Abstract
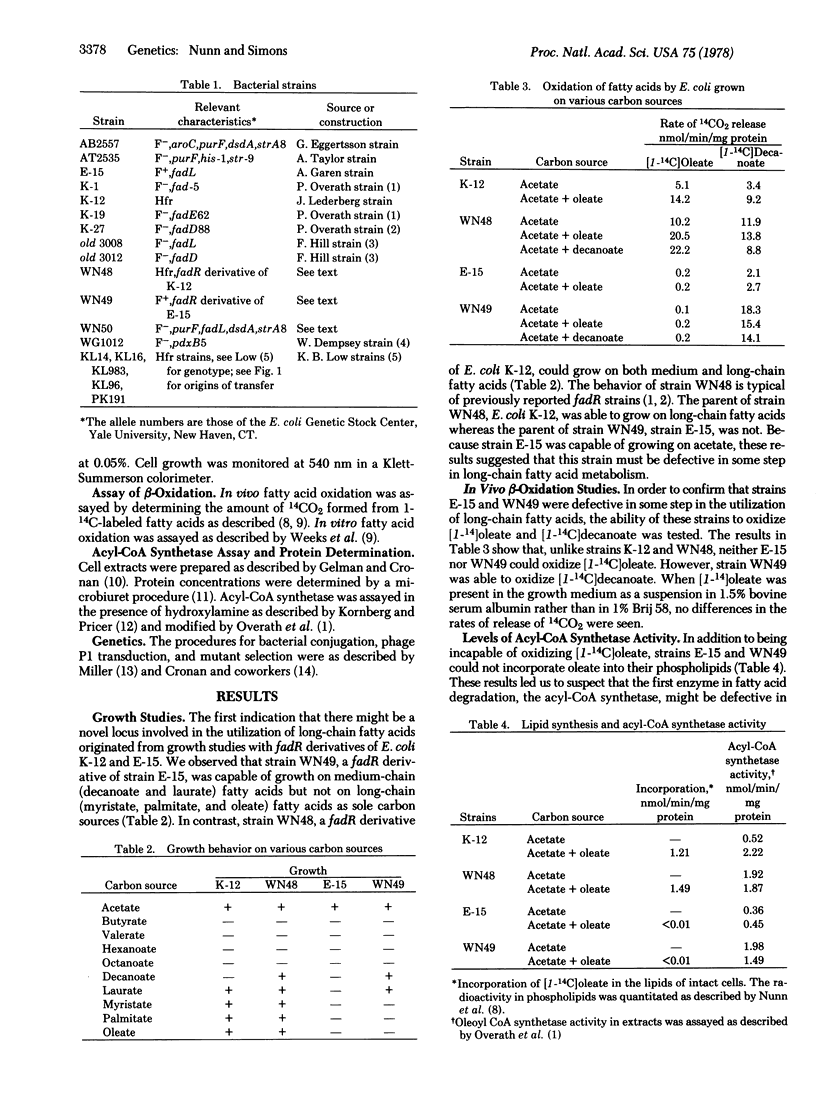
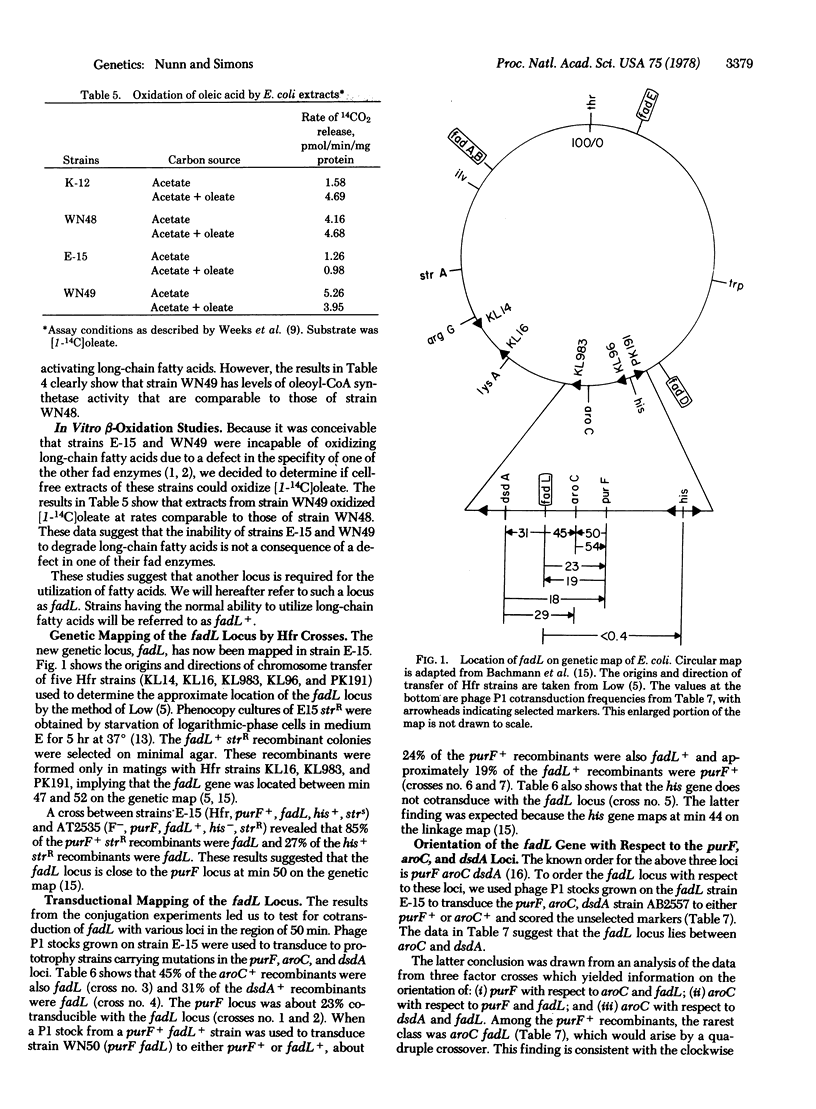
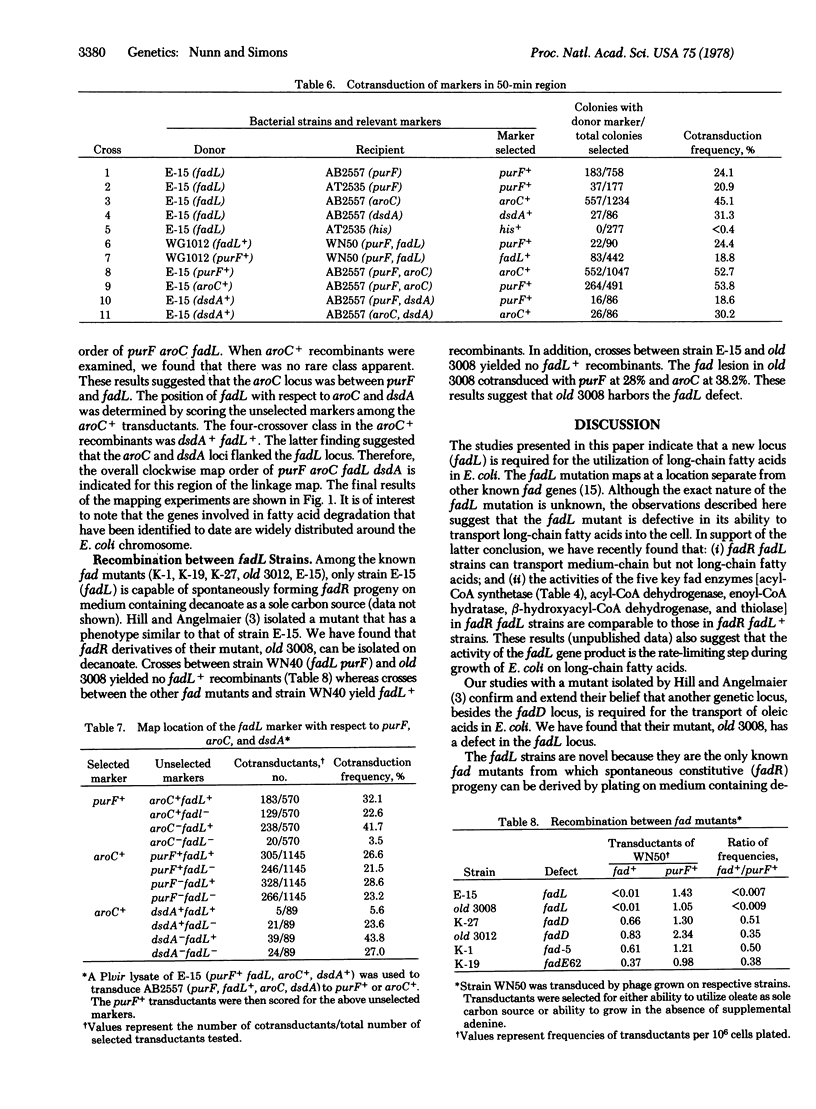
A new locus (fadL) that is required for the utilization of long-chain fatty acids has been mapped and partially characterized in an Escherichia coli mutant. The fadL locus has been mapped at 50 min on the chromosome. A mutant bearing a defect in this locus cannot utilize long-chain fatty acids as a sole carbon source. Derivatives of this mutant that can grow on decanoate (termed fadR) are capable of growth on medium-chain but not long-chain fatty acids. It is believed that the fadL mutants is defective in the transport of long-chain fatty acids into the cell for the following reasons: (i) fadR fadL strains can oxidize in vivo decanoate but not oleate; (ii) neither fadL nor fadR fadL strains can incorporate oleate into their membrane lipids; (iii) the activity of the acyl-CoA synthetase (EC 6.2.1.3) in fadR fadL strains is comparable to the acyl-CoA synthetase activity in fadR fadL+ strains; and (iv) in vitro extracts from fadR fadL+ strains. If the above hypothesis is correct, the uptake of long-chain fatty acids by E. coli requires at least two gene products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Birge C. H., Vagelos P. R. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1969 Nov;100(2):601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Characterization of pyridoxine auxotrophs of Escherichia coli: chromosomal position of linkage group I. J Bacteriol. 1969 Oct;100(1):295–300. doi: 10.1128/jb.100.1.295-300.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann E. P., Cronan J. E., Jr Mutant of Escherichia coli deficient in the synthesis of cis-vaccenic acid. J Bacteriol. 1972 Oct;112(1):381–387. doi: 10.1128/jb.112.1.381-387.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill F. F., Angelmaier D. Specific enrichment of mutants of Escherichia coli with an altered acyl CoA synthetase by tritium suicide. Mol Gen Genet. 1972;117(2):143–152. doi: 10.1007/BF00267611. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- McFall E. Mapping of the d-serine deaminase region in Escherichia coli K-12. Genetics. 1967 Jan;55(1):91–99. doi: 10.1093/genetics/55.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Cheng P. J., Deutsch R., Tang C. T., Tropp B. E. Phenethyl alcohol inhibition of sn-glycerol 3-phosphate acylation in Escherichia coli. J Bacteriol. 1977 May;130(2):620–628. doi: 10.1128/jb.130.2.620-628.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. J., Frerman F. E. Evidence for a complex of three beta-oxidation enzymes in Escherichia coli: induction and localization. J Bacteriol. 1977 Nov;132(2):532–540. doi: 10.1128/jb.132.2.532-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


