Abstract
The pattern of integration of spleen necrosis virus (SNV) DNA in DNA from a large population of SNV-infected chicken cells was studied by nucleic acid hybridization with iodinated viral RNA by the blotting technique of Southern. SNV DNA was found to be integrated at multiple sites in acutely infected chicken cells. Concomitant with the transition from acute to chronic infection, a shift in the pattern of integration was observed. The majority of integrated SNV DNA found in acutely infected cells was absent from chronically infected cells. This result is consistent with the hypothesis that the cell death that occurs after infection of avian cells with reticuloendotheliosis viruses is a consequence of the multiple integrations of the provirus. Viral DNA was also integrated at multiple sites in chronically infected cells. However, infectious viral DNA molecules in chronically infected cells migrated in a uniform manner in agarose gel electrophoresis after EcoRI digestion (which does not cut viral DNA), indicating that not all integrated SNV copies are equally infectious.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula N., Temin H. M. Infectious DNA of spleen necrosis virus is integrated at a single site in the DNA of chronically infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):281–285. doi: 10.1073/pnas.74.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
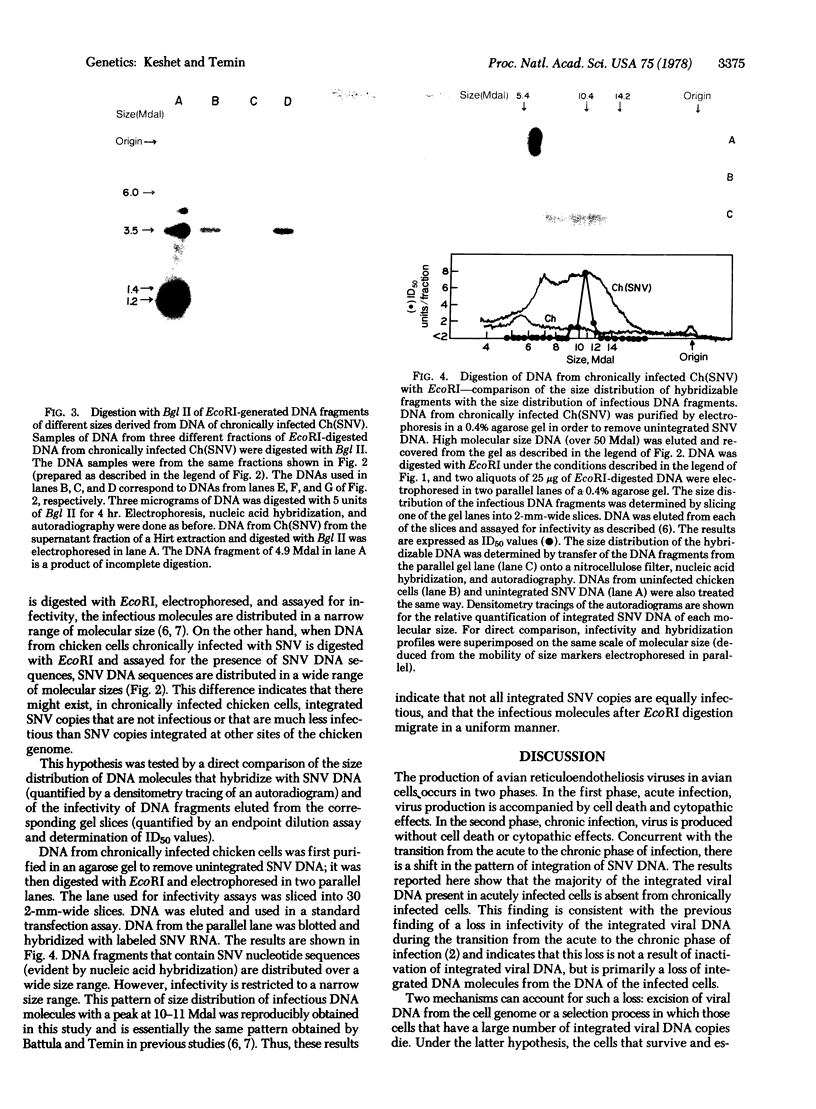
- Battula N., Temin H. M. Sites of integration of infectious DNA of avian reticuloendotheliosis viruses in different avian cellular DNAs. Cell. 1978 Feb;13(2):387–398. doi: 10.1016/0092-8674(78)90207-6. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastoor M. N., Shoyab M., Baluda M. A. Variations in integration site of avian oncornaviruses in different hosts. J Virol. 1977 Feb;21(2):541–547. doi: 10.1128/jvi.21.2.541-547.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Breedveld D., Hossenlopp P., Chambon P. A rapid procedure for purification of EcoRI endonuclease. Biochem Biophys Res Commun. 1977 May 9;76(1):78–85. doi: 10.1016/0006-291x(77)91670-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: chronic infection. J Gen Virol. 1975 Jun;27(3):267–274. doi: 10.1099/0022-1317-27-3-267. [DOI] [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]