SUMMARY
Selective autophagy functions to specifically degrade cellular cargo tagged by ubiquitination, including bacteria. Strains of the Burkholderia cepacia complex (Bcc) are opportunistic pathogens that cause life-threatening infections in patients with cystic fibrosis (CF) and chronic granulomatous disease (CGD). While there is evidence that defective macrophage autophagy in a mouse model of CF can influence B. cenocepacia susceptibility, there have been no comprehensive studies on how this bacterium is sensed and targeted by the host autophagy response in human macrophages. Here, we describe the intracellular life cycle of B. cenocepacia J2315 and its interaction with the autophagy pathway in human cells. Electron and confocal microscopy analysis demonstrates that the invading bacteria interact transiently with the endocytic pathway before escaping to the cytosol. This escape triggers the selective autophagy pathway, and the recruitment of ubiquitin, the ubiquitin-binding adaptors p62 and NDP52 and the autophagosome membrane-associated protein LC3B, to the bacterial vicinity. However, despite recruitment of these key autophagy pathway effectors, B. cenocepacia blocks autophagosome completion and replicates in the host cytosol. We find that a pre-infection increase in cellular autophagy flux can significantly inhibit B. cenocepacia replication and that lower autophagy flux in macrophages from immunocompromised CGD patients could contribute to increased B. cenocepacia susceptibility, identifying autophagy manipulation as a potential therapeutic approach to reduce bacterial burden in B. cenocepacia infections.
INTRODUCTION
The Gram negative bacterium Burkholderia cenocepacia is a member of the Burkholderia cepacia complex (Bcc), a group of seventeen phenotypically similar species that are found ubiquitously in nature but not usually as part of the normal mammalian flora (Vandamme et al., 1997, Coenye et al., 2003, Vandamme et al., 2003, Mahenthiralingam et al., 2005, Vanlaere et al., 2008, Vanlaere et al., 2009). Once acquired from the environment, B. cenocepacia can cause devastating infections in immunocompromised individuals, especially in patients suffering from cystic fibrosis (CF) and chronic granulomatous disease (CGD) (Vandamme et al., 1997, Nzula et al., 2002, Bernhardt et al., 2003). Although macrophages form a critical first line of defense against bacterial infection, the majority of prior studies on the pathogenesis of this bacterium in mammalian host cells have been done in either neutrophils or epithelial cells (Burns et al., 1996, Martin et al., 2000, Bylund et al., 2005, Sajjan et al., 2006). Moreover, prior reports of B. cenocepacia infection in macrophages and epithelial cells, have suggested that virulent strains of the bacteria remain in a membranous compartment, avoid host degradation by delaying endosomal maturation, and form a replicative vacuole (Martin et al., 2000, Sajjan et al., 2006, Lamothe et al., 2007, Lamothe et al., 2008, Huynh et al., 2010, Abdulrahman et al., 2011).
Autophagy is an evolutionarily conserved eukaryotic process by which cytoplasmic components are engulfed and sequestered by multi-membraned autophagosomes that subsequently fuse to lysosomes for degradation (Deretic et al., 2009). In response to nutrient-starvation conditions, ‘general’ autophagy serves a catabolic function by mediating nonselective degradation of organelles to generate substrates for energy metabolism and protein synthesis (Deretic et al., 2009). In contrast, ‘selective’ autophagy functions to specifically remove protein aggregates and specific organelles via ubiquitin-mediated targeting (Youle et al., 2011, Ding et al., 2012). Recently, selective autophagy has been shown to play an important role in innate defense against invading intracellular pathogens (Deretic et al., 2009), eliminating microbes that enter into the cytosol by sequestering them into autophagosomes, and delivering them to autophagolysosomes for degradation (Reggiori et al., 2002, Otto et al., 2004, Yoshimori, 2004, Swanson, 2006). Studies of Salmonella enterica serovar Typhimurium infection have shown that bacteria that avoid the endosomal pathway and enter the cytosol are ubiquitinated and delivered to autophagosomes via recognition by the cytosolic autophagy receptors p62/SQTSM1 and NDP52 (Thurston et al., 2009, Zheng et al., 2009). Recent reports in macrophages derived from a murine model of CF have suggested that defective autophagy underlies increased B. cenocepacia replication in these cells, due both to sequestration of key autophagy adapters in CFTR aggregates, and through an infection-dependent decrease in autophagy gene expression (Abdulrahman et al., 2011, Abdulrahman et al., 2013). However, these studies suggested that autophagy evasion in this murine CF model is primarily driven by the host cell genetic defect, and aside from the observation of altered autophagy gene expression, they did not address whether the bacteria itself can actively subvert the autophagy pathway (Abdulrahman et al., 2011, Abdulrahman et al., 2013).
To counter the host selective autophagy response, several pathogens have been found to employ autophagy evasion mechanisms that are critical for long-term, persistent infection (Kudchodkar et al., 2009). Shigella flexneri, Legionella pneumophila, Francisella tularensis and Listeria monocytogenes can actively subvert autophagic targeting and survive within host cells (Ogawa et al., 2005, Ogawa et al., 2011). However, it should be noted that all the autophagy subversion mechanisms reported to date are classified in three categories: prevention of autophagic induction, suppression of autophagosome maturation, or evasion of pathogen recognition by the autophagic machinery (Orvedahl et al., 2009).
Since B. cenocepacia is an important human pathogen, and comprehensive studies on how human macrophages may sense B. cenocepacia and initiate the autophagic pathway are still lacking, we investigated the intracellular life cycle of B. cenocepacia and its interplay with the autophagy pathway in this key host cell type. We show that in contrast to reports suggesting B. cenocepacia remain in a membranous vacuole throughout infection, bacteria of the clinically relevant B. cenocepacia J2315 strain interact only transiently with the endocytic pathway in human macrophages, before compromising their phagocytic vacuoles and escaping to the cytosol. Once exposed in the cytosol, B. cenocepacia elicits a ubiquitin-mediated host autophagy response, but unexpectedly, targeting of the bacteria for autophagy-mediated clearance rarely results in autophagosome formation. While the specific mechanism of autophagy subversion remains unclear, the bacteria ultimately escape the autophagic process despite efficient recruitment of LC3B to a high percentage of intracellular bacteria, suggesting a novel strategy of host evasion. Importantly from a clinical perspective, we also find that pre-stimulation of autophagy prior to infection can dramatically suppress bacterial replication. This indicates that intracellular B. cenocepacia which are targeted by the mammalian autophagy pathway are not effectively cleared unless the host cell autophagy flux is increased prior to infection. We extend these findings to a clinically relevant B. cenocepacia infection scenario using macrophages derived from CGD patients and a CGD mouse model, and show that pharmacological induction of autophagy flux prior to infection successfully controls bacterial replication in these cells. These data may have important implications for the development of therapeutics that could provide protection from B. cenocepacia infection in immunocompromised patients.
RESULTS
Intracellular growth of B. cenocepacia in human and murine macrophages
To establish a macrophage model for infection in human cells and to gain a better understanding of the pathogenesis of B. cenocepacia in these important target cells, we infected either human monocyte-derived macrophages (hMDMs), prepared using cells from the blood of healthy donors, or mouse bone marrow-derived macrophages (BMDMs), prepared from C57BL/6 mice, with live wild-type (WT) J2315 at a multiplicity of infection (MOI) of 1. After 1h of infection, all extracellular bacteria were removed by 2h of antibiotic treatment (see Experimental Procedures for detailed infection protocol). The WT J2315 strain displayed continuous growth kinetics with 15 to 127 and 6 to 89-fold increase in the colony forming units (CFU) recovered at 8 and 24h from hMDMs and BMDMs respectively (Fig. S1A). This indicates that both human and murine macrophages are permissive for intracellular bacterial replication. We then tested whether the human monocytic THP-1 cell line differentiated into a macrophage-like state is a viable model for infection. We infected THP-1 with WT J2315 at MOI 1, and found comparable growth kinetics to primary macrophages (Fig. S1A).
To determine the optimal MOI for THP-1, we infected cells at bacteria per cell MOIs of 0.1, 1, 10 and 30 and assessed the level of intracellular replication (Fig. S1B). We found that MOI = 1 showed more consistent infectivity than 0.1 and better macrophage survival at 24h than 10 or 30, indicating that MOI = 1 represents an optimal bacterial dose for subsequent experiments, including quantification of replication using confocal microscopy. To determine whether the intracellular growth we measured was due to widespread replication in all infected cells or only in a subset, we performed single cell analysis by confocal microscopy using THP-1 cells infected with a DsRed-expressing strain of J2315 (Fig. S1C, D). At 5 min and 3h after infection, approximately 90% of the infected cells harbored 1–2 organisms (Fig. S1C, D). After 8h of infection, approximately 90% of the infected cells contained 3 to 15 bacteria, while after 24h, bacterial counts per cell exceeded 15 in almost all infected cells (Fig. S1C, D). In these confocal studies, we found that the percentage of infected cells does not vary significantly between experiments (approximately 70–80%) and importantly in each single experiment; the percentage of infected cells was invariant. We conclude that the replication of J2315 in THP-1 cells was relatively homogeneous, clearly intracellular and that there were very few bacteria unable to replicate.
Intracellular trafficking of B. cenocepacia within human macrophages
It has previously been shown in studies with the CF IB3 epithelial cell line and mouse macrophages that live B. cenocepacia survives within a modified vacuole that acquires late endosomal but not lysosomal markers (Sajjan et al., 2006, Lamothe et al., 2007). To evaluate the intracellular localization of B. cenocepacia within THP-1 cells, we stained for the early endosomal protein EEA-1, the late endosomal/ lysosomal protein LAMP-2, and the luminal lysosomal enzyme Cathepsin-D (Fig. 1A–C). Formalin-killed (FK) bacteria, which traffic without delay to phagolysosomes, were used as a positive control for lysosomal degradation. We found that at 5 and 30 min post-infection, phagosomes containing either WT or FK bacteria showed approximately 25 and 50% co-localization, respectively, with the EEA1 marker (Fig. 1A). After 1h of infection, while 30% of the WT bacteria remained colocalized with EEA-1, FK bacteria showed only 8% co-localization, likely reflecting maturation of the FK-containing vacuoles to late endosomes (Fig. 1A, B). At all time points up to 8h post-infection, only a moderate proportion of the WT bacteria colocalized with LAMP-2 (20–30%) and Cathepsin D (20–40%), while the FK bacteria showed increasing co-localization of both markers with time to over 80% by 8h post-infection (Fig. 1B, C). These observations suggest that while killed bacteria are processed effectively through the endo-lysosomal pathway, metabolically active bacteria are able to subvert the endocytic process.
Figure 1. Intracellular trafficking of B. cenocepacia J2315 in macrophages.
(A–C) Percentage colocalization of B. cenocepacia in THP-1 cells with early endosomal EEA1 (A), late endosomal Lamp2 (B) and lysosomal Cathepsin-D (C) at the indicated time points post-infection. At least 100 infected cells from multiple coverslips were examined in each experiment. All results shown in A through C are representative of three independent experiments, each performed in duplicate. Data represent mean ± standard deviation. Asterisks represent statistically significant difference (p value <0.05). (D–H) TEM of THP-1 cells infected with B. cenocepacia at MOI 1: (D) Bacteria residing in intact vacuoles at 1h post infection. (E) Bacteria residing in compromised vacuoles or (F) free in the cytosol at 3h post infection. (G) Formalin killed (FK) bacterium residing in intact vacuole at 1h post infection. (H) WT bacteria replicating in the cytosol at 8h post infection. Scale bars shown are equal to 100nm (D, G), 500 nm (E, F), 1 um (H). Representative images from four independent TEM infection experiments each performed in duplicate are shown. (I) Quantification from TEM images of the percentage of bacteria residing in intact vacuoles, in compromised vacuoles/ free in the cytosol, or autophagosome-like vacuoles at various time points post-infection. Cells were scored from analysis of at least 50 infected cells at each time point. Data represent mean ± standard deviation.
B. cenocepacia resides transiently in single-membrane phagosomes before escaping to the cytosol
To directly test the previously reported hypothesis that the bacteria replicate in a modified vacuole (Sajjan et al., 2006, Lamothe et al., 2007), which could correspond to the endocytic compartment in which we find the bacteria to reside several hours after infection, we utilized transmission electron microscopy (TEM) to examine intracellular B. cenocepacia during the early stages of human macrophage infection. When the integrity of the phagosome was examined by TEM (Checroun et al., 2006, Jarry et al., 2006),. a bacteria-containing vacuole (BCV) was considered intact when the membrane had no visible disruptions (Checroun et al., 2006). A BCV was considered compromised when apparent physical disruptions of the phagosomal membrane were present and/or when host cell cytoplasmic elements, such mitochondria, vesicles, and amorphous material, were visible next to the bacteria without a limiting membrane or with remnants of membrane surrounding the organism. We observed that at 1h post-infection the majority of the bacteria (70%) were inside single-membrane vacuoles (Fig. 1D–I), consistent with phagocytic uptake. Surprisingly, 3h after infection, the BCV started to lose its integrity and, in a large proportion of infected cells (77%) (Fig. 1I)., the bacteria were surrounded by compromised vacuolar membranes (Fig. 1E) or completely exposed to the cytosol (Fig. 1F). In contrast, ~90% of FK bacteria were surrounded by an intact phagosomal membrane (Fig. 1G). These data provide clear evidence that the lack of colocalization of live bacteria with the late endosomal and lysosomal markers is not due to an arrest in vacuole maturation but rather to a clear escape of the bacteria from the endocytic trafficking pathway, with >95% of bacteria replicating in the macrophage cytosol 8h post-infection (Fig. 1H, I).
To further analyze B. cenocepacia escape from the phagocytic vacuole, we used Galectin 3 (Gal-3) as a marker for vacuole compromise (Paz et al., 2010). Gal-3, a cytosolic lectin, is a danger receptor that monitors endosomal integrity and detects bacterial invasion by binding host glycans exposed on damaged bacteria-containing vacuoles (Paz et al., 2010). At 1h post-infection, we found Gal-3 to be colocalized with B. cenocepacia in approximately 40% of infected cells (Fig. 2B, Fig. S1E), increasing to >80% at 3h post-infection (Fig. 2A, B). At both time points, cells with Gal-3/WT B. cenocepacia overlap showed almost complete colocalization, while very little or no colocalization was observed with FK bacteria (Fig. S1E, Fig. 2A, B), consistent with our TEM observation that live bacteria-containing vacuoles begin to lose integrity as early as 1h post infection. To further confirm that the BCV are disrupted, we used a previously described technique using selective antibody labeling under conditions that leave that vacuolar membranes intact (Creasey et al., 2012). After fixation with 4% paraformaldehyde, the macrophage cell membrane is sufficiently permeable to allow immunostaining of cytosolic proteins, but not proteins located in the lumen of organelles (Creasey et al., 2012). As expected, intracellular FK B. cenocepacia could not be detected by immunostaining under these conditions (Fig. 2C, D). In contrast, by 1h and 3h post-infection, the WT could be detected without further permeabilization of the cells (Fig. 2C, D). These data indicate disruption of the integrity of the vacuolar membrane after WT B. cenocepacia infection, lending further support to the TEM observations and the Gal-3 data. The physical escape of the bacteria from the degradative endocytic compartments by means of rupturing the vacuolar membrane and replication within the cytoplasm is often accompanied by cytosolic actin recruitment (Gouin et al., 1999, Checroun et al., 2006, Paz et al., 2010). To further verify that B. cenocepacia is residing in the cytosol, we analyzed the actin distribution in the infected cells. At 3h post-infection, we found actin recruitment to B. cenocepacia in approximately 67% of infected cells (Fig. 2E, F) while very little or no colocalization was observed with FK bacteria (Fig. 2E, F), further suggesting that live bacteria are free in the cytosol as early as 3h post infection.
Fig. 2. B. cenocepacia resides in compromised vacuoles before escaping to the cytosol.
(A) Confocal images of THP-1 macrophages infected with WT or FK DsRed B. cenocepacia at MOI 1 for 3h post infection. Infected cells were labeled with an anti-Gal-3 antibody (green) prior to visualization. Bacterial colocalization with Gal-3 was quantified using Imaris and is shown in the fourth panel. Imaris calculates the % of voxel colocalization between the 2 channels in the 3D region of interest (ROI) reconstructed from the planes of the confocal image. The ROI in this case is the infected cell. (B) Colocalization of the bacteria-containing vacuoles (BCVs) with Gal-3 at the indicated time points post-infection. (C) Confocal images of THP-1 macrophages infected with WT or FK DsRed B. cenocepacia at MOI 1 for 3h post infection selectively antibody-labeled for the bacteria residing in damaged/compromised vacuoles under conditions that leave the vacuolar membrane intact. Note that the intracellular WT bacterium is antibody-labeled (green) while only the extracellular FK bacterium is accessible to the antibody. (D) Percentage of disrupted vacuoles as determined by selective antibody labeling of bacteria at the indicated time points post-infection. (E) Confocal images of THP-1 macrophages infected with WT or FK DsRed B. cenocepacia at MOI 1 for 3h post infection with cytosolic actin labeled using phalloidin 568 (Red). Bacterial colocalization with actin was quantified using Imaris and is shown in the fourth panel. (F) Percentage of actin colocalization at the indicated time points post-infection. In panels B, D, and F error bars represent the standard deviation of the % mean value averaged from 100 cells over 3 experiments. Asterisks represent statistically significant difference (p value <0.05).
B. cenocepacia recruits endoplasmic reticulum (ER) markers and is targeted by the host autophagy pathway
Our TEM analysis revealed a close proximity of endoplasmic reticulum (ER) with the bacteria (dashed arrows; Fig. S2A). To evaluate this further, we measured colocalization of the KDEL ER marker with both WT and FK B. cenocepacia. At 1h post-infection, 50% of the WT bacteria colocalized with the KDEL marker, whereas the FK bacteria showed only marginal KDEL co-localization (~ 10%; Fig. S2B, C). Acquisition of the KDEL marker by WT bacteria increased during the course of infection, with 85% colocalization by 8h (Fig. S2B).
Since ER membrane has been proposed as a potential source of autophagosomal membrane for the host autophagy pathway, the presence of ER cisternae adjacent to the bacteria (dashed arrows; Fig. S2A) might suggest an attempt by the infected host cell to incorporate the bacteria into an autophagosome-like compartment. Therefore, to determine if autophagy pathway effectors colocalized with B. cenocepacia after human macrophage infection, we used a THP-1 cell line stably expressing a GFP-tagged fusion of the key autophagy protein LC3B (Shi et al., 2012). Following infection of cells with B. cenocepacia at MOI 1, we evaluated changes in the cellular localization of LC3B (Fig. 3A, C). One hour after infection, ~20% of infected cells showed WT bacteria colocalized with GFP-LC3B, increasing to ~80% at 6h post-infection before decreasing to 25% by 24h post-infection (Fig. 3C). No LC3B puncta formation or recruitment to the bacteria was observed during infection with FK B. cenocepacia (Fig. 3A). Three-dimensional confocal imaging of these cells revealed that GFP-LC3B surrounds the live bacteria (Movie S1).
Fig. 3. B. cenocepacia cytosolic escape triggers the autophagy pathway in macrophages.
(A) Confocal images of THP-1 macrophages expressing GFP-LC3B left untreated or infected with wild type (WT) or formalin killed (FK) DsRed B. cenocepacia at MOI 1 for 1h and 4h post infection. (B) Confocal images of GFP-LC3B expressing THP-1 cells infected for 4hrs with DsRed J2315 at MOI 1 and immunostained with FK1 anti-ubiquitin antibody (magenta) and anti-NDP52 or anti-p62 (cyan). For panels A and B, representative images from three independent experiments are shown. (C) Quantification and kinetics of bacterial colocalization with LC3B, p62, NDP52, and ubiquitin during infection. (D) Percentage of B. cenocepacia showing triple positive colocalization with LC3B, ubiquitin, and p62 throughout the infection time course. For panels C and D, at least 100 bacteria were counted at each time point and the results shown are mean ± standard deviation of three experiments.
It has been suggested that many bacteria that reside in damaged vacuoles or gain access to the cytosol are modified with ubiquitin (Perrin et al., 2004, Birmingham et al., 2006, Collins et al., 2010), and that this represents an initial step in activation of the autophagy response. We evaluated ubiquitin colocalization with B. cenocepacia using the FK1 anti-ubiquitin antibody that recognizes both mono and polyubiquitin (Fig. 3B, C). The bacteria were surrounded by GFP-LC3B and had strong ubiquitin labeling at both 4h (53%) and 6h (81%) post-infection (Fig. 3B, C). This suggests that following compromise of the phagocytic membrane (Fig. 1, E, F, Fig. 2), B. cenocepacia that are exposed to the cytoplasm are detected and marked by the ubiquitin conjugation system.
Autophagy adaptor proteins such as p62 and NDP52 recognize ubiquitinated cargo through a ubiquitin-binding domain (Thurston et al., 2009, von Muhlinen et al., 2010, Von Muhlinen et al., 2011), and can subsequently recruit LC3B as part of the autophagy initiation process. We examined whether p62 and NDP52 are recruited during the host cell attempt to target the bacteria to autophagosomes. As shown in (Fig. 3B), LC3B-positive B. cenocepacia was found to colocalize with both p62 and NDP52. Colocalization of B. cenocepacia with both adapters peaked at 4h post-infection with 70–80% of total intracellular bacteria associated, before dropping to approximately 24% at 24h post-infection (Fig. 3C). The levels of p62 and NDP52 colocalization were comparable to the ubiquitin recruitment to the bacteria over the same time course (Fig. 3C). Kinetics of recruitment of LC3B, both adaptors and ubiquitin to B. cenocepacia are summarized in Fig. 3C. Dual-labeling experiments showed that ~55–70% of the LC3B+ B. cenocepacia also colocalized with both ubiquitin and p62 (Fig. 3D). Thus, as with S. Typhimurium and Mycobacterium tuberculosis (Cemma et al., 2011), both autophagy adaptors are involved in autophagy initiation after infection with B. cenocepacia.
Moreover, knockdown of p62 expression in GFP-LC3B THP-1 using small interfering RNA (siRNA) resulted in a dramatic decrease in LC3B colocalization with WT B. cenocepacia (Fig. S3A, B). Importantly, ubiquitin recruitment was unaffected in the p62-silenced cells (Fig. S3B), indicating that ubiquitin recruitment to B. cenocepacia precedes p62. These data suggest a model whereby cytosol-exposed bacteria are first marked with ubiquitin, recruit ubiquitin-binding adapter proteins such as p62, and this signals the subsequent recruitment of LC3B.
B. cenocepacia evades selective autophagy
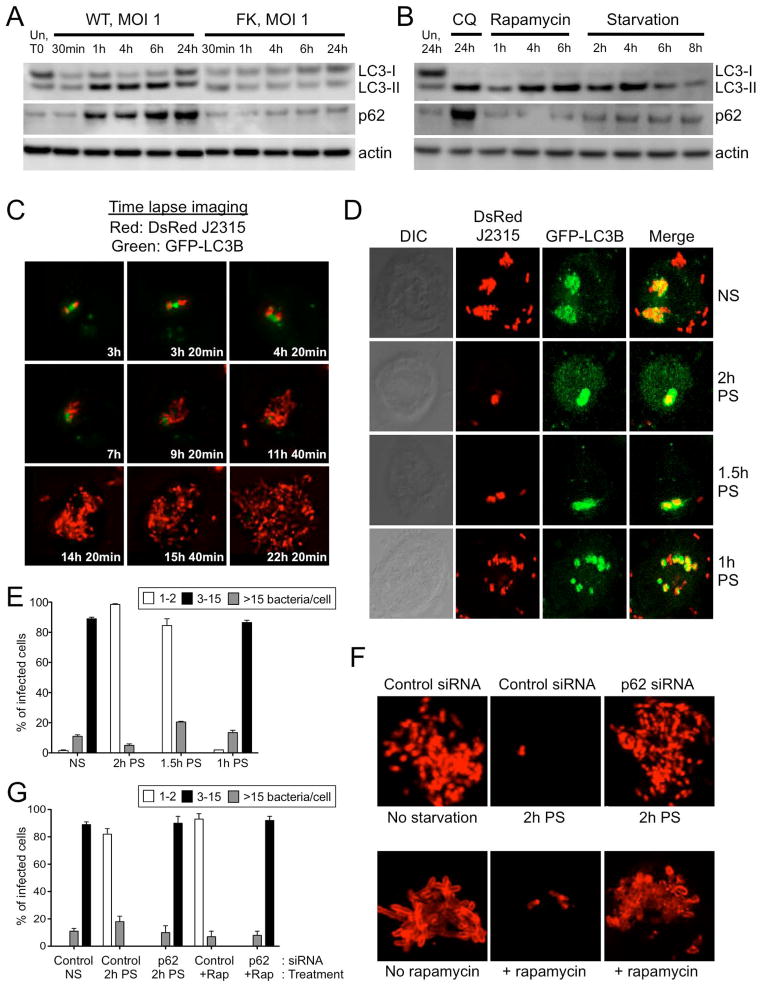
Despite recruitment of all the key autophagy initiation components in the first 6h after infection (Fig. 3), B. cenocepacia ultimately replicate efficiently in the host cells at later time points (Fig. 1H, S1C, D), and this coincides with a reduction in autophagy marker colocalization (Fig. 3C, D). To test for possible autophagy pathway interference by the bacteria, we measured cellular levels of the LC3B and p62 markers to monitor host cell autophagy flux. Initiation of the autophagy pathway involves conversion of LC3B from an 18 kDa cytosolic form (LC3B-I), to a 16 kDa processed form (LC3B-II) that localizes to the autophagosomal membrane (Kabeya et al., 2000, Reggiori et al., 2002, Kuma et al., 2007, Mizushima et al., 2007). To first determine whether B. cenocepacia infection leads to increased LC3B conversion, we monitored LC3B-I and LC3B-II levels in infected THP-1 cells by Western blotting (Fig. 4A). As soon as 1h post infection with WT bacteria, there was a significant increase in the conversion of LC3B-I to LC3B-II. This effect lasted up to 6h post-infection but decreased towards 24h post-infection. FK bacteria did not induce this increase in LC3B-II levels (Fig. 4A). An increase in LC3B-II levels could be caused either by an increase in autophagic flux, or by a block in the maturation of the autophagosome (Orvedahl et al., 2009). To distinguish between these possibilities, we measured p62 levels in B. cenocepacia infected cells, as this protein is a selective substrate for autophagy (Kuma et al., 2007). As such, the level of p62 should decrease with increased autophagic flux, while a block in autophagosome maturation will lead to p62 accumulation (Kuma et al., 2007). Treatment of THP-1 cells with chloroquine (CQ), which is known to block autophagosomal maturation (Chiu et al., 2009, Min et al., 2009), led to the expected increase in both the conversion of LC3B-I to LC3B-II, and accumulation of p62 (Fig. 4B). We show that upon WT B. cenocepacia infection, p62 protein accumulates gradually beginning 1h post-infection and peaks at 24h post-infection, while no change in p62 level is observed with FK bacteria (Fig. 4A). While B. cenocepacia infection induces an increase in p62 mRNA, this does not occur until 24h after infection (Fig. S4A) suggesting that transcriptional upregulation does not contribute significantly to the p62 protein accumulation observed at the early time points. Therefore, these data show that infection with WT B. cenocepacia leads to a block in the cellular autophagy cycle, and suggest that suppression of cellular autophagy may be an important requirement for productive B. cenocepacia infection. In contrast to the autophagic block induced by B. cenocepacia infection, conventional induction of autophagy by either cellular starvation or rapamycin treatment leads to a different pattern of changes in the levels of the LC3B and p62 autophagic markers (Jacinto et al., 2003). While there is still an increased conversion of LC3B-I to LC3B-II by both treatments, the increased consumption of autophagy substrates leads to a decrease in p62 levels (Fig. 4B).
Fig. 4. B. cenocepacia evades selective autophagy to establish a replicative niche in macrophages.
(A) Western blot of LC3B and p62 levels: Shown are the LC3B-I (cytosolic) and LC3B-II (lipid conjugated) forms of LC3B, as well as the autophagic flux marker p62 and actin as a loading control. Cells were either left untreated or infected with wild-type (WT) J2315 or formalin killed (FK) bacteria at MOI 1 for the indicated times. (B) Western blot of LC3B-I, LC3B-II, p62 and actin from cells starved or treated with 50 ug/ml rapamycin for the indicated time points, or +/− 50 uM chloroquine treatment for 24h. (C) Time-lapse imaging of the course of infection with DsRed-J2315 and THP1 cells expressing GFP-LC3B. (D–E) THP-1 macrophages expressing GFP-LC3B were either left untreated (non starved, NS) or pre-starved (PS) for the indicated time points. All cells were infected with WT DsRed B. cenocepacia at MOI 1, and cells were fixed and processed for confocal microscopy at 24h post-infection. Representative confocal images (D) and quantification of the percentage of THP-1 cells harboring various bacterial loads (E) are shown. Uncountable bacteria present in a heavily infected cell were considered as >15 bacteria. (F–G) THP-1 macrophages were transfected with non-targeting control siRNA or p62-siRNA for 48hr. Cells were either left untreated (No starvation or no rapamycin), pre-starved (PS) for 2h or treated with rapamycin (+rapamycin), then infected with the WT DsRed B. cenocepacia at MOI 1. At 24h post-infection, cells were fixed and processed for confocal microscopy. Representative images (F) and quantification (G) of the percentage of THP-1 cells harboring various bacterial loads are shown. Uncountable bacteria in heavily infected cells were considered as >15 bacteria. For the imaging experiments shown, infected cells from multiple cover slips were examined in each experiment. All data are representative of three independent experiments. For panels E and G, at least 100 bacteria were counted at each time point and the results shown are mean ± standard deviation of three experiments.
To measure autophagic flux during infection, we infected the cells in the presence bafilomycin A1, a lysosomal inhibitor which prevents the autophagic degradation of p62 (Kawai et al., 2007). We find that p62 accumulates to higher levels in infected cells treated with bafilomycin A1 compared to those infected in the absence of bafilomycin A1 (Fig. S4B). This demonstrates autophagic flux is blocked during B. cenocepacia infection, however it implies that the effect of infection on autophagic flux is not complete, and that residual flux can be more fully blocked with the bafilomycin A1 treatment.
To understand how B. cenocepacia is able to replicate despite being targeted by the selective autophagy process, we performed time-lapse imaging of GFP-LC3B expressing THP1 cells infected with DsRed-labeled bacteria (Movie S2, and Fig. 4C). GFP-LC3B puncta appear initially in close contact with B. cenocepacia, however as replication begins at 6–8h post-infection, the bacteria clearly separate from the LC3B and the localized GFP-LC3B signal dissipates as the bacteria rapidly multiply. Around 20–24h post-infection, the heavily replicating bacteria appear to begin exiting the cell (Movie S2). These data show that LC3B actively associates with B. cenocepacia in the early stages of infection, but later dissociates from the majority of bacteria that replicate in an LC3B-negative environment.
Pre-infection stimulation of autophagy restricts B. cenocepacia replication in macrophages
It has been demonstrated that autophagy is a mechanism by which bacterial survival within macrophages can be restricted (Gutierrez et al., 2004b), so we tested whether stimulation of autophagy is detrimental for productive B. cenocepacia infection. We induced autophagy by the physiological trigger of cell starvation for a 2h period, which we have shown is an effective method of increasing autophagy flux (Fig. 4B), infected the cells with WT B. cenocepacia, and then performed time-lapse imaging of live infected cells (Movie S3). GFP-LC3B puncta appeared in close contact with B. cenocepacia similar to the non-starved cells. However, over the course of a 24h infection, the bacteria remained within the LC3B positive compartment, without any evidence of replication. In addition, we performed confocal imaging analysis to assess bacterial replication in pre-starved THP-1 cells (Fig. S5, S6). Cell starvation did not affect phagocytosis or the infectivity rate, as 96% of the cells had 1–2 bacteria 1h after infection, similar to the untreated cells (Fig. S5A, S6A). However, the increased autophagy flux markedly decreased the levels of B. cenocepacia replication. At 24h post-infection, only 35% of the cells contained 3–15 bacteria, in contrast to the untreated cells where 85% of the cells contained more than 15 bacteria (Fig. S5D, S6D). In addition, we utilized TEM to examine intracellular B. cenocepacia during starvation conditions (Fig. S7A). We show that at 4h post infection the bacterium is surrounded by multiple membranes indicating that it resides in an autophagosome-like compartment, while apparent autophagosome-lysosome fusion takes place at later time points (Fig. S7B). Therefore, we conclude that pre-stimulation of autophagy can prevent B. cenocepacia replication in macrophages.
These data suggest that there may be a threshold of minimal autophagy flux required for the host macrophage cell to control B. cenocepacia infection. To determine the shortest time required to reach this threshold through cellular starvation, we exposed the THP-1 cells expressing GFP-LC3B to various periods of pre-starvation before infection with B. cenocepacia (Fig. 4D, E). Reducing the time of pre-exposure to starvation conditions to 1.5h was still sufficient to abrogate bacterial replication, however decreasing this period of pre-starvation to 1h generated a replication phenotype similar to the non-starved cells where 90% and 80% of the cells contained more than 15 bacteria respectively (Fig. 4D, E). This indicates that a minimum period of 1h to 1.5h of autophagy induction is required to increase autophagy flux to a level that can protect macrophages from a productive infection.
Similar results were observed when we induced autophagy using rapamycin, a pharmacological inhibitor of the mTor pathway. Rapamycin treatment of the cells 2h prior to infection also significantly decreased the number of replicating B. cenocepacia (Fig. S5, S6). In contrast to these effects of rapamycin pretreatment, adding rapamycin directly to bacterial cultures in LB broth did not alter B. cenocepacia survival (data not shown) and did not affect the initial rate of infectivity (Fig. S5A, S6A). Thus, the basal autophagy state of the infected cell is critical to the outcome of infection, and manipulation of cellular autophagy flux prior to infection can effectively block bacterial replication.
Role of p62 in the autophagy-mediated restriction of B. cenocepacia replication
Since increased autophagy flux prior to infection establishes host resistance to B. cenocepacia, we asked whether the knockdown of a key component of the autophagy process would alter this protective effect. Both control and p62 siRNA-treated cells were starved for 2hr prior to infection, and cells were processed for confocal imaging analysis at 24h post-infection. Initial phagocytosis and infectivity rate were not affected by p62 silencing when compared to the control siRNA-treated cells (data not shown). At 24h post-infection, p62 siRNA-treated cells that were pre-starved for 2h prior to infection, showed a significantly greater number of intracellular bacteria than the control siRNA-treated cells, with similar replication levels to non-starved cells treated with control siRNA (Fig 4F, G). Similar results were observed when cells were treated with rapamycin to induce autophagy (Fig. 4F, G). This supports the hypothesis that increased host cell autophagy is responsible for the protection from B. cenocepacia infection and replication observed in pre-starved cells, as silencing of a key autophagy pathway component rescues the bacterial replication phenotype.
Induction of autophagy by starvation promotes lysosomal maturation of bacteria-containing phagosomes in infected macrophages
Induction of autophagy has been shown to inhibit mycobacterial survival and replication in macrophages by speeding maturation of the bacterial phagosome and its fusion with the lysosome (Gutierrez et al., 2004a). To further investigate the mechanism by which increased autophagy flux is able to perturb B. cenocepacia survival and replication, we tested the effect of pre-infection starvation on B. cenocepacia endocytic trafficking. We observed a significant increase in the colocalization of Lamp-2 and Cathepsin-D with bacteria in the pre-starved cells 24h post-infection (Fig. S8). These findings show that a pre-infection increase in autophagic flux leads to host cell control of the infection by rendering the phagocytic bacterial vacuole more susceptible to lysosomal fusion.
Macrophages derived from CGD patients and a CGD mouse model support increased B. cenocepacia replication
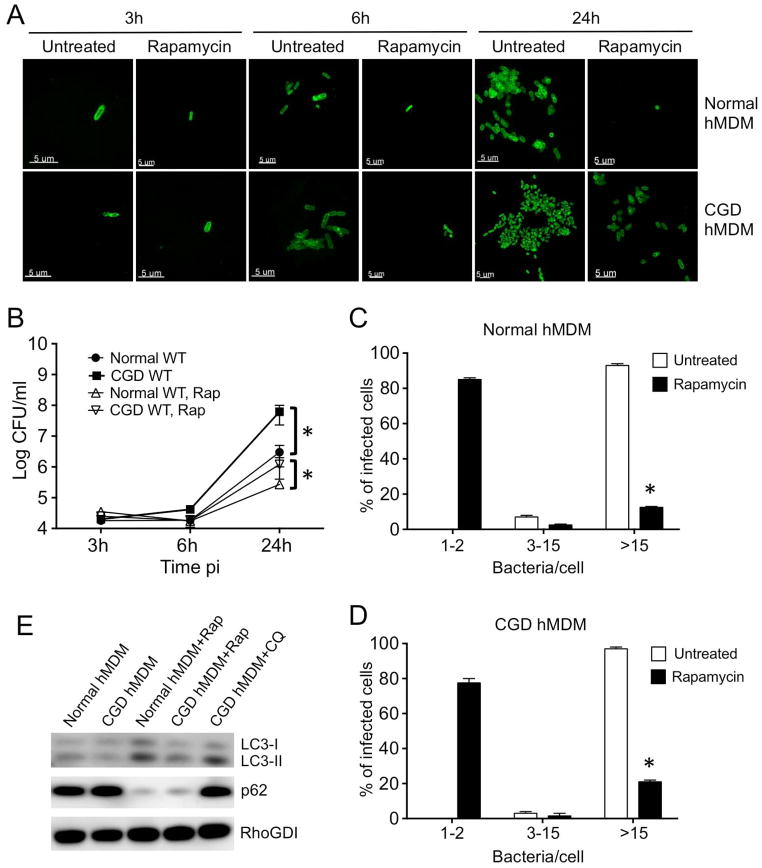
To investigate the potential clinical relevance of our finding that host cell autophagy flux influences B. cenocepacia infection outcome, we studied bacterial pathogenesis and infection control in human macrophages derived from patients with CGD. Various studies have looked at the infection of CGD patient-derived neutrophils with B. cenocepacia (Gao et al., 2002, van de Loo et al., 2003, Bylund et al., 2005, Bylund et al., 2006), but the bacterial burden in macrophages has not been carefully examined. Therefore, we infected primary macrophages derived from CGD patients compared to macrophages isolated from healthy donors to first determine whether B. cenocepacia had a replication advantage in the former (Fig. 5A, B). The WT J2315 strain displayed continuous growth with 166-fold increase in the CFU recovered at 24h in normal hMDMs (Fig. 5B). While the initial uptake of the bacteria was similar in normal and CGD hMDM (Fig. 5A, 1st panel, 5B), we found that 20-fold more bacteria were recovered from the CGD hMDMs compared to the normal hMDMs at 24h post-infection (Fig. 5A, 5th panel, 5B). Next, we performed single cell analysis to assess the number of bacteria associated with each cell type (Fig. 5A). At 3 and 6h after infection, approximately 90% of the infected cells harbored 1–2 and 3–15 organisms respectively, while at 24h post infection, bacterial counts per cell exceeded 15 in almost all infected cells, with a clearly higher burden at 6 and 24h in the CGD hMDMs (Fig. 5A, C). Thus, the imaging data are consistent with the CFU measurements, suggesting that the CGD macrophages are more permissive to B. cenocepacia compared to normal cells.
Fig. 5. Human macrophages derived from CGD patients support increased B. cenocepacia survival and replication but autophagy stimulation prior to infection improves bacterial clearance.
hMDMs derived from normal donors or CGD patients were either left untreated or pretreated with rapamycin for 2h prior to infection with WT J2315. The infection was carried out in triplicate with MOI 1, for 1h, followed by 2h of gentamicin/ceftazidime treatment to kill extracellular bacteria. (A) Confocal images are shown of hMDMs fixed at various times post-infection and labeled with B. cenocepacia-specific antibody (green) prior to visualization. Scale bar shown is 5 μm. (B) Intracellular growth kinetics of WT J2315 in normal and CGD hMDMs infected as described above. The infected monolayers were lysed at different time intervals and plated onto agar plates for colony enumeration. (C, D) Quantification of the percentage of cells from normal donors (C) or from CGD patients (D) harboring various bacterial loads at 24h post-infection. Uncountable bacteria present in a heavily infected cell were considered as >15 bacteria. (E) Western blot of LC3B-I and II, and p62 from normal and CGD hMDMs left untreated or treated with rapamycin for 2h or with 50 μM chloroquine for 24h. RhoGDI was used as a loading control. For all imaging experiments shown, infected cells from multiple cover slips were examined in each experiment. All data are representative of three independent experiments, each performed in triplicate. Data represent mean ± standard deviation. Asterisks represent statistically significant difference (p value <0.05).
X-linked CGD arises due to mutations in the gp91phox gene and is responsible for 65–70% of the clinical cases of CGD (Rajakariar et al., 2009). We also infected at MOI 1 BMDMs isolated from the gp91phox−/− mouse model for CGD and from C57BL/6 control mice with WT J2315 (Fig. S9). The WT displayed continuous growth with 53-fold increase in the CFU recovered at 24h compared to 6h in normal BMDMs (Fig. S9B). While the initial uptake of the bacteria was similar in both CGD and normal BMDMs (Fig. S9A, 1st panel, S9B), we found that 5-fold more bacteria were recovered from the CGD BMDMs compared to the control BMDMs at 24h post-infection (Fig. S9A, 5th panel, S9B). Single cell analysis confirmed the CFU count, with more bacterial replication observed at both 6 and 24h post-infection in the CGD BMDMs compared to the control BMDMs at the indicated time points (Fig. S9A). This indicates that similar to human macrophages, murine CGD macrophages are more susceptible to bacterial replication compared to normal macrophages.
Autophagy induction by rapamycin reduces bacterial burden in macrophages derived from CGD patients and a CGD mouse model
We next examined whether autophagy induction by rapamycin can reduce bacterial burden in CGD macrophages. Initially, we verified that rapamycin treatment can activate autophagy in primary macrophages by measuring levels of the LC3B and p62 autophagic markers (Jacinto et al., 2003). We observed an increased conversion of LC3B-I to LC3B-II in cells treated with rapamycin, and a decrease in p62 levels indicating an effectively increased autophagic flux (Fig. 5E). We find that rapamycin treatment of the cells significantly decreases the number of replicating B. cenocepacia in both human and murine normal macrophages (Fig. 5, S9). This effect was observed as soon as 6h post infection, and lasted up to 24h after infection where only 10–20% of the hMDMs and BMDMs contained more than 15 bacteria (Fig. 5C, D, S9C, D). These data provide important primary cell validation of our observations in THP1 cells, confirming that increased host macrophage autophagy flux can provide protection from B. cenocepacia infection. Significantly, a similar effect of rapamycin was observed in macrophages derived from CGD patients and the CGD mouse model, where rapamycin treatment led to a dramatic reduction in bacterial replication (Fig. 5A–D, S9A–D). Thus, although CGD cells contained a much larger bacterial burden, rapamycin treatment was not only effective, but also reduced the B. cenocepacia infection to a level comparable to normal cells. Therefore, therapies that increase autophagic flux could be a promising approach to improving B. cenocepacia clearance in CGD patients.
DISCUSSION
Infection with B. cenocepacia, especially in patients with CF and CGD, is often fatal. To elucidate this bacterium’s immune evasion strategy, it is critical to understand pathogenesis in host macrophages, as they represent a first line of defense against many infectious agents. At a physiologically relevant MOI of 1, we show that the B. cenocepacia J2315 strain replicates intracellularly in either primary macrophages or the human macrophage-like THP-1 cell line with similar kinetics to mouse macrophages. Previous reports have shown that this bacterium is able to prevent maturation of its vacuole in macrophages by delaying its fusion with the late endosomal compartments for almost 4 hours (Lamothe et al., 2007). However when we assessed the intracellular localization of WT B. cenocepacia within THP-1 cells, we saw transient interaction with an early endosomal marker but minimal subsequent colocalization with late endosomal or lysosomal markers, indicating a subversion rather than a delay of the endocytic trafficking process. Given this initial information, and to further test the previously reported hypothesis that the bacteria can replicate in a modified vacuole (Sajjan et al., 2006), we utilized TEM to examine intracellular B. cenocepacia during the early stages of human macrophage infection. Surprisingly, our TEM studies revealed that in the first hour after infection, B. cenocepacia is found either free in the cytosol or in compromised phagosomes, indicating that the lack of colocalization with the late endosomal and lysosomal markers is not due to an arrest in maturation but rather to a clear escape of the bacteria from the endocytic pathway. We further confirmed these findings using selective antibody labeling under conditions that leaves the vacuolar membrane intact and the danger receptor, Gal-3, which we show is recruited specifically to bacteria-containing vacuoles that lose integrity soon after entry. While Gal-3 is considered as a reliable marker associated with the initial process of vacuole lysis, the recruitment of actin is a late phenomenon, observed long after the vacuole had been lysed and it further confirms the cytosolic residence of the bacteria. While a recent report suggests that the B. cenocepacia type VI secretion system mediates the escape of secreted bacterial proteins into the cytoplasm of infected macrophages (Rosales-Reyes et al., 2012), bacterial escape from the phagosome is a mechanism of avoidance of the host endocytic trafficking pathway that has not previously been described for B. cenocepacia.
Confocal imaging and further TEM analysis revealed a close proximity of ER cisternae to the cytosolic bacteria with very few bacteria enclosed within a multi-membrane autophagosome. Using both live cell imaging of infection and immunofluorescence analysis of THP-1 macrophages stably expressing a fluorescently tagged autophagy effector, GFP-LC3B (Shi et al., 2012), we found that WT bacteria colocalized with GFP-LC3B between 1 to 6h post-infection before decreasing by 24h post-infection, indicating that WT B. cenocepacia is initially recognized and targeted by the host autophagy pathway, but that this diminishes as the bacteria replicate. While LC3B colocalization with the bacteria could also result from LC3B-associated phagocytosis (LAP), this process does not depend on initial recruitment of ubiquitin and ubiquitin-binding autophagy adapters, which we show are a pre-requisite for bacteria/LC3B colocalization. Although the mechanism of cytosolic bacterial recognition remains unknown, recent evidence indicates that bacteria exposed or released into the cytosol are targeted by the host ubiquitin conjugation system. A study with S. flexneri has shown that vacuolar membrane remnants are polyubiquitinated, recognized by p62 and targeted for autophagic degradation (Dupont et al., 2009). This raises the possibility that the remnants of vacuolar membrane observed around B. cenocepacia in our TEM analyses may be a signal for ubiquitination and recruitment of selective autophagy adaptors.
Autophagy plays a protective role in defense against intracellular pathogens such as M. tuberculosis (Gutierrez et al., 2004a), Shigella spp (Ogawa et al., 2005, Ogawa et al., 2006) and others. Accordingly, pathogens have evolved multipronged strategies to avoid and/or to dampen this pathway at its various stages (Gutierrez et al., 2004a). These strategies include preventing the induction of autophagy, which although more common in viral infections (reviewed in (Deretic et al., 2009)), has also been shown for L. monocytogenes. Certain microbes also use secreted effectors to avoid autophagic capture (such as L. pneumophila RavZ, B. pseudomallei BopA, and S. flexneri IcsB (Cullinane et al., 2008, Gong et al., 2011) while Coxiella burnettii and M. tuberculosis have been shown to suppress autophagolysosomal maturation to create an intracellular replicative niche within the autophagosome (Gutierrez et al., 2004a, Deretic et al., 2009, Choy et al., 2012).
In this report, we find that following endocytic escape, B. cenocepacia are targeted by the host autophagy pathway. Yet, despite ubiquitin tagging and the subsequent recruitment of autophagy adapters and LC3B to the vicinity of the escaped bacteria, host cell control through autophagy ultimately fails in a high proportion of infected cells. This is demonstrated most clearly by live cell imaging, where LC3B/bacteria colocalization dissipates abruptly when the bacteria begin to proliferate. The most likely site of action in the autophagy process targeted by B. cenocepacia is completion of the autophagosome. This is supported by a very low frequency of autophagosome formation in our TEM analysis and western blot protein levels of LC3B-II and p62 that indicate a block in the autophagy cycle.
Recently, the B. pseudomallei TssM protein was identified as exhibiting deubiquitinase activity (Tan et al., 2010), which was proposed to lead to a reduced recognition by the machinery of selective autophagy. It seems unlikely that B. cenocepacia uses a comparable strategy, as the bacteria appears to be efficiently tagged by ubiquitin after cytosolic escape. Another evasion model has been exhibited by S. flexneri, whose secreted IcsB effector acts at a later stage in the autophagy process by preventing Atg5 binding to IcsA and thus inhibiting LC3B recruitment and sequestration of bacteria in autophagosomes (Ogawa et al., 2005). More recently, Choy et al showed that L. pneumophila has evolved a specific mechanism to interfere with host autophagy by directly targeting LC3B (Choy et al., 2012). The Legionella RavZ protein inhibits host autophagy by functioning as a cysteine protease that specifically targets lipid-conjugated LC3B-II proteins and generates a deconjugated LC3B-I product that lacks the essential C-terminal glycine required for reconjugation (Choy et al., 2012). RavZ can both prevent LC3B from accumulating on the membranes of phagophores and inactivate the LC3B protein during the deconjugation reaction (Choy et al., 2012).
We have been unable to identify homologues for either of these effectors in the B. cenocepacia genome, suggesting that an effector acting at a more distal step in the autophagy pathway would be required to perturb bacterial sequestration while permitting LC3B recruitment. Identifying a mechanistic basis for how B. cenocepacia exposed to the cytosol is resistant to attack by selective autophagy will be an important topic for future investigation.
Our infection studies with B. cenocepacia suggest a novel mechanism by which the cytosolic bacteria can manipulate autophagic flux in order to create a permissive niche for replication in macrophages. This brings up a pertinent question of why this bacterium is not more hazardous to immune competent individuals. It is possible that there is a minimal threshold of autophagy pathway flux that is protective for B. cenocepacia infection, and that immunocompromised individuals are rendered susceptible due to lower autophagy pathway turnover in cells such as macrophages that are a primary target for initial infection. This is supported by lower basal LC3B-II levels in CGD hMDMs, and a significantly higher bacterial burden in these cells compared to normal hMDMs. Importantly, we show that pre-stimulation of the autophagy pathway prior to infection can reduce bacterial replication by targeting the bacteria to autophagosomes. In cells pre-treated with rapamycin, bacterial co-localization with the autophagy effector LC3B was sustained and bacterial proliferation was inhibited. More importantly from a clinical application perspective, we find that increasing autophagic flux can dramatically reduce bacterial burden in macrophages derived from either CGD patients, or from a murine CGD model. This suggests that the basal autophagy level in a B. cenocepacia infected cell is a critical determinant of the infection outcome, and we propose that manipulation of autophagic flux could be a promising therapeutic approach to improving B. cenocepacia clearance in immunocompromised patients.
EXPERIMENTAL PROCEDURES
A more detailed description of materials and methods can be found in the Extended Experimental Procedures in supplementary information.
Bacterial strains and cell cultures
B. cenocepacia J2315 was used as WT strain and FK bacteria was used as a control. The human monocytic cell line THP-1, or a clonal line stably expressing GFP-LC3B, were used where indicated. After written informed consent, normal donor and CGD (age ≥18 yrs) blood was obtained at the NIH under IRB approved protocols. Primary macrophages were differentiated from human peripheral blood or from mouse bone marrow following standard procedures.
Infection protocol
Both primary macrophages and THP-1 cells were infected with bacteria at MOI 1 unless otherwise indicated. To synchronize the infection, the plates were centrifuged for 5 min at 1000 rpm and then incubated at 37°C in 5% CO2. Infection times up to 1 hr did not receive antibiotic treatment as extracellular bacterial growth would not affect data interpretation in this time frame. For infection times greater than 1 hr, the cells were washed 3 times with the culture medium to remove extracellular bacteria at 1 hr post-infection, and incubated with an antibiotic combination of 250 μg/ml gentamicin (Sigma) and 500 μg/ml of ceftazidime (Sigma) for 2h to kill the remaining extracellular bacteria. (Sajjan et al., 2008). The infection was stopped at this stage for the 3 hr infection time point, or media was changed and the infection continued for later time points.
Antibodies
To label the bacteria we generated an anti-B. cenocepacia rabbit polyclonal antibody (Cocalico Biologicals). For the trafficking studies anti-EEA1 (Abcam), anti-Gal-3 antibodies (Novus Biologicals), anti-LAMP-2 (H4B4) (Developmental Studies Hybridoma Bank) anti-Cathepsin D (BD Transduction) and anti-KDEL (StressGen) were used. For the autophagy studies a mouse monoclonal antibody against ubiquitin (Enzo), mouse anti-p62 and rabbit anti-NDP52 (Novus) were used.
Microscopy
Approximately 5 × 105 cells were grown on circular glass coverslips in 24-well culture plates for confocal and on Thermanox® coverslips for TEM. After infection, cells were washed three times with the culture medium, and processed for confocal microscopy as we described previously (Al-Khodor et al., 2008). Live cells were imaged for 21h at 40× using the Leica Episcope, an inverted fluorescence microscope. For TEM, cells were fixed in 2.5% glutaraldehyde in processed as described previously (Burtnick et al., 2008).
Western blotting and p62 siRNA Knockdown
THP-1 cells were infected with WT B. cenocepacia J2315 strain at MOI 1. Cell starvation, to induce autophagy, was performed by incubating the cells for 2h in RPMI without addition of glutamine or FBS. Rapamycin 50ug/ml (Sigma) was used as a pharmacological inducer of autophagy. SQSTM1 (p62) (no. M-010230–00) and control siRNA (no. D-001206–14–20) were from Thermo Fisher. THP-1 cells were transfected with 50nM each siRNA 48h before infection using HiPerfect (Qiagen).
Statistical analysis
All experiments were performed at least three times and representative data are shown. To analyze for statistically significant differences between different sets of data, two-tail Student t-test was used and P values were calculated using Prism software.
Supplementary Material
Acknowledgments
This work was generously supported by the National Institute of Allergy and Infectious Diseases Division of Intramural Research (S.A-K, V.N, L.D and I.D.C.F.). K.M-B. and D.E.G. were supported by UT Southwestern Medical Center. We are grateful to Dr. John Kehrl for the LC3B-GFP expressing THP-1 cell line, to Dr. Steve Holland for access to CGD patient blood samples and bone marrow from CGD model mice, and to David Dorward for assistance with TEM image processing. We thank colleagues in the Laboratory of Systems Biology for helpful discussions and critical reading of the manuscript. The authors declare that they have no relevant conflicts of interest.
References
- Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DH, et al. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7:1359–1370. doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulrahman BA, Khweek AA, Akhter A, Caution K, Tazi M, Hassan H, et al. Depletion of the ubiquitin-binding adaptor molecule SQSTM1/p62 from macrophages harboring cftr DeltaF508 mutation improves the delivery of Burkholderia cenocepacia to the autophagic machinery. J Biol Chem. 2013;288:2049–2058. doi: 10.1074/jbc.M112.411728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y. A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol. 2008;70:908–923. doi: 10.1111/j.1365-2958.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt SA, Spilker T, Coffey T, LiPuma JJ. Burkholderia cepacia complex in cystic fibrosis: frequency of strain replacement during chronic infection. Clin Infect Dis. 2003;37:780–785. doi: 10.1086/377541. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Burns JL, Jonas M, Chi EY, Clark DK, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun. 1996;64:4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick MN, Brett PJ, Nair V, Warawa JM, Woods DE, Gherardini FC. Burkholderia pseudomallei type III secretion system mutants exhibit delayed vacuolar escape phenotypes in RAW 264.7 murine macrophages. Infect Immun. 2008;76:2991–3000. doi: 10.1128/IAI.00263-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund J, Burgess LA, Cescutti P, Ernst RK, Speert DP. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J Biol Chem. 2006;281:2526–2532. doi: 10.1074/jbc.M510692200. [DOI] [PubMed] [Google Scholar]
- Bylund J, Campsall PA, Ma RC, Conway BA, Speert DP. Burkholderia cenocepacia induces neutrophil necrosis in chronic granulomatous disease. J Immunol. 2005;174:3562–3569. doi: 10.4049/jimmunol.174.6.3562. [DOI] [PubMed] [Google Scholar]
- Cemma M, Kim PK, Brumell JH. The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy. 2011;7:341–345. doi: 10.4161/auto.7.3.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Soni S, Kulp SK, Curry H, Wang D, Gunn JS, et al. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J Biomed Sci. 2009;16:110. doi: 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy A, Dancourt J, Mugo B, O’Connor TJ, Isberg RR, Melia TJ, Roy CR. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, LiPuma JJ. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology. 2003;149:77–88. doi: 10.1099/mic.0.25850-0. [DOI] [PubMed] [Google Scholar]
- Collins CA, Brown EJ. Cytosol as battleground: ubiquitin as a weapon for both host and pathogen. Trends Cell Biol. 2010;20:205–213. doi: 10.1016/j.tcb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci U S A. 2012;109:3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, Wolvetang E, et al. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–753. doi: 10.4161/auto.6246. [DOI] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biological chemistry. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, et al. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe. 2009;6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, et al. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- Gong L, Cullinane M, Treerat P, Ramm G, Prescott M, Adler B, et al. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One. 2011;6:e17852. doi: 10.1371/journal.pone.0017852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, et al. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112 (Pt 11):1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004a;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004b;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Huynh KK, Plumb JD, Downey GP, Valvano MA, Grinstein S. Inactivation of macrophage Rab7 by Burkholderia cenocepacia. Journal of innate immunity. 2010;2:522–533. doi: 10.1159/000319864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- Jarry TM, Cheung AL. Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun. 2006;74:2568–2577. doi: 10.1128/IAI.74.5.2568-2577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Uchiyama H, Takano S, Nakamura N, Ohkuma S. Autophagosome-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- Kudchodkar SB, Levine B. Viruses and autophagy. Reviews in medical virology. 2009;19:359–378. doi: 10.1002/rmv.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy. 2007;3:323–328. doi: 10.4161/auto.4012. [DOI] [PubMed] [Google Scholar]
- Lamothe J, Huynh KK, Grinstein S, Valvano MA. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol. 2007;9:40–53. doi: 10.1111/j.1462-5822.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- Lamothe J, Valvano MA. Burkholderia cenocepacia-induced delay of acidification and phagolysosomal fusion in cystic fibrosis transmembrane conductance regulator (CFTR)-defective macrophages. Microbiology. 2008;154:3825–3834. doi: 10.1099/mic.0.2008/023200-0. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia cepacia. Infect Immun. 2000;68:24–29. doi: 10.1128/iai.68.1.24-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y, Xu W, Liu D, Shen S, Lu Y, Zhang L, Wang H. Autophagy promotes BCG-induced maturation of human dendritic cells. Acta Biochim Biophys Sin (Shanghai) 2009;42:177–182. doi: 10.1093/abbs/gmq006. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Nzula S, Vandamme P, Govan JR. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J Antimicrob Chemother. 2002;50:265–269. doi: 10.1093/jac/dkf137. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Mimuro H, Yoshikawa Y, Ashida H, Sasakawa C. Manipulation of autophagy by bacteria for their own benefit. Microbiol Immunol. 2011;55:459–471. doi: 10.1111/j.1348-0421.2011.00343.x. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Sasakawa C. Shigella and autophagy. Autophagy. 2006;2:171–174. doi: 10.4161/auto.2829. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto GP, Wu MY, Clarke M, Lu H, Anderson OR, Hilbi H, et al. Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum. Mol Microbiol. 2004;51:63–72. doi: 10.1046/j.1365-2958.2003.03826.x. [DOI] [PubMed] [Google Scholar]
- Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 2010;12:530–544. doi: 10.1111/j.1462-5822.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- Perrin AJ, Jiang X, Birmingham CL, So NS, Brumell JH. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr Biol. 2004;14:806–811. doi: 10.1016/j.cub.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Rajakariar R, Newson J, Jackson EK, Sawmynaden P, Smith A, Rahman F, et al. Nonresolving inflammation in gp91phox−/− mice, a model of human chronic granulomatous disease, has lower adenosine and cyclic adenosine 5′-monophosphate. J Immunol. 2009;182:3262–3269. doi: 10.4049/jimmunol.0801739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Reyes R, Aubert DF, Tolman JS, Amer AO, Valvano MA. Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages. PLoS One. 2012;7:e41726. doi: 10.1371/journal.pone.0041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan SU, Carmody LA, Gonzalez CF, LiPuma JJ. A type IV secretion system contributes to intracellular survival and replication of Burkholderia cenocepacia. Infect Immun. 2008;76:5447–5455. doi: 10.1128/IAI.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan US, Yang JH, Hershenson MB, LiPuma JJ. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell Microbiol. 2006;8:1456–1466. doi: 10.1111/j.1462-5822.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS. Autophagy: eating for good health. J Immunol. 2006;177:4945–4951. doi: 10.4049/jimmunol.177.8.4945. [DOI] [PubMed] [Google Scholar]
- Tan KS, Chen YH, Lim YC, Tan GYG, Liu YC, Lim YT, et al. Suppression of Host Innate Immune Response by Burkholderia pseudomallei through the Virulence Factor TssM. Journal of Immunology. 2010;184:5160–5171. doi: 10.4049/jimmunol.0902663. [DOI] [PubMed] [Google Scholar]
- Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- van de Loo FA, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LA, et al. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. The American journal of pathology. 2003;163:1525–1537. doi: 10.1016/S0002-9440(10)63509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P, Holmes B, Coenye T, Goris J, Mahenthiralingam E, LiPuma JJ, Govan JR. Burkholderia cenocepacia sp. nov.--a new twist to an old story. Res Microbiol. 2003;154:91–96. doi: 10.1016/S0923-2508(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, et al. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- Vanlaere E, Baldwin A, Gevers D, Henry D, De Brandt E, LiPuma JJ, et al. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. International journal of systematic and evolutionary microbiology. 2009;59:102–111. doi: 10.1099/ijs.0.001123-0. [DOI] [PubMed] [Google Scholar]
- Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, Mahenthiralingam E, et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58:1580–1590. doi: 10.1099/ijs.0.65634-0. [DOI] [PubMed] [Google Scholar]
- von Muhlinen N, Thurston T, Ryzhakov G, Bloor S, Randow F. NDP52, a novel autophagy receptor for ubiquitin-decorated cytosolic bacteria. Autophagy. 2010;6:288–289. doi: 10.4161/auto.6.2.11118. [DOI] [PubMed] [Google Scholar]
- Von Muhlinen N, Thurston T, von Muhlinen N, Wandel M, Akutsu M, Foeglein AA, et al. How the Autophagy Receptor NDP52 Defends the Cellular Cytosol against Bacterial Invasion. Glycobiology. 2011;21:1486–1486. [Google Scholar]
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochemical and biophysical research communications. 2004;313:453–458. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.