Abstract
Purpose
This randomized controlled trial examined the quality-of-life benefits of an expressive writing (EW) intervention for patients with renal cell carcinoma (RCC) and identified a potential underlying mechanism of intervention efficacy.
Patients and Methods
Patients (N = 277) with stage I to IV RCC were randomly assigned to write about their deepest thoughts and feelings regarding their cancer (EW) or about neutral topics (neutral writing [NW]) on four separate occasions. Patients completed the Center for Epidemiologic Studies Depression Scale (CES-D), MD Anderson Symptom Inventory (MDASI), Brief Fatigue Inventory (BFI), Pittsburgh Sleep Quality Index (PSQI), Medical Outcomes Study Short Form-36 (SF-36), and Impact of Event Scale (IES) at baseline and 1, 4, and 10 months after the intervention.
Results
The mean age of participants (28% stage IV; 41% female) was 58 years. Multilevel modeling analyses, using a Bonferroni-corrected α = .021 for six outcomes adjusted for the correlation among outcomes, revealed that, relative to the NW group, patients in the EW group reported significantly lower MDASI scores (P = .003) and higher physical component summary scores on the SF-36 (P = .019) at 10 months after the intervention. Mediation analyses revealed that significant group differences for MDASI scores at 10 months were mediated by lower IES scores at 1 month after the intervention in the EW group (P = .042). No significant group differences were observed in the BFI, CES-D, PSQI, and mental component summary of the SF-36.
Conclusion
EW may reduce cancer-related symptoms and improve physical functioning in patients with RCC. Evidence suggests that this effect may occur through short-term improvements in cognitive processing.
INTRODUCTION
A cancer diagnosis may be experienced as a traumatic event, eliciting trauma symptoms such as intrusive thoughts (unbidden, distressing thoughts and images) and avoidance behaviors (consciously recognized avoidance of certain thoughts and feelings).1–3 Some intrusive thoughts are an adaptive part of processing and integrating traumas4; however, they often elicit negative affect (eg, depression)1,2,5–8 and somatic symptoms (eg, fatigue, sleep disturbances).8–11 Managing patients' psychological responses to their cancer experience may be an important aspect of effective patient care considering that depression has been related to tumor progression and decreased survival.12–18
Expressive writing (EW) is a brief and simple intervention that may help patients cognitively and emotionally process the cancer experience. The EW paradigm is designed to induce processing of a traumatic event by prompting participants on several occasions to briefly write about their deepest thoughts and feelings regarding the experience; this processing may help convert disorganized emotions into organized thoughts.19 Through this integration of thoughts and feelings, patients may develop a coherent narrative of the experience, create meaning, and eventually derive benefit from the experience.20–22
Most studies examining the effectiveness of EW interventions have been conducted in healthy populations, but some equivocal evidence of benefit can also be seen in patients with cancer. Stanton et al23 demonstrated that written emotional disclosure for patients with early-stage breast cancer with a low-avoidance coping style significantly reduced distress, physical symptoms, and number of medical appointments for cancer-related morbidities at 3 months after the intervention. Others have found beneficial effects in terms of fewer sleep disturbances24 and somatic symptoms25 in patients with metastatic disease. Yet some trials have failed to demonstrate significant findings regarding improved quality-of-life (QOL) outcomes.26–28
Investigations involving nononcologic populations have focused primarily on cognitive/linguistic models as mechanisms of EW.22,29–32 The underlying mechanisms in cancer are relatively unexplored, although Low et al33 revealed that within writing sessions, heart rate habituations and greater use of negative emotion words mediated the effects of EW on the physical symptom decline of patients with breast cancer. Because cognitive processes such as intrusive thoughts and avoidance have been associated with increased psychological and physical symptoms in various cancer populations,8–11 we examined a cognitive-processing mediation model.
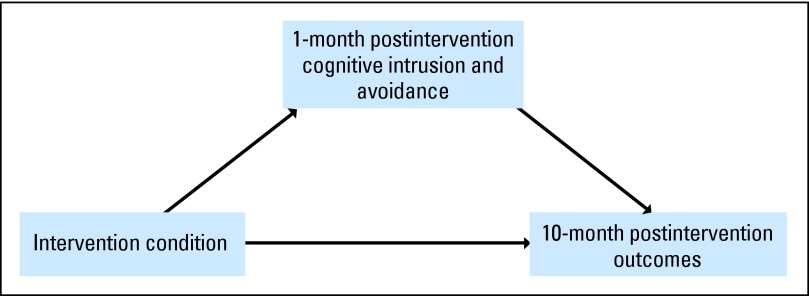
To address the limitations of previous investigations (ie, small sample sizes, short follow-up periods, and lack of mechanistic models), we conducted a large, randomized controlled trial (RCT) with a longer follow-up period and an a priori hypothesized intervention mechanism. Because previous work primarily focused on women with breast cancer,23,25,26,28,33 we evaluated the benefits of EW in a non–sex-specific cancer, renal cell carcinoma (RCC). Furthermore, RCC is an immunogenic cancer,34 and previous research found that EW modulates the immune system.35–37 We hypothesized that over a 10-month period, patients assigned to the EW group would report better QOL (ie, fewer cancer-related and depressive symptoms, less fatigue, fewer sleep disturbances, and better overall QOL, both physical functioning and mental health) compared with patients in a neutral writing (NW) group (CONSORT diagram, Fig 1). We also hypothesized that long-term effects on QOL would be mediated by early intervention effects (ie, 1 month) on reducing intrusive thoughts and avoidance behaviors (hypothesized intervention mediation model, Fig 2).
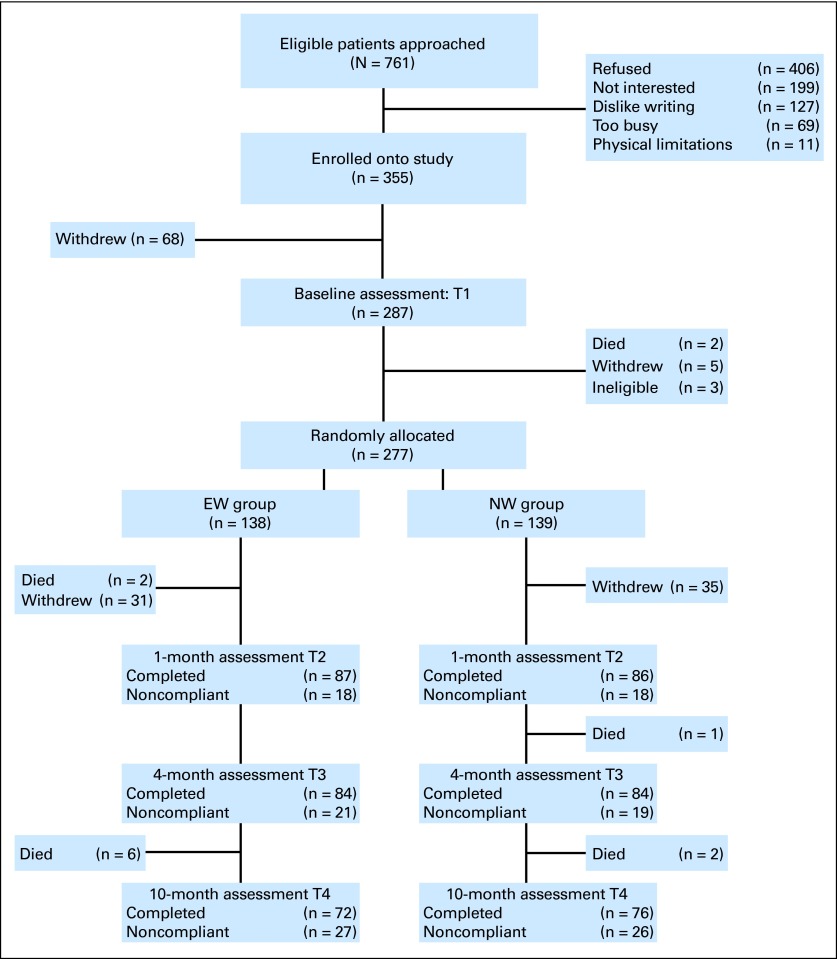
Fig 1.
CONSORT flow diagram. EW, expressive writing; NW, neutral writing.
Fig 2.
Hypothesized intervention mediation model.
PATIENTS AND METHODS
Study Population
Newly diagnosed patients with stage I to IV RCC, a Zubrod performance status of ≤ 2, and no serious intercurrent medical illness requiring hospitalization and who were at least 18 years old and able to read, write, and speak English were eligible to participate in the study. Patients who were unable to provide consent, on immunosuppressive drugs, currently receiving psychological (ie, nonpharmacologic) interventions, and/or had a history of primary or secondary immunodeficiency were excluded from the study. The trial was conducted at MD Anderson Cancer Center (MDACC) between 2006 and 2009.
Procedures
After providing written informed consent, participants completed baseline (T1) questionnaires approximately 6 months after their surgical procedure or at the time of their initial consult before receiving systemic treatment. Participants were then randomly assigned to either the EW or NW group through a form of adaptive random assignment called minimization,38 ensuring that the groups were balanced on stage, sex, age, systemic treatment (yes v no), and surgery (yes v no). Participants in both groups completed three additional assessments at 1 (T2), 4 (T3), and 10 months (T4) later. Questionnaires were returned via mail. Participants also provided blood and saliva samples (biologic data and other self-reported secondary outcomes will be discussed in later reports). Participants received a $20 gift card for completing each assessment. MDACC's Institutional Review Board approved the protocol.
Description of Intervention
We followed the general writing procedures as outlined by Pennebaker and Beall19 with modifications based on our pilot work.24,27 Participants in the EW group were asked to write about their deepest emotions and thoughts regarding their cancer experience with slightly different probes at each session (eg, how the diagnosis and treatment interfere with their lives; treatment-related decision making; and fears about the future). Participants in the NW group were prompted to write about the following four neutral topics: dietary behaviors, physical activity and exercise behaviors, attitudes toward smoking and other substance use, and sleep habits. Participants in both groups were asked to complete four 20-minute writing assignments in their home over a 10-day period, with at least 1 day and no more than 3 days between sessions. A research assistant prompted participants with a phone call to begin and stop writing. Participants were provided with envelopes for mailing each writing sample as soon as it was completed.
Measures
Demographic and medical data were collected and extracted from medical records.
Intervention outcomes.
Cancer-related symptoms were assessed with the MD Anderson Symptom Inventory (MDASI),39 which asks patients to rate the severity of 13 core symptoms common across all cancer diagnoses and treatments and the extent to which these symptoms interfere with daily activities. Higher scores denote greater severity and interference. We report on the combined scales.
Fatigue was measured with the Brief Fatigue Inventory (BFI),40 a nine-item questionnaire asking participants to rate the severity of their fatigue and the degree to which it interferes with their lives. Higher scores represent worse fatigue, and a score of greater than 3 indicates clinically significant fatigue.
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D),41 a 20-item self-report measure focusing on the affective component of depression. A score of ≥ 16 indicates the need to screen for a depressive disorder.
Sleep disturbances were measured with the Pittsburgh Sleep Quality Index, an 18-item questionnaire that includes seven subscales and a total score assessing sleeping problems over the past month.42 Higher scores represent greater problems with sleep, and the total score is presented, with a score of ≥ 5 associated with clinically significant sleep disturbances.
Overall QOL was assessed with the Medical Outcomes Study Short Form-36 (SF-36),43 a generic QOL instrument assessing several distinct domains. The standardized mental component summary (MCS) and physical component summary (PCS) scores are presented, with higher scores denoting better QOL.
Hypothesized intervention mediator.
Intrusive thoughts and avoidance behaviors were measured with the Impact of Events Scale (IES), a 15-item scale assessing thought intrusion and avoidance during the past week.44 Higher scores represent greater intrusive thoughts and avoidance behaviors, and the total score is reported.
Data Analyses
The primary outcomes were the 10-month QOL indices (ie, cancer-related and depressive symptoms, fatigue, sleep disturbances, and overall QOL). A sample size of approximately 140 patients per group provided 80% power (two-sided α; P = .05) to detect an effect of 0.39 standard deviation (SD) units24 assuming 20% attrition. Main analyses were performed with multilevel modeling using PROC MIXED (SAS, 9.2.2 version; SAS Institute, Cary, NC). We specified an unstructured covariance matrix and a random intercept. Because age, sex, and stage at diagnosis have been associated with QOL outcomes in RCC,9,45–47 these factors were included as a priori covariates in all main analyses. In case some participants withdrew consent, we tested for systematic differences between completers and noncompleters and controlled for these characteristics in the main analyses. To evaluate the effectiveness of the EW intervention, we used an intent-to-treat approach and examined the group-time interaction effect while controlling for main effects and baseline scores of the outcome. A Bonferroni correction for six outcomes adjusted for a correlation among outcomes of r = 0.52 yields an α = .021.48,49 We used CONTRAST statements within the mixed procedure to test for group differences at each time point. PROC MIXED uses a likelihood-based estimation method for missing data so that attrition is less of a concern50; however, we also examined whether our findings would replicate using a multiple imputation strategy in SAS (PROC MI and PROC MIANALYZE)51 in case of missing data.
To test for mediation, we examined IES group differences at T2 using the general linear model controlling for IES baseline scores and aforementioned covariates. If a group difference at P ≤ .05 was found, we proceeded to examine indirect effects for significant T4 intervention outcomes. Because of recent criticism of classical mediation,52–54 we calculated indirect effects using the bias-corrected bootstrap procedure of Preacher and Hayes55 with the PROCESS macro of Hayes56 to test whether long-term (ie, 10-month; T4) benefits were mediated by earlier (ie, 1-month; T2) intervention effects on cognitive processing (IES scores). We then followed up with the Sobel test57 to provide a significance test of the indirect effect. Lastly, we examined whether intervention efficacy was moderated by dose or sex.
RESULTS
Baseline Characteristics of Sample
We approached 761 eligible patients; 355 patients consented to participate, and 287 completed baseline measures (Fig 1). Three patients became ineligible (misdiagnosis of RCC), resulting in a sample of 284 participants. Five patients withdrew before random assignment (death in family, n = 2; too busy, n = 3), 58 passively withdrew before initiation of the intervention, 18 passively withdrew after the intervention (unable to reach participant after repeated attempts), and 14 died over the course of the study; the dropout rate was similar in each group. χ2 tests and t tests comparing baseline demographic and medical characteristics and outcome measures of study completers versus noncompleters revealed no significant differences except for education (χ2 = 14.63, P = .005) and IES scores (t = 2.14, P = .023); completers were more likely to have had higher education and lower IES scores at baseline compared with noncompleters. No significant group differences were found in regard to demographic and medical factors (Table 1) or any of the baseline study variables. Regarding clinical cutoff scores, 20.5% of the sample met the CES-D criterion for caseness (mean score, 10.7; SD, 9.3), 36.1% experienced clinical levels of fatigue (mean score, 2.61; SD, 2.2), and 57% reported sleep disturbances (mean score, 6.89; SD, 4.2).
Table 1.
Participant Demographics and Clinical Characteristics by Group
| Demographic or Clinical Characteristic | EW (n = 138) |
NW (n = 139) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .69 | ||||
| Mean | 58.1 | 57.6 | |||
| SD | 9.8 | 9.9 | |||
| Range | 31-81 | 34-82 | |||
| Male | 83 | 60.1 | 86 | 61.9 | .81 |
| Race/ethnicity | .41 | ||||
| White | 105 | 76.1 | 113 | 81.3 | |
| Hispanic/Latino | 19 | 13.8 | 11 | 7.9 | |
| African American/Black | 4 | 2.9 | 5 | 3.6 | |
| Asian/Pacific Islander | 1 | 0.7 | — | — | |
| Native American | — | — | 5 | 3.6 | |
| Other | 7 | 5.1 | 1 | 0.7 | |
| Missing | 2 | 1.4 | 4 | 2.9 | |
| Marital status: married | 98 | 71.0 | 99 | 71.2 | .88 |
| Highest level of education: some college or higher | 99 | 72.7 | 109 | 79.0 | .26 |
| Income | .36 | ||||
| < $50,000 | 45 | 32.6 | 40 | 28.8 | |
| ≥ $50,000 | 85 | 61.6 | 89 | 64.0 | |
| Missing | 8 | 5.8 | 10 | 7.2 | |
| Employment status | .18 | ||||
| Full-time | 82 | 59.4 | 69 | 49.6 | |
| Part-time | 13 | 9.4 | 8 | 5.8 | |
| Unemployed | 2 | 1.4 | 6 | 4.3 | |
| Retired | 39 | 28.3 | 50 | 36.0 | |
| Missing | 2 | 1.4 | 6 | 4.3 | |
| Stage* | .99 | ||||
| I | 50 | 36.2 | 49 | 35.3 | |
| II | 18 | 13.0 | 18 | 12.9 | |
| III | 26 | 18.8 | 25 | 18.0 | |
| IV | 40 | 29.0 | 42 | 30.2 | |
| Missing | 4 | 2.9 | 5 | 3.6 | |
| Treatment | |||||
| Surgery | 98 | 71.0 | 97 | 69.8 | .60 |
| Systemic treatment | 52 | 37.7 | 51 | 36.7 | .87 |
| Cell type: clear cell | 107 | 79.9 | 108 | 80.6 | .89 |
Abbreviations: EW, emotional writing; NW, neutral writing; SD, standard deviation.
Group stage is based on the 2002 TNM staging of the American Joint Committee on Cancer and the International Union Against Cancer as suggested by Ng et al.58
Completion of Intervention
Two hundred five participants (72% of baseline sample) completed all four writing sessions, nine participants completed three sessions, seven completed two sessions, and seven completed only one session. The completion of writing sessions did not differ based on group assignment (χ2 = 3.74, P = .48), but it did differ by sex (χ2 = 10.97, P = .03), with women showing greater compliance than men.
Manipulation Check
On the basis of linguistic word count analyses using the Linguistic Inquiry and Word Count59 software, the writing samples of participants in the EW group revealed significantly more emotional content than did those in the NW group (F = 93.17, P < .001).
Intervention Efficacy
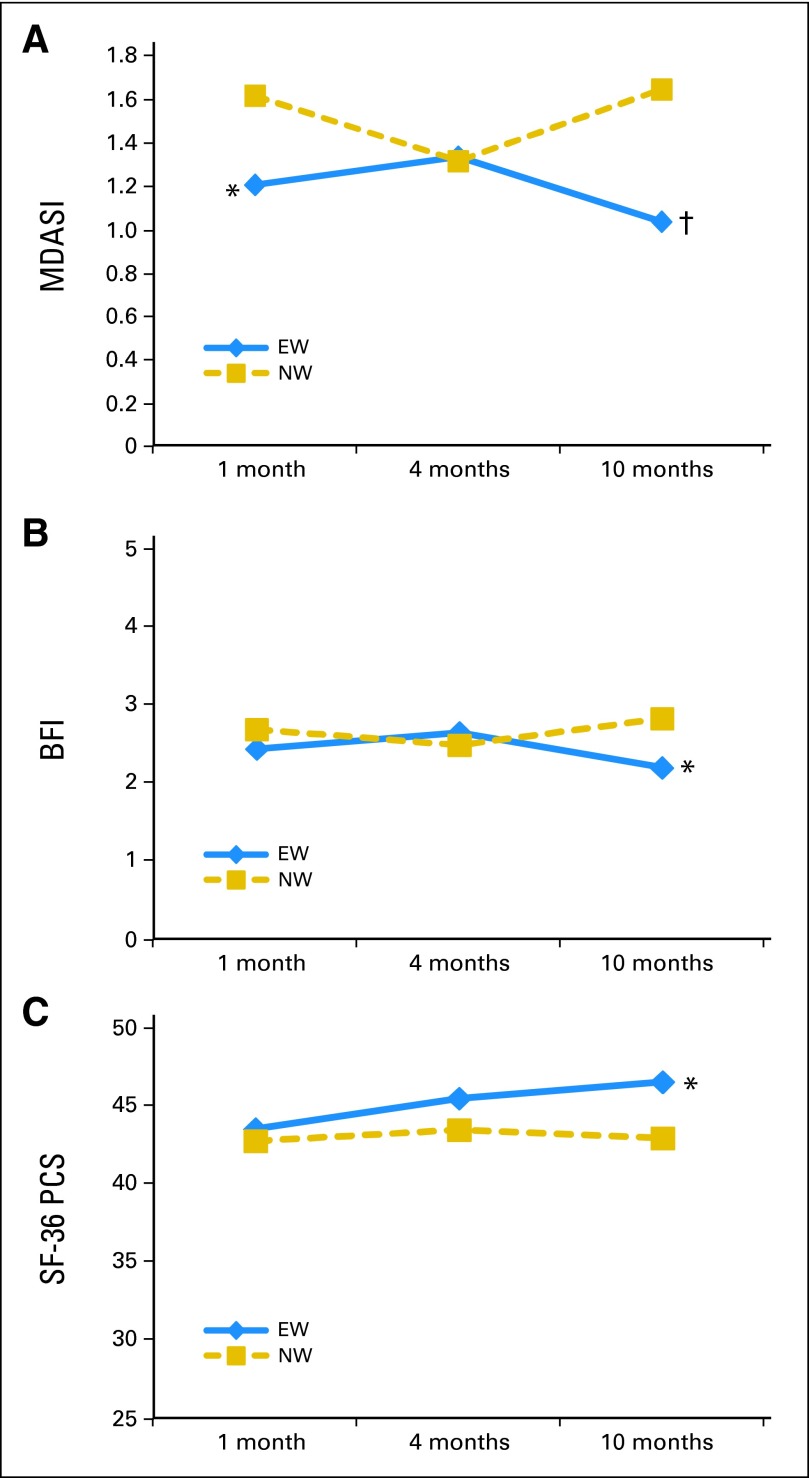
Table 2 lists the raw group means and SDs for outcomes at each assessment point. Figure 3 portrays the least square means at each assessment adjusted for baseline levels of the outcome and covariates (sex, age, stage, education, and baseline levels of the IES).
Table 2.
Outcome Measures by Group at Each Assessment Point
| Measure | Baseline (T1) |
1 Month (T2) |
4 Months (T3) |
10 Months (T4) |
Effect Size (d)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EW |
NW |
EW |
NW |
EW |
NW |
EW |
NW |
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| MDASI | 1.52 | 1.53 | 1.48 | 1.59 | 1.22 | 1.51 | 1.62 | 1.52 | 1.31 | 1.48 | 1.40 | 1.44 | 1.09 | 1.42 | 1.64 | 1.64† | 0.40 |
| BFI | 2.7 | 2.3 | 2.6 | 2.0 | 2.6 | 2.3 | 3.0 | 2.3 | 2.7 | 2.2 | 2.9 | 2.2 | 2.5 | 2.2 | 3.2 | 2.3‡ | 0.32 |
| CES-D | 10.3 | 9.7 | 11.3 | 8.9 | 8.8 | 8.6 | 10.2 | 6.7 | 9.9 | 10.1 | 9.5 | 7.1 | 9.5 | 9.6 | 10.5 | 7.4 | 0.10 |
| PSQI | 7.0 | 4.3 | 6.9 | 4.1 | 6.4 | 3.9 | 6.0 | 3.3 | 6.4 | 4.0 | 6.4 | 3.6 | 6.3 | 4.4 | 6.4 | 3.5 | 0.04 |
| SF-36 MCS | 49.6 | 8.7 | 47.8 | 9.1 | 44.8 | 47.7 | 48.7 | 8.2 | 48.9 | 9.3 | 48.4 | 7.3 | 48.2 | 8.7 | 48.8 | 7.0 | 0.10 |
| SF-36 PCS | 39.5 | 11.6 | 39.4 | 10.6 | 43.2 | 12.04 | 42.2 | 11.4 | 46.3 | 11.1 | 42.8 | 11.8 | 46.2 | 11.1 | 42.3 | 12.6ठ| 0.44 |
| IES | 17.8 | 15.1 | 19.6 | 15.0 | 11.3 | 13.7 | 15.9 | 14.0‖ | 12.0 | 12.9 | 13.4 | 14.4 | 12.7 | 14.7 | 13.8 | 14.3 | 0.03 |
Abbreviations: BFI, Brief Fatigue Inventory; CES-D, Center for Epidemiologic Studies Depression Scale; EW, emotional writing; IES, Impact of Events Scale; MCS, Mental Component Summary; MDASI, MD Anderson Symptom Inventory; NW, neutral writing; PCS, Physical Component Summary; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation; SF-36 Medical Outcomes Study Short Form-36.
Effect size, Cohen's d,60 for group differences at 10 months after the intervention (T4) using least square means adjusted for baseline levels, stage, age, sex, and education and baseline levels of IES and pooled SDs. Small effect, d = 0.2; medium effect, d = 0.5; and large effect, d = 0.8. Significant group differences for EW v NW are noted.
P < .01 from multilevel modeling analyses.
P < .05 from multilevel modeling analyses.
Clinically significant improvement from baseline.
P = .05 from multilevel modeling analyses.
Fig 3.
Least square means for (A) cancer-related symptoms (MD Anderson Symptom Inventory [MDASI]); (B) fatigue (Brief Fatigue Inventory [BFI]); and (C) physical function aspects of quality of life (Medical Outcomes Study Short Form-36, Physical Component Summary [SF-36 PCS]). Higher scores represent greater symptoms (MDASI and BFI) or better quality of life (SF-36 PCS). Group mean difference: (*) P < .05; (†) P < .01. EW, expressive writing; NW, neutral writing.
Cancer-related symptoms.
The group main effect was not significant after correcting for multiple comparisons (F = 4.31, P = .039). A significant group-time interaction effect (F = 5.16, P = .006) was found, and contrast comparisons revealed a significant group difference at T4 (F = 9.14, P = .003), indicating that participants in the EW group reported lower MDASI total scores compared with those in the NW group (Fig 3A). The group difference at T2 (F = 4.49, P = .035) was not significant after correcting for multiple comparisons.
Fatigue.
The group main effect (F = 1.03, P = .31) and the group-time interaction (F = 3.69, P = .027; contrast comparisons at T4: F = 4.49, P = .035) were not significant at the Bonferroni-corrected α level (Fig 3B).
Depressive symptoms and sleep disturbances.
The main effects, the group-time interaction, and the contrast comparisons for time were not significant for the CES-D and Pittsburgh Sleep Quality Index scores.
Overall QOL.
Neither the main effects nor the group-time interaction were significant for the MCS and PCS of the SF-36. Although none of the contrast comparisons for time were significant for the MCS of the SF-36, a significant group difference was seen at T4 for the PCS (F = 5.55, P = .019), with participants in the EW group reporting higher scores relative to the NW group (Fig 3C). For patients in the EW group, improvements from baseline to T4 were greater than half an SD, indicating clinically significant improvements not seen in the NW group.61 All findings were replicated (with some larger effects) when the imputed data were analyzed.
Dose response and sex differences.
Neither number of sessions completed (dose) nor sex moderated intervention efficacy for any of the outcomes.
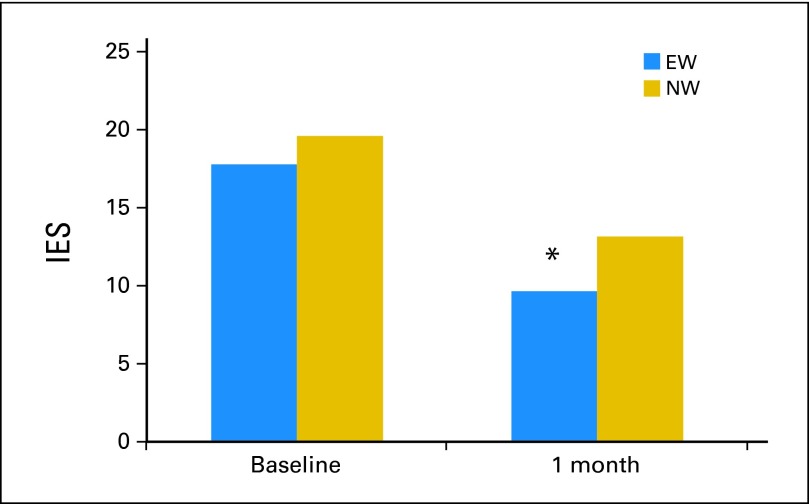
Intrusive Thoughts and Avoidance Behaviors As Intervention Mediator
Marginally significant group differences were found in IES scores at T2 (F = 3.77, P = .053), with participants in the EW group reporting lower scores than those in the NW group (Fig 4). Because of the a priori, theory-driven mediation hypothesis, we proceeded to examine IES at T2 as a mediator of significant between-group differences for intervention outcomes at T4 at P ≤ .05 (MDASI, BFI, and PCS). For MDASI scores, bootstrapping of 5,000 random sample simulations generated a bootstrap estimate of 1.92 (SE, 1.30) with a 95% bias-corrected and accelerated CIs (BCa) of 0.03 and 5.25. Because the BCa did not cross zero and the Sobel test was significant (z = 2.07, P = .042), mediation was established. Similarly, for BFI scores, bootstrapping resulted in an estimate of 0.17 (SE, 0.12) with 95% BCa of 0.003 and 0.48 and a significant Sobel test (z = 1.93, P = .050) establishing evidence for mediation. There was no evidence that IES mediated the intervention effect for PCS scores.
Fig 4.
Baseline and 1-month (T2) postintervention group differences for intrusive thoughts and avoidance behaviors (Impact of Events Scale [IES]). At baseline, raw means are depicted. At T2, least square means controlling for baseline levels, stage, age, sex, and education are depicted. Group mean difference: (*) P = .53. EW, expressive writing; NW, neutral writing.
DISCUSSION
The current EW trial, to our knowledge the largest conducted to date in an oncology population, found that EW reduced cancer-related symptoms and improved physical functioning and possibly fatigue in patients receiving treatment for RCC. The most pronounced group differences emerged 10 months after the intervention. This may be explained by our hypothesized mediation model, which suggested that later outcomes are mediated by the early intervention effects on intrusive thoughts and avoidance behaviors. More specifically, the current data revealed that patients who wrote about their deepest concerns related to the cancer experience reported fewer intrusive thoughts and avoidance 1 month after completing the writing sessions compared with those in the NW group, which was, in turn, related to fewer long-term cancer-related symptoms and possibly less fatigue.
Although previous research on this subject focused primarily on women with breast cancer, we demonstrated that EW seems to be equally beneficial for men and women with RCC. Similar to previous findings, this research is consistent with EW trials that have reported somatic symptom relief and fewer cancer-related physician visits.23,25,62 Unlike other work, this investigation revealed overall group differences in cancer-related symptoms as opposed to differences in only a subset of the participants (eg, low avoidance coping,23 recently diagnosed, lacking social support25). Nevertheless, in future secondary analyses, we will explore whether subsets of patients (eg, those lacking social support or low in avoidance coping or high in distress) might benefit more from EW than others. Such moderation analyses may explain our null findings regarding depression and sleep, which are consistent with the null findings of previous trials.26–28
Additionally, future research is needed to examine the mediating role of intrusive thoughts and avoidance behaviors on EW's effects on cancer symptoms and fatigue. Intrusive thoughts may cause hypervigilance/hyperarousal, which diminishes mental energy and increases catastrophizing and leads to cancer-related symptoms.8,63 Evidence also suggests that post-traumatic stress symptoms, such as intrusive thoughts, lead to increased activity in endocrine and inflammatory pathways,64–68 which play a role in cancer-related symptoms69–71 and fatigue.17,72,73 Our next step is to examine the biologic outcomes from blood (eg, immune function) and saliva samples (eg, cortisol) to potentially uncover shared biologic pathways establishing a biobehavioral process as explanatory mechanisms.
Although treatment effects were generally moderate, clinically significant improvements in PCS scores were associated with EW. Considering the findings in light of an entirely self-administered, brief, safe, and virtually no-cost intervention, EW seems to be a promising supportive care approach. However, further research that uses a distress eligibility criterion is needed that will allow researchers to rigorously examine the impact of EW on distress and compare EW with other behavioral/psychosocial programs such as cognitive behavioral therapy or expressive-disclosure groups.74 Additionally, future research is needed to explain why EW may improve some measures of mental health (eg, IES) but not others (eg, CES-D).
Our study had some limitations. Only 47% of approached patients consented to participate. Of these, 19% withdrew before baseline assessment, and 73% completed the 10-month assessment. This completion rate is somewhat lower than a previous large RCT of EW in cancer,23 and attrition is a limitation; however, our trial included a longer follow-up period than previous studies. Additionally, we performed multiple imputation analyses for the missing data, which replicated findings involving observed data, so that a bias introduced by attrition is less of a concern. Although most participants completed all four writing sessions, EW may not be an acceptable program for all patients. In fact, a dislike for writing was the main reason for study refusal. Additionally, patients with lower education and higher IES scores were more likely to withdraw from the study. This may mean that educated people and people who are less distressed are more comfortable with writing. Results regarding fatigue need to be interpreted with caution because group differences reached the conventional but not the Bonferroni-corrected significance level; nevertheless, a medium-size treatment effect was found. Finally, although representative of the patient population at MDACC, our sample's ethnic diversity was restricted.
In conclusion, this RCT found that EW is a safe, brief, and cost-effective therapeutic approach that may improve cancer-related symptoms and physical functioning, with benefits emerging 10 months after EW. This finding may be possibly explained via early improvement in cognitive processing, which in turn are associated with long-term improvements in cancer-related symptoms.
Supplementary Material
Footnotes
Supported in part by National Cancer Institute Grant No. R01CA090966 (L.C.).
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2012, Chicago, IL; and the 9th International Conference of the Society for Integrative Oncology, October 8-10, 2012, Albuquerque, NM.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00505310.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Nizar Tannir, Novartis (C), Pfizer (C), AVEO Pharmaceuticals (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: Nizar Tannir, Pfizer, GlaxoSmithKline Research Funding: Nizar Tannir, Pfizer, GlaxoSmithKline Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Christopher Wood, Nizar Tannir, Louis Pisters, Lorenzo Cohen
Collection and assembly of data: Amy Spelman, Surena F. Matin, Qi Wei
Data analysis and interpretation: Kathrin Milbury, Eric Jonasch, Qi Wei, Lorenzo Cohen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Redd WH, DuHamel KN, Johnson Vickberg SM, et al. Long-term adjustment in cancer survivors: Integration of classical-conditioning and cognitive processing models. In: Baum A, Andersen BL, editors. Psychosocial Interventions for Cancer. Washington, DC: American Psychological Association; 2001. pp. 77–97. [Google Scholar]
- 2.Green BL, Epstein SA, Krupnick JL, et al. Trauma and medical illness: Assessing trauma-related disorders in medical settings. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD. New York, NY: The Guilford Press; 1997. pp. 160–191. [Google Scholar]
- 3.Cordova MJ, Andrykowski MA, Kenady DE, et al. Frequency and correlates of posttraumatic stress disorder–like symptoms after treatment for breast cancer. J Consult Clin Psychol. 1995;63:981–986. doi: 10.1037//0022-006x.63.6.981. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg MA. Cognitive processing of traumas: The role of intrusive thoughts and reappraisals. J Appl Soc Psychol. 1995;25:1262–1296. [Google Scholar]
- 5.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: Relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13:211–220. doi: 10.1002/pon.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horowitz M. Stress Response Syndromes (ed 2) Northvale, NJ: Aronson; 1986. [Google Scholar]
- 7.Whitaker KL, Brewin CR, Watson M. Intrusive cognitions and anxiety in cancer patients. J Psychosom Res. 2008;64:509–517. doi: 10.1016/j.jpsychores.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Dupont A, Bower JE, Stanton AL, et al. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol. doi: 10.1037/a0031131. [epub ahead of print on February 4, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker PA, Swartz R, Fellman B, et al. Comprehensive assessment of quality of life and psychosocial adjustment in patients with renal tumors undergoing open, laparoscopic and nephron sparing surgery. J Urol. 2012;187:822–826. doi: 10.1016/j.juro.2011.10.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine D, Parker PA, Fouladi RT, et al. The association between social support, intrusive thoughts, avoidance, and adjustment following an experimental cancer treatment. Psychooncology. 2003;12:453–462. doi: 10.1002/pon.656. [DOI] [PubMed] [Google Scholar]
- 11.Manne S, Glassman M, Du Hamel K. Intrusion, avoidance, and psychological distress among individuals with cancer. Psychosom Med. 2001;63:658–667. doi: 10.1097/00006842-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: Role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 14.Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirl WF, Greer JA, Traeger L, et al. Depression and survival in metastatic non-small-cell lung cancer: Effects of early palliative care. J Clin Oncol. 2012;30:1310–1315. doi: 10.1200/JCO.2011.38.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennebaker JW, Beall SK. Confronting a traumatic event: Toward an understanding of inhibition and disease. J Abnorm Psychol. 1986;95:274–281. doi: 10.1037//0021-843x.95.3.274. [DOI] [PubMed] [Google Scholar]
- 20.Roberts KJ, Lepore SJ, Helgeson V. Social-cognitive correlates of adjustment to prostate cancer. Psychooncology. 2006;15:183–192. doi: 10.1002/pon.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepore SJ. Expressive writing moderates the relation between intrusive thoughts and depressive symptoms. J Pers Soc Psychol. 1997;73:1030–1037. doi: 10.1037//0022-3514.73.5.1030. [DOI] [PubMed] [Google Scholar]
- 22.Slatcher RB, Pennebaker JW. Emotional processing of traumatic events. In: Cooper C, editor. Handbook of Stress, Medicine and Health. ed 2. Cambridge, MA: Cambridge Press; 2005. [Google Scholar]
- 23.Stanton AL, Danoff-Burg S, Sworowski LA, et al. Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. J Clin Oncol. 2002;20:4160–4168. doi: 10.1200/JCO.2002.08.521. [DOI] [PubMed] [Google Scholar]
- 24.de Moor C, Sterner J, Hall M, et al. A pilot study of the effects of expressive writing on psychological and behavioral adjustment in patients enrolled in a phase II trial of vaccine therapy for metastatic renal cell carcinoma. Health Psychol. 2002;21:615–619. doi: 10.1037//0278-6133.21.6.615. [DOI] [PubMed] [Google Scholar]
- 25.Low CA, Stanton AL, Bower JE, et al. A randomized controlled trial of emotionally expressive writing for women with metastatic breast cancer. Health Psychol. 2010;29:460–466. doi: 10.1037/a0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker BL, Nail LM, Croyle RT. Does emotional expression make a difference in reactions to breast cancer? Oncol Nurs Forum. 1999;26:1025–1032. [PubMed] [Google Scholar]
- 27.de Moor JS, Moyé L, Low MD, et al. Expressive writing as a presurgical stress management intervention for breast cancer patients. J Soc Integr Oncol. 2008;6:59–66. [PubMed] [Google Scholar]
- 28.Mosher CE, Duhamel KN, Lam J, et al. Randomised trial of expressive writing for distressed metastatic breast cancer patients. Psychol Health. 2012;27:88–100. doi: 10.1080/08870446.2010.551212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth JM, Stone AA, Hurewitz A, et al. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: A randomized trial. JAMA. 1999;281:1304–1309. doi: 10.1001/jama.281.14.1304. [DOI] [PubMed] [Google Scholar]
- 30.Pennebaker JW, Francis ME. Cognitive, emotional, and language processes in disclosure. Cognition Emotion. 1996;10:601–626. [Google Scholar]
- 31.Campbell RS, Pennebaker JW. The secret life of pronouns: Flexibility in writing style and physical health. Psychol Sci. 2003;14:60–65. doi: 10.1111/1467-9280.01419. [DOI] [PubMed] [Google Scholar]
- 32.Klein K, Boals A. Expressive writing can increase working memory capacity. J Exp Psychol Gen. 2001;130:520–533. doi: 10.1037//0096-3445.130.3.520. [DOI] [PubMed] [Google Scholar]
- 33.Low CA, Stanton AL, Danoff-Burg S. Expressive disclosure and benefit finding among breast cancer patients: Mechanisms for positive health effects. Health Psychol. 2006;25:181–189. doi: 10.1037/0278-6133.25.2.181. [DOI] [PubMed] [Google Scholar]
- 34.Tang X, Liu T, Zang X, et al. Adoptive cellular immunotherapy in metastatic renal cell carcinoma: A systematic review and meta-analysis. PLoS One. 2013;8:e62847. doi: 10.1371/journal.pone.0062847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry DS, Pennebaker JW. Nonverbal and verbal emotional expression and health. Psychother Psychosom. 1993;59:11–19. doi: 10.1159/000288640. [DOI] [PubMed] [Google Scholar]
- 36.Rivkin ID, Gustafson J, Weingarten I, et al. The effects of expressive writing on adjustment to HIV. AIDS Behav. 2006;10:13–26. doi: 10.1007/s10461-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 37.Koschwanez HE, Kerse N, Darragh M, et al. Expressive writing and wound healing in older adults: A randomized controlled trial. Psychosom Med. 2013;75:581–590. doi: 10.1097/PSY.0b013e31829b7b2e. [DOI] [PubMed] [Google Scholar]
- 38.Pocock SJ. Clinical Trials: A Practical Approach. New York, NY: John Wiley & Sons; 1983. [Google Scholar]
- 39.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Radloff LS. The CES-D scale: A new self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 42.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Ware J, Jr, Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide, The Health Institute. Boston, MA: New England Medical Centre Hospitals; 1993. [Google Scholar]
- 44.Horowitz M, Wilner N, Alvarez W. Impact of Events Scale: Measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Downs TM, Schultzel M, Shi H, et al. Renal cell carcinoma: Risk assessment and prognostic factors for newly diagnosed patients. Crit Rev Oncol Hematol. 2009;70:59–70. doi: 10.1016/j.critrevonc.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poulakis V, Witzsch U, de Vries R, et al. Quality of life after surgery for localized renal cell carcinoma: Comparison between radical nephrectomy and nephron-sparing surgery. Urology. 2003;62:814–820. doi: 10.1016/s0090-4295(03)00687-3. [DOI] [PubMed] [Google Scholar]
- 47.Stark D, Kiely M, Smith A, et al. Anxiety disorders in cancer patients: Their nature, associations, and relation to quality of life. J Clin Oncol. 2002;20:3137–3148. doi: 10.1200/JCO.2002.08.549. [DOI] [PubMed] [Google Scholar]
- 48.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 50.Wolfer R, Sang C. Comparing the SAS GLM and MIXED Procedures for Repeated Measures. Proceedings of the Twentieth Annual SAS Users Group Conference; Cary, NC: SAS Institute; 1995. [Google Scholar]
- 51.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 52.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 53.MacKinnon DP, Lockwood CM, Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- 55.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Ins C. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 56.Hayes A. PROCESS: A versatile computational tool for observed variable moderation, mediation and conditional process modeling. http://www.afhayes.com/public/process2012.pdf.
- 57.MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivar Behav Res. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng CS, Wood CG, Silverman PM, et al. Renal cell carcinoma: Diagnosis, staging, and surveillance. AJR Am J Roentgenol. 2008;191:1220–1232. doi: 10.2214/AJR.07.3568. [DOI] [PubMed] [Google Scholar]
- 59.Pennebaker JW, Booth RJ, Francis ME. Linguistic Inquiry and Word Count (LIWC) http://languageinquiry.com/
- 60.Cohen J. Statistical Power Analysis for the Behavioral Sciences (ed 2) Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 61.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: A phase 1-2 trial. Lancet Oncol. 2012;13:395–402. doi: 10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frisina PG, Borod JC, Lepore SJ. A meta-analysis of the effects of written emotional disclosure on the health outcomes of clinical populations. J Nerv Ment Dis. 2004;192:629–634. doi: 10.1097/01.nmd.0000138317.30764.63. [DOI] [PubMed] [Google Scholar]
- 63.Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72:355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–459. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 65.Spivak B, Shohat B, Mester R, et al. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- 66.Vidoviæ A, Gotovac K, Vilibiæ M, et al. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation. 2011;18:199–211. doi: 10.1159/000322869. [DOI] [PubMed] [Google Scholar]
- 67.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mundy-Bosse BL, Thornton LM, Yang HC, et al. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 70.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 72.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carmack CL, Basen-Engquist K, Yuan Y, et al. Feasibility of an expressive-disclosure group intervention for post-treatment colorectal cancer patients: Results of the Healthy Expressions study. Cancer. 2011;117:4993–5002. doi: 10.1002/cncr.26110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.