Abstract
General anesthetic photolabels are used to reveal molecular targets and molecular binding sites of anesthetic ligands. After identification, the relevance of anesthetic substrates or binding sites can be tested in biological systems. Halothane and photoactive analogs of isoflurane, propofol, etomidate, neurosteroids, anthracene, and long chain alcohols have been used in anesthetic photolabeling experiments. Interrogated protein targets include the nicotinic acetylcholine receptor, GABAA receptor, tubulin, leukocyte function-associated antigen-1, and protein kinase C. In this review, we summarize insights revealed by photolabeling these targets, as well as general features of anesthetics, such as their propensity to partition to mitochondria and bind voltage-dependent anion channels. The theory of anesthetic photolabel design and the experimental application of photoactive ligands are also discussed.
Keywords: photoaffinity labeling, photolabeling, GABAA receptor, Torpedo nicotinic acetylcholine receptor, VDAC, voltage-dependent anion channel, leukocyte function-associated antigen, tubulin, PKC, protein kinase C, halothane, neurosteroid, propofol, etomidate, octanol, aminoanthracene, isoflurane, optoanesthesia
Introduction
General anesthetics are a chemically diverse class of drugs that cause clinically important endpoints through multiple molecular and neuronal mechanisms. The prevalence of general anesthetics in modern medicine mandates a greater understanding of their pharmacology. At clinical concentrations, these drugs can affect the function of proteins. Characterizing the interactions of anesthetics with proteins is a biochemical and biophysical challenge, especially as many targets are membrane proteins of lower abundance or without high-resolution structures. Injectable general anesthetics are typically of higher potency than the volatile type, suggesting higher affinities for protein targets that influence consciousness; however, micromolar dissociation constants are common for all anesthetic-macromolecule interactions. Micromolar dissociation constants indicate rapid ligand unbinding, and one experimental approach that overcomes these dissociation kinetics is photoaffinity labeling, or simply “photolabeling”.
General anesthetic photolabeling experiments have been performed on purified proteins, enriched subcellular fractions, tissue sections, and whole organisms, with variations on each biological system. After equilibration with a molecule, the biological system contains ligand-bound macromolecules, the concentration of which depends on the ligand concentration and the dissociation constants associated with the substrates in the system. Photolabeling specifies that the ligand undergo photolysis upon light exposure, generating chemically unstable intermediates that covalently attach to the macromolecules, converting ligand off-rates infinitely towards zero. This attachment (or “adduction”) provides a tag in a site that can then be identified with mass spectrometry, or else traced if the ligand is radiolabeled. Anesthetic photolabeling has been used to identify protein targets and their binding sites, to study functional effects of ligand binding, and for discovery-based proteomic or distribution studies.
Anesthetic pharmacology encompasses mechanisms of hypnosis as well as those of numerous side effects, and therefore the photolabeling literature is broadly cast. Some consensus targets of general anesthetics have been extensively studied with photolabeling (e.g., gamma aminobutyric acid type A [GABAA] receptors and nicotinic acetylcholine receptors [nAChRs]). Certain experimental approaches and some proteins, however, have been analyzed with specific ligands, particularly halothane, which was the first compound applied in anesthetic photolabeling experiments. The diversity of the photolabeling studies is reflected by incomplete information for all anesthetic chemotypes in this review. Herein, we overview the molecules used for anesthetic photolabeling, summarize studies utilizing the photolabeling technique, and describe molecular insights revealed with photoactive anesthetics. Future approaches, including ex vivo and in vivo photolabeling, are also discussed.
Development and detection of anesthetic photolabels
With ~254 nm exposure, halothane undergoes photodecomposition, primarily to reactive chlorotrifluoroethyl and bromine radicals, which permits its use as a photolabel [1,2]. An advantage of halothane photolabeling is the use of an unmodified clinical compound; however, the primary disadvantage is that the high energy UV that induces photolysis can be destructive to biological macromolecules. The chlorotrifluoroethyl radical, the distinguishing label, also demonstrates reaction selectivity towards aromatic residues, and the released bromine atom can propagate further radical reactions [3–9].
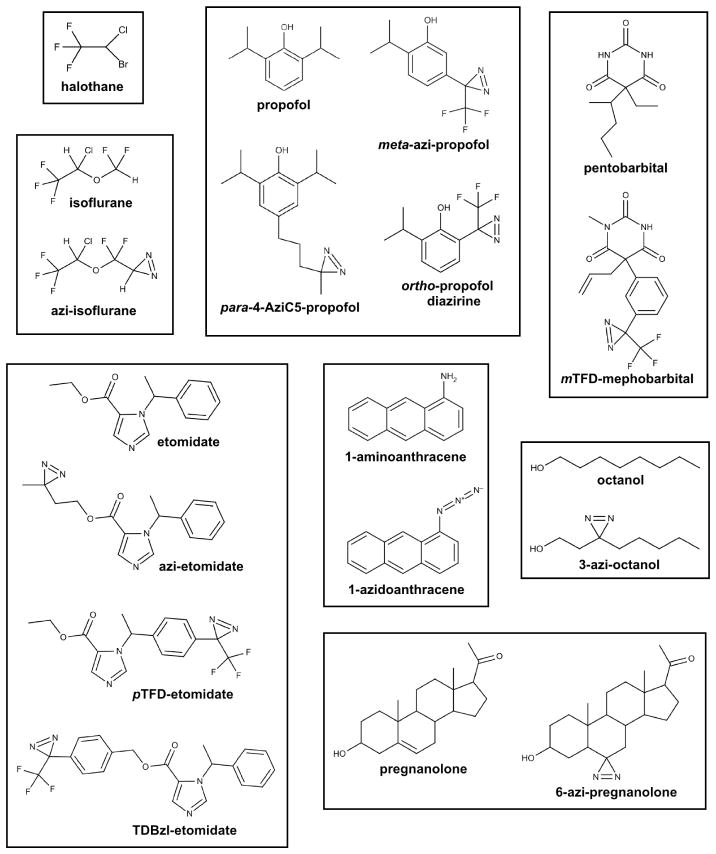
To overcome these problems, and further broaden application, photoactive anesthetic analogs containing diazirine or aryl azide moieties have been synthesized for a range of anesthetics. The chemotypes represented by these photolabels include haloalkanes [10], long chain alcohols [11,12], etomidate [13–16], neurosteroids [17], haloethers [18,19], anthracene [20], alkylphenols [21–23], and barbiturates [24] (Figure 1). Incorporated photoactive groups undergo photolysis at lower energy wavelengths than halothane (~300–375 nm), limiting damage to biological samples upon irradiation. The chemical and pharmacologic properties of these analogs, most notably general anesthetic potency, are initially characterized to ensure reasonable mimicry of the parent compounds. Photolabeling studies with analogs are strengthened by confirmation that pharmacologic properties are conserved with the parent compound through parallel functional assays or competition experiments. Competition experiments hint at target specificity or saturability, easing subsequent experimental investigations, but not necessarily designating a relevant role for the functional effects of the compounds.
Figure 1.
Chemical structures of general anesthetics and their photoactive analogs. With the exception of halothane, a diazirine or azido group serves as the photoactive moiety.
The diazirine moiety has generally been favored in anesthetic photolabel design. Diazirine photolysis ideally results in a single reactive intermediate, a singlet carbene, and an inert by-product, dinitrogen. A singlet carbene is a neutral divalent carbon species having an empty p-orbital and a hybrid orbital that contains two paired valence electrons [25]. By virtue of this electronic arrangement, the carbene intermediate simultaneously displays carbocation and carbanion characteristics, allowing both electrophilic and nucleophilic reactions, and enabling covalent attachment to proteins orders-of-magnitude faster than carbon-centered radicals (e.g., chlorotrifluoroethyl radical) [26,27]. While initially formed in the singlet state, many carbenes will convert to a triplet state that has one electron of the same spin in the two valence orbitals. This species will act as a diradical and has much lower reactivity than the singlet carbene. The promiscuous reactivity of singlet carbenes is desired to reliably photolabel equilibrium binding sites, which can consist of chemically diverse side chain and backbone atoms. Additionally, photolysis of diazirines often generates alternative intermediates, including diazonium and carbocation species that display undesired selectivity for nucleophilic residues [22,28–30]. Placing the diazirine on an electron deficient carbon or adjacent to an electron withdrawing group, especially a trifluoromethyl group, promotes the desired singlet carbene reactivity; this feature is integrated into multiple photoactive ligands of diverse chemotypes (Figure 1). Photolabel reactivity is suggested by recovering a broad range of adducted residues, including aliphatic amino acids [19,21,24,30,31].
In addition to suggesting broad reactivity of the photolysis intermediate, photolabel attachment to multiple and diverse residues lining a pocket indicates a considerably dynamic ligand in the site. It is likely that general anesthetics favorably assume multiple orientations, defined primarily by steric hindrance, in a pocket where interactions are mediated by hydrophobic or van der Waals forces. An experimental method to more completely probe an anesthetic cavity is photolabeling with isomers or analogs that contain the substituted photoactive group in different positions of the molecule [12]. In addition to defining steric limitations, this approach can provide evidence for critical higher-energy interactions, such as an intermolecular hydrogen bond, if a consistent relationship (e.g., molecular distance) is identified between the location of the adducted residues, the placement of the photoactive group, and the predicted intermolecular interaction.
To detect residue-level attachment of a photolabel to a protein site, Edman degradation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) are most commonly used. Edman degradation is a technique in which N-terminal residues are chemically cleaved from a peptide in repeated cycles, and the released, derivatized amino acids and peptide fragments can be isolated, identified, and scintillation counted. A radioactive probe is essential for identifying a residue with an attached photolabel using Edman degradation. LC-MS/MS avoids the use of radioactivity and identifies photolabeled peptides by mass (note: mass spectrometers actually detect mass-to-charge ratios [m/z], but “mass” suffices for this discussion). In LC-MS/MS, peptide masses are detected before trapping and fragmenting the peptides into random pieces, the masses of which are also detected. Computer algorithms assist manual interpretation of mass spectra by identifying peptides and amino acids with a mass modification that is equivalent to that of the adducted ligand.
A primary consideration for both sequencing methods is the length of peptide that can be analyzed. Edman degradation is limited to peptides ~30 amino acids or less, due to the efficiency of the chemical reaction, while LC-MS/MS optimally sequences peptides ~7–20 amino acids in length; therefore, proteases or chemical cleavage methods must be chosen to optimize peptide length and also to maximize sequence coverage of the analyzed protein. Further, the hydrophobic nature of anesthetic binding sites often leads to hydrophobic, sometimes insoluble peptides to analyze for adducts. This is particularly problematic for mass spectrometry, which only detects charged, and presumably soluble, peptides and fragments. Elaborate methodologies, aided in part by advances in mass spectrometer technology, have been developed to comprehensively sequence some membrane proteins with LC-MS/MS [32]. The accuracy and throughput of LC-MS/MS makes this technique important for the future of photo-adduct analysis, yet the reliability of the more laborious Edman degradation has been critical thus far in identifying photolabel binding sites.
Supramolecular binding of a volatile anesthetic
Halothane, an early anesthetic photolabel, has been used to characterize tissue-level distribution of volatile anesthetics. Halothane photolabeling of brain slices ex vivo demonstrated widespread anatomic binding with specificity in synapse-dense regions compared to cell bodies and white matter [33]. This reflects concentrated binding of the anesthetic in a protein-rich compartment. At least 15% of neuronal proteins are photolabeled by halothane, in agreement with the number of proteins that contain cavities large enough to accommodate the ligand [34]. Subcellular halothane binding and lipid distribution are not correlated [35], although membrane proteins can increase anesthetic binding to surrounding lipids [6]. Together, these suggest preferential binding of the general anesthetic to proteins, with lipid binding generally nonspecific, with the exception of lipids at some protein-membrane interfaces.
In agreement with widespread binding, no intracellular microenvironment becomes saturated within the clinical range of halothane (0–300 μM) [35]. Interestingly, halothane preferentially and saturably partitions to mitochondria [36]. Protein components of respiratory complexes and multiple mitochondrial enzymes have been identified with photolabeling as specific binding partners of halothane [37,38]. General anesthetics affect mitochondrial respiration [39,40], and genetic modulation of respiratory complexes alters mammalian sensitivity to anesthetics, including injectable drugs [41]. This suggests mitochondrial energetics modulate sensitivity to anesthesia, with specific ligand-protein interactions a plausible mechanism of altering mitochondrial energetics.
Despite localization of halothane to mitochondria, anesthetics also elicit their endpoints through mechanisms that do not involve energetic depression. Most current theories emphasize additive or synergistic interactions with multiple targets, each contributing variably to a given endpoint. Thus, bulk partitioning and/or saturable binding by themselves do not specify relevance, as a high concentration of ligand may be required for a small contribution to an endpoint and vice versa, on both molecular and physiologic levels. Further, isoflurane was relatively ineffective at competing halothane binding in brain slices or synaptosomes, arguing against a unitary mechanism of anesthesia dependent on bulk binding [2,33,34].
Protein targets
Photolabeling enriched subcellular fractions or purified protein allows interrogation of general anesthetic targets, including ligand specificity and binding site character. Significant findings regarding specific proteins are summarized here.
nAChR
Nicotinic acetylcholine receptors are heteromeric ligand-gated cation channels. Neuronal subtypes are widely distributed in the central nervous system, but with low abundance and diverse subunit stoichiometries. The readily available Torpedo muscle-type nAChRs with a fixed stoichiometry and medium resolution structure has served as a model to study anesthetic interactions [42]. The subunits are symmetrically arranged around the pore in the order of α*–γ–α*–δ–β, and extracellular interfacial acetylcholine sites are located between subunits denoted by the asterisks.
Halothane photolabeling of Torpedo nAChRs identified the transmembrane helices as the preferential anesthetic binding domain on Cys-loop receptors [3]. Halothane labeling was saturable and partially inhibited by isoflurane. Intrasubunit labeling was concentrated in the α subunit, but the identification of residue-level binding sites was confounded by the chemical selectivity of the photolysis intermediate [9]. Intrasubunit binding was demonstrated for azi-isoflurane and m-azi-propofol in a water-solubilized version of the nAChR α subunit transmembrane domain [43]; an analogous site for desflurane and propofol was crystallographically identified in a homologous cationic bacterial Cys-loop channel [44]. Indeed, photolabeling of Torpedo receptors in native membranes identified a δ intrasubunit site for m-azi-propofol and pTFD-etomidate [45,46].
M-azi-propofol and pTFD-etomidate, as well as TDBzl-etomidate, also bind an intersubunit site, likely at the γ–α non-agonist interface [45–47]. Both the intra- and inter-subunit sites might enhance or inhibit channel function, depending on the ligand. The most common mechanism for channel inhibition by anesthetics, however, is pore block. M-azi-propofol, pTFD-etomidate, TDBzl-etomidate, azi-etomidate, and 3-azi-octanol all bind within the nAChR ion channel [29,45–48]. The inhibitory influence of binding to pore residues was further demonstrated electrophysiologically by photolabeling functional receptors with 3-azi-octanol and azi-etomidate to produce irreversible pore block [49,50].
The Torpedo muscle-type nAChR has been critical in establishing the presence of multiple specific binding sites for anesthetics on Cys-loop receptors. However, translating this information to mammalian neuronal-type nAChRs will be challenging, especially without high-resolution structures of multiple receptor subtypes. In addition, the most potent anesthetic analog discussed (TDBzl-etomidate) actually potentiates this excitatory receptor [47], in contrast to etomidate and most clinical general anesthetics and their analogs [16,51]. This limits the usefulness of muscle-type nAChR inhibition as model for anesthetic pharmacology, and demonstrates limitations associated with subtle changes in the chemical structure of the ligands.
GABAA receptor
Most intravenous and volatile anesthetics positively modulate the anionic GABAA receptor, also a Cys-loop ligand-gated channel. Rational design of a general anesthetic ligand would likely consider GABAA specificity, and receptor modulation is a standard validation of photolabel pharmacology. Neuroanatomic expression of GABAA subunits essentially reflects receptor function. In phasic receptors, synaptic inhibition is largely mediated through (α1–3)2(β)2(γ2) subunit stoichiometries, while (α4–6)2(β)2(δ) subtypes mediate tonic, extrasynaptic inhibition [52]. Anesthetic-sensitive GABAA receptors have subunits arranged in order of β*–α–β*–α–γ/δ, with extracellular interfacial GABA sites denoted by the asterisk, and a benzodiazepine site specific to the α–γ interface.
Similar to nAChRs, anesthetics bind the GABAA receptor transmembrane domain. Site identification and competition experiments are complicated by allosteric interactions between multiple transmembrane sites, which also couple to the extracellular GABA site. GABA enhances azi-etomidate and mTFD-mephobarbital photolabeling of receptors [53,54]; similarly, propofol, pentobarbital, isoflurane, octanol, etomidate, as well as azi-etomidate, TDBzl-etomidate, and mTFD-mephobarbital, enhance muscimol binding to the agonist sites [30,54,55]. Positive allosteric coupling has also been demonstrated with halothane photolabeling of brain slices co-incubated with GABA [56].
In contrast to nAChRs, a GABAA intrasubunit site has not been identified with anesthetic photolabeling; however, intersubunit binding is conserved amongst ligands. Azi-etomidate and TDBzl-etomidate photolabel residues at the β*–α interfaces in γ-containing receptors, with competitive binding by etomidate and isoflurane [30,53–55]. Alternatively, mTFD-mephobarbital photolabels analogous sites at the α–β and γ–β non-agonist interfaces, with photolabeling inhibited by pentobarbital and phenobarbital [54]. Interestingly, pTFD-etomidate preferentially binds to the mTFD-mephobarbital site, while bulkier barbiturates such as thiopental and brallobarbital show decreasing preference for any subunit interface [54]. Propofol inhibits both azi-etomidate and mTFD-mephobarbital binding, suggesting propofol could bind to at least four subunit interfaces [54]. Photolabeling with ortho-propofol diazirine confirmed alkylphenols bind at least to interfacial sites that contain a β subunit [23]. Neurosteroids, including allopregnanolone and alphaxalone, enhance azi-etomidate and mTFD-mephobarbital binding specifically in the absence of GABA [54,57]; lack of competition by neurosteroids is consistent with the identification of a 6-azi-pregnanolone site on a β transmembrane helix, located deeper in the membrane towards the cytoplasm [31].
Chiara and colleagues clearly demonstrated that general anesthetics have varying affinities for the cavities photolabeled by azi-etomidate and mTFD-mephobarbital [54]. Structural features of these interfaces are conserved, and it is likely that the allosteric mechanisms of potentiation overlap. The allosteric energetics of TDBzl-etomidate are particularly interesting, as this ligand potentiates the cationic nAChR and anionic GABAA receptor by binding to non-agonist and agonist interfaces, respectively. Essential will be the extension of GABAA photolabeling to other ligands and receptor subtypes, including δ-containing receptors believed to contribute to the hypnotic effects of injectable drugs [58].
VDAC
Voltage-dependent anion channels (VDACs) are ubiquitous targets of general anesthetics. These β-barrel porins, typically in an open conformation, gate metabolites and ions traversing the mitochondrial outer membrane. VDAC isoforms bind halothane [37,38], neurosteroids [17,31,59], long-chain alcohols [11,48], alkylphenols [22,60], anthracene [20], and etomidate [13,14,16,29,45]. Saturable, competitive binding has been demonstrated for all chemotypes, and VDAC has been photolabeled in tissue originating from mammals (including humans), amphibians, insects, and fish. Anesthetic effects on VDAC function have not been reported; characterization and measurement of this mitochondrial ion channel is technically challenging and requires specialized systems. VDAC and cys-loop receptors associate in vitro [17,59,61,62], though the in vivo relevance of this is unclear, and knockout of VDAC isoforms 1 and 3 does not affect rodent sensitivity to neurosteroids [59]. The necessity of VDAC2 for cellular viability renders these channels difficult to study in vivo, as the isoforms compensate for the loss of each other, and a variety of specific pharmacologic modulators are not yet available.
Tubulin
As the primary component of microtubules, α- and β-tubulin isoforms are expressed in every cell type, and have been proposed as relevant targets for all general anesthetics [63]. 6-azi-pregnanolone binds neuronal β-tubulin in a site conserved with colchicine, a potent inhibitor of microtubule stability [64]. 6-azi-pregnanolone and its parent anesthetics, pregnanolone and allopregnanolone, also inhibit tubulin polymerization, suggesting modulation of neuronal microtubule dynamics or stability by endogenous neurosteroids. The fluorescent anesthetic 1-aminoanthracene [65] and its analog 1-azidoanthracene also bind tubulin, occupying the colchicine site and destabilizing microtubules [20]. Co-administration of the potent microtubule stabilizer epothilone D increased the concentration of allopregnanolone or 1-aminoanthracene required to achieve the immobility endpoint in tadpoles [20]. This suggests microtubule polymerization dynamics can alter general anesthetic sensitivity, albeit through mechanisms that remain undefined. Halothane also photolabels tubulin [37,38], yet stabilizes microtubule polymerization similar to propofol and isoflurane [20,66], casting uncertainty as to whether tubulin represents a unitary target for anesthetic hypnosis.
Protein Kinase Cδ
The protein kinase C (PKC) family of proteins consists of serine/threonine kinases involved in signal transduction, including regulation of pre- and post-synaptic ion channels and GPCRs. “Conventional” and “novel” PKC isoforms contain four domains, termed C1–C4. The C1 domain consists of conserved subdomains, C1A and C1B, which each bind diacyglycerol and phorbol esters (PE) with varying affinities [67]. Diacylglycerol and PE sufficiently activate novel isoforms while conventional PKCs also require calcium, which binds the C2 domain. Conventional and novel PKCs, in addition to “atypical” PKCs that are both calcium and diacylglycerol/PE independent, contain a C3 ATP binding domain and a C4 catalytic domain [68].
General anesthetics variably affect PKC activity, causing inhibition or activation depending on the isoform, anesthetic, and experimental conditions [68]. The isolated catalytic domains of PKCs appear unaffected by anesthetics [69]. In contrast, anesthetics, most notably alcohols, allosterically interact with the diacylglycerol/PE sites on the C1 domain [70]. Photolabeling experiments with azi-alcohols have been performed with PKCδ, a “novel” isoform, in part because a high-resolution structure of its C1B subdomain is available.
Separately, the PKCδ C1A and C1B subdomains were isolated and photolabeled with 3-azi-octanol, 7-azi-octanol, and 3-azi-butanol, and specific binding sites allosteric to the diacylglycerol/PE sites were identified on each subdomain [71,72]. The allosteric coupling between butanol or octanol and a phorbol ester was maintained even for the isolated C1B domain [71]. The azi-alcohols photolabeled a single residue in C1B, corresponding to tyrosine-236 of the full-length protein, nearby the PE binding cleft [71]. Further demonstrating the importance of this site, PKCδ C1B was crystallized in complex with the anesthetic cyclopropylmethanol [73], which non-competitively inhibits PE stimulated PKCδ activity, and was revealed to hydrogen bond with tyrosine-236. Mutation of this residue abolished binding of cyclopropylmethanol and its inhibitory effects on PE stimulated activity [73].
Unraveling the complex interactions of alcohols with PKCδ has only begun. For instance, while azi-octanol, azi-butanol, and cyclopropylmethanol bind an identical site on C1B, the binding modes likely differ. This is first suggested by the positive coupling between butanol or octanol and PE, and the conversely negative coupling between cyclopropylmethanol and PE [71,73]. Additionally, binding modes conserved with cyclopropylmethanol were not observed in structures of PKCδ C1B co-crystallized with longer chained alcohols [73]. The structural and functional effects of clinical general anesthetics on PKCδ as well as other PKC isoforms should be the subject of further investigation.
LFA-1
Leukocyte function-associated antigen-1 (LFA-1) is a heterodimeric adhesion protein, composed of αL and β2 subunits, that mediates leukocyte arrest at endothelial inflammatory sites. The activated, “open” conformation of this integrin has an exposed intercellular adhesion molecule (ICAM) binding domain, also known as the αI domain, on the αL subunit. The β2 subunit is homologous to αL, but functions as a regulatory subunit to facilitate conformational changes of the protein, including transitions from the resting, “closed” conformation to the activated form. General anesthetics, especially the volatile type, have been shown to suppress leukocyte accumulation at inflammatory sites [74,75]. A critical antagonistic mechanism is suggested by in vitro inhibition of LFA-1 binding to ICAM by volatile anesthetics and propofol [76–78].
NMR and x-ray crystallography studies with isoflurane and sevoflurane identified an anesthetic binding site on the αI domain of the αL subunit, allosteric to the ICAM binding site, that is photolabeled by azi-isoflurane and m-azi-propofol and is conserved with other LFA-1 antagonists (e.g., lovastatin) [19,77,79–81]. Azi-isoflurane photolabeling also identified a homologous binding site on the β2 subunit in the βI domain [76]. Subsequent in vitro experiments suggested that isoflurane inhibits leukocyte arrest by binding to both the αL and β2 subunits and stabilizing the LFA-1 closed conformation [76].
In addition to LFA-1, other adhesion molecules, including platelet integrins that mediate clot formation and stability, are also inhibited by volatile anesthetics and have been probed with azi-isoflurane [82]. Identifying mechanisms associated with volatile anesthetic integrin inhibition, and the apparent lack of inhibition by propofol, is of clinical interest [82].
Photoactivation of ligands in vivo
The in vivo efficacy of anesthetic analogs is routinely tested in tadpoles by dissolving the compound in water, from which it is absorbed by passive diffusion. In these tadpole assays, animal immobility is the conventional and fairly unambiguous anesthetic endpoint [83]. In a recent study, tadpoles anesthetized with m-azi-propofol were photolabeled in vivo [60]. This assured photolabeled targets were functional and equilibrated with ligand concentrations aligned to the anesthetic endpoint. Covalent attachment of ligand in target sites dramatically lengthens drug off-rates, thereby increasing apparent affinity and potency of a compound [60]. Similarly, light-induced attachment of 1-azidoanthrance to tadpole forebrain targets converted this relatively inefficacious anesthetic analog into a potent immobilizer [20]. In both cases, “optoanesthesia” was reversible, indicating that in vivo mechanisms exist for terminating even covalent drug action.
Ex vivo photolabeling of GABAA receptors with azi-etomidate demonstrated that covalent attachment of ligand can irreversibly modulate the ion channel [84]. This included potentiation of GABA currents after washout of unattached compound, as well as increased receptor sensitivity to direct activation by etomidate and propofol [84]. Similarly, in vivo attachment of m-azi-propofol to relevant targets, likely including the GABAA receptor, prolonged emergence of anesthetized tadpoles following washout of unattached compound, and after emergence, tadpoles were more sensitive to propofol [60]. Time-resolved proteomic identification of proteins photolabeled in vivo provides molecular candidates associated with the immobility endpoint of general anesthesia.
In vivo photolabeling in mammalian systems has not been reported in the literature; however, the potency of photoactive analogs in rodents indicates this is a feasible approach. Propofol and etomidate, administered intravenously, anesthetize mice with two to three times the potency of their corresponding analogs, m-azi-propofol, ortho-propofol diazirine and azi-etomidate (our unpublished data and [23, 85]). Photolabeling defined anatomic coordinates in vivo in rodents or tadpoles could identify higher and lower brain structures that are functionally related to specific anesthetic endpoints.
Conclusions
The continued and improved application of anesthetic photolabeling techniques will further the goal of unraveling the pharmacology of these drugs. Through photolabeling, progress has been made in understanding anesthetic-protein interactions and the complex mechanisms that cause general anesthesia. The relevance of revealed targets and binding sites must continue to be examined through in vitro functional and in vivo physiologic assays, including through mutagenesis and animal knock-in/knockout approaches. To comprehensively understand the causes of hypnosis and drug side effects of any single chemotype is ambitious, especially as research naturally transitions to currently used clinical compounds. However, it is clear that the information generated from anesthetic photolabeing experiments will be invaluable in rational drug design and the continued elucidation of general anesthetic mechanisms.
Acknowledgments
The authors are supported in part by NIH grants GM055876 (RGE), NS080519 (BPW), and GM008076 (KAW).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Brian P. Weiser, Kellie A. Woll, William P. Dailey, and Roderic G. Eckenhoff declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Brian P. Weiser, Email: bweiser@mail.med.upenn.edu.
Kellie A. Woll, Email: kwoll@mail.med.upenn.edu.
William P. Dailey, Email: dailey@sas.upenn.edu.
Roderic G. Eckenhoff, Email: roderic.eckenhoff@uphs.upenn.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bösterling B, Trevor A, Trudell JR. Binding of halothane-free radicals to fatty acids following UV irradiation. Anesthesiology. 1982;56:380–4. doi: 10.1097/00000542-198205000-00010. [DOI] [PubMed] [Google Scholar]
- 2.el-Maghrabi EA, Eckenhoff RG, Shuman H. Saturable binding of halothane to rat brain synaptosomes. Proc Natl Acad Sci USA. 1992;89:4329–32. doi: 10.1073/pnas.89.10.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckenhoff RG. An inhalational anesthetic binding domain in the nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1996;93:2807–10. doi: 10.1073/pnas.93.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckenhoff RG. Amino acid resolution of halothane binding sites in serum albumin. J Biol Chem. 1996;271:15521–6. doi: 10.1074/jbc.271.26.15521. [DOI] [PubMed] [Google Scholar]
- 5.Eckenhoff RG, Petersen CE, Ha CE, Bhagavan NV. Inhaled anesthetic binding sites in human serum albumin. J Biol Chem. 2000;275:30439–44. doi: 10.1074/jbc.M005052200. [DOI] [PubMed] [Google Scholar]
- 6.Tang P, Eckenhoff RG, Xu Y. General anesthetic binding to gramicidin A: the structural requirements. Biophys J. 2000;78:1804–9. doi: 10.1016/S0006-3495(00)76730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckenhoff RG, Pidikiti R, Reddy KS. Anesthetic stabilization of protein intermediates: myoglobin and halothane. Biochemistry. 2001;40:10819–24. doi: 10.1021/bi010691r. [DOI] [PubMed] [Google Scholar]
- 8.Ishizawa Y, Pidikiti R, Liebman PA, Eckenhoff RG. G protein-coupled receptors as direct targets of inhaled anesthetics. Mol Pharmacol. 2002;61:945–52. doi: 10.1124/mol.61.5.945. [DOI] [PubMed] [Google Scholar]
- 9.Chiara DC, Dangott LJ, Eckenhoff RG, Cohen JB. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–67. doi: 10.1021/bi0351561. [DOI] [PubMed] [Google Scholar]
- 10.Eckenhoff RG, Knoll FJ, Greenblatt EP, Dailey WP. Halogenated diazirines as photolabel mimics of the inhaled haloalkane anesthetics. J Med Chem. 2002;45:1879–86. doi: 10.1021/jm0104926. [DOI] [PubMed] [Google Scholar]
- 11.Husain SS, Forman SA, Kloczewiak MA, Addona GH, Olsen RW, Pratt MB, et al. Synthesis and properties of 3-(2-hydroxyethyl)-3-n-pentyldiazirine, a photoactivable general anesthetic. J Med Chem. 1999;42:3300–7. doi: 10.1021/jm9806300. [DOI] [PubMed] [Google Scholar]
- 12.Addona GH, Husain SS, Stehle T, Miller KW. Geometric isomers of a photoactivable general anesthetic delineate a binding site on adenylate kinase. J Biol Chem. 2002;277:25685–91. doi: 10.1074/jbc.M201303200. [DOI] [PubMed] [Google Scholar]
- 13.Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, et al. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–65. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 14.Husain SS, Nirthanan S, Ruesch D, Solt K, Cheng Q, Li G-D, et al. Synthesis of trifluoromethylaryl diazirine and benzophenone derivatives of etomidate that are potent general anesthetics and effective photolabels for probing sites on ligand-gated ion channels. J Med Chem. 2006;49:4818–25. doi: 10.1021/jm051207b. [DOI] [PubMed] [Google Scholar]
- 15.Bright DP, Adham SD, Lemaire LCJM, Benavides R, Gruss M, Taylor GW, et al. Identification of anesthetic binding sites on human serum albumin using a novel etomidate photolabel. J Biol Chem. 2007;282:12038–47. doi: 10.1074/jbc.M700479200. [DOI] [PubMed] [Google Scholar]
- 16.Husain SS, Stewart D, Desai R, Hamouda AK, Li SG-D, Kelly E, et al. p-Trifluoromethyldiazirinyl-etomidate: a potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels. J Med Chem. 2010;53:6432–44. doi: 10.1021/jm100498u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darbandi-Tonkabon R, Hastings WR, Zeng C-M, Akk G, Manion BD, Bracamontes JR, et al. Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. J Biol Chem. 2003;278:13196–206. doi: 10.1074/jbc.M213168200. [DOI] [PubMed] [Google Scholar]
- 18.Xi J, Liu R, Rossi MJ, Yang J, Loll PJ, Dailey WP, et al. Photoactive analogues of the haloether anesthetics provide high-resolution features from low-affinity interactions. ACS Chem Biol. 2006;1:377–84. doi: 10.1021/cb600207d. [DOI] [PubMed] [Google Scholar]
- 19.Eckenhoff RG, Xi J, Shimaoka M, Bhattacharji A, Covarrubias M, Dailey WP. Azi-isoflurane, a photolabel analog of the commonly used inhaled general anesthetic isoflurane. ACS Chem Neurosci. 2010;1:139–45. doi: 10.1021/cn900014m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Emerson DJ, Weiser BP, Psonis J, Liao Z, Taratula O, Fiamengo A, et al. Direct modulation of microtubule stability contributes to anthracene general anesthesia. J Am Chem Soc. 2013;135:5389–98. doi: 10.1021/ja311171u. An approach to uncovering anesthetic mechanisms (i.e., probe synthesis, target identification, and in vivo implication of the target) is implemented here. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall MA, Xi J, Lor C, Dai S, Pearce R, Dailey WP, et al. m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J Med Chem. 2010;53:5667–75. doi: 10.1021/jm1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart DS, Savechenkov PY, Dostalova Z, Chiara DC, Ge R, Raines DE, et al. p-(4-Azipentyl)propofol: a potent photoreactive general anesthetic derivative of propofol. J Med Chem. 2011;54:8124–35. doi: 10.1021/jm200943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip GMS, Chen Z-W, Edge CJ, Smith EH, Dickinson R, Hohenester E, et al. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol. 2013 doi: 10.1038/nchembio.1340. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savechenkov PY, Zhang X, Chiara DC, Stewart DS, Ge R, Zhou X, et al. Allyl m-trifluoromethyldiazirine mephobarbital: an unusually potent enantioselective and photoreactive barbiturate general anesthetic. J Med Chem. 2012;55:6554–65. doi: 10.1021/jm300631e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilchrist TL, Rees CW. Carbenes, Nitrenes and Arynes. The Pitman Press; Bath: 1969. [Google Scholar]
- 26•.Gerbig D, Ley D. Computational methods for contemporary carbene chemistry. WIREs Comput Mol Sci. 2013;3:242–72. This provides a review of computational practices for predicting carbene reactivity within ligand design. [Google Scholar]
- 27.De Frémont P, Marion N, Nolan SP. Carbenes: Synthesis, properties, and organometallic chemistry. Coord Chem Rev. 2009;253:862–92. [Google Scholar]
- 28.Brahms DLS, Dailey WP. Fluorinated carbenes. Chem Rev. 1996;96:1585–632. doi: 10.1021/cr941141k. [DOI] [PubMed] [Google Scholar]
- 29.Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J Biol Chem. 2004;279:17640–9. doi: 10.1074/jbc.M313886200. [DOI] [PubMed] [Google Scholar]
- 30•.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. Consideration of the chemical reactivity of photolysis intermediates when interpreting binding site identification is addressed here. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z-W, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, et al. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABA(A) receptor. Mol Pharmacol. 2012;82:408–19. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Chen Z-W, Fuchs K, Sieghart W, Townsend RR, Evers AS. Deep amino acid sequencing of native brain GABAA receptors using high-resolution mass spectrometry. Mol Cell Proteomics. 2012;11:M111.011445. doi: 10.1074/mcp.M111.011445. A procedure to comprehensively sequence GABAA receptors with mass spectrometry is described here, and this has subsequently been implemented in anesthetic photo-adduct identification (ref. 23 and 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckenhoff MF, Eckenhoff RG. Quantitative autoradiography of halothane binding in rat brain. J Pharmacol Exp Ther. 1998;285:371–6. [PubMed] [Google Scholar]
- 34.Eckenhoff MF, Chan K, Eckenhoff RG. Multiple specific binding targets for inhaled anesthetics in the mammalian brain. J Pharmacol Exp Ther. 2002;300:172–9. doi: 10.1124/jpet.300.1.172. [DOI] [PubMed] [Google Scholar]
- 35.Eckenhoff RG, Shuman H. Localization of volatile anesthetic molecules at the subcellular and molecular level. Ann N Y Acad Sci. 1991;625:755–9. doi: 10.1111/j.1749-6632.1991.tb33910.x. [DOI] [PubMed] [Google Scholar]
- 36.Eckenhoff RG, Shuman H. Subcellular distribution of an inhalational anesthetic in situ. Proc Natl Acad Sci USA. 1990;87:454–7. doi: 10.1073/pnas.87.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xi J, Liu R, Asbury GR, Eckenhoff MF, Eckenhoff RG. Inhalational anesthetic-binding proteins in rat neuronal membranes. J Biol Chem. 2004;279:19628–33. doi: 10.1074/jbc.M313864200. [DOI] [PubMed] [Google Scholar]
- 38.Pan JZ, Xi J, Tobias JW, Eckenhoff MF, Eckenhoff RG. Halothane binding proteome in human brain cortex. J Proteome Res. 2007;6:582–92. doi: 10.1021/pr060311u. [DOI] [PubMed] [Google Scholar]
- 39.Rottenberg H. Uncoupling of oxidative phosphorylation in rat liver mitochondria by general anesthetics. Proc Natl Acad Sci USA. 1983;80:3313–7. doi: 10.1073/pnas.80.11.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayser E-B, Suthammarak W, Morgan PG, Sedensky MM. Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg. 2011;112:1321–9. doi: 10.1213/ANE.0b013e3182121d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintana A, Morgan PG, Kruse SE, Palmiter RD, Sedensky MM. Altered anesthetic sensitivity of mice lacking Ndufs4, a subunit of mitochondrial complex I. PLoS ONE. 2012;7:e42904. doi: 10.1371/journal.pone.0042904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–89. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Cui T, Mowrey D, Bondarenko V, Tillman T, Ma D, Landrum E, et al. NMR structure and dynamics of a designed water-soluble transmembrane domain of nicotinic acetylcholine receptor. Biochim Biophys Acta. 2012;1818:617–26. doi: 10.1016/j.bbamem.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–31. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 45.Hamouda AK, Stewart DS, Husain SS, Cohen JB. Multiple transmembrane binding sites for p-Trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor. J Biol Chem. 2011;286:20466–77. doi: 10.1074/jbc.M111.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayakar SS, Dailey WP, Eckenhoff RG, Cohen JB. Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog. J Biol Chem. 2013;288:6178–89. doi: 10.1074/jbc.M112.435909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nirthanan S, Garcia G, 3rd, Chiara DC, Husain SS, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem. 2008;283:22051–62. doi: 10.1074/jbc.M801332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem. 2000;275:29441–51. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- 49.Forman SA, Zhou QL, Stewart DS. Photoactivated 3-azioctanol irreversibly desensitizes muscle nicotinic ACh receptors via interactions at alphaE262. Biochemistry. 2007;46:11911–8. doi: 10.1021/bi701287a. [DOI] [PubMed] [Google Scholar]
- 50.Chiara DC, Hong FH, Arevalo E, Husain SS, Miller KW, Forman SA, et al. Time-resolved photolabeling of the nicotinic acetylcholine receptor by [3H]azietomidate, an open-state inhibitor. Mol Pharmacol. 2009;75:1084–95. doi: 10.1124/mol.108.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Olsen RW, Sieghart W International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G-D, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, et al. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J Biol Chem. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. This paper thoroughly characterizes GABAA receptor binding selectivity of multiple injectable anesthetic chemotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G-D, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–20. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eckenhoff M, Eckenhoff R. γ-Aminobutyric acid enhancement of halothane binding in rat cerebellum. Neurosci Lett. 2000;286:111–4. doi: 10.1016/s0304-3940(00)01105-8. [DOI] [PubMed] [Google Scholar]
- 57.Li G-D, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–5. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, et al. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci. 2013;33:7264–73. doi: 10.1523/JNEUROSCI.5475-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darbandi-Tonkabon R, Manion BD, Hastings WR, Craigen WJ, Akk G, Bracamontes JR, et al. Neuroactive steroid interactions with voltage-dependent anion channels: lack of relationship to GABAA receptor modulation and anesthesia. J Pharmacol Exp Ther. 2004;308:502–11. doi: 10.1124/jpet.103.058123. [DOI] [PubMed] [Google Scholar]
- 60.Weiser BP, Kelz MB, Eckenhoff RG. In vivo activation of azipropofol prolongs anesthesia and reveals synaptic targets. J Biol Chem. 2013;288:1279–85. doi: 10.1074/jbc.M112.413989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bureau MH, Khrestchatisky M, Heeren MA, Zambrowicz EB, Kim H, Grisar TM, et al. Isolation and cloning of a voltage-dependent anion channel-like Mr 36,000 polypeptide from mammalian brain. J Biol Chem. 1992;267:8679–84. [PubMed] [Google Scholar]
- 62.Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Chernyshov V, Kryukova E, et al. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS ONE. 2012;7:e31361. doi: 10.1371/journal.pone.0031361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hameroff S, Nip A, Porter M, Tuszynski J. Conduction pathways in microtubules, biological quantum computation, and consciousness. BioSystems. 2002;64:149–68. doi: 10.1016/s0303-2647(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 64.Chen Z-W, Chen L-H, Akentieva N, Lichti CF, Darbandi R, Hastings R, et al. A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis. 2012;33:666–74. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butts CA, Xi J, Brannigan G, Saad AA, Venkatachalan SP, Pearce RA, et al. Identification of a fluorescent general anesthetic, 1-aminoanthracene. Proc Natl Acad Sci USA. 2009;106:6501–6. doi: 10.1073/pnas.0810590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Craddock TJA, St George M, Freedman H, Barakat KH, Damaraju S, Hameroff S, et al. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS ONE. 2012;7:e37251. doi: 10.1371/journal.pone.0037251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slater SJ, Ho C, Kelly MB, Larkin JD, Taddeo FJ, Yeager MD, et al. Protein kinase Calpha contains two activator binding sites that bind phorbol esters and diacylglycerols with opposite affinities. J Biol Chem. 1996;271:4627–31. doi: 10.1074/jbc.271.9.4627. [DOI] [PubMed] [Google Scholar]
- 68.Rebecchi MJ, Pentyala SN. Anaesthetic actions on other targets: protein kinase C and guanine nucleotide-binding proteins. Br J Anaesth. 2002;89:62–78. doi: 10.1093/bja/aef160. [DOI] [PubMed] [Google Scholar]
- 69.Slater SJ, Cox KJ, Lombardi JV, Ho C, Kelly MB, Rubin E, et al. Inhibition of protein kinase C by alcohols and anaesthetics. Nature. 1993;364:82–4. doi: 10.1038/364082a0. [DOI] [PubMed] [Google Scholar]
- 70.Slater SJ, Kelly MB, Larkin JD, Ho C, Mazurek A, Taddeo FJ, et al. Interaction of alcohols and anesthetics with protein kinase Calpha. J Biol Chem. 1997;272:6167–73. doi: 10.1074/jbc.272.10.6167. [DOI] [PubMed] [Google Scholar]
- 71.Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW. Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cdelta. J Biol Chem. 2004;279:37964–72. doi: 10.1074/jbc.M405137200. [DOI] [PubMed] [Google Scholar]
- 72.Das J, Zhou X, Miller KW. Identification of an alcohol binding site in the first cysteine-rich domain of protein kinase Cdelta. Protein Sci. 2006;15:2107–19. doi: 10.1110/ps.062237606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shanmugasundararaj S, Das J, Sandberg WS, Zhou X, Wang D, Messing RO, et al. Structural and functional characterization of an anesthetic binding site in the second cysteine-rich domain of protein kinase Cδ*. Biophys J. 2012;103:2331–40. doi: 10.1016/j.bpj.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kowalski C, Zahler S, Becker BF, Flaucher A, Conzen PF, Gerlach E, et al. Halothane, isoflurane, and sevoflurane reduce postischemic adhesion of neutrophils in the coronary system. Anesthesiology. 1997;86:188–95. doi: 10.1097/00000542-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 75.Möbert J, Zahler S, Becker BF, Conzen PF. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–81. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 76.Yuki K, Bu W, Xi J, Sen M, Shimaoka M, Eckenhoff RG. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 2012;26:4408–17. doi: 10.1096/fj.12-212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 2010;113:600–9. doi: 10.1097/ALN.0b013e3181e89a77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuki K, Soriano SG, Shimaoka M. Sedative drug modulates T-cell and lymphocyte function-associated antigen-1 function. Anesth Analg. 2011;112:830–8. doi: 10.1213/ANE.0b013e31820dcabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, et al. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–16. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Astrof NS, Liu J-H, Wang J, Shimaoka M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 2009;23:2735–40. doi: 10.1096/fj.09-129908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuki K, Bu W, Xi J, Shimaoka M, Eckenhoff R. Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth Analg. 2013 doi: 10.1213/ANE.0b013e3182a00ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuki K, Bu W, Shimaoka M, Eckenhoff R. Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin αIIbβ3. PLoS ONE. 2013;8:e60415. doi: 10.1371/journal.pone.0060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABAA receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–51. [PubMed] [Google Scholar]
- 84.Zhong H, Rüsch D, Forman SA. Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of γ-aminobutyric acid type A receptors. Anesthesiology. 2008;108:103–12. doi: 10.1097/01.anes.0000296074.33999.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, et al. R (+) Etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor β3 subunit. Anesth Analg. 2005;101:131–5. doi: 10.1213/01.ANE.0000153011.64764.6F. [DOI] [PubMed] [Google Scholar]