Abstract
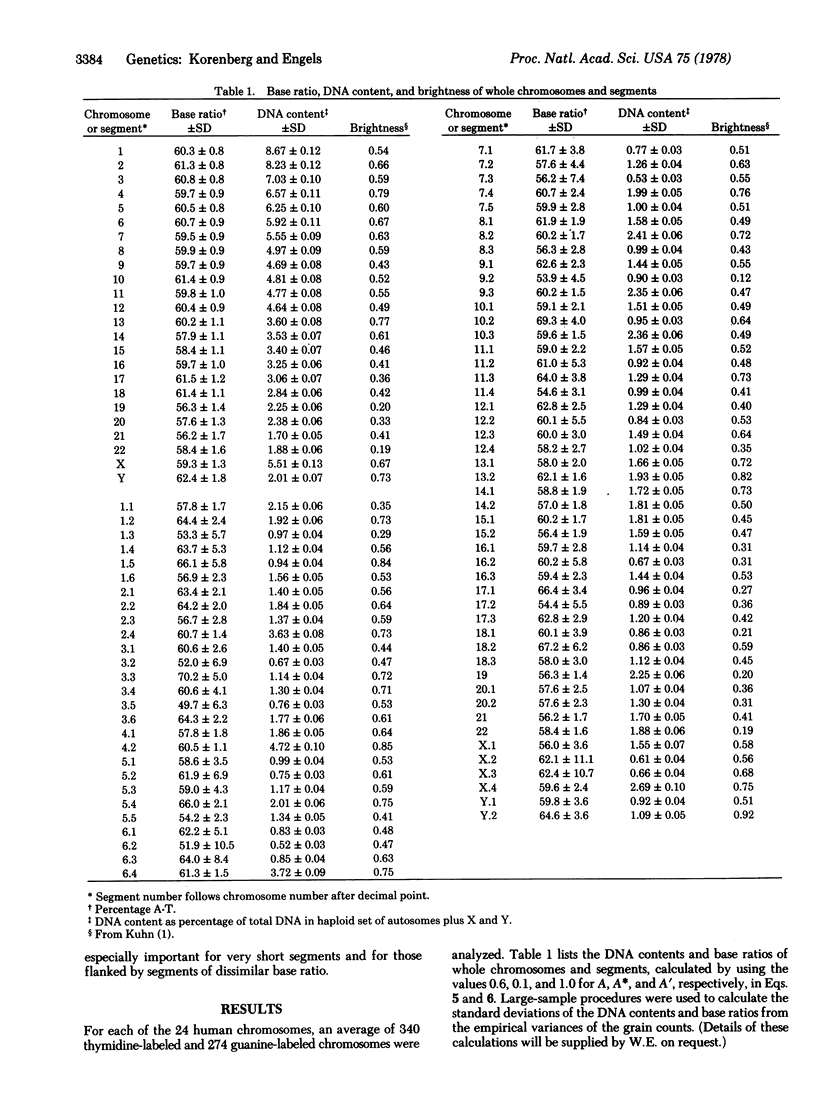
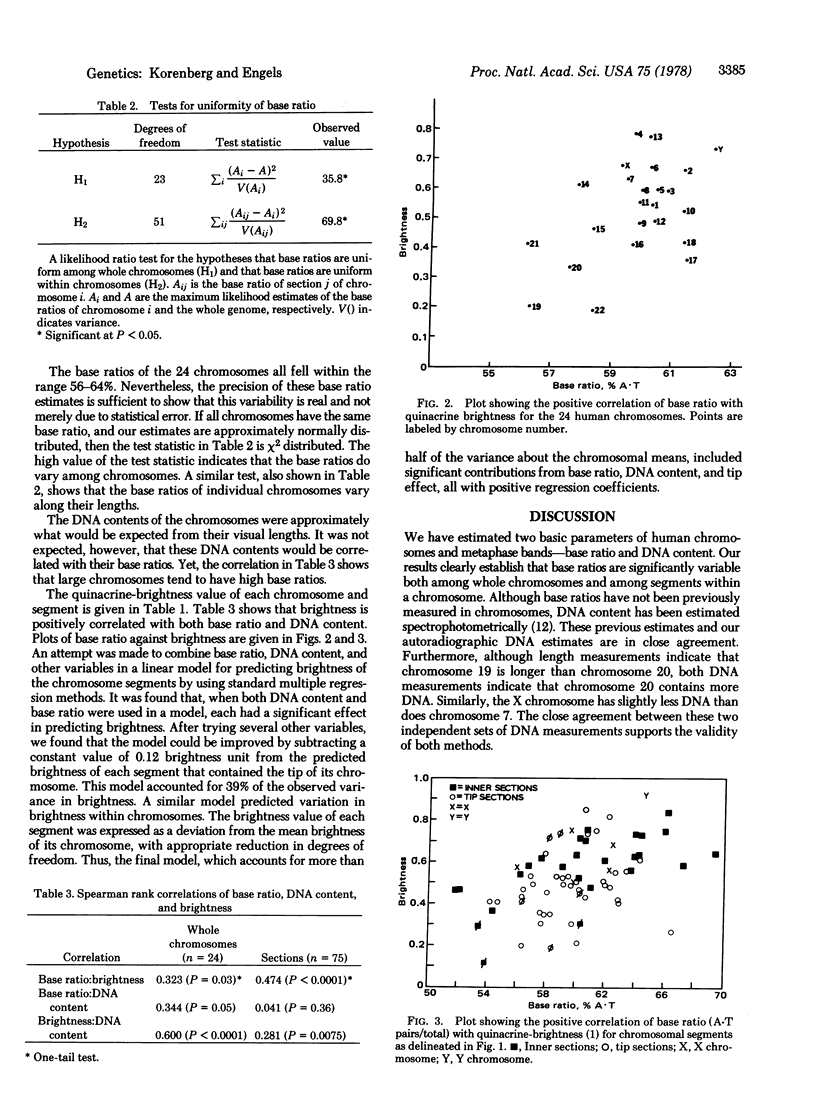
Human chromosomes were labeled with base-specific radioactive DNA precursors and examined autoradiographically to measure their DNA content and base ratio (percentage A-T base pairs). The requirement that incorporation of labeled bases be uniform during DNA synthesis was met by the use of inhibitors of de novo synthesis of DNA precursors. The genome was subdivided into 75 segments based on quinacrine banding, and the base ratio of each was calculated by a method that corrects for bias due to the scatter of grains about their source. Estimates of base ratio are shown to be sufficiently precise to detect variability among chromosomes and among segments within a chromosome. Analysis of these data and of measurements of the quinacrine fluorescence intensity of segments leads to the following conclusions. Base ratio is positively correlated with brightness, as predicted from independent in vitro studies. Larger chromosomes tend to have higher base ratios and to be brighter than smaller ones. The best prediction of the brightness of a segment must take into account not only its base ratio but also its DNA content. To explain these results, we suggest an evolutionary model in which chromosomes containing repeated sequences of A-T-rich DNA tend to grow by means of unequal sister chromatid and meiotic exchanges.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll D., Brown D. D. Adjacent repeating units of Xenopus laevis 5S DNA can be heterogeneous in length. Cell. 1976 Apr;7(4):477–486. doi: 10.1016/0092-8674(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E., Drets M. E. Mechanisms of chromosome banding. IX. Are variations in DNA base composition adequate to account for quinacrine, Hoechst 33258 and daunomycin banding? Chromosoma. 1976 Jul 8;56(3):199–211. doi: 10.1007/BF00293185. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganner E., Evans H. J. The relationship between patterns of DNA replication and of quinacrine fluorescence in the human chromosome complement. Chromosoma. 1971;35(3):326–341. doi: 10.1007/BF00326282. [DOI] [PubMed] [Google Scholar]
- Holmberg M., Jonasson J. Preferential location of x-ray induced chromosome breakage in the R-bands of human chromosomes. Hereditas. 1973;74(1):57–67. doi: 10.1111/j.1601-5223.1973.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Kuhn E. M. Localization by Q-banding of mitotic chiasmata in cases of Bloom's syndrome. Chromosoma. 1976 Aug 4;57(1):1–11. doi: 10.1007/BF00292945. [DOI] [PubMed] [Google Scholar]
- Lozovskaya E. R., Slesinger S. I., Prokofieva-Belgovskaya A. A. Comparative study of human chromosome replication in primary cultures of embryonic fibroblasts and in cultures of peripheral blood leucocytes. III. Distribution of AT- and GC-nucleotide pairs along the length of chromosomes 1, 2, 3, and 16 in the two types of human cells. Chromosoma. 1977 Mar 7;60(1):69–79. doi: 10.1007/BF00330412. [DOI] [PubMed] [Google Scholar]
- Mendelsohn M. L., Mayall B. H., Bogart E., Moore D. H., 2nd, Perry B. H. DNA content and DNA-based centromeric index of the 24 human chromosomes. Science. 1973 Mar 16;179(4078):1126–1129. doi: 10.1126/science.179.4078.1126. [DOI] [PubMed] [Google Scholar]
- Schreck R. R., Warburton D., Miller O. J., Beiser S. M., Erlanger B. F. Chromosome structure as revealed by a combined chemical and immunochemical procedure. Proc Natl Acad Sci U S A. 1973 Mar;70(3):804–807. doi: 10.1073/pnas.70.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 1976 Nov 29;58(4):307–324. doi: 10.1007/BF00292840. [DOI] [PubMed] [Google Scholar]
- Tobia A. M., Schildkraut C. L., Maio J. J. Deoxyribonucleic acid replication in synchronized cultured mammalian cells. I. Time of synthesis of molecules of different average uanine + cytosine content. J Mol Biol. 1970 Dec 28;54(3):499–515. doi: 10.1016/0022-2836(70)90122-1. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Fluorescent probes of chromosomal DNA structure: three classes of acridines. Cold Spring Harb Symp Quant Biol. 1974;38:441–449. doi: 10.1101/sqb.1974.038.01.048. [DOI] [PubMed] [Google Scholar]



