in 1.5 s of reinforcement and the 10 s ITI. Each session was composed of 100 trials in 10 ten trial blocks, in which each pair was randomly presented twice and the location of the stimuli was counterbalanced between the left and right pecking keys. Mixed pair training continued until a correct choice was made on 70% of the trials involving each pair (14 out of 20 trials per pair) or 84% correct overall. To encourage the development of some variation in accurate discrimination of the premise pairs, we trained the pigeons for a maximum of 70 sessions including both successive pair training and mixed pair training. This was done to determine the extent that TI depends on discrimination accuracy prior to testing.
Testing
After completion of mixed pair training, the pigeons were given two sessions with test pairs BD, BE, and CE. Each test session consisted of 96 trials (32 trials per pair) in 16 six trial blocks in which each pair was randomly presented twice and counterbalanced between the left and right pecking keys. One peck to either stimulus presented constituted a choice. To promote continued responding but without differential reinforcement, choice of either stimulus resulted 1.5 s of reinforcement on a random half of the trials. Test trials were separated by a 10 s ITI.
Results
Successive-Pair Training
Criterion for each phase of successive pair training was 90% correct choice (86 out of 96 trials) for two consecutive sessions and a minimum of three sessions . Thus, the minimum number of sessions needed to complete successive pair training was 15 sessions, which is the time it took 19306 to reach criterion. Pigeons 10742 and 19831 acquired the discriminations almost as quickly (16 and 17 sessions, respectively). Only one pigeon, 19276, took appreciably longer (32 sessions) to reach criterion.
Transfer to Mixed-Pair Training
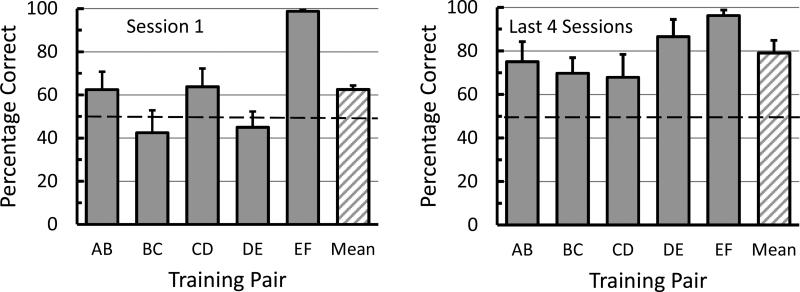
Considering the level of discrimination accuracy during successive pair training, overall transfer of learning the simultaneous discriminations from successive pair training to mixed pair training was not very good (M = 62.5%; SE = 1.89). However, when pooled over the premise pairs this level of transfer was statistically significant (M = 62.5%, SE = 1.89), t(3) = 6.60, p = .007. A graph of the data from the first session of mixed pair training for individual premise pairs appears in Figure 1 (left). A single sample t test was computed for the data plotted in Figure 1; it revealed that the transfer to mixed pair training was significantly above chance for the endpoint pair EF (the pair that they were trained on last, M = 98.8%, SE = 1.2), t(3) = 6.60, p = .007. The AB pair (the first trained pair, M = 62.5%, SE = 8.3) and the CD pair (M = 63.8%, SE = 8.5), were also above chance; however, not significantly, t(3) = 1.51, p = .23 and t(3) = 1.62, p = .20, respectively.
Fig. 1.
Mean percentage correct on the first session (left) and the last four sessions (right) of mixed pair training for each training pair and the mean pooled over all training pairs. Error bars = one standard error of the mean.
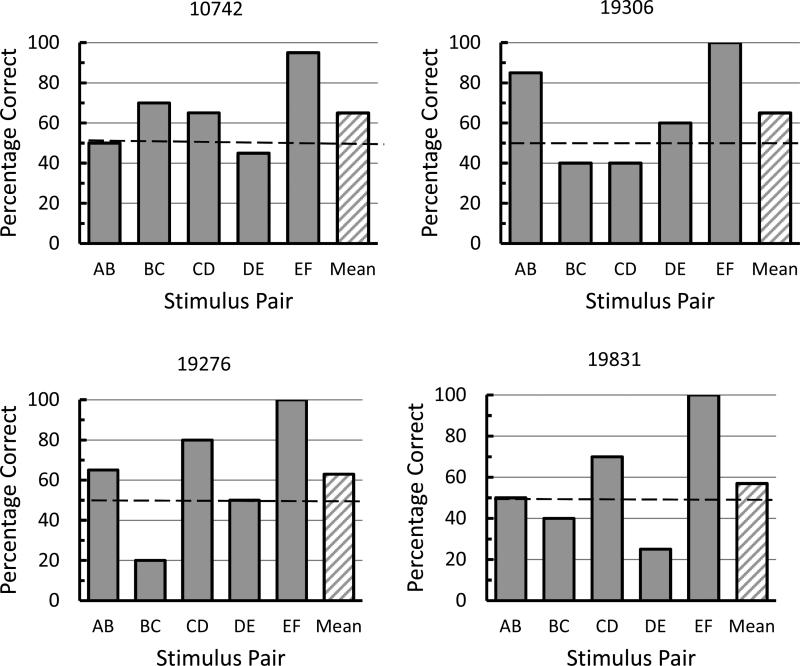
First session data from individual pigeons appears in Figure 2. Inspection of Figure 2 reveals why pairs AB and CD, when averaged over subjects, were not significantly above chance. Pigeons 19306 and 19276 were correct on pair AB more than 50% of the time (85% and 65%, respectively) whereas pigeons 10742 and 19831 were correct on AB at or below chance levels (50% and 45%, respectively). Finally, it is clear that all pigeons transferred well above chance levels for pair EF, with accuracy on pair EF being greater than 90% for all subjects.
Fig. 2.
Mean percentage correct for each pigeon on the first session of mixed pair training for each training pair and the mean pooled over all training pairs.
Acquisition of Mixed-Pair Training
The criterion for mixed pair training was either making a correct choice on 70% of the trials involving each pair (14 out of 20 trials per pair; i.e., 84% correct overall for two consecutive sessions) or completing a total of 70 sessions (successive pair and mixed pair training combined), whichever came first. Pigeons 10742, 19306, and 19831 reached the 80% correct criterion on mixed pair training in 22, 32, and 44 sessions, respectively. Pigeon 19276 failed to reach the 80% correct criterion and training was terminated after 70 total sessions. Pigeon 19276 spent 38 sessions on mixed pair training.
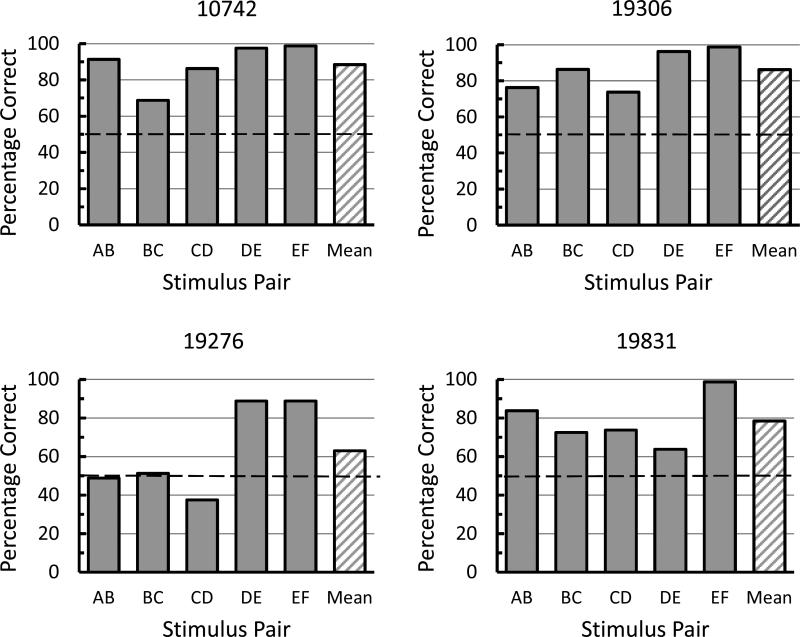
Over the course of mixed pair training the overall accuracy on the premise pairs increased to 79.1% by the last four sessions of training, however there was a considerable range of discrimination accuracy (88.5% to 63.0%) among the pigeons. A graph of the mean data from the last four sessions of mixed pair training appears in Figure 1 (right). Inspection of Figure 1 shows that accuracy on the training pairs was above chance on each of the training pairs.
The data from the last four sessions of mixed pair training from individual subjects appears in Figure 3. Visual inspection reveals that there were large individual differences among the subjects in the accuracy on the training pairs but all of the pigeons were performing above chance on all of the training pairs by the end of training with the exception of Pigeon 19276 on training pairs AB, BC, and CD.
Fig, 3.
Percentage correct for each pigeon over the last four sessions of mixed pair training on each training pair and the mean pooled over all training pairs.
Testing: Pairs BD, BE, and CE
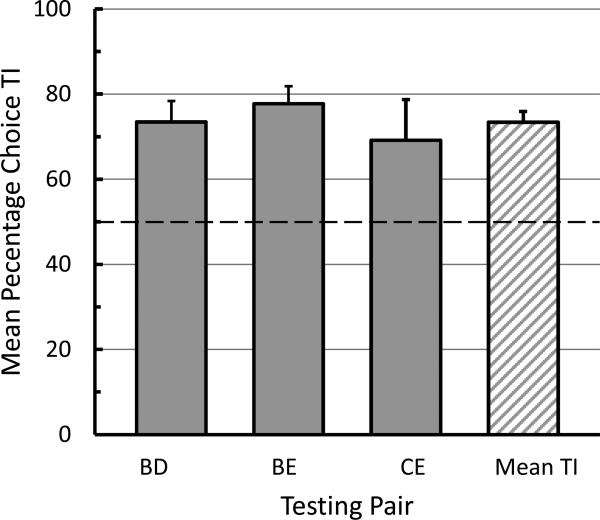
All four pigeons showed evidence of TI performance. Mean percentage choice of B over D was 73.4% (SE = 6.1), choice of B over E was 77.7% (SE = 6.3), and choice of C over E was 69.2% (SE = 9.5). A graph of the data pooled over the two test sessions for each of the test pairs appears in Figure 4. When all choices were pooled over the three different test pairs, a single sample t test revealed that the mean percentage of transitive choices, 73.4% (SE = 3.5) was significantly different than chance, t(3) = 6.4, p = .007. Single sample t tests also revealed that choice of B over D and choice of B over E differed significantly from chance, t(3) = 3.77, p = .003 and t(3) = 4.33, p = .002, respectively; whereas, choice of C over E did not, t(3) = 1.96, p = .14.
Fig. 4.
Mean TI effect for each testing pair (BD, BE, and CE) and the mean of the TI effect pooled over all three testing pairs (error bars = one standard error of the mean).
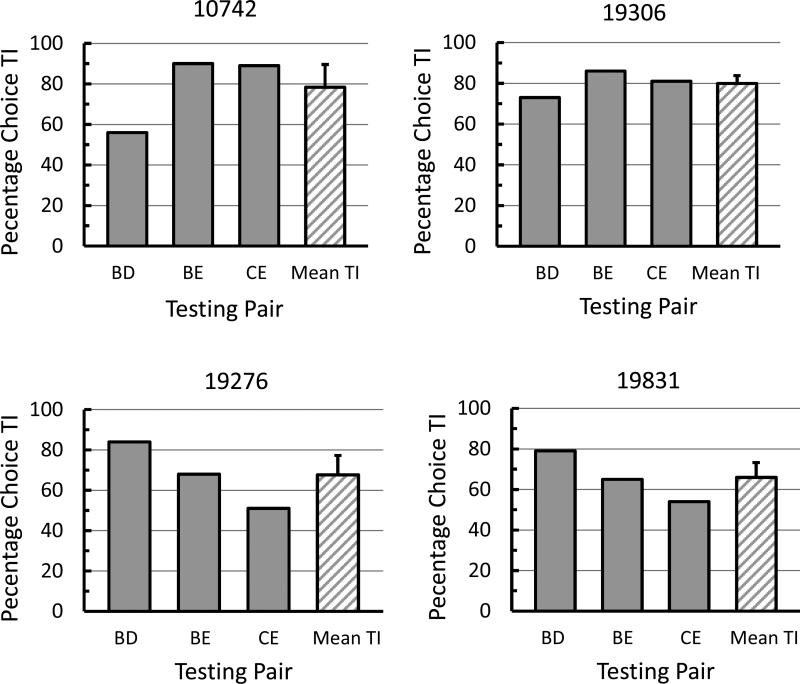
A graph of each of the pigeons’ choices on the individual test pairs appears in Figure 5. As can be seen in the figure, TI performance was poorest with the BD test pair for Pigeons 10742 and 19306 (56% and 73%, respectively) whereas it was poorest with the CE test pair for Pigeons 19276 and 19831 (51% and 54%, respectively). A binomial test, p ≤ .0001, indicated that pigeon 19306 showed significant TI on all three test pairs whereas a binomial test, p ≤ .008, indicated that Pigeons 10742, 19831, and 19276 showed significant TI on only two out of the three test pairs; pairs BE and CE for Pigeon 10742 and pairs BD and BE for Pigeons 19831 and 19276.
Fig. 5.
TI effect for individual pigeons on each testing pair (BD, BE, and CE) and the mean of the TI effect pooled over all three testing pairs (error bars = one standard error of the mean).
Correlation Between Mixed-Pair Accuracy and TI Performance
Although the sample size was very small, there was a strong correlation between the overall accuracy on the training pairs at the end of mixed pair training and the overall TI effect (r = .77) (see Table 1). Furthermore, if one considers only the “interior” three pairs of stimuli (BC, CD, and DE), stimuli that served as both S+ and S− during training and were represented in the test stimuli, the correlation was even higher (r = .90) Thus, TI test performance on novel test pairs can be predicted from training accuracy on the premise pairs prior to testing.
Table 1.
Percentage correct on the last four sessions of mixed-pair training compared with the percentage TI effect in testing for each individual pigeon.
| Subject | TI Effect (% correct) | End of Mixed-Pair Training (% correct) |
|---|---|---|
| 10742 | 78.3 | 88.5 |
| 19306 | 80.0 | 86.2 |
| 19276 | 67.7 | 63.0 |
| 19831 | 66.0 | 78.5 |
Analysis of Reinforcement and Nonreinforcement
Given that training accuracy on premise pairs can predict TI performance, it may be that reinforcement history can account for our results. According to a general theory of reinforcement history, preference for one stimulus over another should be directly related to the number of reinforced or nonreinforced responses (Steirn et al., 1995; Weaver et al., 1997). For example, in novel test pair BD, preference for B over D should be the result of more reinforced choices of B, or conversely, more nonreinforced choices of D. In order to test the hypothesis that reinforcement history could account for our results, we calculated proportions of relative reinforcement and nonreinforcement for the stimuli involved in all three novel test pairs: BD, BE, and CE.
Single sample t tests revealed that none of the proportions of relative reinforcement (M = .47, SE = 0.02) nor nonreinforcement (M = .53, SE = 0.02) were significantly different from chance (0.50), all ts < 1.5, all ps > 0.1. Additionally, there was not a significant correlation between any of the proportions of relative reinforcement and nonreinforcement and TI performance, all ts < 1.5, all ps > 0.2. This suggests that reinforcement history is not likely to account for our data (see Table 2 for each bird's proportion of relative reinforcement and nonreinforcement for each test pair).
Table 2.
Proportions of relative reinforcement and nonreinforcement in training for the stimuli in each novel test pair for each individual pigeon.
| Subject | |||||||
|---|---|---|---|---|---|---|---|
| Test Pair | Ratio | 10742 | 19306 | 19831 | 19276 | Mean | |
| Reinforced | BD | B/(B+D) | .47 | .47 | .54 | .57 | .51 |
| BE | B/(B+E) | .41 | .43 | .43 | .52 | .45 | |
| CE | C/(C+E) | .41 | .41 | .46 | .56 | .46 | |
| Nonreinforced | BD | D/(D+B) | .58 | .65 | .48 | .55 | .56 |
| BE | E/(E+B) | .49 | .54 | .68 | .53 | .56 | |
| CE | E/(E+C) | .42 | .43 | .58 | .48 | .48 | |
Inspection of Table 2 reveals a few notable exceptions to the statistical results. For example, Pigeon 19306 for pair BD had proportions of relative reinforcement and nonreinforcement of .47 and .65, respectively. Although .47 is below chance, .65 is above chance and suggests that choice of B over D could have occurred because D received more nonreinforced responses than B. Additionally, Pigeon 19831 had proportions of relative reinforcement and nonreinforcement of .43 and .68, respectively for pair BE. Again, it is possible that choice of B over E occurred because E received more nonreinforced responses than B. The most notable exception was for Pigeon 19276, which for five out of six proportions had values greater than chance. These proportions suggest that responding to any of the test pairs may have resulted from the fact that the TI consistent response was reinforced more than the alternative response and that the alternative response (except in pair CE) received more nonreinforced responses than the TI consistent response. However, even with these exceptions, for most of the comparisons reinforcement history does not appear to account for the pigeons’ choice behavior.
Discussion
The main purpose of this experiment was to train pigeons with a novel training procedure on a six term TI task such that they could be tested on three untrained interior stimulus pairs (BD, BE, CE). The data clearly show that the novel procedure of successive pair training and then transferring to mixed pair training before testing has three advantages over alternative procedures. First, although the mean number of training sessions provided in the present experiment (M = 54.0) was more training than is typically provided by the successive pair procedure (e.g. Weaver et al., 1997), it is comparable to the mean number of sessions needed in the procedure used by Lazareva and Wasserman (2012) and substantially less than that required with the mixed pair procedure used by Fersen et al. (1991). Furthermore, because in the present procedure the pigeons received mixed pair training before testing, the present procedure avoids differential delays between training each pair and testing (e.g. Weaver et al.). It also avoids potential reinforcement biases that may emerge when stimulus pairs are added to already trained pairs (e.g. Lazareva & Wasserman). Finally, by training with five stimulus pairs rather than four, the present procedure allows for a more robust test of TI by allowing for three novel non endpoint test pairs (BD, BE, and CE). In fact, the TI effect for each pigeon was above 50% for each test pair and for each of the four pigeons a significant TI effect was found for at least two of the three test pairs. Thus, good evidence for a TI effect was found in the present experiment using a novel training procedure with more than one test pair.
Another purpose of the present experiment was to determine if first being trained on the successive pair procedure would transfer learning of the simultaneous discriminations to the mixed pair procedure. Although positive transfer was found (62.5% overall), transfer was not uniformly positive. This result suggests that the two training procedures do not result in similar learning. This finding is somewhat curious because both procedures have been shown to give rise to a significant TI effect, at least with four premise pairs in training. It may be, however, that the intervening sessions between training of a pair and the first session of mixed pair training contributed to the lack of transfer for earlier trained pairs. This would explain why significant transfer was found only for the most recently trained pair. Although if the time between successive training of the pair and mixed pair training was responsible for the poor transfer, then one would expect a linear increase in level of transfer, with pair AB transferring most poorly and EF transferring best. This was not the case for overall transfer or for individual pigeons. Thus, it does not appear that the duration of the delay between a training pair and the completion of single pair training can account for the poor transfer effects. Nonetheless, it demonstrates that one of the weaknesses of successive pair training is the delay that intervenes between single pair training and transfer to mixed pair training.
On the other hand, if a reasonable level of mixed pair accuracy is considered important to avoid biases, prior training with the individual premises appears to reduce the number of mixed pair training sessions required. We gave a total of only 70 sessions of training (successive pair and mixed pair training combined) with five premise pairs, whereas Fersen et al. (1991) gave 125 sessions of training with only four premise pairs and 190 further sessions of training with an additional two premise pairs. Thus, the present procedure allows for efficient training with unbiased tests of reinforcement history, while providing multiple tests of TI. Additionally, it could be useful for examining the acquisition of TI over training by introducing nonreinforced probe trials containing novel test pairs at various points in training. Moreover, it would be valuable to know to what degree different procedures result in similar learning; it may be that different procedures lead to different learning and different mechanisms of choice behavior during testing.
Another important finding in the present experiment was that pigeons that were more accurate at the end of mixed pair training also showed a stronger TI effect. Interestingly, even the pigeon that did not meet the mixed pair training criterion showed a 67.6% TI effect and for that pigeon the TI effect was statistically significant for two of the three test pairs. For three of the pigeons, the S+ from training with the poorest accuracy was also the test stimulus that showed the poorest TI effect. Thus, it appears that the TI effect can be predicted from discrimination accuracy on the premise pairs following mixed pair training.
Consistent with this finding, it has been suggested that TI performance may result from differential reinforcement and nonreinforcement of the original stimuli in training (Wynne et al., 1992). Several studies have shown that during training, the proportions of relative reinforcement and nonreinforcement to the stimuli involved in the test pairs are not adequate to account for the TI effect (e.g. Lazareva & Wasserman, 2006; Steirn et al., 1995; Weaver et al., 1997) and a similar analysis conducted in the present experiment produced similar results; for most pigeons the proportions of relative reinforcement and nonreinforcement were close to chance (0.5) and were uncorrelated with TI performance. However, there were several exceptions to this rule that statistical analysis may have masked, in which behavior may have been under control of reinforcement history. For example, the pigeon that did not complete mixed pair training with above chance accuracy on all training pairs—19276—had the greatest proportions of relative reinforcement and nonreinforcement above chance. This was also the case for two other pigeons on at least one pair (19306 on the BD pair and 19831 on the BE pair). It might be that individual pigeons use different strategies on individual pairs; however, the overall finding suggests that reinforcement history cannot easily account for the TI behavior demonstrated by most of the pigeons on most of the pairs, especially those pigeons that showed the largest TI effect. Furthermore, the fact that pigeons that were more accurate at the end of mixed pair training also showed a stronger TI effect would seem to be a logical prediction of any account of the transitive inference effect.
Value transfer theory has also been proposed to account for the TI effect (Fersen et al., 1991). This theory posits that Stimulus A (which never serves as an S−) has more value to transfer to stimulus B than stimulus C (which serves as both an S− in the BC pair and as an S+ in the CD pair) has to transfer to D. If this were true, in the present experiment, there should have been very little TI found with the CE test pair because value transfer from B to C in the BC training pair should have been about the same as value transfer from D to E in the DE training pair. Only two of the pigeons showed little or no preference for C over E (19831 and 19276); the two others (10742 and 19306) showed a strong preference for C over E. Furthermore, pigeon 10742 showed a very weak preference for B over D, which according to VTT should have been strong. Thus, although value transfer may have contributed to the TI effect for two of the pigeons it cannot account for all of the results, nor can it account for the results of previously published TI results (Weaver et al. 1997).
At present, the basis for the TI effect is not clear. Some have argued that TI has evolved as a way of guiding foraging behavior (Weiss, Kehmeier, & Schloegl, 2010) or as a means of establishing the dominance ranks of members of a group of animals without having to interact with each member of the group (Bond, Kamil, & Balda, 2003; Bond, Wei, & Kamil, 2010; Grosenick, Clement, & Fernald, 2007; MacLean, Merrit, & Brannon, 2008; Paz-y-Miňo, Bond, Kamil, & Balda; 2004). For example, if a target animal has found that it is subordinate to animal B and it observes that animal B is subordinate to animal A, then if the target animal can make a transitive inference, it would not have to interact with animal A to know that it is subordinate to animal A, thus avoiding a confrontation and wasted time or possible injury. The fact that pigeons (not known for their strong sociality), show TI performance with arbitrary stimuli (colors) in a nonsocial context, and under a variety of conditions, suggests that social hierarchies are not likely to be the basis of TI performance.
Several researchers have proposed a more cognitive account of the transitive inference effect (Bryant & Trabasso, 1971; Gillan, 1981; Roberts & Phelps, 1994). During training, animals may learn a representation of the underlying linear series (A>B>C>D>E>F). Ostensibly, animals first locate the end points (A and E) and then over the course of training progressively fill in the middle of the series with the other stimuli according to their relative value (Daniels et al., 2013; Roberts and Phelps, 1994;Vasoncelos 2008). During testing, animals then use this linear representation to determine the correct solution on test pairs.
A prediction of the linear representation account is that the further apart on the linear representation two test stimuli are, the larger should be the transitive inference effect (i.e., there. should be a symbolic distance effect). That is, choice of B over E should be greater (because there are presumed to be two stimuli, C and D, between them) than choice of B over D or choice of C over E (because for those pairs there are presumed to be only one stimulus between them). Although for one of the pigeons (19306) choice of B over E is clearly greater than choice of B over D and choice of C over E. For pigeon 10742 choice of B over E was similar to choice of C over E and for the other two pigeons (19276 and 19831) the transitive inference effect was stronger for the BD pair than for the BE pair.
Thus, currently there is no theory that can adequately account for the present results or the transitive inference effect as widely distributed among different species as it is and under the diverse training conditions in which it has been found. However, given the benefits of training with the hybrid procedure used in the present experiment, it should be easier to investigate the potential mechanisms and dynamics of TI. Future TI research should focus on how TI is acquired, identifying the conditions under which TI occurs, and the mechanisms responsible for its occurrence.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 63726 and by National Institute of Child Health and Development Grant 60996.
References
- Bryant PE, Trabasso T. Transitive inferences and memory in young children. Nature. 1971;232:456–458. doi: 10.1038/232456a0. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal Behaviour. 2003;65:479–487. [Google Scholar]
- Bond AB, Wei CA, Kamil AC. Cognitive representation in transitive inference: a comparison of four corvid species. Behavioural Processes. 2010;85:283–292. doi: 10.1016/j.beproc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers M, McGonigle B. Are children any more logical than monkeys on the five-term series problem? Journal of Experimental Child Psychology. 1984;37:355–377. doi: 10.1016/0022-0965(84)90075-4. [DOI] [PubMed] [Google Scholar]
- Couvillon PA, Bitterman ME. A conventional conditioning analysis of “transitive inference” in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:308–310. [Google Scholar]
- Daniels CD, Laude JR, Zentall TR. Transitive inference by pigeons: Representation of the stimulus order or reinforcement history? 2013 Manuscript submitted for publication. [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus). Journal of Comparative Psychology. 1992;106:342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- Delius JD, Siemann M. Transitive responding in animals and humans: Exaptation rather than adaptation? Behavioural Processes. 1998;42:107–137. doi: 10.1016/s0376-6357(97)00072-7. [DOI] [PubMed] [Google Scholar]
- von Fersen L, Wynne CDL, Delius JD, Staddon JER. Transitive inference formation in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:334–341. [Google Scholar]
- Gillan DJ. Reasoning in the chimpanzee: II. Transitive inference. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:150–164. [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Higa JJ, Staddon JER. “Transitive inference” in multiple conditional discriminations. Journal of the experimental analysis of behavior. 1993;59(2):265–291. doi: 10.1901/jeab.1993.59-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareva OF, Smirnova AA, Bagozkaja MS, Zorina ZA, Rayevsky VV, Wasserman EA. Transitive responding in hooded crows requires linearly ordered stimuli. Journal of the Experimental Analysis of Behavior. 2004;82:1–19. doi: 10.1901/jeab.2004.82-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA. Effects of stimulus orderability and reinforcement history on transitive responding in pigeons. Behavioural Processes. 2006;76:161–172. doi: 10.1016/j.beproc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA. Nonverbal transitive inference: Effects of task and awareness on human performance. Behavioural Processes. 2010;83:99–112. doi: 10.1016/j.beproc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA. Transitive inference in pigeons: measuring the associative values of Stimuli B and D. Behavioural Processes. 2012;89:244–255. doi: 10.1016/j.beproc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Merritt DJ, Brannon EM. Social complexity predicts transitive reasoning in prosimian primates. Animal Behaviour. 2008;76(2):479–486. doi: 10.1016/j.anbehav.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267:694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Paz-y-Miňo C, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Piaget J. Judgment and reasoning in the child. Kegan Paul, Trench, & Trubner; London: 1928. [Google Scholar]
- Piaget J. The child's construction of reality. Routledge & Kegan Paul; London: 1955. [Google Scholar]
- Roberts WA, Phelps MT. Transitive inference in rats: a test of the spatial coding hypothesis. Psychological Science. 1994;5(6):368–374. [Google Scholar]
- Scarf D, Colombo M. Representation of serial order: A comparative analysis of humans, monkeys, and pigeons. Brain Research Bulletin. 2008;76:307–312. doi: 10.1016/j.brainresbull.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Scarf D, Colombo M. Knowledge of the ordinal position of list items in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:483. doi: 10.1037/a0023695. [DOI] [PubMed] [Google Scholar]
- Steirn JN, Weaver JE, Zentall TR. Transitive inference in pigeons: Simplified procedures and a test of value transfer theory. Animal Learning & Behavior. 1995;23:76–82. [Google Scholar]
- Terrace HS. A nonverbal organism's knowledge of ordinal position in a serial learning task. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12(3):203. [Google Scholar]
- Trabasso T, Riley CA. The construction and use of representations involving linear order. In: Solso RL, editor. Information processing and cognition: The Loyola Symposium. Erlbaum; Hillsdale, NJ: 1975. pp. 381–410. [Google Scholar]
- Vasconcelos M. Transitive inference in non-human animals: An empirical and theoretical analysis. Behavioural Processes. 2008;78(3):313–334. doi: 10.1016/j.beproc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Weaver JE, Steirn JN, Zentall TR. Transitive inference in pigeons: Control for differential value transfer. Psychonomic Bulletin & Review. 1997;4:113–117. [Google Scholar]
- Weiss BM, Kehmeier S, Schloegl C. Transitive inference in free-living graylag geese, Anser anser. Animal Behaviour. 2010;79:1277–1283. [Google Scholar]
- Wright BC, Howells D. Getting one step closer to deduction: Introducing an alternative paradigm for transitive inference. Thinking & Reasoning. 2008;14(3):244–280. [Google Scholar]
- Wynne CDL, von Fersen L, Staddon JER. Pigeons’ inferences are transitive and the outcome of elementary conditioning principles: A response. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:313–315. [Google Scholar]
- Zentall TR, Sherburne LM. Transfer of value from S+ to S- in a simultaneous discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:176–183. doi: 10.1037//0097-7403.20.2.176. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Sherburne LM, Roper KL, Kraemer PJ. Value transfer in a simultaneous discrimination appears to result from within-event Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:68–75. doi: 10.1037//0097-7403.22.1.68. [DOI] [PubMed] [Google Scholar]