Abstract
Cements for maxillofacial reconstruction of jaw defects through calcification of rotated muscle have been tested. The objective of this study was to investigate the visibility of loading of two types of commercially available cements, Cerament™ Spine Support and Cerament Bone Void Filler with mesenchymal cells and cytokines (bone morphogenetic protein) to act as a biomimetic scaffolding for future clinical application. Determination of basic biocompatibility (cell viability) using methyl thiazolyl tetrazolium and live/dead assay was carried out using MG-63 cells at various time points. Next, in order to inform potential subsequent in vivo experiments, a collagen tissue mimic was used for characterization of rabbit mesenchymal stromal cells using immunofluorescent cytoskeleton staining, and simultaneous and then sequential injection of Cerament Spine Support cement and cells into collagen gels. Results indicated that Cerament Spine Support was more biocompatible and that sequential injection of cement and then rabbit mesenchymal stromal cells into the tissue mimics is an optimal approach for clinical applications.
Keywords: Calcium sulphate, hydroxyapatite, injectable, cell culture, mesenchymal stromal cell
Introduction
The field of tissue engineering holds great promise for the repair or regeneration of damaged and diseased tissue. Researchers in bone tissue engineering are working to develop alternatives to allogeneic and autologous bone grafts in order to address the growing clinical needs and to avoid the morbidities associated with harvesting bone graft. The introduction of mesenchymal stromal cells (MSCs) has been tested in the repair of large bony defects in animal models and in humans, with variable degrees of success.1–3 The inclusion of undifferentiated mesenchymal cells and/or growth factors to biological scaffolds should enhance the bone regeneration abilities of the material.1,4 One of the main challenges of the reconstruction of the created defects of the jaw bones following the resection for oral cancer is the deficient blood supply. This deficiency is due to either the pathological condition that affects the blood vessels in the head and neck region or fibrosis of the vascular bed resulting from post-operative radiotherapy. Therefore, it is a major challenge to apply the recent advances in tissue bioengineering for bone regeneration in the oro-facial regions.
Recently, the potential for skeletal muscle to form bone has been explored.5 This is seen most dramatically in the genetic disease fibrodysplasia ossificans progressiva (FOP), where patients develop a second skeleton as muscle spontaneously ossifies.6 Heterotopic ossification of muscle is also common after joint replacement surgery and following blast injuries.7,8 Although these are troubling medical conditions, they suggest novel opportunities for regenerative medicine to address circumstances where it is necessary to grow large amounts of new bone which requires a rich blood supply as seen in muscular tissue. Therefore, there is enormous interest in developing a biomimetic scaffold that could be injected in muscular tissue to stimulate osteogenesis for potential reconstruction of critical-size bony defects in the oro-facial region, which will be the next step of this study.
Various bio-scaffolds have been designed to replace resected tissue in the oro-facial region. The potential option of using an injectable scaffold is an attractive choice for clinicians. Injectable materials would reduce surgical invasiveness, minimizing the risk of infection, scar formation and the cost of treatment.9 The ideal injectable scaffold should be injected in a liquid form and undergo a gelation or setting process once in situ. In open surgery, the use of such a material allows surgeons to fill to reconstruct bone defects.10
Calcium sulphate (CS), or plaster of Paris, has been used as a bone substitute for over 100 years and has been proved to be safe and biocompatible.11 However, it has been shown that the resorption of CS is faster than the formation of new bone, which precludes its use in the reconstruction of large bony defects.12 To overcome this limitation, composite injectable cements have been developed by adding more slowly resorbing materials to CS cements.12,13 A composite injectable material based on 60% calcium sulphate hemihydrate (CSH) and 40% hydroxyapatite (HA) has been developed (Cerament™).13 When this material is mixed with a liquid phase, it forms a resorbable phase consisting of calcium sulphate dihydrate (CSD) and unreacted HA. The CSD phase is absorbed in the body creating pores, thereby permitting new bone ingrowth. The HA phase lasts longer (9–12 months), hence promoting osteoconduction and potentially osseoinduction of bone into the defect. The compressive strength of the material after setting was estimated to be 20 MPa and reached 13 MPa after 10 days of immersion at simulated body fluid (SBF).13,14
Cerament cement is available commercially for spinal fusion (Cerament Spine Support) and as filler for bone cavities (Cerament Bone Void Filler) with different formulations, setting speeds and mechanical properties.13–20 To our knowledge, there are no published data testing these two materials at the cellular level. The biocompatibility testing was carried out by implanting the material at ectopic and orthotropic sites using rat model.14 The application of the material as a biomimetic injectable scaffold (loaded with cytokines and seeded with undifferentiated mesenchymal cells) for maxillofacial reconstruction has not been considered, and the suitability of the material for this application has not yet been tested. This in vitro study is part of a comprehensive research programme to take the material from the laboratory to clinical maxillofacial application. This is an essential step before applying the material in a preclinical model. This model has been established by our team for construction of critical-size osteoperiosteal continuity defect of the mandible.21 Thus, this is the first study conducted to characterize the interaction between Cerament and the undifferentiated rabbit mesenchymal stromal cells (rMSCs) and to inform the optimum surgical approach.
Therefore, the aims of this study are to characterize the interaction between two types of Cerament and the undifferentiated rMSCs and to assess the overall suitability of the materials as biomimetic scaffolding for potential clinical applications.
Materials and methods
Cement mixing and sample preparation
The materials tested in this experiment have the same powder consisting of 60% α-CSH and 40% HA, provided by Bone Support AB (Lund, Sweden). The difference lies in the powder-to-liquid ratio and the viscosity of the liquid phase, which is an iodine solution. In Cerament Bone Void Filler, the liquid phase is Cerament |C-Tru with iodine concentration of 180 mg/mL; the powder was mixed at a ratio of 1 g/0.43 mL of liquid. Cerament Spine Support powder was mixed with Cerament |C-Tru with iodine concentration of 300 mg/mL as an alternative radiopacifier and mixed in a powder-to-liquid ratio of 1 g/0.5 mL. The material is metastable below 40°C at atmospheric pressure and hydrates on contact with water to form CSD as in equation (1). The crystals of CSD which form are about 4–6 µm long and flat in shape, and crystal growth occurs in all directions forming a matrix of well-interconnected crystals. The setting reaction of the cement is slightly exothermic, at 37°C; the setting time was 7 min and the pH of the injected cement was 7.5.14,16
| (1) |
Once the cement reached a paste-like consistency, 0.5 mL of cement was evenly spread over a glass slide. Before the material was set, it was divided into 5 × 5 × 1 mm3 squares, which were used for cell culture, cytotoxicity and proliferation tests. The samples were prepared in triplicate; nine samples were prepared and examined at each time point for both cements.
Biocompatibility assays using MG-63
Methyl thiazolyl tetrazolium
MG-63 cells were cultured in filtered Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% foetal calf serum (FCS; Life Technologies, Paisley, UK) and a 2% a mixture of streptomycin, penicillin, fluconazole and glutamine (Life Technologies) and maintained at 95% humidity at 37°C and 5% CO2. When the cells reached confluence, they were passaged from the tissue culture flasks; this involved removal of the culture media and washing them in Dulbecco’s phosphate buffered saline (DPBS) twice before removing the cells with a trypsin/ethylenediaminetetraacetic acid (EDTA) solution (Sigma–Aldrich, UK) for 5 min at 37°C. The trypsin was inactivated by the addition of an equal volume of DMEM supplemented with 10% FCS, and the cells were collected by centrifugation. At this point, the cells were resuspended in 1 mL of DMEM supplemented with 10% FCS. The prepared cement squares of both Cerament Bone Void Filler and Cerament Spine Support were placed in 24-well plates. The prepared cell suspension was seeded on the surface of the prepared squares at a cell density of 1 × 103 cells/cm2, 15 min after mixing the cement. Wells containing cells only (no cement) were used as controls. The plates were incubated and maintained at 95% humidity at 37°C and 5% CO2. Cell viability was determined after 1, 3 or 7 days culture using the standard colorimetric methyl thiazolyl tetrazolium (MTT) assay to detect the presence of living cells.22,23 Within active, living cells, the yellow MTT substrate is reduced by mitochondrial enzymes to form an insoluble purple formazan crystal product. The reduction of this yellow MTT substrate only takes place when mitochondrial enzymes are active, and therefore, conversion is often used as a measure of viable (living) cells; 100 µL of MTT (Sigma–Aldrich, UK) was added into all wells (the experimental and control) in a plate. The plate was incubated for 1.5 h. Then, the supernatant of each well was removed, and 200 µL dimethyl sulphoxide (DMSO) was added into all wells. An optical density (OD) reading was obtained to correlate directly with cell number and was used to determine the relative number of live cells on each cement, that is, the absorbance is directly proportional to the cell numbers. The plate reader (Dynatech MR7000, Channel Islands, UK) was adjusted to a wavelength of 550 nm.
Live/dead assay
MG-63 cells were plated onto the prepared cement discs for both cements at a density of 1 × 104 cells/mL in 2 mL of culture medium. The plate was incubated at 37°C and 5% CO2 for three time points: 1, 3 and 7 days. Live/dead stain was prepared by adding 2 µmol/L acetomethoxy derivate of calcein (calcein-AM) and 2 µmol/L ethidium homodimer-1 per millilitre of media. The construct was then left in the incubator for 30 min; afterwards, the dye was removed and replaced with 1 mL of DMEM. The examination was carried out on the same day using fluorescent microscopy (Leica-Letiz DM IRB, Wetzlar, Germany), three random fields of view were chosen at low objective 5× magnification and then five areas were magnified and imaged at 10× objective using an Axiovision camera (Zeiss, Jena, Germany; total of 3 construct yielded 15 images for each cement).24,25 Mean live and mean dead cell numbers were estimated from the five images. Then, the percentage of viable cell was counted by dividing the mean number of live cells by the sum of the total cell count (live and dead cells).
rMSCs harvesting, isolation, characterization and osteogenic potential testing
rMSCs harvesting, culturing and characterization
Bone marrow was aspirated from the anterior iliac spine of sedated rabbits using 20-mL heparinized syringes. The mononuclear cells were separated by centrifuging at 900g for 10 min at room temperature to remove the fat and debris and then lysed. The remaining cells were loaded into 12-mL tubes containing Ficoll-paque, a density medium (GE Healthcare Life Sciences, Buckinghamshire, UK) and centrifuged at 800g for 30 min. A cell count was performed using a Neubauer haemocytometer, and then, the cells were resuspended in 10 mL of Alpha Minimum Essential Medium (α-MEM; Sigma–Aldrich, UK).26 The rMSCs, at a density of 2 × 106 cells/mL, were cultured into filtered α-MEM supplemented with 10% FCS; Life Technologies) and 2% of a mixture of streptomycin, penicillin, fluconazole and glutamine (Life Technologies) and maintained at 95% humidity at 37°C and 5% CO2. The cells took 3–5 weeks to reach the number of cells required for potential implantation.
Once rMSCs with a typical fibroblast-like morphology had reached confluence, they were passaged from the tissue culture flasks by removing the culture media and washed in DPBS (Sigma–Aldrich, UK) twice before removing the cells with a 2% trypsin-EDTA solution (Sigma–Aldrich, USA) for 5 min at 37°C. Cells characterization was carried out for specific rMSCs protein surface markers CD44 (Abbiotec, CA, USA), CD166 (Abcam, UK) and negative for CD34 (Abbiotec, San Diago, USA) using immunofluorescent stains as described below.27 Cells were left for 3 days in conventional culture medium, namely, DMEM culture medium, supplemented with 10% FCS, before being checked for osteogenic potential using osteogenic medium, where they were cultured for 28 days before the assessment was performed. The osteogenic medium was prepared by using 500 mL of α-MEM supplemented with 10% foetal bovine serum (FBS), 100 nmol/L dexamethasone (Sigma–Aldrich, UK) and 50 µmol/L ascorbic acid-2-phosphates (Sigma–Aldrich, UK). Then, the differentiation potential of rMSCs was assessed using an immunofluorescent stain for osteocalcin (OCN; AbD Serotec, USA). Furthermore, gene expressions for OCN (Invitrogen, UK) and osteopontin (OPN; Invitrogen, UK) were also carried out.28
Messenger RNA (mRNA) expression profiles to test the osteogenic potential of rMSCs
Total RNA was isolated from rMSCs after 21 days of cell culture in osteogenic medium as described above. RNA was isolated using a Qiagen RNeasy Mini Kit (Life Technologies). Synthesis and amplification of DNA were performed with the Qiagen one-step reverse transcriptase–polymerase chain reaction (RT-PCR) kit. Six pairs of primers were tested; their sequences are listed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. DNA amplification was carried out through different thermal consecutive cycles (Thermal cycle, Master Cycle personal, Eppendorf, Germany) with an annealing temperature of 45°C and incubation for 30 s. PCR reactions were resolved on 1.2% agarose gel in tris-borate-EDTA (TBE) buffer.29
Table 1.
Primer gene sequence for the assessed genes, their size and annealing temperatures.
| Genes | Primer sequence (5′–3′) (forward/reverse) | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| OPN | Forward: G A C A G C C A G G A G A A G G A C A G | 169 | 50 |
| Reverse: T C T T C A C T C T T C G G C T C G A T | |||
| OCN | Forward: G T G C A G A G T C T G G C A G A G G | 153 | 50 |
| Reverse: G G T T G A G C T C G C A C A C C T | |||
| GAPDH | Forward: T G C C C T G G G G T G G G A A T G G A | 200 | 45 |
| Reverse: A G G G G T G A G G G A C A C G A G G C |
OPN: osteopontin; OCN: osteocalcin; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
rMSCs response upon direct cell seeding
A direct seeding approach was used to assess early rMSC response before in-gel seeding to test cell adhesion and proliferation on the surface of the cement. Cement constructs were prepared using Cerament Spine Support due to superior biocompatibility, which was mixed with 0.4 mg/mL of bone morphogenetic protein-7 (BMP-7).30 BMP-7 has been selected because it has reported successful clinical applications in the maxillofacial regions.22,31 Nine constructs of each cement were prepared for rMSC seeding. After 1 or 3 days of cell culture, rMSCs on the test material were prepared according to an established protocol for immunofluorescent cytoskeletal staining.27 After 3 days of culture, rMSCs were fixed in 4% formaldehyde/phosphate buffered saline (PBS) with 1% sucrose, at 37°C for 15 min to allow the viewing of individual cells. Once fixed, the samples were washed with PBS, and a permeabilising buffer (10.3 g sucrose, 0.292 g NaCl, 0.06 g MgCl2·6H2O, 0.476 g 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) buffer and 0.5 mL Triton X, in 100 mL water, pH 7.2) was added and kept at 4°C for 5 min. The samples were then incubated at 37°C for 5 min in 1% bovine serum albumin (BSA)/PBS. This was followed by the addition of anti-vinculin primary antibody (Antibodies-online.com; 1:100 in 1% BSA/PBS, mouse monoclonal anti-human cross-reacted with rabbit IgG for 1 h at 37°C). Simultaneously, rhodamine-conjugated phalloidin (Invitrogen, Carlsbad, CA, USA) was added for the duration of this incubation (1:100 in 1% BSA/PBS; Molecular Probes, OR, USA). The samples were next washed in 0.5% Tween 20/PBS (3 × 5 min). A secondary, biotin-conjugated antibody (1:50 in 1% BSA/PBS, monoclonal anti-mouse IgG; Vector Laboratories, UK) was added for 1 h (37°C) followed by washing. A fluorescein isothiocyanate (FITC)-conjugated streptavidin third layer was added (1:50 in 1% BSA/PBS; Vector Laboratories) at 4°C for 30 min and given a final wash. Finally 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) nucleus stain was added before mounting the cultures on glass microscope slides. Samples were then viewed by fluorescent microscopy.
Scanning electron microscopy
Cement constructs were prepared first, by mixing Cerament Spine Support powder to liquid ratio of 1 g/0.5 mL. BMP-7 was added at a concentration of 0.4 mg/mL of cement.29 Nine constructs were prepared for rMSC seeding. After 1 or 3 days of cell culture, rMSCs on the test material were prepared according to a standard protocol for scanning electron microscopy (SEM) examination to allow the viewing of individual cells.27 The cells were fixed with 1% glutaraldehyde (Sigma–Aldrich, USA) buffered in 0.1 M sodium cacodylate (Agar Scientific, Stanted, UK) at 4°C. The cells were then post-fixed in 1% osmium tetroxide (Agar Scientific, UK) and 1% tannic acid (Agar Scientific, UK) and then dehydrated through a series of alcohol concentrations (20%, 30%, 40%, 50%, 60%, 70%, 90%, 96% and 100%). The final dehydration was in hexamethyl-disilazane (Sigma–Aldrich, UK), followed by air-drying. Once dry, the samples were sputter-coated with gold before examination with a Carl Zeiss Sigma VP Oxford Micro-analysis S800 or S4700 field emission SEM at an accelerating voltage of 10 kV.
rMSCs in collagen gel model
Collagen preparation
Collagen gels were used to mimic clinical tissue into which the material will eventually be injected. Collagen was prepared by using a rat-tail collagen type-I that was treated with chloroform with a protein concentration of 2.05 mg/mL added to 0.6% acetic acid (Millipore Ltd, Temecula, CA, USA); 0.5 mL of 10× DMEM was added to 0.5 mL FCS, and then, 2.5 mL of the collagen was added simultaneously with 1 mL of 0.1 M NaOH. The addition of 0.1 M NaOH was needed to adjust the acidity of the collagen mixture to neutral until the colour turns to red; the universal tube was placed on an ice bath. Then, 1.5 mL of the mixture was poured in each well plate, using triplicates for each experiment. The mixture was then incubated at 37°C before cement/cell injection.
Injection of rMSCs and cement into collagen
For scaffold preparation, Cerament Spine Support was prepared by mixing 3 g of the calcium sulphate/hydroxyapatite (CS/HA) (60:40) powder with 0.629 mL Cerament |C-Tru (opacifier, BONESUPPORT, Lund, Sweden) solution and 103/0.6 mL cells suspension (in culture medium). Then, resultant formed paste was loaded into a 5-mL syringe. The liquid scaffold was injected into the prepared collagen using an 18-gauge needle (Figure 1). The scaffold inside the collagen was incubated at 37°C and 5% CO2 overnight, and then, the collagen was detached from the sides of the well using a needle; then, fresh media was added to feed the cells. A live/dead stain was performed as described earlier.
Figure 1.
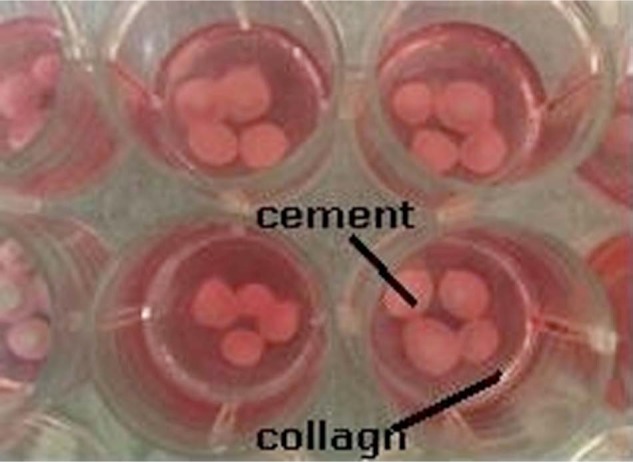
The well plate which contains the prepared collagen constructs and the injectable cement: collagen construct (red) and the injected scaffold with cells (white).
Injection of cement into collagen followed by rMSCs seeding
Cerament Spine Support powder was mixed with Cerament |C-Tru, with a powder-to-liquid ratio of 1 g/0.5 mL, and immediately injected into the collagen. After 15 min, 0.6 mL of rMSC suspension was injected between the CS/HA bone cement and incubated at 37°C and 5% CO2 overnight. Then, live/dead staining was carried out using calcein-AM to stain viable cells and ethidium homodimer to stain compromised cells (Biolab, UK), following the same protocol described earlier.
Testing the osteogenic potential of CS/HA on rMSCs differentiation potential
The same collagen model was used as above: Cerament Spine Support powder was mixed with Cerament |C-Tru, with a powder-to-liquid ratio of 1 g/0.5 mL, and immediately injected into the collagen; 0.6 mL of rMSC suspension was injected around the cement. The constructs were incubated for 21 days, at 95% humidity, 37°C and 5% CO2; the culture medium was changed twice a week. At day 21, immunofluorescent staining was carried out for rMSCs seeded on collagen using an OCN (AbD Serotec) primary antibody to assess the osteoinductive potential of the cement on the seeded cells following the same protocols as in the immunofluorescent cytoskeletal assessment.27 Cells were assessed and photographed using fluorescent microscopy, and random fields of view were chosen and imaged using Axiovision camera (Zeiss) as explained earlier. The osteogenesis was quantified by counting the percentage of cells labelled OCN with respect to a total cell count, which was performed by counting all the labelled nuclei which were stained by Hoechst stains (DNA stain).32
Results
Biocompatibility test
MTT
The percentage of viable MG-63 cells on the surface of the scaffold was similar for the two cements after the first 24 h. However, after 1 week of cell culture, the percentage of viable cells were 90.6% ± 12% and 77.0% ± 7% for Cerament Spine Support and Cerament Bone Void Filler cement, respectively. However, this difference in cell metabolism was not statistically significant (p > 0.05) when mean MTT values were compared (Figure 2).
Figure 2.
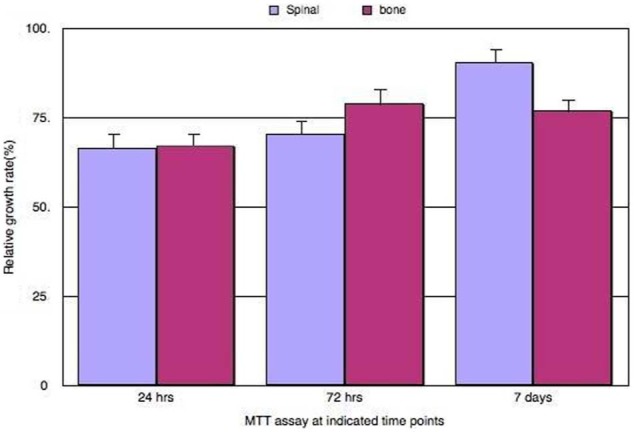
Biocompatibility MTT toxicity assay using MG-63 cell line; the graph shows the percentage of proliferating cells based on mitochondrial enzyme activity of the cultured MG-63 live cells seeded on both cements, Cerament Bone Void Filler and Cerament Spinal Support, at different time points. On the x-axis is the different treatments done at 24 h, 72 h and at 1 week; the y-axis shows the percentage of proliferating cells calculated in different treatments. Purple and pink bars represent the Cerament Bone Void Filler cement and Cerament Spine Support cement, respectively. The figure indicates that at 1 week of cell culture, there was no statistically significant difference between the percentage of active proliferating live cells between Cerament Bone Void Filler and Cerament Spinal support (p > 0.05), after analysis using student t-test on SPSS software (error bar = SD, n = 3).
MTT: methyl thiazolyl tetrazolium; SD: standard deviation.
Live and dead assay
The percentages of viable MG-63 cells were 85.6% ± 11.7% and 82.0% ± 2% in the first 24 h for Cerament Spine Support cement and Cerament Bone Void Filler cement, respectively. At day 7 of cell culture, the mean value of viable cells were 145 ± 16.9 and 105 ± 25.09 cells in well plate with Cerament Spine Support and Cerament Bone Void Filler, respectively; this difference was statistically significant (p < 0.05). Viable cells were found at a distance away from both cements, and typical images are shown in Figure 3.
Figure 3.
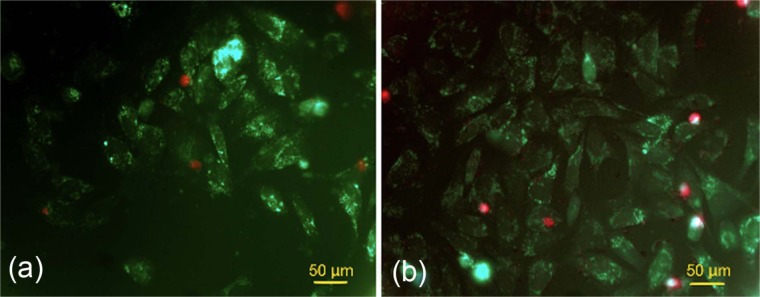
Live and dead assays of (a) MG-63 cell proliferation and growth around Cerament Bone Void Filler and (b) MG-63 cell proliferation around Cerament Spine Support (scale bars = 70 µm).
Based on the biocompatibility testing, we decided to focus further studies on Cerament Spine Support cement as our preferred material due to the greater cell viability.
rMSCs harvesting, culturing and characterization
The methods used to isolate and expand the rMSCs required an average of 5 weeks for the cells to reach sub-confluent status (approximately 1,000,000 cells/mL). Immunofluorescent staining for cell characterization showed positive staining for CD166 and CD44 and negative staining for CD34 surface markers, which indicated that the cells had mesenchymal stem cell characteristics. With regard to cell osteogenic potential, the immunofluorescent stain for OCN, which is a late protein marker specific to osteoblastic expression, was positive on day 21 of treatment with osteogenic media (Figure 4(a)). Quantitatively, cells were seeded at day 0 at cell density of 104 cells/cm2 at each cover slip and were cultured on osteogenic media; their count at day 21 was 381 × 104 cells/cm2.
Figure 4.
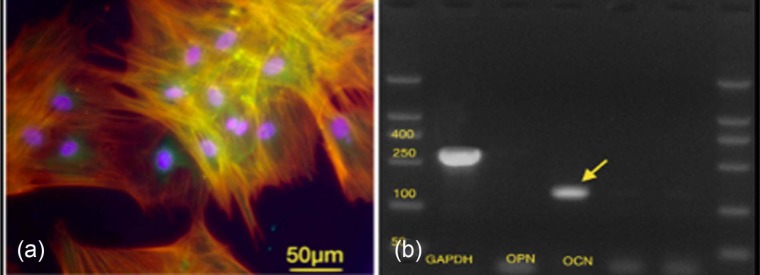
(a) Cytoskeleton immunofluorescent images showing positive osteocalcin expression, actin (red) and DAPI (blue) stains at 21 days of rMSCs culture (marker bars = 50 µm). (b) A scan image of gel RT-PCR of osteogenic lineage marker of rMSCs harvested after 21 days of cell culture showing a strong signal for osteocalcin (arrows) and weak signal for osteopontin gene expression.
DAPI: 4′,6-diamidino-2-phenylindole; RT-PCR: reverse transcriptase–polymerase chain reaction; rMSCs: rabbit mesenchymal stromal cells.
mRNA expression profiles to test the osteogenic potential of rMSCs
Osteogenic differentiation potential was further confirmed by strong expression OCN using RT-PCR at 150 bp and negative expression of OPN (Figure 4(b)). Prior to testing in collagen gel tissue mimics, we undertook short-term biocompatibility (cytoskeletal and morphological) analysis of rMSCs on the Cerament Spine Support cement.
Immunofluorescent staining for cytoskeleton
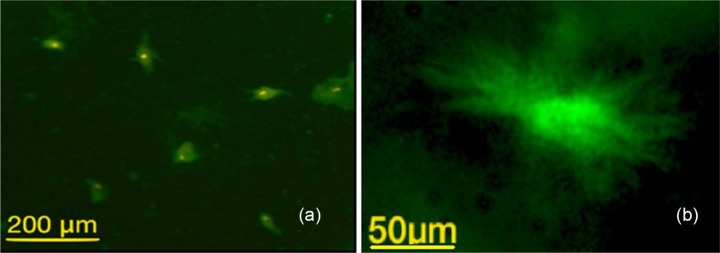
rMSCs on the Cerament Spine Support attained a spread morphology on the surface of the material (Figure 5). rMSCs attained the required shape, size and adherence on the surface of the material.
Figure 5.
Cytoskeleton immunofluorescent image of rMSCs after 3 days of culture showing adherent and spread MSCs on the surface of Cerament Spine Support (marker bar = 50 µm).
rMSCs: rabbit mesenchymal stromal cells.
SEM
A large number of rMSCs were adherent and were clearly visible on the surface of the Cerament Spine Support cement at all time points. At day 1, the rMSCs had started to adhere to the surface of the material and interact with the cement crystals (Figure 6). After day 3 of rMSC culture, there were areas where cells had proliferated well and aggregated with each other. Cells attained a typical size (60–160 µm in length), and they were polygonal or fusiform in shape (Figure 7). These cells had pseudopodia interacting with the crystals of the CS/HA cement (Figure 8). Cells showed accompanying filamentous fibres forming on the surface; this may be evidence for extracellular matrix production (Figure 8). After ascertaining that the surfaces supported rMSC growth, we moved to the three-dimensional (3D) model.
Figure 6.

Scanning electron microscopy image showing rMSC (arrows) at day 1 of cell culture. The cell is covering micropore and is adherent onto the surface of the Cerament Spine Support cement (scale bar = 1 µm).
rMSCs: rabbit mesenchymal stromal cells.
Figure 7.
Scanning electron microscopy images of rMSCs attaining normal size (60–160 µm) and shape (polygonal and fusiform) on the surface of Cerament Spine Support after 3 days of culture (scale bars: (a), (b) and (d) = 10 µm and (c) 2 µm).
rMSCs: rabbit mesenchymal stromal cells.
Figure 8.

Scanning electron microscopy images showing filopodia and lamellipodia of rabbit BMSCs (arrows) (a) extended over the filamentous fibres on surface of Cerament Spine Support and (b) attached and advancing over the crystal of Cerament Spine Support at 72 h (scale bars: (a) = 1 nm and (b) = 200 µm).
BMSCs: bone marrow stromal cells.
rMSCs in collagen model
Simultaneous injection of rMSCs and cement into collagen
The live/dead assay of the constructs where the rMSCs and the cement were injected simultaneously into the collagen showed only dead cells in close apposition to the cement (Figure 9).
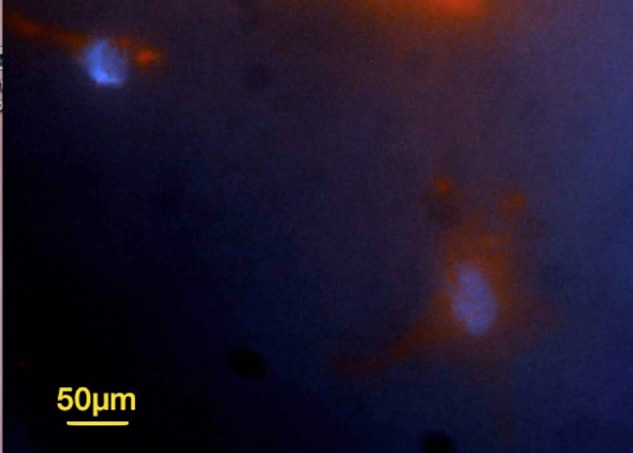
Figure 9.
Live/dead assay (calcein-AM and ethidium homodimer) shows that compromised cells (red) are trapped inside cement on collagen construct after 24 h of cell culture (scale bar = 100 µm).
calcein-AM: acetomethoxy derivate of calcein.
Injecting cement into collagen followed by rMSC seeding
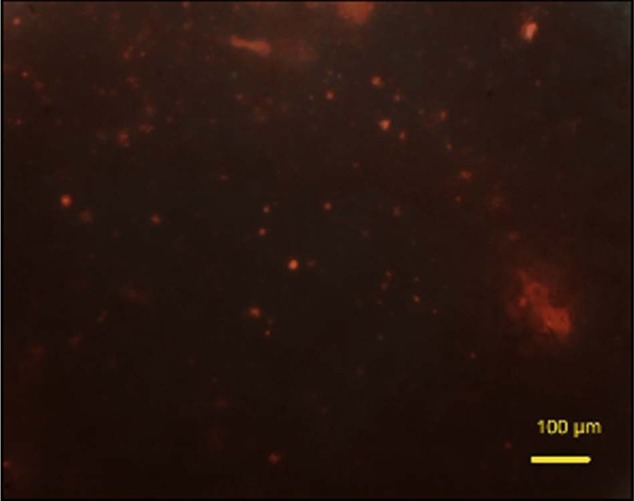
In this collagen construct, many live cells were noted in the collagen gel with cell distribution when cell suspension was injected into the collagen between the cement, and live cells appeared green in colour (calcein-AM, Biolab), the percentage of live cells was 69.7% ± 12% after 24 h of cell seeding (Figure 10(a)). To provide a control for this, only experiment cells were injected into a collagen construct which showed even distribution of live cells inside collagen (Figure 10(b)). Understanding that sequential rather than parallel injection provided greater viability, we then investigated osteogenesis in vitro.
Figure 10.
Photomicrographs for live and dead staining (calcein-AM and ethidium homodimer) for rMSCs in collagen after 24 h of cell culture: (a) presence of live rMSCs around the cement (Cerament Spine Support; green) after 24 h of cell culture (scale bare = 100 µm); (b) live cells inside a rat-tail collagen without cement (control) after 24 h of cell culture (scale bar = 50 µm). Result confirms presence of live cell evenly distributed around the injected cement.
calcein-AM: acetomethoxy derivate of calcein; rMSCs: rabbit mesenchymal stromal cells.
Testing osteogenic potential of CS/HA cement on rMSCs osteospecific differentiation
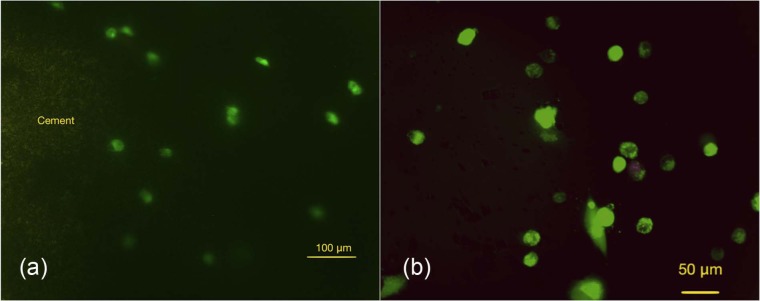
Fluorescent microscopy showed positive expression for OCN, which indicates osteoblastic differentiation of the rMSCs injected around the Cerament Spine Support cement loaded within collagen. The percentage of the differentiated cells was 42.3% ± 17% (Figure 11(a)).
Figure 11.
Photomicrography showing immunofluorescent stain for rMSCs showing positive staining to OCN, an antibody: (a) presence of multiple rMSCs with positive intake to OCN antibodies (scale bar = 200 µm); (b) on-cell ith higher magnification (scale bar = 50 µm), which indicates the pre-osteoblast differentiation of the MSCs after seeding into the collagen around the CS/HA cement.
OCN: osteocalcin; CS/HA: calcium sulphate/hydroxyapatite; rMSCs: rabbit mesenchymal stromal cells.
Discussion
The application of Cerament Spine Support or Cerament Bone Void Filler cement in combination with BMP-7 with rMSCs to stimulate muscle osteogenesis to reconstruct bone defects in the maxillofacial region is a novel concept, and hence was investigated in vitro. The potential potency of this technique is likely to stem from its ability to provide simultaneously the three major requirements of tissue repair: space-filling scaffold, a source of progenitor cells and an inductive protein, BMP-7. Therefore, comprehensive assessments of bio-scaffolds include the assessment of cell viability, and adhesion pattern on their surfaces are required.
Bone mineral–based composites remain an attractive choice for use as bone substitutes. Various mechanisms have been proposed for the role of CS in osteoconduction and/or osteoinduction in bone regeneration. While still not fully understood, it has been suggested that CS particles bind to adjacent bone and then resorb, providing a mechanism to guide bone growth.33 Other studies indicate that CS increases the microvascular density in the CS-treated defects, suggesting a positive effect on angiogenesis. The resultant increase in vascularity may, in part, account for the biological effects of CS implants.34 A study by Walsh et al.35 showed that CS exerts a chemotactic effect. They also found increased concentrations of BMP-2 and BMP-7, transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF), which play roles in connective tissue regeneration. Alternatively, calcium ions are released during dissolution of CS, leading to local increases in calcium ion concentration, which may affect osteoblast genesis, function and differentiation. Therefore, the elevated Ca2+ concentrations may inhibit osteoclast activity and tip the bone balance towards formation, similar to the mechanism following the resorptive phase of bone remodelling.36
There is evidence that calcium metaphosphate has been shown to stimulate the osteoblastic differentiation of human bone marrow stromal cells.37 It has also been shown by Lazary et al.38 that the proliferation and gene expression of mouse pre-osteoblastic cells on CS is also associated with increased alkaline phosphatase activity and gene expression associated with bone healing. The authors suggested that these effects might be due to the released calcium ions and might be mediated via SMAD3 (mothers against decapentaplegic homolog 3) receptors.39 An indirect mechanism of dissolution of CS was postulated to cause localized demineralization due to the increased acidity, thereby releasing growth factors previously incorporated into the bone matrix. Release of biomolecules, such as the BMPs, would enhance osteoblastic differentiation of MSCs and would consequently increase bone formation.35
Taken together, MTT and live/dead evaluation of biocompatibility after incubation of an osteoblast cell line, MG-63, on the cements showed significantly better results with regard to cell survival for Cerament Spine Support compared to the Cerament Bone Void Filler version of the CS/HA cement. This may be due to the toxic effect of the unreacted CS particles that causes local fluctuation in pH or ionic strength. Cerament Bone Void Filler has a lower liquid-to-powder ratio, which may have left some unreacted CSH powder. A similar concept was reported with calcium phosphate cement (CPC) when a lower powder-to-liquid ratio was used.40,41 The literature supports the use of in vitro cell culture as a reliable and sensitive approach to evaluate the biocompatibility of the bio-scaffolds.12,24,39,42 In this study, MG-63s were chosen because of the high proliferation rate of this cell line, hence it was possible to evaluate the viability of the cells within the first week of cell culture.22
Assessment of rMSCs was carried out using a collagen model, which proved to be an effective model to guide to the best seeding technique to be used at the next preclinical stage of this study. These constructs provided a 3D in vitro mimic of the muscle to examine what might happen to the cells inside the scaffold when injected in vivo. The results showed that rMSCs could not be applied simultaneously with the injection of Cerament with retention of good viability. The cells, however, could be successfully applied after 15 min from injection of the scaffold, allowing the material to start the setting process prior to the addition of the cells.
Qualitatively, rMSC viability assessments using direct cell seeding onto the materials and SEM and cytoskeleton evaluation showed that a large number of cells were adherent and proliferating and also attained the typical morphological features of MSCs on the surface of the Cerament Spine Support. Similar SEM findings have been demonstrated when rMSCs were seeded on surface of nano-HA/polyamide 6 constructs.43 Furthermore, the cells went on to express OCN, typical of maturing osteoblasts.
This is the first multidisciplinary assessment to characterize cell adhesion and function on these two types of CS/HA cements, and it highlights the superiority of one composition over the other for our purposes. We appreciate and acknowledge that the materials are commercially available for clinical use and have passed the necessary biocompatibility tests. However, we are aspiring to achieve a novelty in the application of this material as a biomimetic injectable scaffold for future application in the reconstruction and replacement of the lost bone in the oro-facial region.
Conclusion
Our results show that Cerament Spine Support is the preferable material for our purposes. The approach of testing the materials by injection into collagen constructs has shown the importance of timing in the application of both a CS-/HA-injectable cement and rMSCs into soft tissue. In addition, this illustrates the need to use sequential injection first of cement and then cells in subsequent in vivo studies, which should lead to development of effective human protocols.
Acknowledgments
The authors thank King Saud University, Ministry of Higher Education, for supporting Randa Alfotawi, and Bone Support AB (Lund, Sweden) for provision of the Cerament™ bone cements. They also thank Carol-Anne Smith for her technical assistance in this work, and Margaret Mullin and Peter Chung for help with SEM.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: Randa Alfotawi was funded by King Saud University, Ministry of Higher Education.
References
- 1. Schliephake H, Knebel JW, Aufderheide M, et al. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: an experimental pilot study in sheep. Int J Oral Maxillofac Surg 2001; 30: 531–537 [DOI] [PubMed] [Google Scholar]
- 2. Schliephake H, Weich HA, Dullin C, et al. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid – an experimental study in rats. Biomaterials 2008; 29: 103–110 [DOI] [PubMed] [Google Scholar]
- 3. Yuan J, Cui L, Zhang WJ, et al. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials 2007; 28: 1005–1013 [DOI] [PubMed] [Google Scholar]
- 4. Lynch S, Marx RE, Nevins M, et al. Tissue engineering application in oral & maxillofacial surgery and periodontics. 2nd ed. Quintessence Publishing, Hanover, 2008, pp. 9–15 [Google Scholar]
- 5. Liu F, Porter RM, Wells J, et al. Evaluation of BMP-2 gene-activated muscle grafts for cranial defect repair. J Orthop Res 2012; 30: 1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shore EM, Laplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone 2008; 43: 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoltny T, Koczy B, Wawrzynek W, et al. Heterotopic ossification in patients after total hip replacement. Ortop Traumatol Rehabil 2007; 9: 264–272 [PubMed] [Google Scholar]
- 8. Covey DC. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg 2006; 14: S10–S17 [DOI] [PubMed] [Google Scholar]
- 9. Hou Q, De Bank P, Shakesheff K. Injectable scaffolds for tissue regeneration. J Mater Chem 2004; 14: 1915–1923 [Google Scholar]
- 10. Huang Z, Tian J, Yu B, et al. A bone-like nano-hydroxyapatite/collagen loaded injectable scaffold. Biomed Mater 2009; 4: 550–555 [DOI] [PubMed] [Google Scholar]
- 11. Rauschmann MA, Wichelhaus TA, Stirnal V, et al. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005; 26: 2677–2684 [DOI] [PubMed] [Google Scholar]
- 12. Hu G, Xiao L, Fu H, et al. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. J Mater Sci Mater Med 2010; 21: 627–634 [DOI] [PubMed] [Google Scholar]
- 13. Nilsson M, Wielanek L, Wang J-S, et al. Factors influencing the compressive strength of an injectable calcium sulfate-hydroxyapatite cement. J Mater Sci Mater Med 2003; 14: 399–404 [DOI] [PubMed] [Google Scholar]
- 14. Nilsson M. Injectable calcium sulphate and calcium phosphate bone substitutes. PhD Thesis, Faculty of Medicine, Lund University, Lund, 2003, Paper II, pp. 1–8 [Google Scholar]
- 15. Masala S, Nano G, Marcia S, et al. Osteoporotic vertebral compression fractures augmentation by injectable partly resorbable ceramic bone substitute (Cerament™|SPINE SUPPORT): a prospective nonrandomized study. Neuroradiology 2012; 54: 589–596 [DOI] [PubMed] [Google Scholar]
- 16. Karr JC. Management in the wound-care center outpatient setting of a diabetic patient with forefoot osteomyelitis using Cerament Bone Void Filler impregnated with vancomycin: off-label use. J Am Podiatr Med Assoc 2011; 2011(101): 259–264 [DOI] [PubMed] [Google Scholar]
- 17. Karr JC, Lauretta J, Keriazes G. In vitro antimicrobial activity of calcium sulfate and hydroxyapatite (Cerament Bone Void Filler) discs using heat-sensitive and non-heat-sensitive antibiotics against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. J Am Podiatr Med Assoc 2011; 101: 146–152 [DOI] [PubMed] [Google Scholar]
- 18. Rauschmann M, Vogl T, Verheyden A, et al. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament™ Spine Support) in vertebral compression fractures due to osteoporosis. Eur Spine J 2010; 19: 887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcia S, Boi C, Dragani M, et al. Effectiveness of a bone substitute (XEPAMENT™) as an alternative to PMMA in percutaneous vertebroplasty: 1-year follow-up on clinical outcome. Eur Spine J 2012; 21(Suppl. 1): S112–S118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatten H, Voor M. Bone healing using a bi-phasic ceramic bone substitute demonstrated in human vertebroplasty and with histology in a rabbit cancellous bone defect model. Interv Neuroradiol 2012; 18: 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Busuttil Naudi K, Ayoub A, McMahon J, et al. Mandibular reconstruction in the rabbit using beta-tricalcium phosphate (beta-TCP) scaffolding and recombinant bone morphogenetic protein 7 (rhBMP-7) – histological, radiographic and mechanical evaluations. J Craniomaxillofac Surg 2012; 40: e461–e469 [DOI] [PubMed] [Google Scholar]
- 22. Allen M, Myer B, Rushton N. In vitro and in vivo investigations into the biocompatibility of diamond-like carbon (DLC) coatings for orthopedic applications. J Biomed Mater Res 2001; 58: 319–328 [DOI] [PubMed] [Google Scholar]
- 23. O’Hare P, Meenan BJ, Burke GA, et al. Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique. Biomaterials 2010; 31: 515–522 [DOI] [PubMed] [Google Scholar]
- 24. Ormsby R, McNally T, O’Hare P, et al. Fatigue and biocompatibility properties of a poly(methyl methacrylate) bone cement with multi-walled carbon nanotubes. Acta Biomater 2012; 8: 1201–1212 [DOI] [PubMed] [Google Scholar]
- 25. Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials 2009; 30: 2675–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liao S, Chen C, Ramakrishna S. Stem cells and biomimetic materials strategies for tissue engineering. Mat Sci Eng C 2008; 28: 1189–1202 [Google Scholar]
- 27. Dalby MJ, Yarwood SJ, Riehle MO, et al. Increasing fibroblast response to materials using nanotopography: morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp Cell Res 2002; 276: 1–9 [DOI] [PubMed] [Google Scholar]
- 28. Lai M, Cai KY, Hu Y, et al. Regulation of the behaviors of mesenchymal stem cells by surface nanostructured titanium. Colloids Surf B Biointerfaces 2012; 97: 211–220 [DOI] [PubMed] [Google Scholar]
- 29. Yim EKF, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 2007; 313: 1820–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kokubo S, Fujimoto R, Yokota S, et al. Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials 2003; 24: 1643–1651 [DOI] [PubMed] [Google Scholar]
- 31. Lo KW, Ulery BD, Ashe KM, et al. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev 2012; 64(12): 1277–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6: 483–495 [DOI] [PubMed] [Google Scholar]
- 33. Coetzee AS. Regeneration of bone in the presence of calcium-sulfate. Arch Otolaryngol 1980; 106: 405–409 [DOI] [PubMed] [Google Scholar]
- 34. Strocchi R, Orsini G, Iezzi G, et al. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. J Oral Implantol 2002; 28: 273–278 [DOI] [PubMed] [Google Scholar]
- 35. Walsh WR, Morberg P, Yu Y, et al. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res 2003; 406: 228–226 [DOI] [PubMed] [Google Scholar]
- 36. Thomas MV, Puleo DA. Calcium sulfate: properties and clinical applications. J Biomed Mater Res B Appl Biomater 2009; 88: 597–610 [DOI] [PubMed] [Google Scholar]
- 37. Park EK, Lee YE, Choi JY, et al. Cellular biocompatibility and stimulatory effects of calcium metaphosphate on osteoblastic differentiation of human bone marrow-derived stromal cells. Biomaterials 2004; 25: 3403–3411 [DOI] [PubMed] [Google Scholar]
- 38. Lazary A, Balla B, Kosa JP, et al. Effect of gypsum on proliferation and differentiation of MC3T3-E1 mouse osteoblastic cells. Biomaterials 2007; 28: 393–399 [DOI] [PubMed] [Google Scholar]
- 39. Jager M, Wilke A. Comprehensive biocompatibility testing of a new PMMA-hA bone cement versus conventional PMMA cement in vitro. J Biomater Sci Polym Ed 2003; 14: 1283–1298 [DOI] [PubMed] [Google Scholar]
- 40. Simon CGJ, Guthrie WF, Wang FW. Cell seeding into calcium phosphate cement. J Biomed Mater Res A 2004; 68: 628–639 [DOI] [PubMed] [Google Scholar]
- 41. Chow L, Takagi S, Costantino P, et al. Self-setting calcium phosphate cements. Mater Res Soc Symp P 1991; 179: 23–24 [Google Scholar]
- 42. Huan Z, Chang J. Self-setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cements. Acta Biomater 2007; 3: 952–960 [DOI] [PubMed] [Google Scholar]
- 43. Khadka A, Li J, Li Y, et al. Evaluation of hybrid porous biomimetic nano-hydroxyapatite/polyamide 6 and bone marrow-derived stem cell construct in repair of calvarial critical size defect. J Craniofac Surg 2011; 22: 1852–1858 [DOI] [PubMed] [Google Scholar]



