Abstract
Atherosclerosis and osteoporosis are the leading causes of mortality and morbidity in the World. Recent epidemiologic studies have demonstrated that these disease processes develop in parallel. Evidence indicates that hyperlipidemia plays a paradoxical role in both disease processes. However, the mechanism is not understood. This prospectus hypothesizes the role of lipids activate atherosclerosis within the bone and the heart to initiate the development of diseases in both of these tissues. The Prospectus on the Lrp 5/6 receptors provides a foundation for the mechanisms involved in the Lrp5/6 mediated disease biology. The LDL-Density-Pressure theory: the Role of Lrp5/6 provides a biological and a hemodynamic approach towards understanding the development of valvular heart disease and the implications in the field of bone molecular biology. This prospectus will review the current literature, provide a basis for the development of valve disease and indicate future therapeutic pathways for this disease process in the future.
Keywords: LIPIDS, Lrp5, Wnt, OSTEOBLASTOGENESIS
The low-density lipoprotein (LDL)-related receptor 5 and 6 (Lrp5 and Lrp6) genes were cloned in 1998 based on their homology with the LDL receptor (LDLR) [Brown et al., 1998; Dong et al., 1998; Hey et al., 1998; Kim et al., 1998]. Mutations in either LRP5 or LRP6 proteins have caused a number of disease processed in the field of bone [Gong et al., 2001; Little et al., 2002], and have been associated with cardiovascular disease [Kim et al., 1998; Fujino et al., 2003; Rajamannan et al., 2005a; Caira et al., 2006]. The most recent perspectives in the field of Lrp5/6 [Williams and Insogna, 2009] in signaling in the bone and Lrp5 in the regulation of bone mass [Johnson and Summerfield, 2005] demonstrate the most comprehensive reviews in this field and provide the background for the structure and function of these co-receptors. The LDL-density-pressure theory combines the structure, function analysis of these co-receptors with the results from the genetic studies to provide a unique hypothesis for the role of these receptors in the heart. The focus of this prospectus is to further combine the structure, function, developmental biology with the genetics studies of the Lrp5/6 co-receptors to further define their role in the development of cardiovascular calcification as related to the bone formation within the heart.
Lrp5/6 AND CANONICAL Wnt SIGNALING IN OSTEOBLASTOGENESIS
The LDL co-receptor Lrp5/6 is a member of the family of structurally closely related cell surface LDLRs that have diverse biological functions in different organs, tissues, and cell types which are important in development and disease mechanisms. The most prominent role in this evolutionary ancient family is cholesterol homeostasis. In humans, cholesterol in the blood is captured by LDL and metabolized by the liver via endocytosis of the LDLR. There is recent evidence that members of the LDLR gene family are active in the cell signaling pathways between specialized cells in many multicellular organisms.
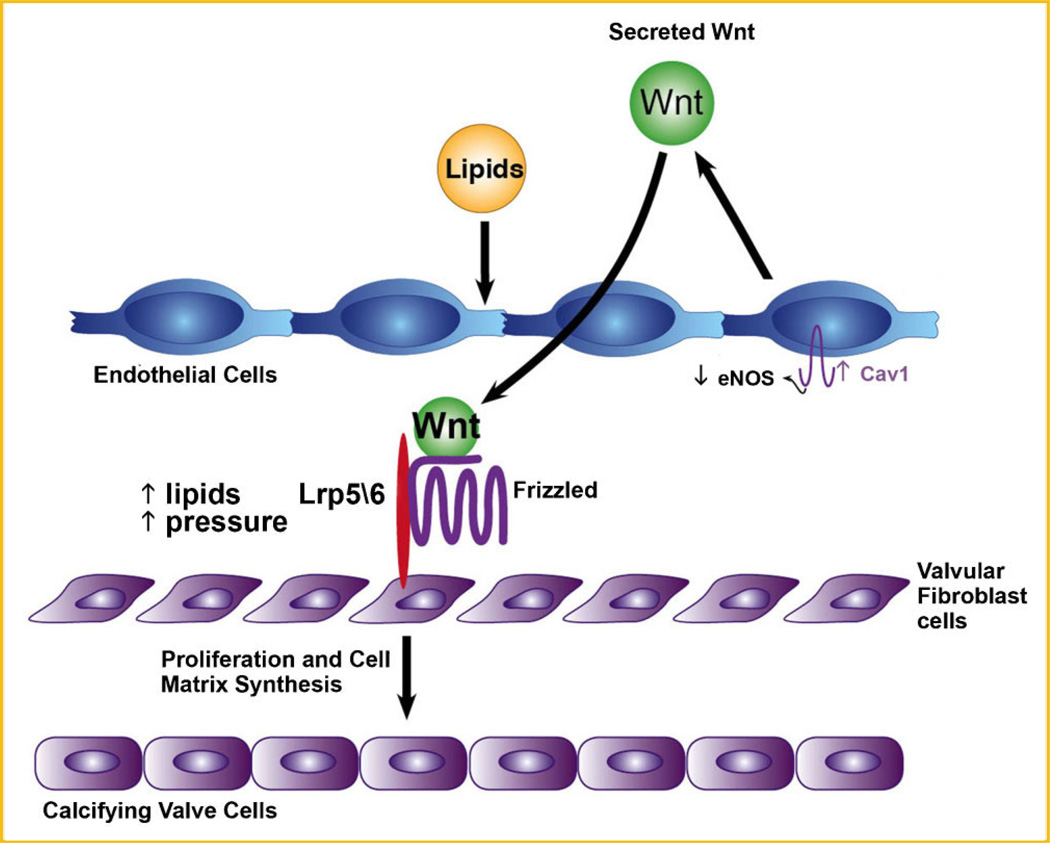
The LRP5 pathway regulates bone formation in different diseases of bone [Gong et al., 2001; Boyden et al., 2002]. The discovery of the LRP5 receptor in the gain of function [Boyden et al., 2002] and loss of function [Gong et al., 2001] mutations in the development of bone diseases, resulted in a number of studies which have shown that activation of the canonical Wnt pathway is important in osteoblastogenesis [Babij et al., 2003; Fujino et al., 2003; Holmen et al., 2004; Westendorf et al., 2004]. Three studies to date have confirmed the regulation of the LRP5/Wnt pathway for cardiovascular calcification in vivo and ex vivo [Shao et al., 2005; Rajamannan et al., 2005a; Caira et al., 2006]. The LRP5 receptor signaling in bone is mediated via the canonical Wnt pathway as shown in Figure 1. In this pathway, Wnt proteins bind to receptors composed of a frizzled protein and either of the LDLR-related proteins LRP5 or LRP6. Signaling via Disheveled and/or Axin then results in inactivation of a multiprotein complex including Axin, adenomatous polyposis coli (APC), and glycogen synthase kinase-3β that normally renders β-catenin unstable. By inhibiting this complex, Wnt signals lead to accumulation of β-catenin in the cytosol and its entry into the nucleus. Once in the nucleus, β-catenin binds to proteins of the T-cell factor/lymphoid enhancer factor-1 family and modulates the expression of several target genes which include Cyclin D, Msx2, Cbfa1, and Sox9. Bone and cartilage are major tissues in the vertebrate skeletal system, which is primarily composed of three cell types: osteoblasts, chrondrocytes, and osteoclasts. In the developing embryo, osteoblast and chrondrocytes both differentiate from common mesenchymal progenitors in situ, whereas osteoclasts are of hematopoietic origin and brought in later by invading blood vessels. Osteoblast differentiation and maturation lead to bone formation controlled by two distinct mechanisms: intramembranous and endochondral ossification, both starting from mesenchymal condensations.
Fig. 1.
Schematic for the mechanism of Lrp5/6 in Canonical Wnt Activation.
Lrp5 has been shown to have an effect on bone mass via the mechanostat effect on regulating bone formation. The findings in the human of the high bone mass gain of function mutation [Little et al., 2002] led to a series of discoveries that Lrp5 regulates bone mass via the mechanical force effect on the receptor [Akhter et al., 2004; Johnson et al., 2004; Johnson and Summerfield, 2005]. Lrp6 also regulates bone but has been found to have a low bone mass effect in patients in which a putative partial loss-of-function mutation in LRP6 was associated with early cardiovascular-related death associated with increased plasma LDL, triglycerides, hypertension, diabetes, and osteoporosis [Mani et al., 2007]. The first paper for the proof of concept demonstrated that Lrp5 and Lrp6 both play an important role in bone formation in the Lrp5 null mice and the Lrp6 heterozygote mice [Holmen et al., 2004]. This paper demonstrated that both receptors are necessary for bone mass and limb development. These data were further corroborated with studies presented at the Sun Valley workshop in 2008 [Zylstra et al., 2008], which indicated that the homozygous LRP6flox mice with the osteocalcin (OC) promoter had significantly low bone mass demonstrating that Lrp6 was required for normal bone acquisition. When the mice were mated with the Lrp5 null mice, the offspring developed severe osteopenia with reduced bone formation and increased bone resorption suggesting that Lrp5 and Lrp6 are required to fully activate β-catenin in mature osteoblasts.
THE STRUCTURAL ROLE OF Lrp5 AND Lrp6 AS SUBCLASS MEMBERS OF LDLR IN LIPOPROTEIN METABOLISM
The LDLR gene family consists of cell surface proteins involved receptor-mediated endocytosis of specific ligands. This class of gene family has similar protein structures and plays a role in lipid metabolism. However the genetic studies in the field of cardiovascular disease have revealed novel signaling and disease mechanisms for the various subclasses of this family of receptors. LDL is normally bound at the cell membrane and taken into the cell ending up in lysosomes where the protein is degraded and the cholesterol is made available for repression of microsomal enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, the rate-limiting step in cholesterol synthesis. At the same time, a reciprocal stimulation of cholesterol ester synthesis takes place. Mutations in this gene cause the autosomal dominant disorder, familial hypercholesterolemia.
Lrp5 co-receptor in lipid metabolism encodes a transmembrane LDLR that binds and internalizes ligands in the process of receptor-mediated endocytosis. This protein also acts as a co-receptor with Frizzled protein family members for transducing signals by Wnt proteins and was originally cloned on the basis of its association with type 1 diabetes mellitus in humans. This protein plays a key role in skeletal homeostasis and many bone density related diseases are caused by mutations in this gene. Mutations in this gene also cause familial exudative vitreoretinopathy. Mutations results in increases in bone mass (gain of function) [Little et al., 2002] and losses in bone mass (loss of function) [Gong et al., 2001].
The role of lipid signaling of the LRP5 receptor has been defined in experimental in vitro and in vivo lipid models of vascular atherosclerosis. LRP5 binds apoE-containing lipoproteins in vitro and is widely expressed in many tissues including hepatocytes, adrenal gland, and pancreas [Kim et al., 1998]. The production of mice lacking LRP5 revealed that LRP5 deficiency led to increased plasma cholesterol levels in mice fed a high-fat diet, secondary to decreased hepatic clearance of chylomicron remants and also marked impaired glucose tolerance [Fujino et al., 2003]. In the LRP5 mice that were not fed the high cholesterol diet, the mice did not develop high cholesterol levels [Magoori et al., 2003]. The investigators went on to define the role of LRP5 in the lipoprotein metabolism by developing a double knockout mouse for ApoE:LRP5. They found that the double KO mouse had approximately 60% higher cholesterol levels compared with the age matched apoE knockout mice. High-performance liquid chromatography analysis of plasma lipoproteins revealed that their was no difference in the apoproteins but the cholesterol levels in the very low density and LDL fractions were markedly increased in the apoE:Lrp5 double KO mice. There was threefold increase in the atherosclerosis indicating that the Lrp5 mediates both apoE-dependent and apoE-independent catabolism of lipoproteins. In 1994, studies demonstrated that the plasma cholesterol levels in the double KO mice lacking both ApoE and LDLR were not significantly different from the levels in the ApoE knockout mice [Ishibashi et al., 1994]. The severe hypercholesterolemia developed in the double knockout mice lacking both apoE and Lrp5 suggests the presence of an alternative pathway for cholesterol catabolism mediated by Lrp5, which appears to be independent of the LDLR pathway. The LRP5 deficient islets also demonstrated a reduction of intracellular ATP and calcium in response to glucose, and thereby decreasing glucose induced insulin secretion [Fujino et al., 2003]. Furthermore, experimental hypercholesterolemia is associated with the increase in LRP5 receptor expression and activation of cell proliferation and extracellular matrix production critical in bone formation [Rajamannan et al., 2005a]. These studies provide evidence that lipoprotein metabolism is regulated by the fifth family member of the LDL co-receptor family LRP5 in these knockout mouse studies.
Lrp6 co-receptor also plays a role in bone formation and lipid metabolism. This gene encodes a member of the LDLR gene family. LDLRs are transmembrane cell surface proteins involved in receptor-mediated endocytosis of lipoprotein and protein ligands. The protein encoded by this gene functions as a receptor or, combines with Frizzled, as co-receptor for Wnt and thereby transmits the canonical Wnt/beta-catenin signaling cascade. Through its interaction with the Wnt/beta-catenin signaling cascade this gene plays a role in the regulation of cell differentiation, proliferation, and migration and the development of many cancer types. This protein undergoes gamma-secretase dependent RIP-(regulated intramembrane proteolysis) processing but the precise locations of the cleavage sites have not been determined. Mutations results in cardiovascular events related to increases in LDL cholesterol [Mani et al., 2007; Tomaszewski et al., 2009]. In 2008, studies demonstrate that mutations in the EGFP domain of the LDLR-related protein 6 impairs cellular LDL clearance. The LRP6 receptor has been extensively studied for it function in regulating embryogenesis and cell proliferation [Pinson et al., 2000; Tamai et al., 2004]. This protein has been localized to lipid rafts [Yamamoto et al., 2006; Komekado et al., 2007]. Furthermore, LRP6 is important in LDL clearance, as shown in in vitro and in vivo Lrp5/6+/− mice [Liu et al., 2008].
Lrp5/6 AND Wnt SIGNALING IN CARDIAC VALVE DEVELOPMENT
Wnt signaling and Lrp5/6 co-receptors are critical in the signaling process in cardiac development [Gessert and Kuhl, 2010]. A recent discovery in Lrp6 research is the role of kremen 2 (KRM2) for neural crest induction, and the in vivo [Hassler et al., 2007]. The study indicates that Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt Signaling. Wnt signaling has also been demonstrated in heart valve development, osteogenic gene induction at highly specific stages of valve development, remodeling of atrioventricular (AV) and semilunar valves [Alfieri et al., 2010]. All of the developmental data provide the importance of this evolutionary signaling pathway in heart valve and cardiac development. After birth, Lrp5/6 expression is low unless it the expression increases in disease processes.
Lrp5/6 SIGNALING IN OSTEOBLASTOGENESIS IN VALVULAR HEART DISEASE
Our group and others have demonstrated that osteoblastogenesis and chrondrogenesis is critical in the development of valvular heart disease. The presence of calcification in the aortic valve is responsible for valve stenosis. Severe aortic stenosis can result in symptomatic chest pain, as well as syncope and congestive heart failure in patients with severe aortic valve stenosis. For years, aortic valve stenosis was thought to be a degenerative process. However, the pathologic lesions of calcified aortic valves demonstrate indicate the presence of complex calcification in these tissues. Furthermore, there are a growing number of descriptive studies delineating the presence of bone formation in the aortic valve [O’Brien et al., 1995a; Mohler et al., 1997, 2001].
Until recently the etiology of valvular heart disease has been thought to be a degenerative process related to the passive accumulation of calcium binding to the surface of the valve leaflet. Recent descriptive studies have demonstrated the critical features of aortic valve calcification, including osteoblast expression, cell proliferation, and atherosclerosis [O’Brien et al., 1995b; Mohler et al., 2001; Rajamannan et al., 2002, 2003b] and mitral valve degeneration, glycosaminoglycan accumulation, proteoglycan expression, and abnormal collagen expression [Whittaker et al., 1987; Wooley et al., 1991; Grande-Allen et al., 2004, 2005]. These studies define the biochemical and histological characterization of these valve lesions. Studies have also shown that specific bone cell phenotypes are present in calcifying valve specimens in human specimens [Jian et al., 2001; Caira et al., 2006]. These data provide the evidence that the aortic valve calcification follows the spectrum of bone formation in calcifying tissues. Genes which code for the bone extracellular matrix proteins in osteoblast cells include alkaline phosphatase (AP), osteopontin (OP), OC, and bone sialoprotein (BSP). These data support a potential regulatory mechanism that these matrix proteins play a role in the development of biomineralization. To date, many of these markers have been shown to be critical in the extracellular mineralization and bone formation that develops in normal osteoblast differentiation. Figure 1 demonstrates the dual role of Lrp5/6 in the activation of Wnt signaling: (1) lipid binding and (2) mechanostat effect in the activation of the Wnt signaling pathway.
CARDIOVASCULAR RISK FACTORS
SPECIFICALLY LIPID BIOCHEMISTRY IN VALVULAR HEART DISEASE
With the decline in the incidence of rheumatic carditis, calcific AS has become the most common indication for surgical valve replacement in the US. Numerous epidemiologic studies have identified risk factors for AS disease development, which are similar to those of vascular atherosclerosis, including smoking, male gender, body mass index, hypertension, elevated lipid and inflammatory markers, metabolic syndrome, and renal failure [Deutscher et al., 1984; Hoagland et al., 1985; Aronow et al., 1987; Mohler et al., 1991; Lindroos et al., 1994; Boon et al., 1997; Stewart et al., 1997; Wilmshurst et al., 1997; Otto et al., 1999; Palta et al., 2000; Chan et al., 2001; Chui et al., 2001; Pohle et al., 2001; Aronow et al., 2001b; Peltier et al., 2003; Briand et al., 2006; Faggiano et al., 2006]. For years this disease process was thought to be due to a degenerative phenomenon by which calcium attaches to the surface of the aortic valve leaflet. Understanding calcification, as the critical end-stage process which causes progression to severe stenosis and leads to poor outcomes [Rosenhek et al., 2000], is becoming important in the results of the randomized trials for treating aortic stenosis with medical therapy.
HEMODYNAMIC PHENOTYPE OF CARDIAC VALVE DISEASE
ROLE OF Lrp5/6 IN CARDIAC VALVE DISEASE
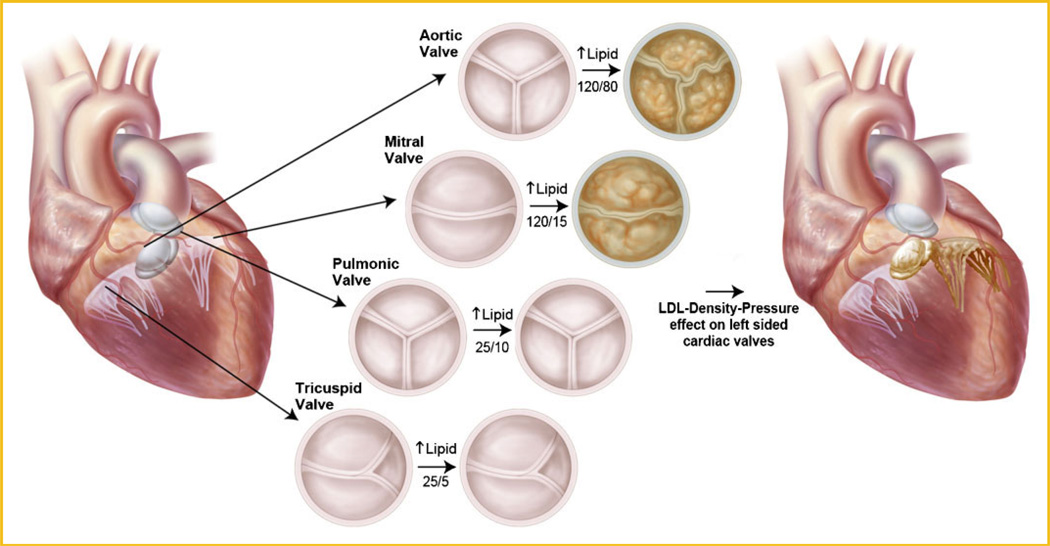
The development of heart valve disease occurs in the left side of the heart the aortic valve and the mitral valve, which manifests as calcific aortic valve disease and myxomatous mitral valve disease. The LDL-density-pressure theory provides a scientific explanation for the manifestation of this phenotype. In experimental hypercholesterolemia the left-sided heart valves aortic [Rajamannan et al., 2001, 2002, 2003a, 2005a,b] and the mitral valve [Makkena et al., 2005] develop the atherosclerotic lesion but not the right-sided heart valves: pulmonic and tricuspid valves. Figure 2 demonstrates the differences in the pressures in the heart and the expression of the phenotype of the heart valve. The lipids bind to the Lrp5/6 receptors to activate the Canonical Wnt pathway and the bone formation. Since the Lrp5 plays a role in the mechanostat theory [Johnson and Summerfield, 2005] this mechanism provides the foundation for the pressure theory on mechanical effects on Lrp5 in the heart. Normal pressures in the heart increase from the right atrium, to the right ventricle, to the left atrium, and finally to the left ventricle for normal cardiac physiology as shown in Figure 2. However, this theory hypothesizes that in the presence of hyperlipidemia the phenotypic expression of the valve changes in response to the different pressures in the heart. Human phenotypic studies of cardiac valve disease have demonstrated that the aortic valve expresses an osteoblast phenotype [Rajamannan et al., 2003c; Caira et al., 2006] and the mitral valve expresses a chondrogenic phenotype [Caira et al., 2006]. It is known that the mitral valve has a lower pressure present in the left atrium as compared to the aorta. Therefore the pressure on the mitral valve is enough to produce cartilage and the pressure on the aortic valve is higher to drive the Lrp5 mechanostat mechanism to form bone. The mitral annulus which has slightly higher pressures than the valve leaflet can form bone if the pressures are high enough causing mitral annular calcification [Boon et al., 1997; Aronow et al., 2001a]. The results of these studies further confirm the mechanostat theory for the role of Lrp5 in the presence of the different pressures in the heart. The highest pressure aortic valve differentiates to form bone and the mitral valve which has lower pressures only develops calcification at the mitral annulus and in the leaflets develops a cartilage phenotype. Even though the lipids are present throughout the systemic circulation, the right-sided valves with the lowest pressures in the hearts do not calcify or form cartilage as shown in Figure 2. The human study [Caira et al., 2006] further confirms the hemodynamic pressures correlating with the expression of Lrp5/6 in the human diseased valves by immunohistochemistry staining with the Lrp5/6 antibody.
Fig. 2.
Schematic for the role of hemodynamic pressures in the heart in the lipid-pressure activation of Lrp5/6 in the valves.
LDL-DENSITY-PRESSURE THEORY
HYPOTHESIS FOR THE ROLE OF Lrp5/6 SIGNALING IN VALVULAR HEART DISEASE
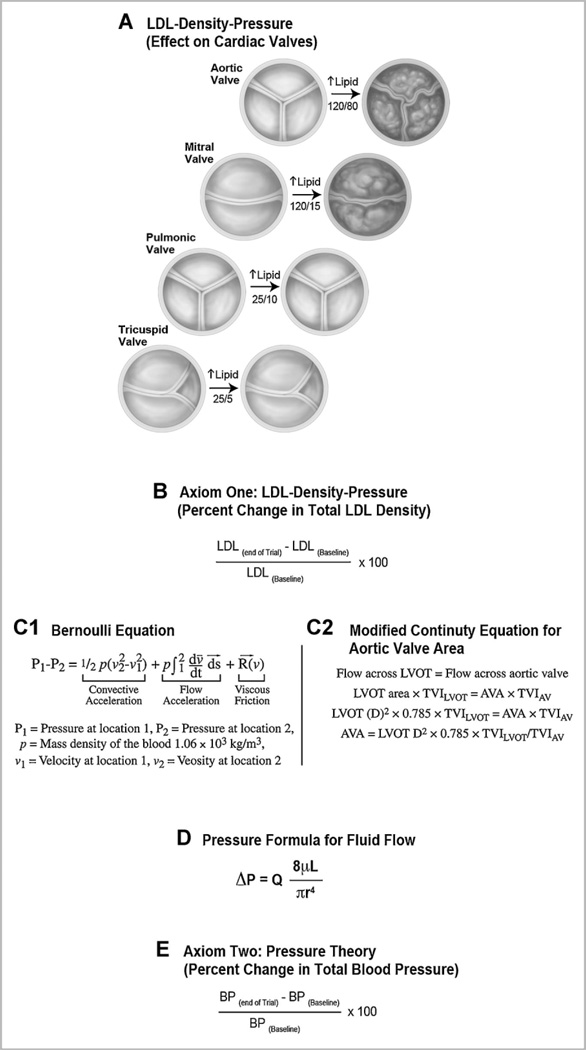
This hypothesis evaluates the effect of pressure in the development of calcification is dependent on two axioms, biology and hemodynamics. The experimental data demonstrate that lipids activate the bone differentiation [Rajamannan et al., 2005a] in the valve. The first axiom is the effect of LDL in atherosclerotic biology. LDL affects the left-sided heart valves, as shown in Figure 3A. In the presence of experimental hypercholesterolemia the LDL only manifests disease in the left-sided valves where the pressure is higher in the heart is overtime, the leaflets fuse which to occlude the aortic valve Figure 3B. Calculation of the percent lowering of LDL density will target specifically the biologic effect of LDL on this disease. Figure 3C demonstrates a formula to calculate the percent reduction of the LDL density before and after therapy.
Fig. 3.
The LDL-Density-Pressure Theory (Effect on Cardiac Valves). The serum lipid levels affect all four heart valves as the lipids circulate throughout the heart. However, the pressure is different depending on the chamber and location in the heart. Panel A: The effect of lipids and the different pressures in the heart. Panel B: Axiom One: LDL-Density-Pressure- percent change in the LDL density. Panel C1: Bernoulli Equation Panel C2: Modified Continuity Equation for Aortic Valve Area Panel D: Pressure Formula for Fluid Flow. Panel E: Axiom Two: Pressure Theory(Percent Change in Total Blood Pressure).
The second axiom is the effect of the radius on fluid flow. The Bernoulli equation [Bernoulli, 1738] Figure 3D1, the formula for flow through a pipe, was modified [Hatle et al., 1980] Figure 3D2, to calculate aortic valve areas by echocardiography using the Doppler technique. The modification of this equation for aortic valve areas includes the deletion for the calculation for the flow acceleration and the viscous friction because the velocity profile in the center of the lumen is usually flat. Thus the viscous friction factor can be ignored in the clinical setting of aortic valve disease. However, the flow in the lumen of a vessel is not flat due to smaller radius, therefore, the viscous friction factor must be taken into account. The importance of the smaller radius is shown in Figure 3E, which is the calculation of resistance of fluid through a pipe. This size of the radius becomes important in the calculation of flow as the inverse r4 dependence of the resistance to fluid flow will increase viscosity by a factor of 16 requiring the effect of viscous friction to become important with smaller radii. Reductions of the LDL density will therefore, have a quicker effect in the reduction of the vascular lesion as it affects the lumen circumference directly as shown in Figure 3A. To measure the treatment effect for blood pressure on aortic valve disease: Figure 3F, is the calculation for the percent improvement in the blood pressure as an effect on the progression of the valve disease. This hypothesis provides a mathematical foundation for treating this disease in the heart.
SUMMARY
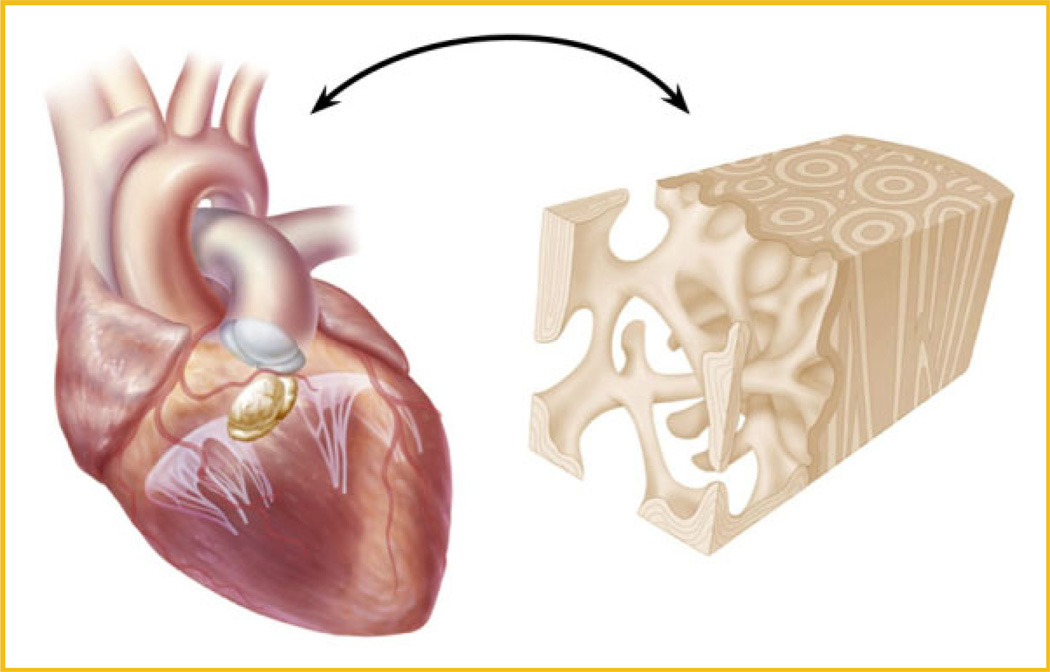
Mathematically and biologically, medical therapy for aortic valve disease can consider the following two axioms for targeting the disease biology in terms of the magnitude of the LDL density to activate the atherosclerotic process and the change in blood pressure in aortic valve disease. This theory provides a biologic-hemodynamic foundation for the mechanism of Lrp5/6 activation in the heart. Figure 4 demonstrates the cardiovascular-bone paradox. This prospectus provides a novel foundation for the role of lipids and blood pressure in the development of bone formation in the heart.
Fig. 4.
The Cardiovascular-Bone Paradox: Atherosclerotic Calcification and Atherosclerotic Osteoporosis.
ACKNOWLEDGMENTS
This work was completed with the support of an American Heart Association Grant-in-Aid (0555714Z) and a grant from the National Institute of Health (5K08HL073927-04, 1R01HL085591-01A1). Nalini M. Rajamannan is an inventor on a patent for the use of statins in degeneration of aortic valve disease. This patent is owned by the Mayo Clinic and Dr. Rajamannan does not receive any royalties from this patent.
REFERENCES
- Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–169. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C, Kronzon I. Association of mitral annular calcium with symptomatic peripheral arterial disease in older persons. Am J Cardiol. 2001a;88:333–334. doi: 10.1016/s0002-9149(01)01657-5. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001b;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- Bernoulli D. Hydrodynamica sive de viribus et motibus fluidorum commentarrii. Strasbourg: Argentoratum; 1738. p. St31. [Google Scholar]
- Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: Characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP, Arsenault M, Couet J, Pibarot P. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–2236. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, Metzker ML, Caskey CT, Todd JA, Hess JF. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. 1998;248:879–888. doi: 10.1006/bbrc.1998.9061. [DOI] [PubMed] [Google Scholar]
- Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Ghani M, Woodend K, Burwash IG. Case-controlled study to assess risk factors for aortic stenosis in congenitally bicuspid aortic valve. Am J Cardiol. 2001;88:690–693. doi: 10.1016/s0002-9149(01)01820-3. [DOI] [PubMed] [Google Scholar]
- Chui MC, Newby DE, Panarelli M, Bloomfield P, Boon NA. Association between calcific aortic stenosis and hypercholesterolemia: Is there a need for a randomized controlled trial of cholesterol-lowering therapy? Clin Cardiol. 2001;24:52–55. doi: 10.1002/clc.4960240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S, Rockette HE, Krishnaswami V. Diabetes and hypercholesterolemia among patients with calcific aortic stenosis. J Chronic Dis. 1984;37:407–415. doi: 10.1016/0021-9681(84)90108-5. [DOI] [PubMed] [Google Scholar]
- Dong Y, Lathrop W, Weaver D, Qiu Q, Cini J, Bertolini D, Chen D. Molecular cloning and characterization of LR3, a novel LDL receptor family protein with mitogenic activity. Biochem Biophys Res Commun. 1998;251:784–790. doi: 10.1006/bbrc.1998.9545. [DOI] [PubMed] [Google Scholar]
- Faggiano P, Antonini-Canterin F, Baldessin F, Lorusso R, D’Aloia A, Cas LD. Epidemiology and cardiovascular risk factors of aortic stenosis. Cardiovasc Ultrasound. 2006;4:27. doi: 10.1186/1476-7120-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML Osteoporosis-Pseudoglioma Syndrome Collaborative G. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: Association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–633. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, Borowski AG, Troughton RW, Houghtaling PL, Dipaola NR, Moravec CS, Vesely I, Griffin BP. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: An extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005;45:54–61. doi: 10.1016/j.jacc.2004.06.079. [see comment]. [DOI] [PubMed] [Google Scholar]
- Hassler C, Cruciat CM, Huang YL, Kuriyama S, Mayor R, Niehrs C. Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development. 2007;134:4255–4263. doi: 10.1242/dev.005942. [DOI] [PubMed] [Google Scholar]
- Hatle L, Angelsen BA, Tromsdal A. Non-invasive assessment of aortic stenosis by Doppler ultrasound. Br Heart J. 1980;43:284–292. doi: 10.1136/hrt.43.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey PJ, Twells RC, Phillips MS, Yusuke N, Brown SD, Kawaguchi Y, Cox R, Guochun X, Dugan V, Hammond H, Metzker ML, Todd JA, Hess JF. Cloning of a novel member of the low-density lipoprotein receptor family. Gene. 1998;216:103–111. doi: 10.1016/s0378-1119(98)00311-4. [DOI] [PubMed] [Google Scholar]
- Hoagland PM, Cook EF, Flatley M, Walker C, Goldman L. Case–control analysis of risk factors for presence of aortic stenosis in adults (age 50 years or older) Am J Cardiol. 1985;55:744–747. doi: 10.1016/0002-9149(85)90149-3. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Jones PL, Li Q, Mohler ER, III, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Summerfield DT. Parameters of LRP5 from a structural and molecular perspective. Crit Rev Eukaryot Gene Expr. 2005;15:229–242. doi: 10.1615/critreveukargeneexpr.v15.i3.50. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: A union made for bone. J Bone Miner Res. 2004;19:1749–1757. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- Kim DH, Inagaki Y, Suzuki T, Ioka RX, Yoshioka SZ, Magoori K, Kang MJ, Cho Y, Nakano AZ, Liu Q, Fujino T, Suzuki H, Sasano H, Yamamoto TT. A new low density lipoprotein receptor related protein, LRP5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. J Biochem. 1998;124:1072–1076. doi: 10.1093/oxfordjournals.jbchem.a022223. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Mani S, Davis NR, Sarrafzadegan N, Kavathas PB, Mani A. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. 2008;103:1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–11336. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- Makkena B, Salti H, Subramaniam M, Thennapan S, Bonow RH, Caira F, Bonow RO, Spelsberg TC, Rajamannan NM. Atorvastatin decreases cellular proliferation and bone matrix expression in the hypercholesterolemic mitral valve. J Am Coll Cardiol. 2005;45:631–633. doi: 10.1016/j.jacc.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: Atherosclerosis risk factors—A causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- Mohler ER, III, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995a;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995b;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [comment]. [DOI] [PubMed] [Google Scholar]
- Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [comment]. [DOI] [PubMed] [Google Scholar]
- Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: Implications for secondary prevention. Circulation. 2000;101:2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- Peltier M, Trojette F, Sarano ME, Grigioni F, Slama MA, Tribouilloy CM. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol. 2003;91:97–99. doi: 10.1016/s0002-9149(02)03010-2. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pohle K, Maffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: Association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–1932. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Sangiorgi G, Springett M, Arnold K, Mohacsi T, Spagnoli LG, Edwards WD, Tajik AJ, Schwartz RS. Experimental hypercholesterolemia induces apoptosis in the aortic valve. J Heart Valve Dis. 2001;10:371–374. [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2260–2265. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Edwards WD, Spelsberg TC. Hypercholesterolemic aortic-valve disease. N Engl J Med. 2003a;349:717–718. doi: 10.1056/NEJMc031360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003b;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003c;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [see comment]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005a;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005b;91:806–810. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tomaszewski M, Charchar FJ, Barnes T, Gawron-Kiszka M, Sedkowska A, Podolecka E, Kowalczyk J, Rathbone W, Kalarus Z, Grzeszczak W, Goodall AH, Samani NJ, Zukowska-Szczechowska E. A common variant in low-density lipoprotein receptor-related protein 6 gene (LRP6) is associated with LDL-cholesterol. Arterioscler Thromb Vasc Biol. 2009;29:1316–1321. doi: 10.1161/ATVBAHA.109.185355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Boughner DR, Perkins DG, Canham PB. Quantitative structural analysis of collagen in chordae tendineae and its relation to floppy mitral valves and proteoglycan infiltration. Br Heart J. 1987;57:264–269. doi: 10.1136/hrt.57.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts went: The exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmshurst PT, Stevenson RN, Griffiths H, Lord JR. A case–control investigation of the relation between hyperlipidaemia and calcific aortic valve stenosis. Heart. 1997;78:475–479. doi: 10.1136/hrt.78.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley CF, Baker PB, Kolibash AJ, Kilman JW, Sparks EA, Boudoulas H. The floppy, myxomatous mitral valve, mitral valve prolapse, and mitral regurgitation. Prog Cardiovasc Dis. 1991;33:397–433. doi: 10.1016/0033-0620(91)90005-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Zylstra CR, Wan C, VanKoevering KK, Sanders AK, Lindvall C, Clemens TL, Williams BO. Gene targeting approaches in mice: Assessing the roles of LRP5 and LRP6 in osteoblasts. J Musculoskelet Neuronal Interact. 2008;8:291–293. [PubMed] [Google Scholar]