Abstract
Glutaminase plays a critical role in the generation of glutamate, a key excitatory neurotransmitter in the CNS. Excess glutamate release from activated macrophages and microglia correlates with upregulated glutaminase suggesting a pathogenic role for glutaminase. Both glutaminase siRNA and small molecule inhibitors have been shown to decrease excess glutamate and provide neuroprotection in multiple models of disease, including HIV-associated dementia (HAD), multiple sclerosis and ischemia. Consequently, inhibition of glutaminase could be of interest for treatment of these diseases. Bis-2-(5-phenylacetimido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) and 6-diazo-5-oxo-L-norleucine (DON), two most commonly used glutaminase inhibitors, are either poorly soluble or non-specific. Recently, several new BPTES analogs with improved physicochemical properties were reported. To evaluate these new inhibitors, we established a cell-based microglial activation assay measuring glutamate release. Microglia-mediated glutamate levels were significantly augmented by tumor necrosis factor (TNF)-α, phorbol 12-myristate 13-acetate (PMA) and Toll-like receptor (TLR) ligands coincident with increased glutaminase activity. While several potent glutaminase inhibitors abrogated the increase in glutamate, a structurally related analog devoid of glutaminase activity was unable to block the increase. In the absence of glutamine, glutamate levels were significantly attenuated. These data suggest that the in vitro microglia assay may be a useful tool in developing glutaminase inhibitors of therapeutic interest.
Keywords: HIV-associated dementia (HAD), Glutamate, Glutamine, Glutaminase, Inflammation, Microglia
Introduction
Microglia play a dual role in the neuroprotection and neurotoxicity associated with various neurodegenerative diseases in the central nervous system (CNS) [1; 2; 3]. As a neuroprotectant, microglia serve as resident sentinels that provide the necessary innate immune response against injury, infection and other adverse stimuli. As a source of neurotoxicity, uncontrolled and excessively activated microglia contribute to neuroinflammation, a hallmark of several neurodegenerative diseases [1]. In response to stimuli, activated microglia produce pro-inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-18, IP-10, PGE2, TNF-α), reactive oxygen species (NO, O2-, H2O2, OH-, NOO-) and excess glutamate that have been shown to injure CNS cells, both in vitro and in vivo [4]. Much attention has been paid to therapeutic strategies aimed at eliminating neurotoxic microglial activation, including the use of enzyme inhibitors, receptor antagonists, natural products and neutralizing antibodies to cytokines [5; 6; 7; 8; 9; 10; 11]. Here, we suggest modulation of excitotoxic glutamate via the inhibition of microglial glutaminase as an alternative therapeutic strategy.
Glutaminase is an enzyme that catalyzes the hydrolysis of glutamine to glutamate and is thought to play a central role in the generation of excitotoxic glutamate in neuroinflammatory CNS disorders [12; 13; 14]. Recent studies have shown that the excess extracellular glutamate is released from CNS-resident activated microglia through gap junctions, after its conversion from glutamine via glutaminase [12; 14; 15]. In fact, in work using HIV-infected human macrophages, prototype glutaminase small molecule inhibitors and glutaminase specific siRNA were able to abrogate the glutamine-dependent increases in glutamate [12]. Glutaminase-mediated glutamate release from microglia was also shown to occur in a model of multiple sclerosis [13]. Thus glutaminase inhibition could be of broad therapeutic interest for neuroinflammatory disorders.
However, to date, there are no known potent and selective glutaminase inhibitors available. The two prototype inhibitors often employed, 6-Diazo-5-oxo-L-norleucine (DON) and bis-2-(5-phenylacetimido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES), are non-specific and insoluble, respectively [16; 17]. Recently, analogs of BPTES were made in an effort to improve on its drug-like properties, including size and solubility while retaining potency [17]. To evaluate these new glutaminase inhibitors, we established a microglial-based assay quantifying glutamate release in response to diverse agents including tumor necrosis factor (TNF)-α, pattern recognition Toll-like receptor (TLR) agonists and phorbol 12-myristate 13-acetate (PMA). We report that glutamate released from microglia is blocked by glutaminase inhibitors, is dependent on glutamine levels and is correlated with glutaminase activity.
Material and Methods
Materials
Tumor necrosis factor (TNF)-α, tripalmitoyl-S-glyceryl-cysteine (Pam3SK4 - TLR 1/2 agonist), polyinosinic-polycytidylic (poly I:C - TLR 3 agonist), lipopolysaccharide (LPS - TLR 4 agonist), CpG oligodeoxynucleotide (GC - TLR 9 agonist) and phorbol 12-myristate 13-acetate (PMA) were all obtained from Invivogen (San Diego, CA). Amplex UltraRed, Dulbecco's Minimum Essential Media (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Grand Island, NY), Horse Radish Peroxidase (HRP) from Worthington Biochemical Corporation (Lakewood, NJ), TRIS from Sigma (St. Louis, MO), Complete Protease Inhibitor Cocktail from Roche (Indianapolis, IN), 96-Well spin columns from Harvard Apparatus (Holliston, MA) and the strong anion ion-exchange resin from BioRad (Hercules, CA). Glutamate oxidase was acquired from either US Biological Life Sciences (Swampscott, MA) or from Sigma (St. Louis, MO). L-[2,3,4-3H]-Glutamine and 96-well LumaPlates were purchased from American Radiolabeled Chemicals (Saint Louis, MO) and Perkin Elmer (Waltham, MA), respectively. Finally, BPTES and its analogs were synthesized in-house [17].
Microglia assay
Single suspension cells were prepared from whole brains of 1 - 2 d old mice, as described previously [18]. Cells were cultured in flasks in high glucose DMEM with 15% FBS. After 7-10 days, microglia were dislodged from adherent cells by shaking the flasks for 1h at 200 rpm. Cells were re-plated at 100,000 cells per well in a 48-well plate and the effects of stimulants and glutaminase inhibitors evaluated in an acute paradigm. One to two days after plating, microglia were stimulated with either TNF-α (100 ng/ml), TLR ligands (Pam3SK4, 1 μg/ml; poly I:C, 10 μg/ml; LPS, 1 μg/ml and GC, 5 μM) or PMA (100 ng/ml). Glutaminase inhibitors (10 μM) were added 10 min prior to the addition of the stimulant. Supernatants were collected 16 - 18h after stimulation and assayed for glutamate.
Glutamate analysis
Glutamate levels were determined using a two-enzyme Amplex UltraRed assay system [19]. The assay was carried out at room temperature in TRIS buffer (pH 7.4), in black, medium bind, Greiner 96-well plates with microclear bottom using glutamate oxidase (0.04 U/mL) and HRP (0.125 U/mL) coupled with fluorogenic Amplex UltraRed (50 μM). The rate of change of fluorescence intensity (at ex 530, em 590) was measured over a 20 min period and correlated to the glutamate levels in the supernatants. Enzyme stocks were constituted in 100 mM TRIS.HCl buffer (pH 7.4) containing 20% glycerol and stored at -80°C until the day of the experiment. Amplex UltraRed stock was made up in 100% anhydrous DMSO.
Glutaminase activity analysis
Glutaminase activity in microglial cells was determined using radiolabeled glutamine (0.076 μM, specific activity 60 Ci/mmol) as substrate [20]. The cells were suspended in ice-cold phosphate buffer (KH2PO4, 45 mM, pH 8.2) containing protease inhibitors (Roche's Complete Inhibitor Cocktail, 1 tablet in 50 ml) and the cells disrupted using Kontes' MicroUltrasonic Cell Disrupter (output control set at 60; 3 pulses each of 10 s duration and on ice). The cell lysate was added to the substrate and the reaction conducted at room temperature (90 min incubation). The assay was terminated upon the addition of 20 mM imidazole buffer (pH 7). 96-Well microplate spin columns packed with strong anion ion-exchange resin were used to separate the unhydrolyzed substrate and the reaction product. Unreacted [3H]-glutamine was removed by washing with imidazole buffer. [3H]-Glutamate, the reaction product, was then eluted with 0.1 M HCl and analyzed for radioactivity.
Results
Glutamate release in microglia is induced by TNF-α, TLR agonists and PMA
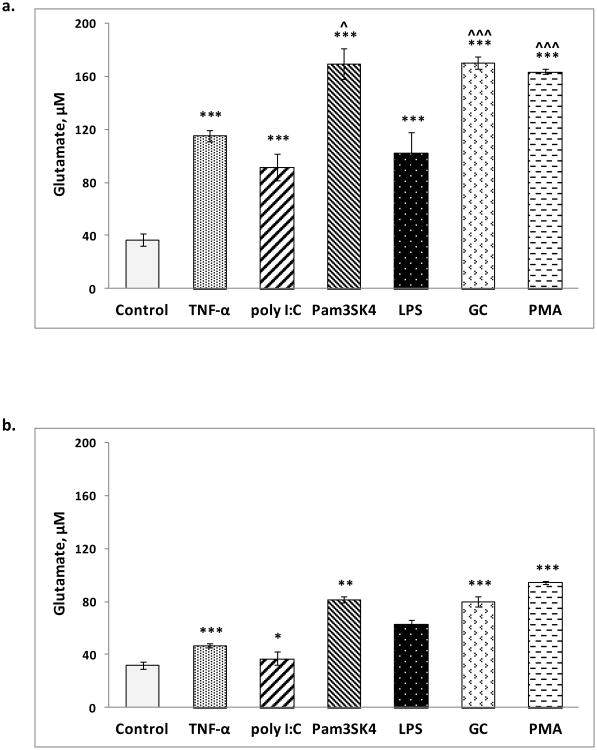
Glutamate levels in unstimulated (control) microglia in the presence and absence of glutamine were 37 ± 5 μM (Fig 1a) and 32 ± 3 μM (Fig 1b), respectively. Following stimulation in the presence of glutamine, glutamate levels were significantly increased by TNF-α (115 ± 4 μM), Pam3SK4 (170 ± 12 μM), poly I:C (91 ± 10 μM), LPS (102 ± 16 μM), GC (171 ± 4 μM) and PMA (164 ± 2 μM) (Fig 1a). Following stimulation in the absence of glutamine, glutamate levels increased to a much lesser degree with TNF-α (47 ± 2 μM), Pam3SK4 (81 ± 2 μM), poly I:C (37 ± 5 μM), LPS (63 ± 3 μM), GC (80 ± 4 μM) and PMA (95 ± 1 μM) (Fig 1b).
Fig 1. Glutamate released upon microglial activation by TNF-α, TLR ligands and PMA.
a. In the presence of glutamine (2 mM); ***p < 0.001 versus control (unstimulated), ˆp < 0.05, ˆˆˆp < 0.001 versus TNF-α and b. In the absence of glutamine; *p < 0.05, **p < 0.01, ***p < 0.001 versus the corresponding glutamate levels in the presence of glutamine. All data are presented as mean ± SEM values of three determinations in two independent experiments. Values were compared using Student's two-population t-test.
Glutamate release in activated microglia is inhibited by glutaminase inhibitors
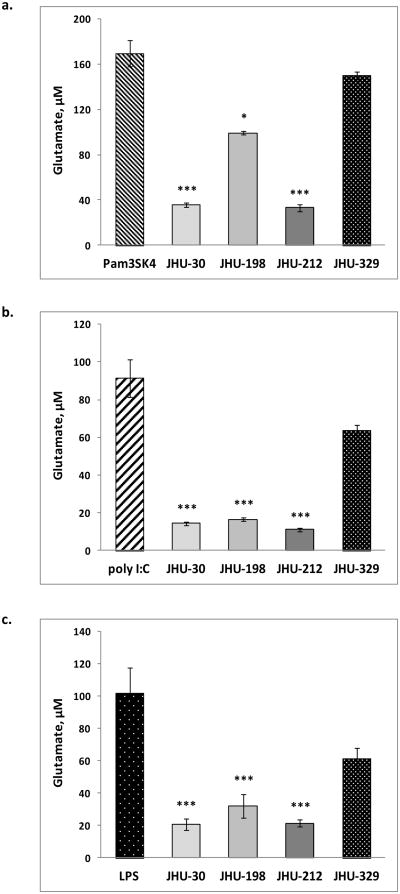
BPTES and its analogs (Table 1) were evaluated for their ability to block TLR-mediated glutamate release. While the active glutaminase inhibitors (BPTES, JHU-198 and JHU-212) abrogated the glutamate release induced by the various TLR agonists, a structurally related analog devoid of glutaminase activity (JHU-329) was unable to block the release (Fig 2).
Table 1. Structure of glutaminase inhibitors employed [17].
| Compound | Molecular Structure | Glutaminase activity (IC50, μM) |
|---|---|---|
| BPTES |
|
3 |
| JHU-198 |
|
4 |
| JHU-212 |
|
30 |
| JHU-329 |
|
>100 |
Fig 2. Glutamate released upon microglial activation by TLR ligands in the presence of glutaminase inhibitors.
a. Pam3SK4 (TLR 1/2 ligand), b. poly I:C (TLR 3 ligand) and c. LPS (TLR 4 ligand)-induced glutamate levels in the presence of glutaminase inhibitors. All data are presented as mean ± SEM values of three determinations in two independent experiments. Values were compared using Student's two-population t-test; *p < 0.05, ***p < 0.001 versus the corresponding microglial activator.
Glutamate release in activated microglia correlates with glutaminase activity
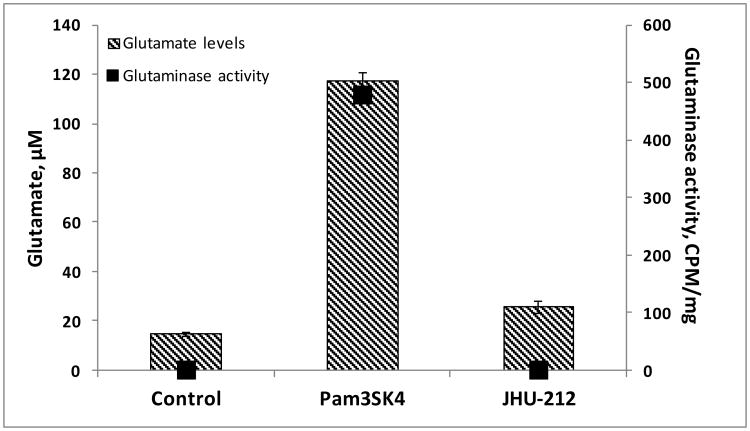
To evaluate if the increased glutamate levels correlated with increased active glutaminase, microglial cells were activated with the potent TLR agonist, Pam3SK4, in the presence and absence of a soluble glutaminase inhibitor, JHU-212. Subsequent to the activation, glutaminase activity was monitored using radiolabeled glutamine. Relative to unactivated microglial cells, glutaminase activity in Pam3SK4-activated microglial cells was increased by approximately 500-fold. Co-treatment of the Pam3SK4-activated microglial cells with the glutaminase inhibitor, JHU-212, blocked the increase in glutaminase activity (Fig 3).
Fig 3. Glutaminase activity in activated microglia.
Glutaminase activity in Pam3SK4-activated microglia in the presence and absence of JHU-212, as measured by the hydrolysis of radiolabeled L-[2,3,4-3H]-glutamine (60 Ci/mmol) at room temperature over a 90 min incubation period. All data are presented as mean ± SEM values of three determinations from a single experiment.
Discussion
We report on the implementation of a cell-based microglial activation assay measuring glutamate release and its utility in evaluating the effects of glutaminase inhibition. We report on the use of TNF-α, PMA and a variety of pattern recognition toll-like receptor (TLR) agonists to elicit glutamate release. We chose TNF-α and LPS, a TLR4 ligand, to validate our cell-based microglial activation assay. Subsequently, we explored the role of other TLR ligands and a TLR2 modulator (PMA) in microglial glutamate release mechanisms and show that the released glutamate comes, in part, from glutamine hydrolysis catalyzed by glutaminase. Inhibition of glutaminase is of considerable interest due to the wide array of neurological diseases where glutaminase and excess glutamate toxicity has been implicated [11; 21; 22; 23].
Confirming previous work [15], we show that microglia-mediated glutamate release is significantly induced by TNF-α (Fig 1a). Activation of the microglia by TLR ligands induced similar glutamate release, yet the magnitude of the effect was significantly more pronounced with Pam3SK4 and GC (Fig 1a). While a direct effect of the TLR ligands at the TNF-receptor is not known, TLR-mediated microglial activation has been shown to induce the release of several proinflammatory cytokines, including TNF-α [24]. The enhanced glutamate release via TLR-mediated microglial activation might be due to a secondary TNF-receptor activation since TNF-α is itself known to exert its effects in an autocrine manner [15]. PMA, a protein kinase C activator and T cell mitogen [25], also induced significantly larger amounts of glutamate than TNF-α (Fig 1). One possible mechanism of PMA-mediated increased glutamate release might be due to over expression and activation of TLR 2 and TLR 4 [26]. Interestingly, PMA activation post TLR stimulation has also been shown to induce significant TNF-α release [26].
The up-regulation of glutaminase has been suggested to be a key mechanism in the release of glutamate upon TNF-α-mediated microglial activation [15]. Results in the absence of glutamine (Fig 1b) and in the presence of glutaminase inhibitors (Fig 2) support this hypothesis. Interestingly, glutamate levels in the absence of glutamine, though significantly lower than when glutamine was present, were not negligible. This could be due to possible alternate sources of glutamate, including the conversion of residual internal glutamine reserves or the biosynthesis of glutamate mediated by various other enzymes [27]. Involvement of glutaminase in increased glutamate release by activated microglia is also supported by studies that have shown increased glutaminase mRNA expression after stimulation with LPS and TNF-α [15]. That glutaminase activity correlates with glutamate release in TLR-activated microglia (Fig 3) not only corroborates these earlier reports [12; 15] but also supports the idea of targeting glutaminase in developing drugs to suppress the toxicity resulting from microglia activation. One caveat is the acute nature of the paradigm in which the glutaminase inhibitors were evaluated. While there is no reason to suspect the efficacy of glutaminase inhibition in chronic paradigms, long term effects of microglia activation on glutaminase, i.e. upregulation, or the consequence of glutaminase inhibition on microglia viability are unknown.
A common intercellular pathway that links microglial activation by diverse agents such as TNF-α, TLR ligands and PMA is their ability to induce the DNA-binding activity of nuclear factor- κB (NF-κB) [25; 28], a crucial transcription factor that regulates the expression of genes involved in immune and inflammatory responses and consequent inflammatory cytokine release [25; 29; 30; 31]. It is conceivable that TNF-α, TLR ligands and PMA-induced glutamate release is mediated through the NF-κB pathway.
Regardless of the precise mechanisms involved, overwhelming evidence suggests that regulation of excess glutamate induced by microglial activation could play an important role in several neurodegenerative disorders [7; 11; 21; 22; 23; 32]. Therefore, any agent that negatively modulates this excess glutamate could potentially alter the clinical course of the disease. However, a large number of randomized controlled trials involving anti-glutamate receptor/transporter drugs have failed to show clinical benefit [22; 32; 33; 34]. In the absence of successful therapies, glutaminase inhibition provides an exciting therapeutic approach for microglia-mediated neurodegenerative diseases and perhaps, other glutamate-mediated disorders. Glutaminase inhibition represents an ‘upstream’ mechanism of glutamate regulation that could reduce transmission at all glutamatergic receptors and minimize any potential adverse events.
Glutamate release in microglia was induced by TNF-α, TLR agonists and PMA.
Glutamate release was blocked by active, small molecule glutaminase inhibitors.
Glutamate release was reduced in the absence of glutamine.
Glutamate release was correlated with glutaminase activity.
Microglia glutamate release assay could be used to evaluate glutaminase inhibitors.
Acknowledgments
This work was supported in part by the Johns Hopkins Brain Science Institute and NIH grant R03DA032470 (to BSS).
Abbreviations
- BPTES
bis-2-(5-phenylacetimido-1,2,4-thiadiazol-2-yl)ethyl sulfide
- CNS
central nervous system
- DON
6-diazo-5-oxo-L-norleucine
- GC
CpG oligodeoxynucleotide
- GLS
glutaminase
- HAD
HIV-associated dementia
- HIV
Human Immunodeficiency Virus
- HRP
horse radish peroxidase
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- Pam3SK4
tripalmitoyl-S-glyceryl-cysteine
- PMA
phorbol 12-myristate 13-acetate
- poly I:C
polyinosinic-polycytidylic
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 2.Kempermann G, Neumann H. Neuroscience. Microglia: the enemy within? Science. 2003;302:1689–90. doi: 10.1126/science.1092864. [DOI] [PubMed] [Google Scholar]
- 3.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 4.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Basith S, Manavalan B, Lee G, Kim SG, Choi S. Toll-like receptor modulators: a patent review (2006-2010) Expert Opin Ther Pat. 2011;21:927–44. doi: 10.1517/13543776.2011.569494. [DOI] [PubMed] [Google Scholar]
- 6.Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–43. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figuera-Losada M, Rojas C, Slusher BS. Inhibition of Microglia Activation as a Phenotypic Assay in Early Drug Discovery. J Biomol Screen. 2013 doi: 10.1177/1087057113499406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illei GG, Lipsky PE. Novel, non-antigen-specific therapeutic approaches to autoimmune/inflammatory diseases. Curr Opin Immunol. 2000;12:712–8. doi: 10.1016/s0952-7915(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 9.McCarty MF. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med Hypotheses. 2006;67:251–69. doi: 10.1016/j.mehy.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25:945–53. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- 11.Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–49. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shijie J, Takeuchi H, Yawata I, Harada Y, Sonobe Y, Doi Y, Liang J, Hua L, Yasuoka S, Zhou Y, Noda M, Kawanokuchi J, Mizuno T, Suzumura A. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J Exp Med. 2009;217:87–92. doi: 10.1620/tjem.217.87. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J Neurochem. 2004;88:169–80. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–8. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 16.Pinkus LM. Glutamine binding sites. Methods Enzymol. 1977;46:414–27. doi: 10.1016/s0076-6879(77)46049-x. [DOI] [PubMed] [Google Scholar]
- 17.Shukla K, Ferraris DV, Thomas AG, Stathis M, Duvall B, Delahanty G, Alt J, Rais R, Rojas C, Gao P, Xiang Y, Dang CV, Slusher BS, Tsukamoto T. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–63. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109:96–107. doi: 10.1016/j.jsbmb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElroy KE, Bouchard PJ, Harpel MR, Horiuchi KY, Rogers KC, Murphy DJ, Chung TD, Copeland RA. Implementation of a continuous, enzyme-coupled fluorescence assay for high-throughput analysis of glutamate-producing enzymes. Anal Biochem. 2000;284:382–7. doi: 10.1006/abio.2000.4740. [DOI] [PubMed] [Google Scholar]
- 20.Engler JA, Gottesman JM, Harkins JC, Urazaev AK, Lieberman EM, Grossfeld RM. Properties of glutaminase of crayfish CNS: implications for axon-glia signaling. Neuroscience. 2002;114:699–705. doi: 10.1016/s0306-4522(02)00357-3. [DOI] [PubMed] [Google Scholar]
- 21.Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 22.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–42. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 23.Marmiroli P, Cavaletti G. The glutamatergic neurotransmission in the central nervous system. Curr Med Chem. 2012;19:1269–76. doi: 10.2174/092986712799462711. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh TK, Mickelson DJ, Fink J, Solberg JC, Inglefield JR, Hook D, Gupta SK, Gibson S, Alkan SS. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Wang Y, Gao L, Zhang J, Shao J, Wang S, Feng W, Wang X, Li M, Chang Z. Expression of toll-like receptors 2 and 4 and CD14 during differentiation of HL-60 cells induced by phorbol 12-myristate 13-acetate and 1 alpha, 25-dihydroxy-vitamin D(3) Cell Growth Differ. 2002;13:27–38. [PubMed] [Google Scholar]
- 27.Goldlust A, Su TZ, Welty DF, Taylor CP, Oxender DL. Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res. 1995;22:1–11. doi: 10.1016/0920-1211(95)00028-9. [DOI] [PubMed] [Google Scholar]
- 28.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–8. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 32.Kostandy BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci. 2012;33:223–37. doi: 10.1007/s10072-011-0828-5. [DOI] [PubMed] [Google Scholar]
- 33.Brigell MG, Taylor CP. ALS defeats gabapentin: reflections on another failed treatment. Neurology. 2001;57:1524–5. doi: 10.1212/wnl.57.8.1524-a. [DOI] [PubMed] [Google Scholar]
- 34.Miller RG, Moore DH, 2nd, Gelinas DF, Dronsky V, Mendoza M, Barohn RJ, Bryan W, Ravits J, Yuen E, Neville H, Ringel S, Bromberg M, Petajan J, Amato AA, Jackson C, Johnson W, Mandler R, Bosch P, Smith B, Graves M, Ross M, Sorenson EJ, Kelkar P, Parry G, Olney R. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:843–8. doi: 10.1212/wnl.56.7.843. [DOI] [PubMed] [Google Scholar]