Abstract
Purpose
Although guidelines recommend in-person counseling before BRCA1/BRCA2 gene testing, genetic counseling is increasingly offered by telephone. As genomic testing becomes more common, evaluating alternative delivery approaches becomes increasingly salient. We tested whether telephone delivery of BRCA1/2 genetic counseling was noninferior to in-person delivery.
Patients and Methods
Participants (women age 21 to 85 years who did not have newly diagnosed or metastatic cancer and lived within a study site catchment area) were randomly assigned to usual care (UC; n = 334) or telephone counseling (TC; n = 335). UC participants received in-person pre- and post-test counseling; TC participants completed all counseling by telephone. Primary outcomes were knowledge, satisfaction, decision conflict, distress, and quality of life; secondary outcomes were equivalence of BRCA1/2 test uptake and costs of delivering TC versus UC.
Results
TC was noninferior to UC on all primary outcomes. At 2 weeks after pretest counseling, knowledge (d = 0.03; lower bound of 97.5% CI, −0.61), perceived stress (d = −0.12; upper bound of 97.5% CI, 0.21), and satisfaction (d = −0.16; lower bound of 97.5% CI, −0.70) had group differences and confidence intervals that did not cross their 1-point noninferiority limits. Decision conflict (d = 1.1; upper bound of 97.5% CI, 3.3) and cancer distress (d = −1.6; upper bound of 97.5% CI, 0.27) did not cross their 4-point noninferiority limit. Results were comparable at 3 months. TC was not equivalent to UC on BRCA1/2 test uptake (UC, 90.1%; TC, 84.2%). TC yielded cost savings of $114 per patient.
Conclusion
Genetic counseling can be effectively and efficiently delivered via telephone to increase access and decrease costs.
INTRODUCTION
Genetic testing has become increasingly central to oncology practice,1–3 and access to genetic counseling is now required by many national organizations for accreditation.4,5 And demand is likely to increase, given recently updated guidelines by the United States Preventive Services Task Force recommending genetic counseling referral for all women whose family history places them at increased risk for a BRCA1 or a BRCA2 mutation.6 Although in-person genetic counseling delivered by trained genetics professionals is standard of care,3,7,8 there are not enough genetics professionals to meet this growing demand, particularly outside metropolitan areas.2,9 In addition, many physicians lack the time and expertise to provide genetic counseling.10 Without increased access, many candidates might not be offered testing, and of those who are tested, fewer might receive comprehensive counseling. Thus, developing and evaluating alternative models of genetic services delivery is critical.11–13
Telephone-based genetic counseling has the potential to extend our ability to provide comprehensive genetic counseling, increase access, and decrease costs.14 However, telephone-based genetic counseling remains controversial because of concerns about patient comprehension and the ability to provide appropriate patient support.2,8,15,16 Despite these concerns, most cancer genetic counselors have provided BRCA1/2 test results by telephone, and 23% have provided pretest telephone counseling.1 At least one for-profit company offers pre- and post-test telephone genetic counseling for multiple conditions,17 and direct-to-consumer genomic testing companies routinely deliver counseling via the telephone or Internet.18,19
Even with increasing clinical use, few studies have evaluated whether telephone-based genetic counseling is as effective as standard counseling.20 Surveys of patients who received BRCA1/2 result disclosures by telephone suggest comparable satisfaction, knowledge, and worry as with in-person disclosure.9,11,15,20 A randomized trial comparing telephone to in-person BRCA1/2 result disclosure found no differences in anxiety, well-being, or satisfaction.21 There have been no randomized trials testing the noninferiority of comprehensive pre- and post-test BRCA1/2 telephone counseling versus standard in-person counseling.
Given the need to identify alternative delivery models and the widespread clinical use of telephone genetic counseling, we conducted the first (to the best of our knowledge) randomized noninferiority trial comparing pre- and post-test telephone BRCA1/2 genetic counseling to standard in-person genetic counseling. We hypothesized that psychosocial outcomes of telephone counseling would be noninferior to standard counseling, that uptake of testing would be equivalent, and that telephone counseling would yield cost savings compared with usual care.
PATIENTS AND METHODS
Participants
From 2005 to 2012, we recruited participants for a parallel group randomized noninferiority trial comparing standard in-person genetic counseling or usual care (UC) to telephone genetic counseling (TC). Participants were recruited from the clinical genetic counseling programs at the Lombardi Comprehensive Cancer Center (Washington, DC), Mount Sinai School of Medicine (New York, NY), University of Vermont Cancer Center (Burlington, VT), and Dana-Farber Cancer Institute (Boston, MA). Eligible participants were women age 21 to 85 years, with a minimum 10% risk for a BRCA1/2 mutation22 who lived within the catchment area of a study site. We excluded participants who had newly diagnosed (< 4 weeks) or metastatic cancer, lacked the cognitive capacity to provide informed consent, or who were candidates for genetic counseling for another hereditary cancer syndrome.
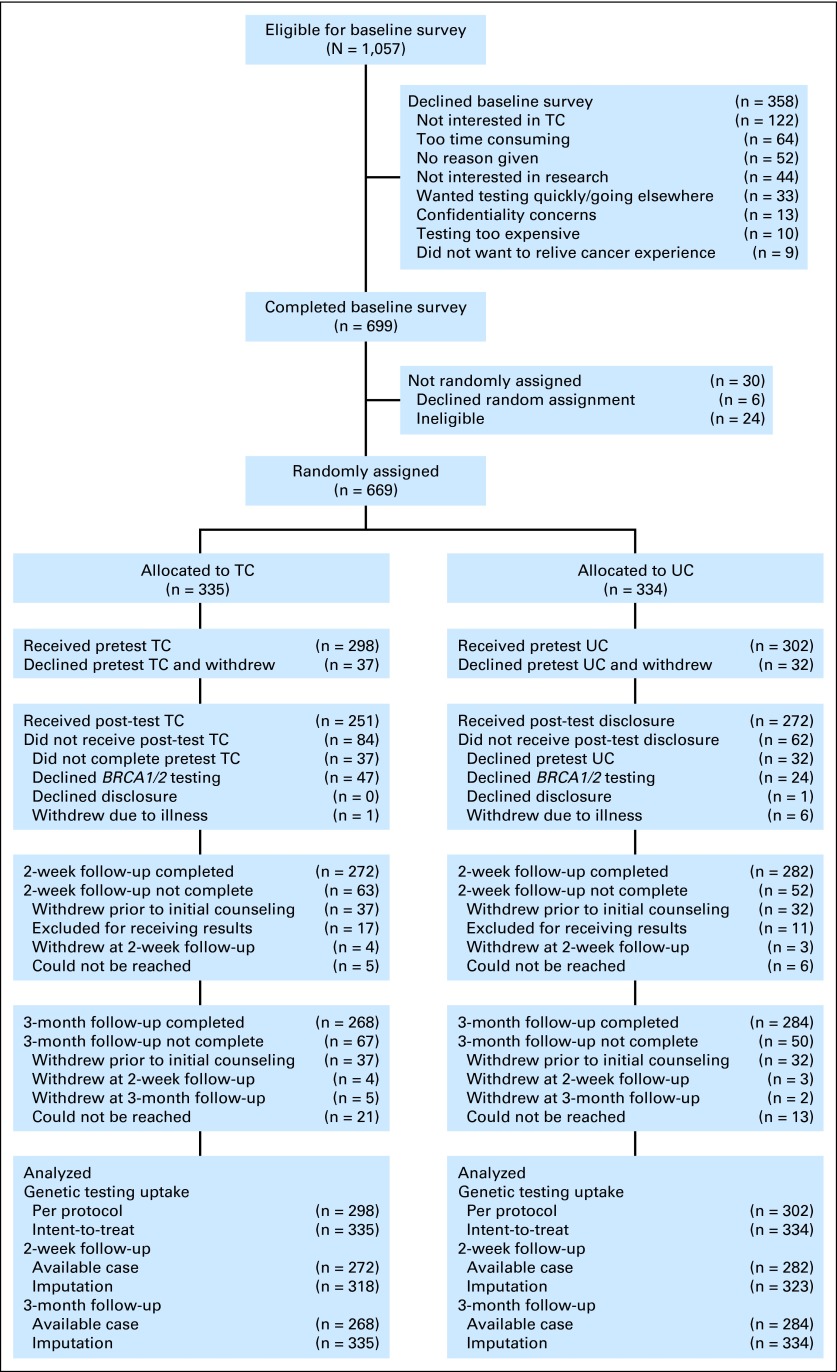
Of 1,057 potentially eligible women, 358 (33.9%) declined the baseline survey. Because we could not collect data on decliners, we do not know how they differed from participants. Of the 699 women who completed a baseline survey, 24 were ineligible and six declined random assignment. Thus, 669 (64.8%) of 1,033 potentially eligible women were randomly assigned to TC (n = 335) versus UC (n = 334). Of the 669 randomly assigned women, 600 (89.7%) completed an initial genetic counseling session (TC, 89.0%; UC, 90.4%; n = 669; Fisher's exact test P = .61) and 69 declined counseling and withdrew from the study. At the 2-week assessment (2 weeks after initial counseling and before result disclosure), we excluded 28 participants who received test results before the assessment. Of the remaining 572 participants, 272 (96.8%) TC and 282 (96.9%) UC participants completed the 2-week assessment (n = 572; Fisher's exact test P = .99). At the 3-month assessment (3 months after random assignment and after result disclosure), 284 UC participants (94.0%) and 268 TC participants (89.9%) completed the assessment (n = 600; Fisher's exact test P = .07; Fig 1).
Fig 1.
Study flow chart. TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Procedure
The institutional review boards at all study sites approved this study. After mailing interested participants an informed consent document to review, a trained research assistant called participants to administer institutional review board–approved verbal consent and complete the baseline (ie, precounseling) telephone interview. During this interview, we collected demographic, cancer history, and psychosocial information. Immediately after the interview, the research assistant randomly assigned participants by using computer-generated random numbers in blocks of four. After returning the signed informed consent document, UC participants received pretest genetic counseling and result disclosure at one of the four clinic sites, and TC participants received pretest genetic counseling and result disclosure by telephone. We conducted follow-up interviews 2 weeks after counseling (pretest disclosure), and 3, 6, and 12 months after random assignment (post-test disclosure). Here, we report on the 2-week and 3-month assessments, which focused on genetic counseling outcomes.
UC.
UC participants received standard BRCA1/2 genetic counseling and result disclosure13,23 delivered in person by board-certified/board-eligible genetic counselors. Participants could provide DNA for testing at the conclusion of the initial counseling session. We mailed participants a clinical summary letter outlining guidelines and recommendations.
TC.
Before the TC session, we mailed visual aids for use during the session. TC was delivered over the telephone by the same genetic counselors who delivered UC and with comparable content to UC.13 After the initial session, participants could provide DNA at a physician's office, a local laboratory (blood kit supplied by the study), or the study site. We mailed participants a clinical summary letter outlining guidelines and recommendations.
Control Variables
We assessed sociodemographics, family history, and personal cancer history. By using personal and family cancer history, we calculated a priori risk with the BRCAPRO model.22
Outcome Variables
BRCA1/2 knowledge was measured at baseline and at the 2-week follow-up assessment with the Breast Cancer Genetic Counseling Knowledge scale.24 Total score was the number of correct responses (Cronbach's alpha = .75 to .80).
Decisional conflict regarding BRCA1/2 testing was measured at all assessments with the 10-item version of the Decisional Conflict Scale (DCS).25 Items were scored on a weighted 3-point scale (yes, 0; unsure, 2; no, 4) with higher scores indicating greater decisional conflict (Cronbach's alpha = .79 to .84).
Genetic counseling satisfaction was measured at 2 weeks with the Genetic Counseling Satisfaction Scale.26 Items were rated on a 5-point Likert scale and summed. Higher scores indicate greater satisfaction (Cronbach's alpha = .83).
Cancer-specific distress was measured at all assessments with the Impact of Event Scale (IES)27 (Cronbach's alpha = .88 to .91). Perceived stress was measured at all assessments with the four-item version of the Perceived Stress Scale (PSS)28 (Cronbach's alpha = .69 to .72).
Quality of life was measured at baseline and at 3 months with the Short Form-12 (SF-12)29 Mental Component Summary (MCS) and Physical Component Summary (PCS). Higher scores reflect better quality of life (Cronbach's alpha = .86 to .87).
We measured costs (in 2012 dollars30) for personnel and patient time and travel, testing, telephone charges, and overhead. We excluded childcare and research and development costs. We estimated staff time via time-motion estimates of counseling elements (scheduling, preparation, pedigree review, follow-up, and provider communications). We valued staff time on the basis of average US wage and benefit rates for each profession31,32; patient time costs were based on average wage rates for women.33 Patient time included travel time for counseling and testing and time in counseling. We estimated travel distance and time from patients' home ZIP codes to the study center by using Google Maps. We assumed all travel was by car; gas and toll costs were based on the Internal Revenue Service allowable rate34; parking was assumed to be $5.00 per trip. We assumed that UC participants provided DNA at the counseling site following their initial session and that TC participants traveled to the clinic to provide DNA. To the extent that TC patients provided DNA at a location closer than the clinic, we would overestimate TC costs.
We estimated costs of BRCA1/2 testing by using charges established by Myriad Genetic Laboratories (Salt Lake City, UT). We assumed that telephone counseling occurred over land lines with costs based on average monthly charges. Overhead costs included space rental and utilities, based on average US rates.35 We tested alternative scenarios in sensitivity analyses.
Statistical Analyses
After confirming the comparability of the groups at baseline, we tested for noninferiority of TC by calculating the group difference on each outcome (with baseline score on the outcome as a covariate) and the one-sided 97.5% confidence limit of this difference. Noninferiority was demonstrated when the one-sided 97.5% confidence limit did not cross the noninferiority limit. We based our noninferiority limits on the literature describing clinically important differences on each outcome (+4 points on the IES and DCS, +1 point on the PSS, −2.5 points on the SF-12).25,27,28,36 For outcomes without guidance (knowledge, satisfaction), we set the limit as the minimum possible change on the scale (ie, 1 point).
Our primary analyses were based on the available sample at each time point.37–39 In sensitivity analyses, we used multiple regression imputation for missing follow-up data. We conservatively substituted the imputed score for UC participants and the imputed score plus or minus the noninferiority margin for TC participants.40 We also conducted sensitivity analyses in which we adjusted for multiple comparisons by using the Holm-Bonferroni approach.41
In a secondary analysis, we used the standard two one-sided test (TOST) approach42 to test for equivalence in genetic testing uptake (equivalence range, ± 7.5%) by using per-protocol and intention-to-treat approaches. We tested for equivalence on genetic testing (rather than noninferiority) because the goal of genetic counseling is to facilitate informed testing decisions, not necessarily to increase testing.
Our sample size calculations for noninferiority assume an alpha of .025. Because power was comparable at 2 weeks and 3 months, we present sample size estimates for the 2-week analyses except for PCS and MCS (which were measured at baseline and 3 months). Our sample sizes of n = 554 and n = 552 at 2 weeks and 3 months, respectively, provide greater than 80% power for all outcomes at 2 weeks and 3 months. The sample sizes required to attain 80% power to detect noninferiority were knowledge (n = 478; noninferiority limit = −1; standard deviation [SD], 3.9), satisfaction (n = 332; noninferiority limit, −1; SD, 3.2), IES (n = 460; noninferiority limit, 4; SD, 15.3), PSS (n = 214; noninferiority limit, 1; SD, 2.6), DCS (n = 348; noninferiority limit, 4; SD, 13.3), PCS (n = 400; noninferiority limit, −2.5; SD, 8.9), and MCS (n = 436; noninferiority limit, −2.5; SD, 9.3). For our secondary outcome of test uptake, 544 participants were required to obtain 80% power to detect equivalence (range, ± 7.5%) to the UC group's 90.1% uptake.
For economic analysis, we followed recommendations of the US Panel on Cost-Effectiveness in Health and Medicine.43 Because the trial included only short-term follow-up, we focused on the costs of intervention delivery and the immediate downstream consequences of counseling. Because counseling and testing have previously been found to be cost-effective and clinically effective in reducing mortality,44,45 we did not examine longer-term costs or life-years saved. We summed costs of counseling and testing to generate an average cost per patient counseled and tested and the incremental differences in costs between arms.
In sensitivity analyses, we varied the following key parameters: counseling probands versus relatives, rural versus urban settings, counselor preparation time, TC patient use of cell phones for counseling, TC patients by using at-home buccal DNA kits to avoid travel, identical overhead in both arms, and actual testing rates. We examined combinations of variables in a multiway sensitivity analysis.
RESULTS
The mean age of participants was 48.0 years (SD, 13.7 years), and their mean a priori mutation risk was 25.0% (SD, 22.9%). The majority were non-Latino white (86.2%), previously affected with breast or ovarian cancer (65.3%), college-educated (80.0%), and married (62.3%). As displayed in Tables 1 and 2, our randomization was effective because the groups were highly similar at baseline.
Table 1.
Sample Characteristics of Women Participating in a Randomized Multicenter Trial Comparing In-Person to Telephone Counseling for BRCA1/2 Testing
| Characteristic | UC (n = 334) |
TC (n = 335) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Mean | 48.4 | 47.7 | ||
| SD | 14.2 | 13.1 | ||
| BRCA1/2 probability | ||||
| Mean | 25.7 | 24.3 | ||
| SD | 24.2 | 21.6 | ||
| Education | ||||
| < College | 69 | 20.7 | 67 | 20.0 |
| College or more | 265 | 79.3 | 268 | 80.0 |
| Employment status | ||||
| Full time | 183 | 54.8 | 199 | 59.4 |
| < Full time | 151 | 45.2 | 136 | 40.6 |
| Race | ||||
| White | 289 | 87.3 | 280 | 85.1 |
| Nonwhite | 42 | 12.7 | 49 | 14.9 |
| Marital status | ||||
| Married/partner | 212 | 63.5 | 205 | 61.2 |
| Single/widow/divorced | 122 | 36.5 | 130 | 38.8 |
| Jewish ethnicity | ||||
| Jewish | 100 | 29.9 | 92 | 27.5 |
| Non-Jewish | 234 | 70.1 | 243 | 72.5 |
| Affected with breast or ovarian cancer | ||||
| Yes | 223 | 66.8 | 214 | 63.9 |
| No | 111 | 33.2 | 121 | 36.1 |
| Proband status | ||||
| Proband | 215 | 64.4 | 211 | 63.0 |
| Relative of known carrier | 119 | 35.6 | 124 | 37.0 |
| BRCA1/2 test result | ||||
| Positive | 51 | 15.2 | 44 | 13.1 |
| Negative | 56 | 16.8 | 57 | 17.0 |
| Uninformative/variant | 165 | 49.4 | 150 | 44.8 |
| Untested | 62 | 18.6 | 84 | 25.1 |
| Recruitment site | ||||
| Lombardi Cancer Center | 214 | 64.1 | 213 | 63.6 |
| Mount Sinai | 65 | 19.5 | 69 | 20.6 |
| University of Vermont | 21 | 6.3 | 21 | 6.3 |
| Dana-Farber Cancer Institute | 34 | 10.2 | 32 | 9.6 |
| Distance to clinic, miles | ||||
| Mean | 24.4 | 20.9 | ||
| SD | 27.1 | 18.0 | ||
Abbreviations: SD, standard deviation; TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Table 2.
Genetic Counseling Outcomes at Baseline, 2 Weeks, and 3 Months After Counseling
| Outcome | UC |
TC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
2 Weeks |
3 Months |
Baseline |
2 Weeks |
3 Months |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Knowledge | 17.0 | 4.8 | 20.1 | 3.9 | — | 17.3 | 4.8 | 20.2 | 3.9 | — | ||
| Decision conflict | 31.1 | 26.3 | 6.7 | 13.2 | 4.0 | 8.6 | 28.5 | 24.5 | 7.5 | 13.4 | 4.9 | 12.2 |
| Cancer distress | 20.7 | 15.5 | 17.0 | 15.5 | 14.0 | 14.7 | 23.2 | 15.1 | 17.3 | 15.1 | 14.8 | 14.9 |
| Perceived stress | 4.5 | 2.6 | 4.3 | 2.6 | 4.0 | 2.5 | 4.4 | 2.4 | 4.0 | 2.6 | 3.9 | 2.6 |
| General counseling satisfaction | — | 27.0 | 3.3 | — | 26.8 | 3.1 | ||||||
| Physical function | 50.7 | 9.2 | — | 50.9 | 9.2 | 51.0 | 8.6 | — | 51.6 | 8.6 | ||
| Mental function | 48.5 | 10.6 | — | 51.0 | 9.3 | 48.9 | 10.3 | — | 50.6 | 9.3 | ||
Abbreviations: SD, standard deviation; TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Predisclosure Outcomes
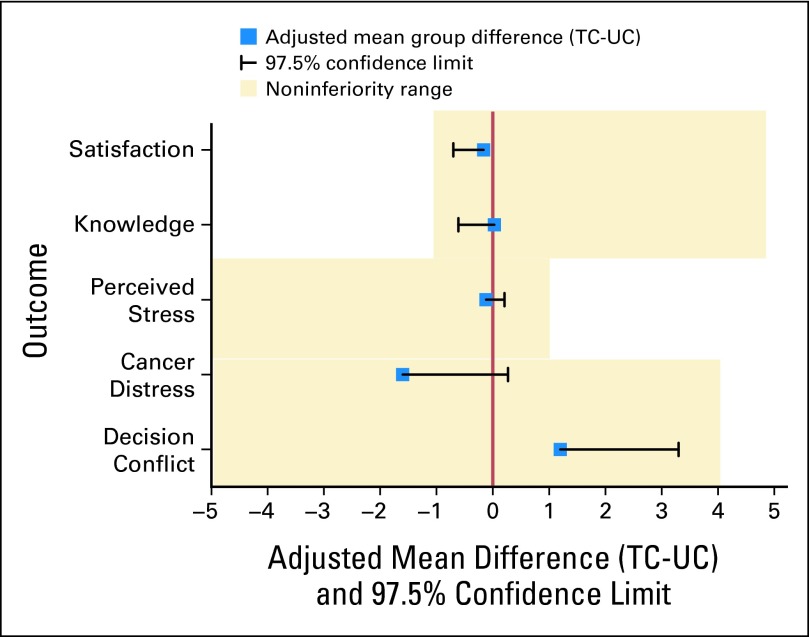
TC was noninferior to UC on all outcomes (Fig 2). The mean adjusted postcounseling knowledge score was 0.03 points higher for TC than for UC participants (d = .03). The lower bound of the one-sided 97.5% CI (−0.61) did not cross the noninferiority limit (−1).
Fig 2.
Noninferiority analyses comparing telephone to standard delivery of genetic counseling at predisclosure (2 weeks). TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Similarly, TC was noninferior on decision conflict (d = 1.2; upper bound one-sided 97.5% CI, 3.3; noninferiority limit, 4); cancer distress (d = −1.6; upper bound one-sided 97.5% CI, 0.27; noninferiority limit, 4); perceived stress (d = −0.12; upper bound one-sided 97.5% CI, 0.21; noninferiority limit, 1), and genetic counseling satisfaction (d = −0.16; lower bound one-sided 97.5% CI, −0.70; noninferiority limit, −1). Sensitivity analyses confirmed noninferiority for all outcomes after adjusting for multiple comparisons by using the Holm-Bonferroni correction41 and imputing for missing follow-up data.
Post-Test Disclosure Outcomes
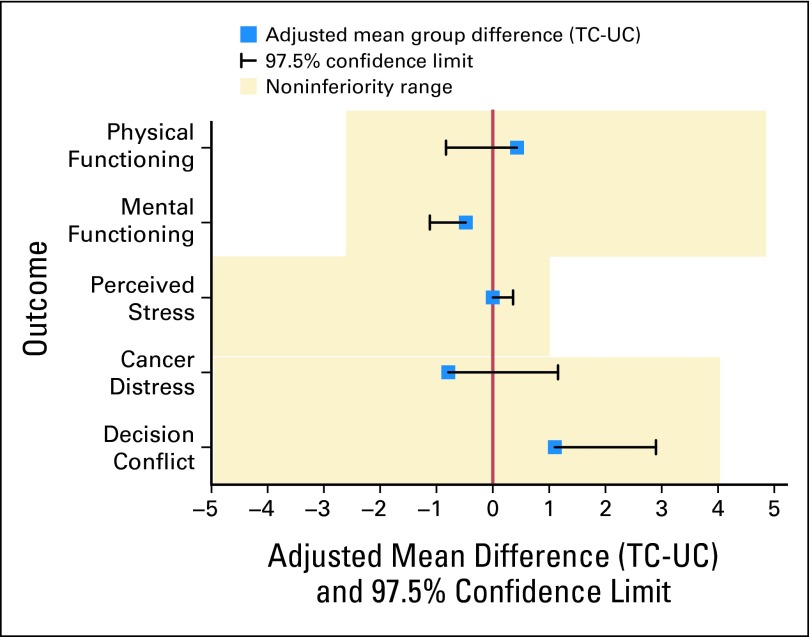
The results at the 3-month assessment were virtually identical with those at predisclosure (Fig 3). TC was noninferior to UC on decisional conflict (d = 1.1; upper bound one-tailed 97.5% CI, 2.9; noninferiority limit, 4), cancer-specific distress (d = −.79; upper bound one-tailed 97.5% CI, 1.16; noninferiority limit, 4), perceived stress (d = .00; upper bound one-tailed 97.5% CI, 0.36; noninferiority limit, 1), physical function (d = .43; lower bound one-tailed 97.5% CI, −0.83; noninferiority limit, −2.5), and mental function (d = −.48; lower bound one-tailed 97.5% CI, −1.8; noninferiority limit, −2.5). As at 2 weeks, sensitivity analyses confirmed noninferiority for all outcomes.
Fig 3.
Noninferiority analysis comparing telephone to standard delivery of genetic counseling at postdisclosure (3 months). TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Genetic Testing
Of those who completed pretest counseling, 90.1% of UC versus 84.2% of TC were tested (relative risk, 0.93; 95% CI, 0.88 to 0.99). By using the TOST approach, the lower bound of the 90% CI (d = −5.9%; 90% CI, −10.3% to −0.01%) fell outside the equivalence range. Results were comparable in the intention-to-treat sample (UC, 81.4% v TC, 74.9%; d = −6.5%; 90% CI, −11.8% to 0.01%).
For economic analyses, we determined that TC costs $114.40 per patient less than UC (Table 3). The lower cost of TC was a result of shorter counseling times, less patient travel, and lower overhead. The lower cost of TC was robust over all assumptions and conditions. Assuming identical overhead decreased the cost savings to $59 per patient. The greatest cost savings ($321.40 per patient) was for rural patients who used in-home buccal DNA kits (Table 4).
Table 3.
Average and Incremental Costs of Telephone Versus In-Person Genetic Counseling and Testing
| Variable | UC |
TC |
Incremental Cost (TC v UC) | ||
|---|---|---|---|---|---|
| Time (minutes) | $ | Time (minutes) | $ | ||
| Initial counseling | |||||
| Staff costs | |||||
| Ancillary personnel (scheduling/administration @ $20.50 per hour)* | 32 | 10.90 | 32 | 10.90 | — |
| Counselor preparation @ $43.60 per hour* | 62 | 45.00 | 62 | 45.00 | — |
| Counseling @ $43.60 per hour* | 76 | 55.10 | 64 | 46.50 | −8.60 |
| Patient costs | |||||
| Round-trip travel @ $22.80 per hour* | 70 | 26.60 | — | — | −26.60 |
| Gasoline + parking† | 15.10 | — | — | −15.10 | |
| Time for initial counseling session* | 76 | 28.80 | 64 | 24.30 | −4.50 |
| Subtotal: initial session | 181.50 | 126.80 | −54.70 | ||
| Testing and disclosure | |||||
| Staff costs | |||||
| Ancillary personnel (scheduling and administration @ $21.90 per hour)* | 15 | 5.10 | 15 | 5.10 | — |
| Counselor preparation @ $44.30 per hour* | 74 | 53.80 | 74 | 53.80 | — |
| Post-test counseling @ $44.30 per hour* | 28 | 20.60 | 24 | 17.20 | −3.40 |
| Round-trip travel: disclosure @ $22.20 per hour* | 70 | 26.60 | — | — | −26.60 |
| Patient costs | |||||
| Gasoline + parking: disclosure† | 15.10 | — | −15.10 | ||
| Round-trip travel for blood draw @ $22.20 per hour* | — | — | 70 | 26.60 | 26.60 |
| Gasoline + parking for blood draw† | — | — | 15.10 | 15.10 | |
| Time for disclosure @ $22.80 per hour* | 28.4 | 10.80 | 23.7 | 9.00 | −1.80 |
| Time for blood draw* | 15 | 5.70 | 15 | 5.70 | — |
| Testing costs | |||||
| Gene sequencing charge‡ | 3,340.00 | 3,340.00 | — | ||
| Blood draw by phlebotomist @ $17.20 per hour* | 10 | 2.90 | 10 | 2.90 | — |
| Phlebotomy materials | 1.00 | 1.00 | — | ||
| Subtotal: testing + disclosure | 3,481.50 | 3,476.30 | −5.20 | ||
| Overhead | |||||
| Office space @ $38.60/sq ft§ (assume 50% of space for telephone counseling) | 110.90 | 55.50 | −55.50 | ||
| Telephone costs‖ (counseling only) | — | 0.96 | 0.96 | ||
| Subtotal: overhead | 110.90 | 56.40 | −54.50 | ||
| Total per patient | 3,774.00 | 3,660.00 | −114.40 | ||
Abbreviations: TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
Cost for genetic counselors' time is based on the national average of salary plus fringe benefit rate of $83,712 per year from 2010 data and adjusted for inflation to estimate 2012 salaries.30–33 The costs of ancillary personnel, patients, and phlebotomists are based on women's earnings from Current Population Survey plus fringe benefit rates31,33 adjusted to 2012 dollars.30 The median average wage and fringe benefit rates for patients, phlebotomists, and ancillary personnel were $22.80, $17.20, and $20.50 per hour, respectively, in 2012 dollars.
Average round-trip mileage for counseling is 43.7 miles (approximately 70 minutes). The standard mileage rate is $0.23 per mile for use of an automobile for medical care.34
We assume all patients receive comprehensive BRCA analysis from Myriad Genetic Laboratories. This assumption is made to isolate any differential costs of counseling related to TC v UC.
New and returning patients are 6 and 2.7 patients per week, respectively, for an estimated 34.8 per month.32 National average office rental rate is $38.60/sq ft,35 and we assume average medical office space is 100 sq ft. Thus, office space cost per patient is $38.60 × 100/34.8 patients = $110.90 per patient.
The phone cost of landline per patient is calculated by the genetic counselors' monthly patient volume: 34.8/per month32 and monthly phone rate of $33.40/month.
Table 4.
Costs of TC Versus UC Genetic Counseling and Testing Under Various Assumptions
| Scenario* and Changed Variable for Sensitivity Analysis | Cost ($) |
||
|---|---|---|---|
| UC | TC | Incremental (compared with UC) | |
| Base case | 3,773.90 | 3,659.50 | −114.40 |
| Scenario 1 | |||
| Proband only | 3,775.20 | 3,665.10 | −110.20 |
| Relative only | 3,755.50 | 3,636.10 | −118.80 |
| Scenario 2 | |||
| Urban area (round-trip, 1 hour)† | 3,763.60 | 3,654.40 | −109.20 |
| Rural area (round-trip, 2 hours)† | 3,826.40 | 3,685.80 | −140.60 |
| Rural area (round-trip, 4 hours)† | 3,952.30 | 3,748.70 | −203.60 |
| Scenario 3 | |||
| Preparation time of genetic counselors in nonresearch setting | 3,718.70 | 3,604.30 | −114.40 |
| Scenario 4 | |||
| Patients use cell phone for counseling in the telephone arm‡ | 3,773.90 | 3,667.30 | −106.60 |
| Scenario 5 | |||
| Both UC and TC arms use buccal swab for DNA collection | 3,783.80 | 3,630.90 | −153.00 |
| Scenario 6 | |||
| Equivalent overhead for TC and UC | 3,773.90 | 3,714.00 | −59.90 |
| Scenario 7 | |||
| Actual rates of genetic testing and disclosure§ | 3,450.80 | 3,099.80 | −351.00 |
| Scenario 8 | |||
| Buccal swab + rural area (round-trip, 2 hours) | 3,826.40 | 3,630.90 | −195.60 |
| Buccal swab + rural area (round-trip, 4 hours) | 3,952.30 | 3,630.90 | −321.40 |
Abbreviations: TC, telephone genetic counseling; UC, usual care (standard in-person genetic counseling).
To isolate the costs and effects of counseling, in the base case and scenarios 1 through 6, we assume that all participants have genetic testing (and post-test disclosure) in both arms. In scenario 7, this assumption is evaluated.
Assuming rural travel time of 75 or 150 miles each way v 30 minutes in urban locations.
The basic rate for Verizon: any time minutes = 450 minutes and monthly access rate = $39.99. The initial and post-testing counseling time for TC is 87.7 minutes (64 + 23.7 minutes). Thus, the cell phone cost is 87.7/450 × $39.99 = $7.80 per patient.
The actual rates of genetic testing and disclosure for UC and TC arms are 90.7% and 83.9%, respectively, so only these proportions of patients accrue testing costs (and post-test disclosure).
DISCUSSION
The results of this randomized noninferiority trial support that telephone BRCA1/2 genetic counseling is not worse than standard in-person counseling. TC outcomes were noninferior to standard in-person UC with a substantial cost savings. However, TC yielded lower rates of genetic testing compared with UC.
The lower testing rate for TC may be a result of the fact that UC participants could provide DNA immediately following their counseling session, whereas TC participants had to travel to the clinic or to a physician's office to provide DNA. This delay between counseling and DNA provision could be a barrier to testing. If this is the case, then using in-home buccal kits could eliminate the delay and increase test uptake following TC. It is also possible that the delay between counseling and DNA provision allowed for added deliberation that led some TC participants to forego testing. This is consistent with the fact that TC participants who declined testing had lower a priori risk scores and were less likely to be considering risk-reducing surgery than those who completed testing (data not shown).
Despite the lower rate of testing, our results provide strong evidence that cancer genetic counseling can be effectively delivered by telephone. We found no evidence that TC led to higher distress, lower satisfaction, or lower comprehension than UC. TC also led to a cost savings of $114 per patient up to $321 per patient for those who live farther from a clinic and who provide DNA via in-home buccal sampling. This is consistent with genetic counselors' perceptions of decreased counseling time, patient travel time, and patient burden associated with TC.1,2
These results have clinical and policy implications. The noninferiority and lower cost of TC compared with UC suggests that TC could broaden the reach and accessibility of BRCA1/2 genetic counseling. Offering TC in rural settings could increase access and maximize cost savings. Because a major barrier to physician use of BRCA1/2 testing is lack of access to appropriate genetic counseling resources,46–49 TC could facilitate such testing. For example, TC might be an efficient and effective way to reach untested individuals from families with known mutations, especially in nonurban areas. These individuals have a high mutation risk but low rates of BRCA1/2 testing.50,51 In the United States, there are about 350,000 adult women who carry a BRCA1/2 mutation, but fewer than 15% have been identified.52 The availability of TC could make it easier to reach and counsel these individuals. By increasing access to genetic testing, cancer mortality could be reduced through the increased use of risk-reducing interventions by newly identified mutation carriers.2,53
These data also provide justification for expanded reimbursement of TC by insurers. At present, insurance reimbursement is a barrier to widespread use of TC. The primary billing codes for genetic counseling are for in-person counseling services.54 However, one of the nation's largest insurers, Aetna, now provides coverage for cancer genetic TC.55 Moreover, the Affordable Care Act requires that insurers cover BRCA1/2 genetic counseling for women at increased risk.56 These data may provide further support for routine reimbursement for genetic TC services.
The study has several limitations. About one third of those approached declined to participate. A primary reason for declining was a preference for in-person counseling. TC is likely to be less effective among women with a strong preference for in-person counseling. However, in women willing to participate in TC, it was reassuring to note that TC performed as well as UC. Furthermore, in this trial, all participants lived within commuting distance of one of the study clinics. Thus, they had the option of declining the study and easily receiving in-person counseling. Women in rural locations may not have this option. Second, our interventions were not blinded, which could have influenced participant responses to outcome assessments. Third, we were not powered to conduct subgroup noninferiority analyses. Thus, we cannot draw any conclusions about specific subgroups who may be better or worse candidates for TC. Future studies should evaluate whether the outcomes of TC vary by test result, cancer-affected status, or other potentially important moderator variables. Finally, our sample comprised individuals seeking counseling at academic medical centers and was predominantly non-Latino white and well educated.
Despite these limitations, this study provides strong evidence for the noninferiority of TC in the BRCA1/2 setting. These results represent an initial step in the development of alternative genetics delivery approaches. As genomic tests proliferate, it will be increasingly critical to develop approaches to extend the reach and efficiency of counseling and lower costs.
Supplementary Material
Acknowledgment
The authors acknowledge the contributions of genetic counselors Tiffani DeMarco, Karen Brown, Andrea Forman, Diana Moglia Tully, Jessica Rispoli Joines, and Morgan Butrick. In addition, we thank Lisa Feeley and Lauren Vanhusen for performing the telephone interviews and Susan Marx for assistance with manuscript preparation. Finally, we thank all of the women who participated in the study.
Glossary Terms
- BRCA1:
A tumor suppressor gene known to play a role in repairing DNA breaks. Mutations in this gene are associated with increased risks of developing breast or ovarian cancer.
- BRCA2:
A tumor suppressor gene whose protein product is involved in repairing chromosomal damage. Although structurally different from BRCA1, BRCA2 has cellular functions similar to BRCA1. BRCA2 binds to RAD51 to fix DNA breaks caused by irradiation and other environmental agents.
- Genomics:
The scientific discipline in which multiple genes, gene products, or regions of the genome are analyzed via large-scale, high-throughput molecular approaches directed to DNA and RNA. This definition is a deviation from that of the original term, which meant an analysis of the whole genome.
- Sensitivity Analyses:
Analyses that evaluate the impact of missing data and possible differences in interval assessments.
Footnotes
See accompanying editorial on page 611
Supported by Grants No. R01 CA108933, U01 CA152958, and P30 CA051008 from the National Cancer Institute; by the Lombardi Comprehensive Cancer Center Biostatistics and Bioinformatics Shared Resource; and by the Jess and Mildred Fisher Center for Familial Cancer Research.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00287898.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Marc D. Schwartz, InformedDNA (U); Judy Garber, Novartis (C), Pfizer (C), TESARO (C), AstraZeneca (C) Stock Ownership: None Honoraria: None Research Funding: Judy Garber, Myriad Genetics, Novartis, Pfizer Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Marc D. Schwartz, Heiddis B. Valdimarsdottir, Beth N. Peshkin, Jeanne Mandelblatt, Rachel Nusbaum, Claudine Isaacs
Provision of study materials or patients: Rachel Nusbaum, Claudine Isaacs, Wendy McKinnon, Judy Garber
Collection and assembly of data: Marc D. Schwartz, Heiddis B. Valdimarsdottir, Rachel Nusbaum, Kristi Graves, Marie Wood, Wendy McKinnon, Judy Garber, Shelley McCormick, Sarah Kelleher, Kara-Grace Leventhal, Patti Vegella, Angie Tong, Lesley King
Data analysis and interpretation: Marc D. Schwartz, Heiddis B. Valdimarsdottir, Beth N. Peshkin, Jeanne Mandelblatt, An-Tsun Huang, Yaojen Chang, Kristi Graves, Claudine Isaacs, Anita Y. Kinney, George Luta
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bradbury AR, Patrick-Miller L, Fetzer D, et al. Genetic counselor opinions of, and experiences with telephone communication of BRCA1/2 test results. Clin Genet. 2011;79:125–131. doi: 10.1111/j.1399-0004.2010.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackenzie A, Patrick-Miller L, Bradbury AR. Controversies in communication of genetic risk for hereditary breast cancer. Breast J. 2009;15(suppl 1):S25–S32. doi: 10.1111/j.1524-4741.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 4.American College of Surgeons. National Accreditation Program for Breast Centers, NAPBC Breast Center Components. Revised August 30, 2010. http://napbc-breast.org/standards/components.html.
- 5.Commission on Cancer, American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient-Centered Care. Version 1.2. http://www.facs.org/cancer/coc/programstandards2012.pdf.
- 6.U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Draft Recommendation Statement. AHRQ Publication No. 12-05164-EF-2. http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/draftrecbrcatest.htm.
- 7.American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: Genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 8.Trepanier A, Ahrens M, McKinnon W, et al. Genetic cancer risk assessment and counseling: Recommendations of the National Society of Genetic Counselors. J Genet Couns. 2004;13:83–114. doi: 10.1023/B:JOGC.0000018821.48330.77. [DOI] [PubMed] [Google Scholar]
- 9.Sutphen R, Davila B, Shappell H, et al. Real world experience with cancer genetic counseling via telephone. Fam Cancer. 2010;9:681–689. doi: 10.1007/s10689-010-9369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wideroff L, Vadaparampil ST, Greene MH, et al. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42:749–755. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumanis L, Evans JP, Callanan N, et al. Telephoned BRCA1/2 genetic test results: Prevalence, practice, and patient satisfaction. J Genet Couns. 2009;18:447–463. doi: 10.1007/s10897-009-9238-8. [DOI] [PubMed] [Google Scholar]
- 12.Calzone KA, Prindiville SA, Jourkiv O, et al. Randomized comparison of group versus individual genetic education and counseling for familial breast and/or ovarian cancer. J Clin Oncol. 2005;23:3455–3464. doi: 10.1200/JCO.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 13.Peshkin BN, Demarco TA, Graves KD, et al. Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: Rationale and development of a randomized controlled trial. Genet Test. 2008;12:37–52. doi: 10.1089/gte.2006.0525. [DOI] [PubMed] [Google Scholar]
- 14.Platten U, Rantala J, Lindblom A, et al. The use of telephone in genetic counseling versus in-person counseling: A randomized study on counselees' outcome. Fam Cancer. 2012;11:371–379. doi: 10.1007/s10689-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughty Rice C, Ruschman JG, Martin LJ, et al. Retrospective comparison of patient outcomes after in-person and telephone results disclosure counseling for BRCA1/2 genetic testing. Fam Cancer. 2010;9:203–212. doi: 10.1007/s10689-009-9303-3. [DOI] [PubMed] [Google Scholar]
- 16.Helmes AW, Culver JO, Bowen DJ. Results of a randomized study of telephone versus in-person breast cancer risk counseling. Patient Educ Couns. 2006;64:96–103. doi: 10.1016/j.pec.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.InformedDNA.com. Quarterly Newsletter–October 2011. http://informeddna.com/newsletter/october2011/index.php.
- 18.Harris A, Kelly SE, Wyatt S. Counseling customers: Emerging roles for genetic counselors in the direct-to-consumer genetic testing market. J Genet Couns. 2013;22:277–288. doi: 10.1007/s10897-012-9548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin E, Riordan S, Klein J, et al. Genetic counseling for personal genomic testing: Optimizing client uptake of post-test telephonic counseling services. J Genet Couns. 2012;21:462–468. doi: 10.1007/s10897-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 20.Pal T, Stowe C, Cole A, et al. Evaluation of phone-based genetic counselling in African American women using culturally tailored visual aids. Clin Genet. 2010;78:124–131. doi: 10.1111/j.1399-0004.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins J, Calzone KA, Dimond E, et al. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;9:487–495. doi: 10.1097/gim.0b013e31812e6220. [DOI] [PubMed] [Google Scholar]
- 22.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20:2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MD, Peshkin BN, Hughes C, et al. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- 24.Erblich J, Brown K, Kim Y, et al. Development and validation of a Breast Cancer Genetic Counseling Knowledge Questionnaire. Patient Educ Couns. 2005;56:182–191. doi: 10.1016/j.pec.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor AM. User Manual–Decisional Conflict Scale (10-item question format) Ottawa, ON, Canada: Ottawa Hospital Research Institute; 1993. (updated 2010). http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- 26.DeMarco TA, Peshkin BN, Mars BD, et al. Patient satisfaction with cancer genetic counseling: A psychometric analysis of the Genetic Counseling Satisfaction Scale. J Genet Couns. 2004;13:293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 29.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Bureau of Labor Statistics, U.S. Department of Labor. Consumer Price Index–December 2012. http://www.bls.gov/news.release/pdf/cpi.pdf.
- 31.Bureau of Labor Statistics, U.S. Department of Labor. Employer costs for employee compensation–September 2012. http://www.bls.gov/opub/ted/2012/ted_20121219.htm.
- 32.National Society of Genetic Counselors. 2012 Professional Status Survey: Salary & Benefits. (May 1, 2012) http://www.nsgc.org/MemberCenter/LeadershipCenter/tabid/190/Default.aspx?EntryId=4245.
- 33.U.S. Department of Labor, Bureau of Labor Statistics. Highlights of Women's Earnings in 2011. Report 1038, October 2012. http://www.bls.gov/cps/cpswom2011.pdf.
- 34.Internal Revenue Service. 2012 standard mileage rates. Notice 2012-1. http://www.irs.gov/pub/irs-drop/n-12-01.pdf.
- 35.Cushman & Wakefield. U.S. CBD office leasing up in 3Q, though down year-over-year. News release October 23, 2012, New York. http://www.cushwake.com/cwglobal/jsp/newsDetail.jsp?Country=FR&Language=EN&repId=c55300017p.
- 36.Norman GR, Sloan JA, Wyrwich KW. Is it simple or simplistic? Med Care. 2003;41:599–600. doi: 10.1097/01.MLR.0000062556.73534.61. [DOI] [PubMed] [Google Scholar]
- 37.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: ICH Harmonised Tripartite Guideline: Statistical Principles for Clinical Trials, E9. Current Step 4 version; 5 February 1998; http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. [Google Scholar]
- 38.Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147–1151. doi: 10.1001/jama.295.10.1147. [DOI] [PubMed] [Google Scholar]
- 39.Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 40.Koch GG. Comments on ‘Current issues in non-inferiority trials’ by Thomas R. Fleming, Statistics in Medicine, DOI: 10.1002/sim. 2855. Stat Med. 2008;27:333–342. doi: 10.1002/sim.2923. [DOI] [PubMed] [Google Scholar]
- 41.Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- 42.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–196. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34:DS197–DS199. [PubMed] [Google Scholar]
- 44.Balmaña J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: Analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112:647–652. doi: 10.1002/ijc.20458. [DOI] [PubMed] [Google Scholar]
- 45.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295:2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 46.Klitzman R, Chung W, Marder K, et al. Attitudes and practices among internists concerning genetic testing. J Genet Couns. 2013;22:90–100. doi: 10.1007/s10897-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: Missed opportunities. Obstet Gynecol. 2010;115:945–952. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussner KM, Jandorf L, Valdimarsdottir HB. Educational needs about cancer family history and genetic counseling for cancer risk among frontline health care clinicians in New York City. Genet Med. 2011;13:785–793. doi: 10.1097/GIM.0b013e31821afc8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivers KF, Baldwin LM, Miller JW, et al. Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: A vignette-based study. Cancer. 2011;117:5334–5343. doi: 10.1002/cncr.26166. [DOI] [PubMed] [Google Scholar]
- 50.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landsbergen K, Verhaak C, Kraaimaat F, et al. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4:115–119. doi: 10.1007/s10689-004-7991-2. [DOI] [PubMed] [Google Scholar]
- 52.Drohan B, Roche CA, Cusack JC, Jr, et al. Hereditary breast and ovarian cancer and other hereditary syndromes: Using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–1737. doi: 10.1245/s10434-012-2257-y. [DOI] [PubMed] [Google Scholar]
- 53.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parman C. Coding & Billing: Genetic Counseling. Oncol Issues. 2010:16–17. [Google Scholar]
- 55.Aetna. DocFind: Telephonic genetic counseling. http://www.aetna.com/docfind/cms/html/telephonic_genetic_counselors.html.
- 56.HealthCare.gov, U.S. Department of Health & Human Services. Preventive services covered under the Affordable Care Act. Fact Sheets posted Sept. 23, 2010. http://www.hhs.gov/healthcare/facts/factsheets/2010/07/preventive-services-list.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.