Abstract
At the time of implantation in the maternal uterus, the mouse blastocyst possesses an inner cell mass comprising two lineages: epiblast (Epi) and primitive endoderm (PrE). Representative stem cells derived from these two cell lineages can be expanded and maintained indefinitely in vitro as either embryonic stem (ES ) or XEN cells, respectively. Here we describe protocols that can be used to establish XEN cell lines. These include the establishment of XEN cells from blastocyst-stage embryos in either standard embryonic or trophoblast stem (TS ) cell culture conditions. We also describe protocols for establishing XEN cells directly from ES cells by either retinoic acid and activin-based conversion or by overexpression of the GATA transcription factor Gata6. XEN cells are a useful model of PrE cells, with which they share gene expression, differentiation potential and lineage restriction. The robust protocols for deriving XEN cells described here can be completed within 2–3 weeks.
Introduction
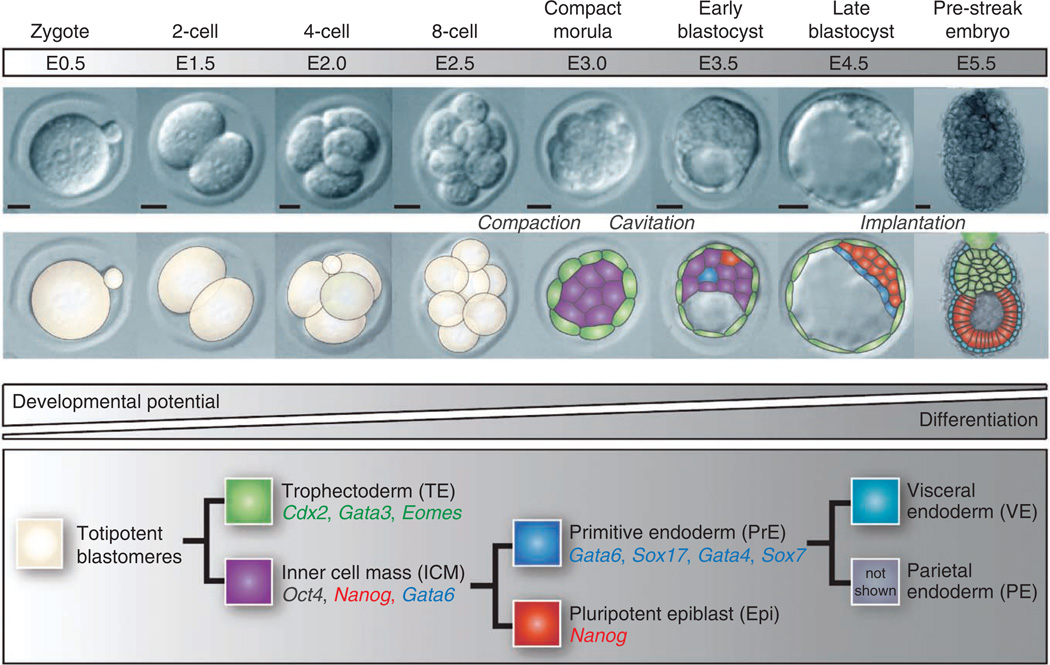
The mouse embryo ~3.5 d after fertilization forms a blastocyst comprising three lineages1: the extraembryonic trophectoderm (TE), the PrE and the pluripotent Epi (Fig. 1) from which cognate ex vivo stem cells can be derived. TS cells are derived from the TE2, XEN cells from the PrE3 and ES cells from the Epi (refs. 4,5; Fig. 2) (reviewed in ref. 6). Notably, each of these stem cell lines is a useful model of the blastocyst cell lineage that they represent. Mouse ES and TS cells have been used successfully for many years to model Epi or TE biology, including the mechanisms of pluripotency maintenance and placental development, respectively. Recently derived XEN cell lines have the distinctive characteristic of cells with at least two morphologies: they are highly refractile as well as epitheliallike3 (Fig. 2), and they are only beginning to be used to understand the mechanisms of PrE development with significance for stem cell and developmental biology.
Figure 1.
Overview of early embryonic development. Proper lineage segregation before implantation is ensured by two cell-fate decisions, with the first giving rise to trophectoderm and inner cell mass, and the second leading to the allocation of primitive endoderm and epiblast. Lineage-associated gene expression is noted below each cell type. After implantation, the PrE differentiates into visceral and parietal endoderm. E: embryonic day.
Scale bars, 50 µm.
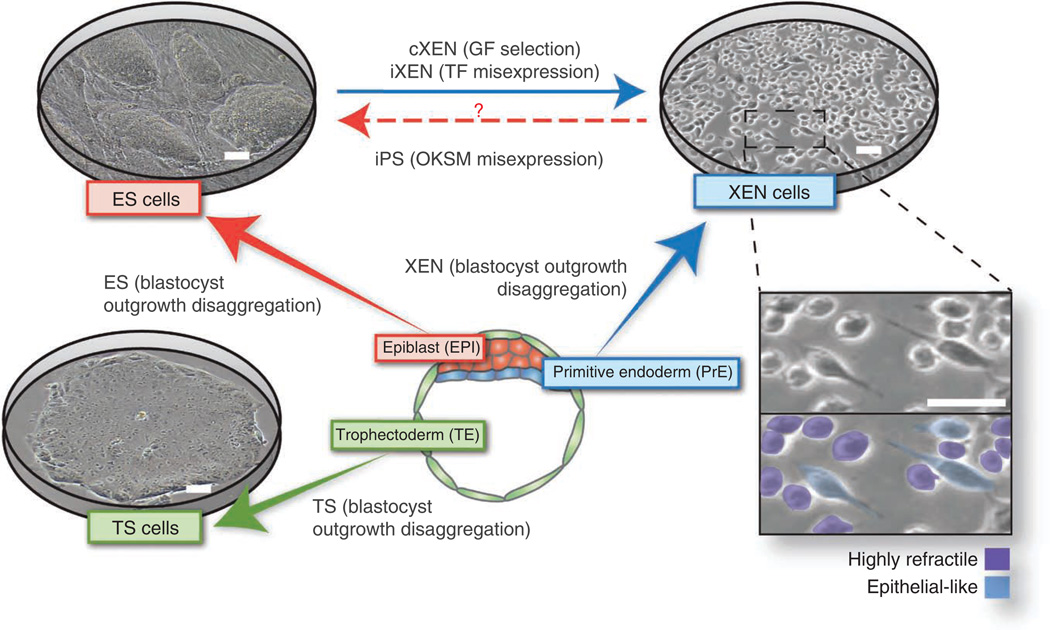
Figure 2.
Stem cell types that can be derived and propagated in culture representing the three blastocyst lineages. Embryonic stem (ES) cells represent the epiblast, trophoblast stem (TS) cells represent the trophectoderm and extraembryonic endoderm (XEN) cells represent the primitive endoderm cell lineage. Heterogeneities in XEN cell morphology are indicated: highly refractile phase-bright and epithelial-like. Cognate embryo– derived stem cells retain the expression of key lineage-associated genes. GF, growth factor; iPS, induced pluripotent stem; OKSM, Oct4, Sox2, Klf4 and c-Myc. Scale bars, 100 µm.
Mouse ES cells can be directed to differentiate into extraembryonic lineages by the overexpression of single transcription factors, such as the caudal-related homeodomain transcription factor Cdx2 (to derive TS cells)7 or the GATA transcription factor Gata6 (to derive XEN cells)8. Retinoic acid treatment of mouse ES cells9–11 or embryoid body aggregation12 has been shown to promote a heterogeneous mixture of XEN-differentiated cells. Notably, these cells have not been demonstrated to self-renew indefinitely, unlike bona fide XEN cell lines. We have recently demonstrated that mouse ES cells can be converted to stable XEN cell lines using retinoic acid together with activin13. In this protocol, we focus on the derivation of XEN cells from embryos and ES cells.
The molecular mechanisms underlying XEN cell establishment and maintenance are beginning to be understood. Robust methods for XEN cell derivation from embryos and ES cells, as well as the concomitant availability of XEN cell lines, will further facilitate and improve our understanding of the key fate decisions that occur within the early embryo, including unraveling mechanisms underlying cellular differentiation and pluripotency14,15. As a stem cell type that can be derived from both embryos and ES cells, XEN cells are emerging as a valuable tool for modeling the XEN lineage.
Applications of XEN cells
XEN cell derivation can be used as a phenotypic tool to assess the requirement of genes for XEN cell specification, maintenance or expansion, as we have previously demonstrated for SRY-box containing gene 17 Sox17)4, platelet-derived growth factor receptor alpha (Pdgfra)11 and fibroblast growth factor 4 (Fgf4)16. Notably, established XEN cell lines serve as a paradigm for XEN biology and for the differentiation of the PrE into derivatives such as visceral17–19 and parietal endoderm.
Given the fact that reciprocal inductive interactions between the pluripotent Epi and its adjacent extraembryonic tissues, including the visceral endoderm (reviewed in refs. 20 and 21), are crucial to patterning the early embryo, XEN cells can be used as an ex vivo tool for teasing apart the underlying mechanisms and for identifying the key molecules involved12. XEN cells can be used as an important in vitro tool for elucidating details of additional patterning activities of the extraembryonic endoderm, such as identifying factors involved in cardiac induction22–24. Moreover, as they can be propagated in large quantities and do not require growth factor supplements to culture media, these cells are a cost-effective, attractive and tractable system for high-throughput analyses. They can be used in screens for PrE-differentiating factors or in proteomics analyses to identify secreted factors that mediate tissue patterning (for example, during cardiac induction)22–24. XEN cells show paternally imprinted X-chromosome inactivation3, and thus they serve as a useful model for understanding the molecular basis of post-translational and epigentic modifications25. Furthermore, as the extraembryonic endoderm has recently been shown to contribute to the gut endoderm in vivo26, XEN cells may also serve as an alternative self-renewing source of definitive endoderm-derived cells and tissues for regenerative medicine. Intriguingly, XEN cells rapidly silence retroviral transcription compared with mouse ES and TS cells and may therefore also represent a useful model to study how viral gene expression is regulated27.
XEN cell establishment from embryos
Several protocols describe the derivation of XEN cells from mouse embryos, including the direct plating of blastocyst stage embryos3 or immunosurgery, to isolate and to plate the inner cell mass28,29. In 2005, Kunath et al.3 reported the first isolation and characterization of XEN cells using methods based on existing protocols of deriving ES28 and TS cells2,30,31. In this case, embryonic day 3.5 blastocysts were cultured on mouse embryonic fibroblast (MEF) cells until they formed an outgrowth that, as in routinely used ES and TS cell derivation protocols, was subsequently disaggregated to promote the proliferation of the different cell types present in the blastocyst. In both procedures, XEN cell outgrowths could be observed alongside ES or TS cells, respectively, suggesting that these conditions are also permissive for XEN cell derivation.
Our experience has been that slight modifications to these protocols (especially media conditions and time of disaggregation) can favor the propagation of XEN cells over that of ES or TS cells. We have also found that, in our hands, TS cell conditions are generally less efficient for the derivation of XEN cell lines compared with ES cell conditions (21% and 56% efficiency, respectively). ES cells start appearing from the outgrowths first and are less resilient, which might facilitate the ability of XEN cells to outcompete them in a culture dish. TS cells, in contrast, reach their proliferative peak around the same time as XEN cells and tend to outcompete XEN cells present in the same cultures, especially in the presence of recombinant FGF4 (ref. 2) or FGF2 (bFGF), the latter of which can elicit the same effect31 and is slightly more cost effective.
XEN cell establishment by transcription factor overexpression
In classical experiments, Davis et al.32 demonstrated that the expression of a single transcription factor, MyoD, was sufficient to convert fibroblasts into myogenic cells. More recently, transcription factor– mediated cell-fate switches have been demonstrated for several other cell types, including the reprogramming of fibroblasts into induced pluripotent stem cells via ectopic expression of the POU domain, class 5, transcription factor 1 (Pou5f1, also known as Oct4), Klf4, Sox2 and Myc (also known as c-Myc)33.
Ectopic transcription factor expression has also been shown to induce the conversion of XEN cells from ES cells (reviewed in ref. 6). Niwa and colleagues8,34 demonstrated that the expression of Gata4 or Gata6 alone is sufficient to induce the conversion of ES cells into XEN cells. Notably, these GATA-derived XEN cells share the molecular and functional characteristics of embryo-derived XEN cells, including contribution to PrE lineages in chimeric embryos34. XEN-like cells have also been generated by ectopic expression of Sox17 (refs. 14,35–37). It is unclear whether Sox17 alone is sufficient to drive XEN cell commitment, as these cells retain the expression of ES cell–associated genes, such as Nanog and Oct4 (refs. 14,35,38), and their contribution to chimeras has not yet been reported. Moreover, it is unclear whether alternative PrE-associated transcription factors can be used to convert ES to XEN cells.
The simplest method used to derive XEN cells from ES cells is the expression of Gata6 either by transfection of a circular plasmid or a linearized DNA using chemical or nonchemical (e.g., electroporation) methods. There are several chemical-based transfection approaches. The most widely used methods for ES cells are lipidbased lipopolyamines39 and cationic polymer–based transfection (reviewed in ref. 40). Lipopolyamines (e.g., lipofection) work by coating a Gata6-cDNA plasmid with a cationic lipid, allowing the DNA to cross the negatively charged phospholipid ES cell membrane by endocytosis41,42. Cationic polymers (e.g., Xfect) act by binding DNA and condensing it, thereby facilitating DNA entry into the cell cytoplasm40,43. An advantage of lipopolyamines and cationic polymers is that they do not require specialized equipment, although the reagents may be cytotoxic and the ratio of DNA to transfection reagent needs to be optimized to the specific cell type42 (reviewed in ref. 44).
Electroporation is the principal alternative to chemical-based transfection approaches and is routinely used for ES cell transfection45–47. Electroporation allows for permeabilization of the ES cell membrane and transfection of a Gata6 cDNA via the application of a transient electrical field. Depending on the purity of the DNA preparation, this can be a highly efficient method and is most commonly used for gene targeting of ES cells. The choice of whether the Gata6 cDNA plasmid is introduced as a circular plasmid or as a linearized DNA can affect transfection efficiency, as supercoiled or open-circular DNA is optimal for transient expression, whereas linearized DNA is more recombinogenic and best suited for stable transfection48–50.
Continued passage of transiently transfected cells will result in the dilution of the exogenous DNA and may restrict the time window for subsequent analysis. Therefore, although it is initially more time consuming, the generation of stable transfected ES cells that have integrated a linearized Gata6 cDNA into the genome can be experimentally more consistent compared with transient transfection. By including a drug-resistance gene in the Gata6 cDNA–targeting plasmid, drug selection can be used to expand only targeted ES cells that retain the ability to overexpress the Gata6 cDNA. Another important consideration is the use of an efficient and robust promoter to drive exogenous Gata6 expression. In mouse ES cells, the cytomegalovirus (CMV) promoter may be silenced51. In contrast, the ubiquitin C (UBC) promoter, elongation factor 1α(EF1A) promoter, and the chicken β-actin (ACTB) promoter coupled with the CMV early enhancer (CAGG) have been reported to be more robustly expressed51.
Although these are not described here, alternative approaches for Gata6 overexpression include viral transduction. Owing to potential toxicity, viral production and transduction require at least a category II tissue culture facility, and the silencing of retroviral transcription in XEN cells may obviate this approach27. Moreover, reversible overexpression of Gata6 can be engineered using an inducible expression system containing a tetracycline-responsive element (controlled by tetracycline or doxycycline), a mutant estrogen-responsive element (controlled by 4-hydroxytamoxifen) or a glucocorticoid-responsive element (controlled by dexamethasone). When combined with drug selection, an inducible system is an effective means of overexpressing Gata6 simultaneously in all cells. Although overexpression of Gata6 may initially be a simpler method to drive XEN differentiation, a major note of caution is that its ubiquitous expression may hinder the ability of converted XEN cells to differentiate into subtypes of XEN cells. One major limitation may be that constitutive Gata6 expression blocks differentiation of induced XEN cells into visceral endoderm subtypes, as in vivo these cells lack Gata6 expression while retaining the expression of Gata4 (refs. 52,53). Here we describe a method to constitutively express Gata6 in mouse ES cells, which can be modified to overexpress other genes such as Gata4 or to introduce an inducible expression system as has been described previously34,54,55. Moreover, transposon systems such as piggyBac56 may also be used, although these methods have not yet been shown to convert ES to XEN cells.
XEN cell establishment by growth factor conversion of ES cells
Although ES cells largely contribute to the Epi in chimera embryos, it has been observed that committed XEN and TS cells also arise within pluripotent culture conditions14,57,58. The presence of TS-and XEN-like cells within ES cell cultures suggests that ES cells may have a broader cell fate potential and are therefore able to differentiate into stable extraembryonic stem cell lines directly. Indeed, ES cell aggregation results in the formation of embryoid bodies with an outer layer of XEN cells12, and growth factors such as retinoic acid have been shown to differentiate embryonic carcinoma and ES cells into XEN-like cells9–11,59. Moreover, the culture of ES cells in serumfree medium on fibronectin-coated dishes at high density has been suggested to promote visceral endoderm differentiation60. However, it was unclear whether self-renewing XEN cells could be differentiated directly from mouse ES cells without gene manipulation. We have recently expanded on these studies by developing a technique to convert ES cells directly into stable XEN (cXEN) cell lines that are equivalent to embryo-derived XEN cells13. We previously demonstrated that cXEN cells are molecularly indistinguishable from embryo-derived XEN and iXEN (i.e., transcription factor induced) cells and are equivalently responsive to differentiation-promoting factors (i.e., bone morphogenetic protein (BMP)-induced visceral endoderm differentiation). Our efforts to generate chimera embryos from cXEN cells resulted in contribution to the parietal endoderm at early postimplantation stages (K.K.N., M. Kang and A.-K.H., unpublished observations), as has been reported for XEN cells derived from embryos3. Here we describe our method for converting mouse ES cells into stable cXEN cell lines using growth factors. Specifically, we use retinoic acid together with activin, which has been demonstrated to promote primitive endoderm development in vivo and in vitro17,61. Notably, although retinoic acid and activin have been shown to differentiate ES cells into neurons and definitive endoderm, respectively, we find that the combination of both factors promotes XEN cell differentiation. We previously tested a range of retinoic acid concentrations from 0 to 100 µM and a range of activin concentrations from 0 to 20 ng ml−1. Although XEN-like cells can emerge in the absence of exogenous retinoic acid and activin14, the proportion of XEN-like cells is enhanced (40.3%) in 0.01 µM retinoic acid and 10 ng ml−1 activin13. We therefore use the lowest dose of retinoic acid and activin that gives us the highest efficacy of XEN cell emergence, although it is presently unclear whether different concentrations of these growth factors influence the identity of the XEN cells obtained. Thus far, we have been able to derive XEN cell lines from any mouse ES cell line and mouse strain unless the cells are mutant for a gene required for XEN establishment and/or maintenance13, although the speed of conversion can vary with any given cell line. An important consideration is the initial heterogeneity that emerges after growth factor treatment. As with the protocol for XEN cell derivation from embryos, selecting cells with XEN cell morphology facilitates the expansion of stable cXEN cells that are able to self-renew indefinitely. This ES cell conversion protocol is particularly useful for determining the genetic requirement for XEN cell function using mutant mouse ES cells without the necessity for mutant mice.
MATERIALS
REAGENTS
Derivation from embryos using modified ES cell conditions
Pregnant mouse, 3.5 days post coitum (d.p.c.) ! CAUTION Experiments involving rodents must conform to all relevant institutional and governmental regulations.
Mitotically inactivated MEF cells (Reagent Setup)
MEF medium (DMEM supplemented with FBS (Gibco, cat. no. 10082147), L-glutamine, β-mercaptoethanol, MEM non-essential amino acids and sodium pyruvate. Optionally, add penicillin-streptomycin; see Reagent Setup)
M2 medium (Millipore, cat. no. MR-015-D)
ES cell medium (DMEM supplemented with FBS (ES cell qualified; Gibco, cat. no. 16141079), L-glutamine (Gibco, cat. no. 25030-081), β-mercaptoethanol (Sigma-Aldrich, cat. no. M6250), MEM non-essential amino acids (Gibco, cat. no. 11140035), sodium pyruvate (Gibco, cat. no. 11360-070), leukemia inhibitory factor (LIF; Millipore, cat. no. ESG1106); optional: penicillin- streptomycin (Invitrogen, cat. no. 15140122); see Reagent Setup)
Ethanol, 70% (vol/vol; Sigma-Aldrich, cat. no. E7023) ! CAUTION Ethanol is flammable and may be harmful if inhaled or ingested.
PBS, 1×, sterile (Ca2+ and Mg2+ free; Gibco, cat. no. 14190-094)
Trypsin-EDTA, 0.25% (wt/vol; Gibco, cat. no. 25200-056)
Derivation from embryos using modified TS cell conditions
Pregnant mouse, 3.5 d.p.c.
Mitotically inactivated MEF cells (Reagent Setup)
TS cell medium (includes advanced RPMI 1640 (Gibco, 12633-012), FBS (Gibco, cat. no. 10082147), L-glutamine, β-mercaptoethanol, sodium pyruvate; optional: 1% (vol/vol) penicillin-streptomycin. Aliquots are supplemented with recombinant FGF2 or FGF4 (Sigma-Aldrich, cat. no. F8424) and heparin (Sigma-Aldrich, cat. no. H3393); see Reagent Setup)
MEF-conditioned medium (Reagent Setup)
M2 medium (Millipore, cat. no. MR-015-D)
Ethanol, 70% (vol/vol; Sigma-Aldrich, cat. no. E7023) ! CAUTION Ethanol is flammable and may be harmful if inhaled or ingested.
PBS, 1×, sterile (Ca2+ and Mg2+ free; Gibco, cat. no. 14190-094)
Trypsin-EDTA, 0.25% (wt/vol; Gibco, cat. no. 25200-056)
Derivation from mouse ES cells by Gata6 overexpression
Mitotically inactivated MEF cells (Reagent Setup)
MEF medium (Reagent Setup)
ES cells (Reagent Setup)
ES cell medium (Reagent Setup)
Ethanol, 70% (vol/vol; Sigma-Aldrich, cat. no. E7023) ! CAUTION Ethanol is flammable and may be harmful if inhaled or ingested.
Gelatin, 0.1% (wt/vol; Sigma-Aldrich, cat. no. G1890) made up in sterile dH2O (Sigma-Aldrich, cat. no. W1503) and autoclaved to dissolve
PBS, 1×, sterile (Ca2+ and Mg2+ free; Gibco, cat. no. 14190-094)
Trypsin-EDTA, 0.05% (wt/vol; Gibco, cat. no. 25300054)
Xfect (Clontech, cat. no. 631320) or Lipofectamine 2000 (Invitrogen, cat. no. 11668030)
Opti-MEM I (Invitrogen, cat. no. 51985)
Bio-Rad Gene Pulser II Xcell eukaryotic system (Bio-Rad, cat. no. 377267)
Cuvette, 4 mm (Cell Projects, cat. no. EP-104)
Agarose (Sigma-Aldrich, cat. no. A9414)
Maxi-prep kit (Qiagen, cat. no. 12262)
Gata6 cDNA vector (Origene, cat. no. MC219384)
Isopropanol (Sigma-Aldrich, cat. no. I9516)
Sodium acetate (Fluka, cat. no. 71196)
Wizard SV genomic DNA purification system (Promega, cat. no. A2360)
Derivation from mouse ES cells using growth factors
cXEN derivation medium (standard XEN medium supplemented with all-trans retinoic acid (Sigma-Aldrich, cat. no. R2625) dissolved in DMSO plus activin A (R & D Systems, cat. no. 338-AC-010); Reagent Setup)
Mitotically inactivated MEF cells (Reagent Setup)
MEF medium (Reagent Setup)
ES cells (Reagent Setup)
ES cell medium (Reagent Setup)
Ethanol, 70% (vol/vol; Sigma-Aldrich, cat. no. E7023) ! CAUTION Ethanol is flammable and may be harmful if inhaled or ingested.
Gelatin, 0.1% (wt/vol; Sigma-Aldrich, cat. no. G1890) made up in sterile dH2O (Sigma-Aldrich, cat. no. W1503) and autoclaved to dissolve
PBS, 1×, sterile (Ca2+ and Mg2+ free; Gibco, cat. no. 14190-094)
Trypsin-EDTA, 0.05% (wt/vol; Gibco, cat. no. 25300054)
EQUIPMENT
Conical tubes (15 ml; BD Falcon, cat. no. 352095)
Conical tubes (50 ml; BD Falcon, cat. no. 352070)
Tissue culture–treated plate (six well; Corning, cat. no. 3516)
Tissue culture–treated plate (12 well; Corning, cat. no. 3512)
Tissue culture–treated plate (24 well; Corning, cat. no. 3524)
Tissue culture–treated dish (100 mm; Corning, cat. no. 430167)
Tissue culture–treated dish (150 mm; Corning, cat. no. 430599)
Tissue culture–treated plate (96-well flat bottom; Corning, cat. no. 3595)
Tissue culture–treated plate (96-well round bottom; Corning, cat. no. 3799)
Plate (four well; Nunc, cat. no. 12566300)
Cryotube vials (Nunc, cat. no. 144444)
Filter, 0.22 µm (Corning, cat. no. 431097)
Flame-pulled glass Pasteur pipettes (unpulled; e.g., Fisher Scientific, 13-678-20D)
Dissecting microscope (e.g., Leica M80)
Inverted microscope (e.g., Olympus CKX41)
Water bath (e.g., Grant JB Aqua 12)
Centrifuge (e.g., Eppendorf Centrifuge 5804)
Hemocytometer (e.g., Hausser scientific, cat. no. 3110)
CO2 incubator (e.g., Sanyo)
Dissection tools
Scissors (e.g., Roboz, cat. no. RS-5910)
Forceps (e.g., Dumont no. 5, Roboz; cat. no. RS-4976)
Syringes with 27-gauge needles (10 ml; BD Biosciences, cat. no. 309623)
Mouth-controlled pipette (Reagent Setup)
Plastic mouthpiece (MedTech, cat. no. 1501P-B4036-2)
Rubber tubing (Fisher Scientific, cat. no. 22-362-772)
Filtered pipette tip, P1000 (USA Scientific, cat. no. 1126-7810)
REAGENT SETUP
Mitotically inactivated MEF cells
Mitotically inactivated MEFs should be plated on pregelatinized cell culture–treated plates 1 d before initiating XEN derivation MEFs can be derived from CF1 mice that are dissected 12.5–13.5 d.p.c.; cells are passaged 3–5 times before inactivation, either by γirradiation or by mitomycin C treatment.
ES cells
ES cells should be thawed ~1–2 weeks before initiating XEN differentiation and passaged at least twice. ES cells are typically maintained on MEFs.
ES cell medium
Prepare a solution of the following: DMEM supplemented with 15% (vol/vol) FBS (ES cell qualified), 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, 0.1 mM MEM non-essential amino acids, 1 mM sodium pyruvate and 103 IU leukemia inhibitory factor. Optionally, add 1% (vol/vol) penicillin-streptomycin. Medium should be prepared in a sterile tissue culture hood and filter sterilized. The medium can be stored for up to 1 month at 4 °C.
MEF medium
Prepare a solution of the following: DMEM supplemented with 15% (vol/vol) FBS, 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, 0.1 mM MEM non-essential amino acids and 1 mM sodium pyruvate. Optionally, add 1% (vol/vol) penicillin-streptomycin. Medium should be prepared in a sterile tissue culture hood and filter sterilized. The medium can be stored for up to 1 month at 4 °C.
TS cell medium
Prepare a solution of the following: advanced RPMI 1640 supplemented with 20% (vol/vol) FBS, 2 mM L-glutamine , 0.1 mM β-mercaptoethanol, 1 mM sodium pyruvate; optional: 1% (vol/vol) penicillinstreptomycin. Medium should be prepared in a sterile tissue culture hood and filter sterilized. The medium can be stored for up to 1 month at 4 °C. Prepare a freshly made aliquot supplemented with 24 ng ml− 1 recombinant FGF2 or FGF4 and 1 µg ml− 1 heparin.
MEF-conditioned medium
Preparation of MEF-conditioned medium should begin at least 10 d before initiating the XEN derivation protocol B. Thaw and culture three 150-mm tissue culture plates of mitotically inactivated MEFs in 25 ml of standard TS cell medium (not supplemented with FGF2/FGF4 and heparin) for 3 d. Collect the medium in 50-ml conical tubes and store the medium at − 20 °C. Add another 25 ml of TS cell medium to each plate and repeat the procedure twice until 225 ml of MEF-conditioned medium have been collected. Thaw the frozen batches and centrifuge the medium at 2,300g for 20 min at room temperature (20–25 °C) to remove debris. Filter-sterilize the medium and store it at 4 °C.
Standard XEN medium
Prepare a solution of advanced RPMI 1640 supplemented with 15% (vol/vol) FBS and 0.1 mM β-mercaptoethanol. Optionally, add 1% (vol/vol) penicillin-streptomycin. Medium should be prepared in a sterile tissue culture hood and filter sterilized. The medium can be stored for up to 1 month at 4 °C. Advanced RPMI 1640 contains glutamine; alternatively, RPMI medium can be used and requires the addition of 2 mM L-glutamine.
cXEN derivation medium
Freshly prepare a solution of standard XEN medium supplemented with 0.01 µM all-trans retinoic acid dissolved in DMSO plus 10 ng ml− 1 activin A. A wide range of retinoic acid concentrations can be used, ranging from 0.01 to 10 µM. The derivation medium can also be supplemented with 24 ng ml− 1 recombinant FGF2 or FGF4 and 1 µg ml− 1 heparin, although this is not required if endogenous Fgf4 is intact. ! CAUTION Retinoic acid is light sensitive and may be harmful if ingested or absorbed.
Freezing medium
Freshly prepare a solution of 10% (vol/vol) DMSO (Sigma-Aldrich, cat. no. D2650) and 90% (vol/vol) FBS. Keep the solution on ice or at 4 °C until immediately before use.
Gata6 cDNA for transfection
Prepared as described in Box 1.
Box 1 | Preparation of Gata6 cDNA for transfection or electroporation ● TIMING ~2 d.
In order to ensure sufficient quantity of plasmid DNA, a large-scale preparation of Gata6 cDNA plasmid by maxiprep is required for transfection. A circular Gata6 cDNA plasmid is generally used for chemical-based transfection, whereas for electroporation, the plasmid should be linearized using a unique restriction enzyme at a non-essential region, which will not affect the function of the gene and drug selection.
If linearized DNA is required then cut one time with a unique restriction enzyme that is in a region of the plasmid that will not affect the function. Linearize 100 µg of Gata6 cDNA plasmid by restriction enzyme digestion overnight at the optimal incubation temperature. Run a small aliquot on a 0.8% (wt/vol) agarose gel to ensure linearization.
For high-purity plasmid preparation, precipitate the linearized Gata6 cDNA with a 1/10-volume of salt (3 M sodium acetate) and 2.5 volumes of 100% ethanol; store at − 20 °C overnight.
The next day, spin the DNA at 16.1 × 103g for 5 min at 4 °C and wash the pellet once with 500 µl of 70% (vol/vol) ethanol.
-
Remove the supernatant and allow any remaining ethanol to evaporate in the hood for several min.
▲ CRITICAL STEP To ensure sterility, work in a sterile tissue culture hood for this and subsequent steps.
-
Resuspend the DNA pellet in 100 µl of sterile PBS without Mg2+ or Ca2+ (a final DNA concentration of 1 µg µl−1).
▲ CRITICAL STEP The presence of salts could be detrimental to transfection efficiency and interfere with electroporation.
■ PAUSE POINT Plasmid DNA preparation can be done any time before transfection. The resuspended DNA can be kept at − 20 °C for several months.
EQUIPMENT SETUP
Mouth-controlled pipette
Assemble a mouth-controlled pipette. This is used for handling embryos and for dissociating blastocyst in vitro–matured outgrowths. Mouth-controlled pipettes can be assembled in a variety of designs (for variations see Nagy et al.28). A simple mouth pipettor can be made by inserting the pointed end of a P1000 filter tip into one end of a cut rubber tube and attaching a plastic mouthpiece to the other. A pulled glass Pasteur pipette can now be attached to the filter tip. Pasteur pipettes can be pulled either over a flame or by using a micropipette puller (Fig. 3).
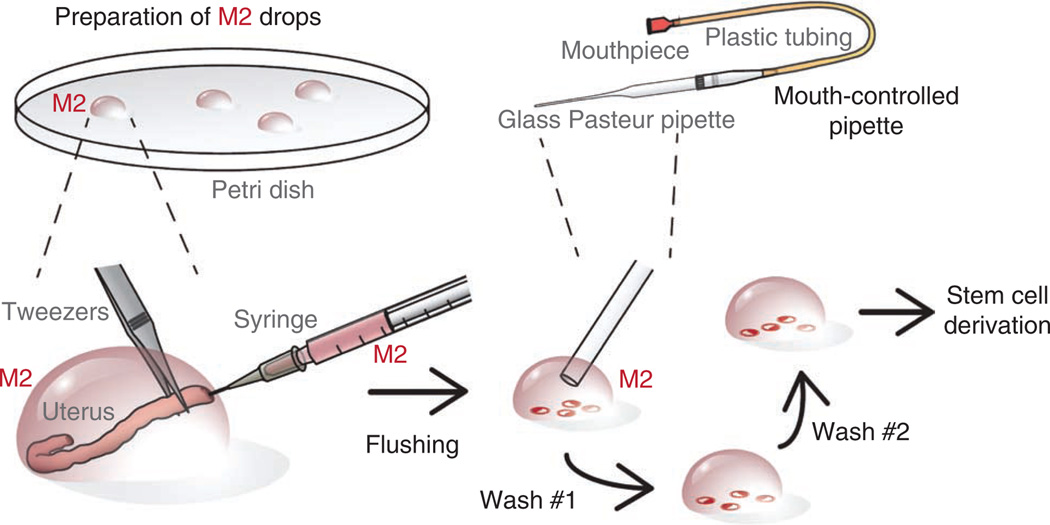
Figure 3.
Recovery of blastocyst-stage embryos from uteri of adult female mice. Several drops of M2 medium are prepared on the lid of a 100-mm dish. The dissected and cleaned uterine horn of a pregnant female (3.5 d.p.c.) is placed in one drop. While securing the uterine horn with forceps, the needle of a 1-ml syringe is inserted and the uterine horn is flushed with ~0.2 ml of M2 medium. The flushed blastocysts are located under high magnification and transferred to a fresh drop of M2 medium using a mouth-controlled pipette. This transfer is repeated at least twice to wash away debris, lipid drops and blood cells.
PROCEDURE
Preparation of MEF feeders ● TIMING 15–30 min
-
1|
Pregelatinize tissue culture plates for >10 min with 1–2 ml of 0.1% (wt/vol) gelatin. Aspirate the gelatin before seeding the cells.
? TROUBLESHOOTING
-
2|
One day before starting the derivation, place a frozen vial of mitotically inactivated MEFs in a 37 °C water bath until nearly thawed and transfer the contents into a 15-ml Falcon tube containing 5 ml of MEF medium. Centrifuge the medium at 200g for 4 min at room temperature and aspirate the supernatant.
? TROUBLESHOOTING
-
3|
Resuspend the cell pellet in MEF medium and plate it at a density of 6 × 104 MEFs per well for a four-well plate, 1.3 × 105 MEFs per well for a six-well plate or 7.8 × 105 cells per 100-mm tissue culture plate, and then incubate the plates overnight at 37 °C. The number of plates to prepare depends on the number of XEN cell derivations to be performed. Generally, four plates (4-well plates) per pregnant mouse for Steps 6A and 6B are sufficient, or a six-well plate for Steps 6C–F.
? TROUBLESHOOTING
-
4|
The morning before starting the derivation, aspirate the medium on the MEF feeders and replace it with ES cell medium for derivation using ES cell conditions (Step 6A), with TS cell medium for derivation using TS cell conditions (Step 6B) or with XEN cell medium for derivation from ES cells (Steps 6C–F). When required, plate MEFs that carry drug-resistance genes, such as DR4 MEFs (resistant to neomycin, hygromycin, puromycin and Zeocin) (Table 1).
? TROUBLESHOOTING
Table 1.
Drug selection.
| Marker | Gene product | Selection method |
|---|---|---|
| Neo | Aminoglycoside phosphotransferase; neo gene from the bacterial transposon Tn5 | Select cells in G418 (0.1–1.0 mg ml−1), an aminoglycoside that blocks protein synthesis and is similar to kanamycin |
| Hyg | Hygromycin-B-transferase; hyg gene from Escherichia coli | Select cells in hygromycin-B (10–300 mg ml−1), an aminocyclitol that inhibits protein synthesis |
| Pac/Puro | Puromycin-N-acetyl transferase; pac gene from Streptomyces alboniger | Select cells in puromycin (0.5–5 mg ml−1), an antibiotic that inhibits protein synthesis |
| Zeo | Bleomycin-binding protein; a zeo gene (a.k.a. blaR) is located on the bacterial transposon Tn5 | Select cells in bleomycin or commercially available Zeo (50–500 mg ml−1), an antibiotic that binds DNA and blocks RNA synthesis |
Cell preparation
-
5|
If you are deriving cells using ES or TS cell conditions, follow option A to collect E3.5 blastocysts. If you are deriving XEN cells from ES cells, follow option B.
(A) Collecting E3.5 blastocysts (flushing)
Dissect out the uterine horns of a pregnant mouse at 3.5 d.p.c. and place them on the lid of a 100-mm culture dish in a drop of M2 medium.
Under a dissection microscope (approximately ×10 total magnification), remove large fat pads and cut away the ovaries and oviducts. Place each of the cleaned uterine horns in a fresh drop of M2 medium.
With a 1-ml syringe, take up 0.5 ml of M2 medium and attach a needle (27 gauge). While securing the uterine horn with forceps, insert the needle and flush the uterine horn with ~0.2 ml of M2 medium (Fig. 3) and remove the uterine horn from the drop. Repeat this process with the second uterine horn.
-
Under higher magnification (~×30–×40), find the flushed blastocysts in both drops.
? TROUBLESHOOTING
By using a mouth-controlled pipette, transfer the blastocysts to a new drop of M2 medium. Repeat the step twice to wash away debris, lipid drops and blood cells.
Proceed with the derivation of XEN cells using either option A (ES cell conditions) or option B (TS cell conditions) in Step 6.
(B) ES cell maintenance in medium with serum
Thaw the ES cells rapidly in a 37 °C water bath and transfer them into a 15-ml Falcon tube containing 5 ml of MEF medium.
Centrifuge the tube at 200g for 4 min at room temperature, aspirate the supernatant and resuspend the ES cell pellet using 1 ml of ES cell medium.
-
Aspirate the medium on the irradiated MEFs and replace it with ES cell medium. Plate ~3 × 105 ES cells onto MEFs in one well of a six-well plate or 2 × 106 ES cells on a 100-mm tissue culture dish. Incubate the cells at 37 °C.
▲ CRITICAL STEP All 37 °C incubations should be carried out in a 5% CO2 humidified incubator.
Change the ES cell medium daily to maintain the cell line and monitor the cells. Proceed to the next step (passage) when they reach ~80% confluency; this typically occurs every 2–3 d.
To passage the ES cells, wash the well that contains the cells with 1 ml of 1× PBS to remove residual proteins. Aspirate the PBS and add 0.25 ml of 0.05% (wt/vol) trypsin for a six-well plate. Rock the plate back and forth to dissociate the cells. Incubate the plate at room temperature for no more than 5 min until all the cells are nearly detached. Do not leave the cells in trypsin for longer than necessary.
Neutralize the trypsin with 1 ml of ES cell medium. Pipette the solution up and down with a P1000 pipette approximately ten times to dissociate the cells into small clumps and single cells.
-
With a P1000 pipette, add 50 µl of the dissociated ES cells onto a freshly prepared MEF well in 2 ml of fresh ES cell medium to continue growing the cell line at a ~1/25 dilution (roughly, a 1/10 dilution should be ready for passaging in 2 d, a 1/25 dilution in 3 d and a 1/40 dilution in 4 d). The trace amount of trypsin on the first day will not adversely affect the cells.
▲ CRITICAL STEP The use of a pipette smaller than P1000 can damage the cells and should be avoided, except to pick colonies or count cells on a hemocytometer. In order to ensure healthy recovery after thawing, ES cells should be passaged at least once before proceeding with the steps below.
If you are electroporating the cells for XEN derivation, passage the cells the day before. If the cells are grown on MEF, MEF-deplete the cells at the same time (see Step 5B(ix)).
MEF-deplete the ES cells. This step is unnecessary if the ES cells are grown in MEF-free conditions. Plate the cells onto a pregelatinized plate in ES cell medium the day before proceeding to XEN derivation. Alternatively, ES cells may be MEF-depleted on the day of derivation by plating them on a pregelatinized 100-mm plate containing 7–10 ml of ES medium (no LIF) for 30 min. After 30 min, the majority of MEFs should have adhered to the pregelatinized plate. Tilt the 100-mm plate to collect the supernatant medium plus ES cells and transfer it into a 15-ml Falcon tube. Do not wash the plate with fresh medium as this may dislodge the attached MEFs.
XEN derivation ● TIMING 15–20 d
-
6|
If you are deriving XEN cells under ES cell conditions, follow option A. If you are deriving XEN cells under TS cell conditions, follow option B. If you are chemically transfecting ES cells with a Gata6 cDNA plasmid using cationic polymer, follow option C. If you are chemically transfecting ES cells with a Gata6 cDNA plasmid using lipopolyamine, follow option D. If you are electroporating ES cells with a Gata6 cDNA plasmid, follow option E. If you are deriving XEN cells from ES cells using growth factors, follow option F.
? TROUBLESHOOTING
(A) XEN cell derivation under ES cell conditions
-
Day 1. Thoroughly clean a dissection microscope with 70% (vol/vol) ethanol. By using a mouth-controlled pipette, place one blastocyst per feeder-covered well in the prepared four-well plates (Fig. 4).
▲ CRITICAL STEP The plates should be incubated in a 5% CO2 humidified incubator at 37 °C, except while performing Step 6A(i–iv), which can be performed under ambient conditions.
? TROUBLESHOOTING
Day 2. Observe the plates under a microscope. The blastocysts should have hatched from the zona pellucida and attached to the feeder layer (Fig. 4).
-
Day 3. Observe the plates under a microscope. The blastocysts should have started to form an outgrowth (Fig. 4). Carefully aspirate the medium and replace it with fresh ES cell medium.
? TROUBLESHOOTING
Day 4. Thoroughly clean a dissection microscope with 70% (vol/vol) ethanol. Dilute 0.25% (wt/vol) trypsin to a sufficient volume of 0.01% (wt/vol) trypsin/1× PBS. Wash the cells with 500 µl of 1× PBS. Aspirate the PBS and add 100 µl of 0.1% (wt/vol) trypsin to each well. Incubate the plates at 37 °C for 5 min. While observing the cells under a cleaned dissection microscope, use a P20 pipette to disaggregate the outgrowth by pipetting up and down several times. Add 400 µl of fresh ES cell medium.
Day 5. Carefully replace the medium with fresh ES cell medium.
Days 6–14. Replace the medium every other day until XEN cell colonies can be observed (Fig. 4).
Day 15–indefinite. When 70% confluency is achieved, passage the cells onto a feeder-covered well of a six-well plate. From this point onward, the cells can be cultured in standard XEN medium. Repeat the step, but passage the cells onto a feeder-covered 100-mm dish. Passage the cells two more times. If desired, remove feeders and freeze the XEN cell line as described in Step 7.
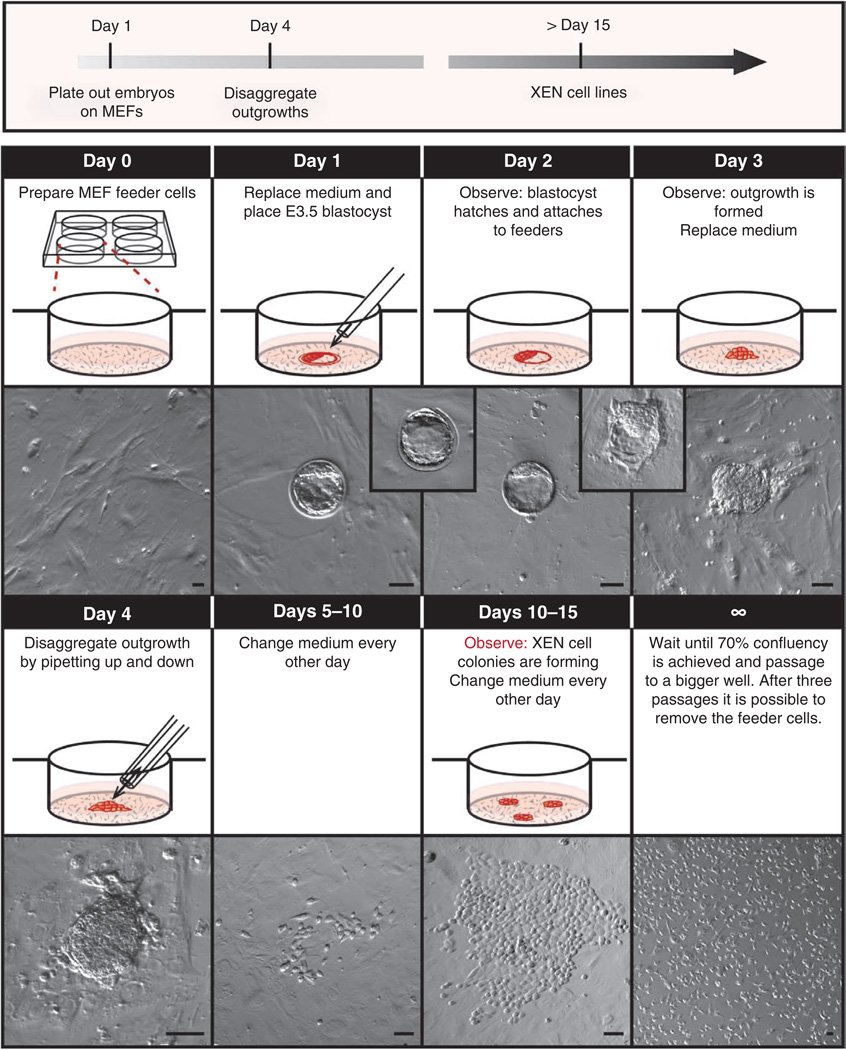
Figure 4.
Timeline for XEN cell derivation from mouse blastocysts. Protocol for the derivation of XEN cells from blastocysts. Day 0: feeder cell–coated four-well plates are prepared. Day 1: medium is replaced and a freshly flushed E3.5 blastocyst is placed in the center of the well. Day 2: the blastocyst hatches and attaches to the feeder cells. Day 3: the blastocyst has formed an outgrowth. Medium is replaced. Day 4: the prominent outgrowth is trypsinized and disaggregated with a P20 pipette. Days 5–10: the medium is replaced every other day and the plate is observed daily. Days 10–15: XEN-like cells with stellate and refractile morphology will emerge. The medium is replaced every other day and the plate is observed daily. Day 15 and onward: After 70% confluency is attained, the XEN cells are passaged onto a well in a six-well plate and subsequently into a 100-mm dish. After three more passages they can be MEF-depleted and the XEN cell line can be frozen. Scale bars, 100 µm.
(B) XEN cell derivation under TS cell conditions
-
Day 1. Thoroughly clean a dissection microscope with 70% (vol/vol) ethanol. By using a mouth-controlled pipette, place one blastocyst per feeder-covered well in the prepared four-well plates (Fig. 4).
▲ CRITICAL STEP The plates should be incubated in a 5% CO2 humidified incubator at 37 °C, except while performing (Step 6B(i–iv)), which can be performed under ambient conditions.
? TROUBLESHOOTING
Day 2. Observe the plates under a microscope. The blastocysts should have hatched from the zona pellucida and attached to the MEF feeder layer (Fig. 4).
-
Day 3. Observe the plates under a microscope. The blastocysts should have started to form an outgrowth (Fig. 4). Carefully aspirate the medium and replace it with fresh TS cell medium.
? TROUBLESHOOTING
Day 4. Thoroughly clean a dissection microscope by wiping it with 70% (vol/vol) ethanol. Dilute 0.05% (wt/vol) trypsin to a sufficient volume of 0.01% (wt/vol) trypsin/1× PBS. Wash the cells with 500 µl of 1× PBS. Aspirate the PBS and add 100 µl of 0.01% (wt/vol) trypsin to each well. Incubate the plates at 37 °C for 5 min. While observing the cells under a dissection microscope, use a P20 pipette to disaggregate the outgrowth by pipetting up and down several times. Add 400 µl of 30% TS cell medium/70% MEF-conditioned medium.
Day 5. Carefully replace the medium with fresh 30% TS cell medium/70% MEF-conditioned medium.
Days 6–14. Replace the medium every other day until XEN cell colonies are observed (Fig. 4).
Day 15–indefinite. When cells reach 70% confluency, passage the cells onto a feeder-covered well of a six-well plate. From this point onward, the cells can be cultured in standard XEN medium. Repeat the step, but passage the cells onto a feeder-covered 100-mm dish. Passage the cells two more times. If desired, remove the feeders and freeze the XEN cell line as described in Step 7.
(C) Chemical transfection of ES cells with a Gata6 cDNA plasmid using a cationic polymer (e.g., Xfect) for transient or stable transfection
Day 1. Re-plate MEF-depleted cells onto pregelatinized plates. Cells plated at a density of 7 × 104 per well of a 24-well plate will be ready for transfection ~12 h after plating.
Day 2. On the day of transfection, ensure that the ES cells are ~80% confluent. All subsequent volumes are for one well of a 24-well plate and may be scaled up or down according to the manufacturer’s recommendations (Clontech).
Prepare two tubes: the first tube contains a 25-µl solution of 1 µg of Gata6 cDNA plasmid diluted with Xfect reaction buffer in a sterile 1.5-µl Eppendorf tube; the second tube contains 0.5 µl of Xfect polymer (prevortexed) plus 24.5 µl of Xfect reaction buffer in a separate 1.5-µl Eppendorf tube. Use an empty plasmid (i.e., typically the same plasmid containing a constitutively active fluorescent reporter instead of Gata6) or sterile dH2O as negative controls.
Vortex each tube, and then add the polymer solution to the DNA solution (total volume = 50 µl) and vortex again. Incubate the DNA-polymer solution for 10 min at room temperature.
To one well of the 24-well plate, add the 50 µl of DNA-polymer solution dropwise. Mix the solution gently by tilting the plate back and forth, and then incubate the plate in a 5% CO2 humidified incubator at 37 °C for 3 h.
-
Aspirate the medium containing the DNA-polymer complex from the ES cells, replace it with 0.5 ml of fresh ES cell medium and incubate the plate in a 5% CO2 humidified incubator at 37 °C overnight.
? TROUBLESHOOTING
Day 3. Replace the ES medium in each well with 0.5 ml of XEN medium and incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
Days 4–10. If the Gata6 cDNA plasmid has been engineered to also express a drug-resistance gene, then drug selection can be used to expand successfully transfected cells. Replace with XEN cell medium supplemented with the appropriate drug selection (Table 1). Replace with fresh XEN medium containing drug selection daily for ~10 d. XEN-like cells should appear within 5 d.
Day 11–indefinite. Enzymatically passage the Gata6 cDNA–transfected cells with 0.05% (wt/vol) trypsin onto a pregelatinized 100-mm dish. Continue selection with drugs in order to establish a XEN cell line. Collect a fraction of the cells for genotyping and freeze the remaining XEN cells as described in Step 7.
(D) Chemical transfection of ES cells with a Gata6-cDNA plasmid using lipopolyamine (e.g., Lipofectamine 2000) for transient or stable transfection
Day 1. Re-plate MEF-depleted cells onto pregelatinized plates. Cells plated at a density of 7 × 104 per well of a 24-well plate will be ready for transfection ~12 h after plating.
Day 2. On the day of transfection, ensure that the ES cells are ~80% confluent. All subsequent volumes are for one well of a 24-well plate and may be scaled up or down according to the manufacturer’s recommendations (Invitrogen).
Prepare two tubes: the first tube contains a 50-µl solution containing 1 µg of Gata6 cDNA plasmid diluted with Opti-MEM I in a sterile 1.5-µl centrifuge tube; the second tube contains 4.5 µl of Lipofectamine 2000 plus 45.5 µl of Opti-MEM I in a separate 1.5-µl centrifuge tube. Use an empty plasmid (i.e., typically the same plasmid containing a constitutively active fluorescent reporter instead of Gata6) or sterile dH2O as negative controls. Incubate the tubes for 5 min at room temperature.
Gently mix the diluted plasmids (Gata6 or control) with the diluted Lipofectamine reagent (total volume = 100 µl) and incubate the tubes for 20 min at room temperature to allow for the formation of DNA-liposome complexes. During the 20-min incubation period, replace the medium of the plated cells with 0.5 ml of fresh ES cell medium without antibiotics.
-
To one well of the 24-well cell culture dish, add 100 µl of DNA-liposome complex dropwise. Mix gently by tilting the plate back and forth, and then incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
? TROUBLESHOOTING
Day 3. Replace the ES medium in each well with 0.5 ml of XEN medium and incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
Days 4–10. If the Gata6-cDNA plasmid has been engineered to also express a drug-resistance gene, then drug selection can be used to expand successfully transfected cells. Replace the medium with XEN cell medium supplemented with the appropriate drug selection (Table 1). Replace with fresh XEN medium containing drug selection daily for ~10 d. XEN-like cells should appear within 5 d.
Day 11–indefinite. Enzymatically passage the Gata6 cDNA–transfected cells with 0.05% (wt/vol) trypsin onto a pregelatinized 100-mm dish. Continue selection with drugs to establish a XEN cell line. Collect a fraction of the cells for genotyping and freeze the remaining XEN cells as described in Step 7.
(E) Electroporation of ES cells with a Gata6 cDNA plasmid for transient or stable transfection
Day 1. On the day of electroporation, ensure that the ES cells are ~80% confluent in one 100-mm tissue culture plate. Aspirate the medium from the 100-mm culture plate and wash the cells with 5 ml of PBS.
Aspirate the PBS and trypsinize the cells with 1 ml of 0.05% (wt/vol) trypsin. Quench the trypsinized cells with 3 ml of MEF medium.
Transfer the ES cells to a 15-ml Falcon tube and pellet the cells at 200g for 4 min at room temperature. Aspirate the MEF medium and resuspend the ES cell pellet with 5 ml of PBS.
Pellet the cells at 200g for 4 min at room temperature. Aspirate the PBS and resuspend the ES cells in 0.7 ml of PBS.
Gently mix the linearized Gata6 cDNA sample with the resuspended cells and incubate the mixture for 5 min at room temperature.
Transfer the DNA-cell mixture to a 4-mm electroporation cuvette. Electroporate the mixture at 500 µF/230 V. The time constant should be between 6 and 8 ms.
Allow the ES cells to recover in the cuvette for 5 min at room temperature and then transfer the cells into 10 ml of ES cell medium; plate the entire volume onto a MEF-coated 100-mm plate. Mix gently by tilting the plate back and forth, and then incubate the plate overnight at 37 °C.
Day 2. Exchange the medium with 10 ml of standard ES cell medium and incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
-
Days 3–9. If the Gata6-cDNA plasmid has been engineered to also express a drug-resistance gene, then drug selection can be used to expand successfully transfected cells. Exchange the medium with 10 ml of standard XEN cell medium supplemented with the appropriate drug selection (Table 1). Replace the medium with fresh XEN medium containing drug selection daily for ~10 d. XEN-like cells should appear within 5 d.
? TROUBLESHOOTING
Day 10–indefinite. Enzymatically passage the Gata6 cDNA–transfected cells with 0.05% (wt/vol) trypsin onto a pregelatinized 100-mm dish. Continue selection with drugs to establish a XEN cell line. Collect a fraction of the cells for genotyping and freeze the remaining XEN cells as described in Step 7.
(F) cXEN cell derivation from ES cells
Day 1. Enzymatically passage MEF-depleted ES cells with 0.05% (wt/vol) trypsin. Inhibit the trypsin with XEN medium.
Centrifuge the ES cells at 200g for 4 min at room temperature and aspirate the supernatant. Be stringent in aspirating (without touching the pellet). This step removes ES medium, which is important as lingering LIF potentially inhibits cXEN derivation.
Resuspend the pellet in ~5 ml of standard XEN medium. Mix the tube well and immediately remove two 20-µl aliquots to count MEF-depleted ES cells on a hemocytometer. Take the average (n = 3) number of cells in one big square (16 little squares), multiply by 104 and multiply by the number of milliliters of medium, which will be equal to the total number of cells.
Remove the gelatin from the six-well cXEN derivation plate and add fresh standard XEN medium. Add the appropriate volume of trypsinized ES cells to start the cXEN derivation procedure for a final density of 1 × 104 cells per cm2 (i.e., 9.6 × 104 cells per well of a six-well plate; Fig. 5). Incubate the plate overnight at 37 °C in standard XEN medium. The cell density is an important determinant of differentiation efficiency, and as different ES cell lines proliferate at different rates, we recommend determining the optimal density for each line.
Day 2. Twenty-four hours after the initial plating, aspirate the standard XEN medium and replace it with 2 ml of cXEN derivation medium per well (Fig. 5). Incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
Day 3. Aspirate the cXEN derivation medium and replace it with fresh cXEN derivation medium. Incubate the plate overnight in a 5% CO2 humidified incubator at 37 °C.
Day 4. Enzymatically passage the cells with 0.05% (wt/vol) trypsin. Quench the trypsin with 0.5 ml of standard XEN medium. Aspirate the MEF medium in a MEF-coated six-well plate and replace it with 2 ml of standard XEN medium per well. Transfer the entire contents of the differentiated cells and distribute the cells evenly into the well of the MEFcoated plate (1:1 dilution; Fig. 5).
Days 5–11. Aspirate the medium from the cXEN cells and replace it with fresh standard XEN medium every day or every other day depending on confluency. XEN-like cells with stellate and refractile morphology will emerge ~5 d after the first passage on MEFs; however, the well will also contain cells of different morphologies. Use standard XEN medium hereafter. During the first (and sometimes second) passage, the cXEN cells recover better when MEFs are present, although they are not a requirement. FGF2/FGF4 and heparin can also be added during the derivation, but they too are not a requirement as long as endogenous Fgf4 is intact.
-
Days 12–19. Manually pick XEN-like cells using a 20-µl pipette under a microscope to facilitate the ES to cXEN cell derivation (Fig. 5). Depending on the density, place the isolated cells in a MEF-coated (low density of XEN-like cells) or pregelatinized plate (high density of XEN-like cells) in XEN medium and continue feeding with standard XEN medium.
? TROUBLESHOOTING
Day 20–indefinite. Once the cells reach 70–90% confluency, passage the cells enzymatically with 0.05% (wt/vol) trypsin and plate them onto a pregelatinized or MEF-coated 100-mm plate or several wells of a six-well plate. The majority of cells should morphologically resemble XEN cells. If there are any contaminating cells that are not XEN-like cells, aspirate, pick away or reciprocally pick the XEN-like cells to enrich for this population in a new plate or well. Passage two more times onto pregelatinized plate or well and freeze the cXEN cell line (Step 7).
Figure 5.
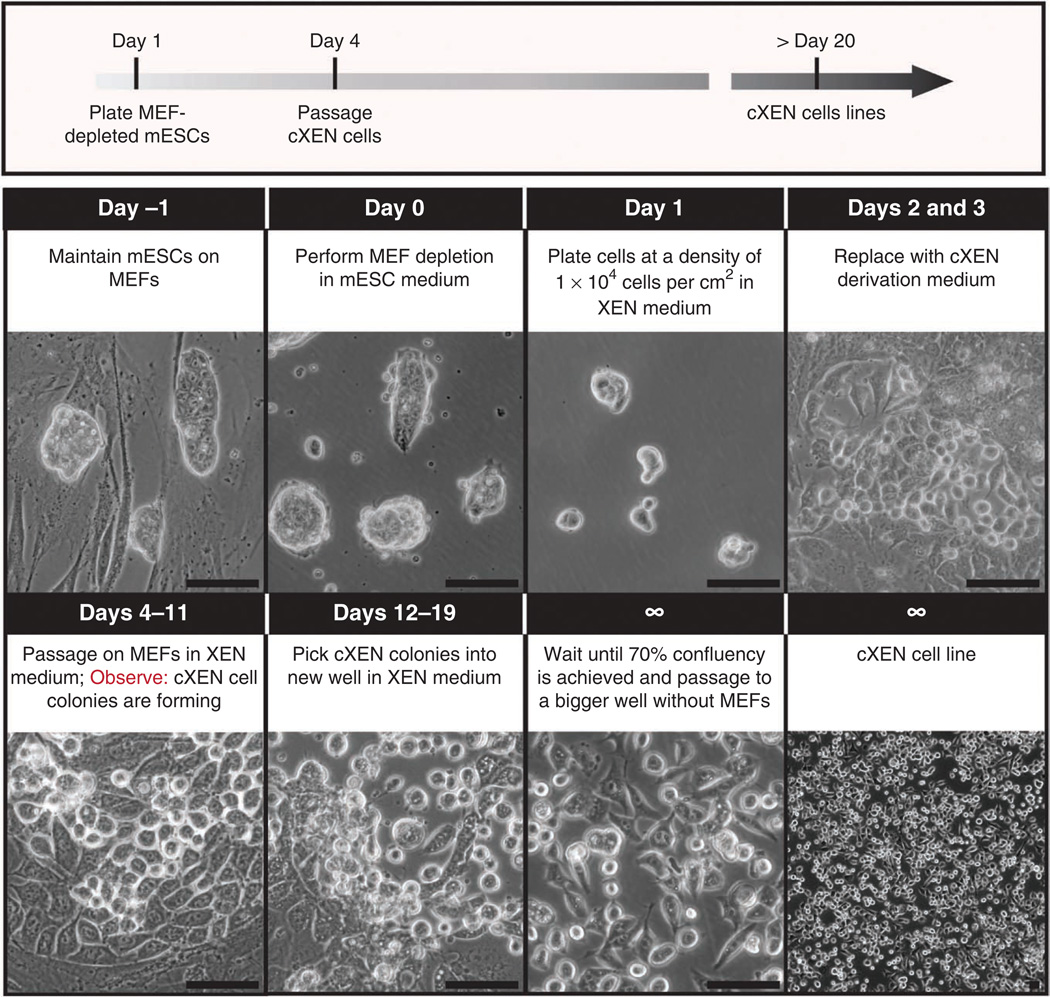
Timeline for cXEN cell derivation from mouse ES cells. Protocol for the conversion of ES cells to cXEN cells using growth factors. Day − 1: ES cells are maintained in ES medium on MEFs. Day 0: ES cells are passaged onto a pregelatinized plate for MEF depletion. Day 1: ES cells are enzymatically passaged with 0.05% (wt/vol) trypsin and plated at a density of 1 × 104 cells per cm2 in standard XEN medium. Days 2 and 3: the medium is replaced with cXEN derivation medium daily (0.01–10 µM retinoic acid plus 10 ng ml− 1 activin A). Days 4–11: cells are enzymatically passaged onto MEFs in XEN medium. The medium is replaced every day or every other day depending on confluency. XEN-like cells with stellate and refractile morphology will emerge within ~5 d. Days 12–19: XEN-like cells are picked manually and placed in a MEF-coated or pregelatinized plate in XEN medium. They are passaged two more times onto pregelatinized plates and the cXEN cell line is frozen. Scale bars, 100 µm.
DNA extraction and freezing cells ● TIMING 1–2 d
-
7|
cXEN or XEN cells can be frozen and thawed using conventional stem cell freeze-thaw protocols in freezing medium. Passage as above and resuspend the trypsinized cell pellet in prechilled (4 °C) freezing medium as described in option A. If you wish to extract DNA from Gata6 cDNA–targeted ES cells, there are a number of different extraction protocols that may be used. Option B describes the Promega SV Wizard purification system.
(A) Freezing cells
Transfer the resuspended cells into prelabeled cryotube vials.
-
Quickly transfer the cryotube vials to − 80 °C freezer overnight covered with Styrofoam.
■ PAUSE POINT For long-term storage, keep cells frozen in a liquid nitrogen dewar.
(B) Lysing cells for genomic DNA extraction
-
Enzymatically passage cells as above and resuspend the cell pellet in the Promega Wizard SV lysis buffer according to the manufacturer’s instructions, and then mix the cell lysate by pipetting.
■ PAUSE POINT The cell lysates can be frozen at − 80 °C until needed.
Transfer each sample lysate from the cell culture plate to a separate Wizard SV minicolumn assembly.
Spin the assembly at 16.1 × 103g for 3 min at room temperature.
Remove the minicolumn from the assembly and discard the liquid in the collection tube. Replace the minicolumn into the collection tube.
Add 650 µl of Wizard SV wash solution (with 95% (vol/vol) ethanol added) to each assembly. Centrifuge the tube at 16.1 × 103g for 1 min at room temperature. Discard the liquid from the collection tube.
Repeat Step 7B(v) for a total of four washes.
Discard the liquid from the collection tube and reassemble the minicolumn assembly. Centrifuge for 2 min at 16.1 × 103g at room temperature to dry the binding matrix.
Transfer the Wizard SV minicolumn to a new 1.5-ml tube. Add 50 µl of room-temperature, nuclease-free water. Incubate the minicolumn for 2 min at room temperature.
Centrifuge the minicolumn at 16.1 × 103g for 1 min at room temperature.
Remove the minicolumn and store the purified DNA at − 20 or − 70 °C for several months or years, respectively.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
Table 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1–6 | Contamination | Poor sterile technique | Ensure that all surfaces are thoroughly cleaned with 70% (vol/vol) ethanol before use and observe proper tissue culture technique |
| 5A(iv) | Finding blastocysts | Lipid drops, blood cells and debris are impeding the view | Adjust magnification and mirror angle to identify blastocysts by their distinctive surrounding zona pellucida |
| 6A(i), 6B(i) | Differentiation | Blastocyst misattachment | Blastocysts should be placed in the center of the well to avoid attachment to the sides of the well |
| 6A(iii), 6B(iii) | Small outgrowth | Insufficient time to develop | If no prominent outgrowth can be observed, wait an additional day before proceeding with the next steps |
| 6 | Low cell density | Low viability and/or contamination | It is imperative to closely observe the cells daily. XEN cell colonies can be observed as early as 10 d but might take up to 15 d to appear. Check for possible contamination |
| 6B | TS cells outcompeting XEN cells | Addition of FGF and heparin; dissociation of the outgrowth | TS cells are more likely to outcompete the XEN cells later on. If this is observed, desist from adding FGF and heparin to the TS cell medium, and dissociate the outgrowth less rigorously. In some cases, it can be beneficial to entirely refrain from dissociating the outgrowth |
| 6C, 6D, 6E | Insufficient expression of exogenous Gata6 | CMV promoter may be silenced in ES cells | We recommend using a plasmid with an EF1A or CAGG promoter to ensure robust expression |
| 6D | Low transfection efficiency | Serum in the medium may interfere with DNA-liposome complex formation | Opti-MEM I can be used during the overnight incubation instead of ES cell medium without penicillin or streptomycin to improve the transfection efficiency; however, this may compromise ES cell viability |
● TIMING
Steps 1–5, preparation of MEF feeders: 15–30 min
Step 6, XEN derivation: 15–20 d
Step 7, DNA extraction and freezing cells: 1–2 d
Box 1, preparation of Gata6 cDNA for transfection or electroporation: ~2 d
ANTICIPATED RESULTS
We have used the protocols described here to establish XEN cell lines from either mouse blastocysts or ES cells. XEN cell lines exhibit characteristic heterogeneous morphology with both highly refractile phase-bright and epithelial-like cells (Figs. 2, 4 and 5). XEN cells can be distinguished from mouse ES cells as the latter form dome-shaped clusters of cells with characteristic high nuclear-to-cytoplasmic ratio4,5, whereas the former grow as individual cells and the nucleus of each cell is not clearly distinguishable (compare Fig. 2). XEN cells are also distinct from TS cells that grow as epithelial colonies comprising cells with distinct nuclei and can differentiate into multinucleated trophoblast giant cells in vitro2 (compare Fig. 2). It was previously demonstrated that XEN cells oscillate between these two morphologies3. All XEN cell lines retain the expression of key XEN-associated genes including the GATA transcription factor Gata4, the SOX factor Sox7 and Disabled homolog 2 Dab2 (Supplementary Fig. 1). Notably, these genes are not homogeneously expressed in all cells within a XEN cell line (Supplementary Fig. 1), and it remains unclear whether heterogeneity in morphology and gene expression reflects a fixed or oscillating heterogeneity representing distinct XEN cell types or substrates present in culture. Notably, mouse XEN cells usually lack the expression of ES cell–associated genes including octamer-binding transcription factor Oct4 (Supplementary Fig. 1) and Nanog. XEN cells self-renew indefinitely in culture in the absence of exogenous growth factors such as FGF2 (refs. 13,16). Moreover, XEN cells can be directed to differentiate into α-fetoprotein (Afp)-expressing visceral endoderm-like cells with the addition of BMP4 (refs. 18,19) and are committed to primitive endoderm-derived lineages in chimeric embryos3,34. Although not reported in the literature, in our own experience the karyotype of XEN cells can change over time and cell lines can acquire karyotypic anomalies with extended passage in culture (N.S. and A.-K.H., unpublished observations). It is therefore preferable to work with cells that are of as low a passage as possible. It should be noted that even clonal XEN cell lines exhibit some degree of variability, for example, in the ratio of cells exhibiting different morphologies and/or in the expression of molecular markers. It is currently not clear whether this inherent variability reflects the cell of origin within an embryo or ES cell culture, cell culture history or an unidentified stochastic or deterministic factor. Furthermore, although there is no ‘gold standard’ XEN cell line that is used across laboratories, as a point of reference, especially when first establishing methods for XEN cell culture within a laboratory, it is advisable to obtain an established and characterized XEN cell line from another laboratory. In contrast to ES cells, no clear strain biases have been reported for the derivation of XEN cells from embryos or conversion from ES cells. This is likely because the protocols for XEN cell derivation, as provided here, are very efficient as compared with most non-inhibitor protocols for ES cell derivation. Notably, even though XEN cells are a relatively new stem cell type, which can be used in a number of applications, in the future, it will be important to compare the differentiation efficiency and molecular identity of XEN cells derived under distinct culture conditions to determine whether there may be biases in potential and/or differences in gene expression. In all, XEN cells are emerging as a useful stem cell model for understanding the convergence of signaling and transcriptional control during XEN cell–fate specification and differentiation.
Supplementary Material
Acknowledgments
We thank A. Foley, M. Kang and S. Wamaitha for comments on this protocol. Work in our laboratories is supported by the Human Frontier Science Program, US National Institutes of Health (NIH) grants RO1-HD052115 and RO1-DK084391 to A.-K.H.; the New York State Department of Health NYSTEM IDEA grant C024318 to A.-K.H.; and the March of Dimes Foundation Research Grant 1-FY11-436 to K.K.N. L.T.Y.C. is supported by a Medical Research Council (MRC) capacity-building studentship. K.K.N. is supported by a Centre for Trophoblast Research Next Generation Fellowship.
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS K.K.N. and A.-K.H. outlined the protocol. K.K.N., N.S. and A.-K.H. wrote the protocol manuscript with the help of L.T.Y.C.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Schrode N, et al. Anatomy of a blastocyst: cell behaviors driving cell fate choice and morphogenesis in the early mouse embryo. Genesis. doi: 10.1002/dvg.22368. Published online; http://dx.doi.org/10.1002/dvg.22368 (25 February 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 3.Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 4.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 5.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artus J, Hadjantonakis AK. Troika of the mouse blastocyst: lineage segregation and stem cells. Curr. Stem Cell Res. Ther. 2012;7:78–91. doi: 10.2174/157488812798483403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwa H, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Fujikura J, et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capo-Chichi CD, et al. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev. Biol. 2005;286:574–586. doi: 10.1016/j.ydbio.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- 11.Artus J, Panthier JJ, Hadjantonakis AK. A role for PDGF signaling in expansion of the extra-embryonic endoderm lineage of the mouse blastocyst. Development. 2010;137:3361–3372. doi: 10.1242/dev.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 13.Cho LT, et al. Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development. 2012;139:2866–2877. doi: 10.1242/dev.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niakan KK, et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010;24:312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim CY, et al. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3:543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kang M, Piliszek A, Artus J, Hadjantonakis AK. FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development. 2013;140:267–279. doi: 10.1242/dev.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruithof-de Julio M, et al. Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling. Development. 2011;138:3885–3895. doi: 10.1242/dev.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paca A, et al. BMP signaling induces visceral endoderm differentiation of XEN cells and parietal endoderm. Dev. Biol. 2012;361:90–102. doi: 10.1016/j.ydbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Artus J, et al. BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity. Dev. Biol. 2012;361:245–262. doi: 10.1016/j.ydbio.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- 21.Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown K, et al. eXtraembryonic ENdoderm (XEN) stem cells produce factors that activate heart formation. PloS ONE. 2010;5:e13446. doi: 10.1371/journal.pone.0013446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Brown K, Legros S, Foley AC. Nodal mutant eXtraembryonic ENdoderm (XEN) stem cells upregulate markers for the anterior visceral endoderm and impact the timing of cardiac differentiation in mouse embryoid bodies. Biol. Open. 2012;1:208–219. doi: 10.1242/bio.2012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtzinger A, Rosenfeld GE, Evans T. Gata4 directs development of cardiac-inducing endoderm from ES cells. Dev. Biol. 2010;337:63–73. doi: 10.1016/j.ydbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senner CE, et al. DNA methylation profiles define stem cell identity and reveal a tight embryonic-extraembryonic lineage boundary. Stem Cells. 2012;30:2732–2745. doi: 10.1002/stem.1249. [DOI] [PubMed] [Google Scholar]
- 26.Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golding MC, Zhang L, Mann MR. Multiple epigenetic modifiers induce aggressive viral extinction in extraembryonic endoderm stem cells. Cell Stem Cell. 2010;6:457–467. doi: 10.1016/j.stem.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: a Laboratory Manual. 3rd edn. Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 29.Lin TP. Microsurgery of inner cell mass of mouse blastocysts. Nature. 1969;222:480–481. doi: 10.1038/222480b0. [DOI] [PubMed] [Google Scholar]
- 30.Himeno E, Tanaka S, Kunath T. Isolation and manipulation of mouse trophoblast stem cells. Curr. Protoc. Stem Cell Biol. 2008;7:1E.4.1–1E.4.27. doi: 10.1002/9780470151808.sc01e04s7. [DOI] [PubMed] [Google Scholar]
- 31.Uy GD, Downs KM, Gardner RL. Inhibition of trophoblast stem cell potential in chorionic ectoderm coincides with occlusion of the ectoplacental cavity in the mouse. Development. 2002;129:3913–3924. doi: 10.1242/dev.129.16.3913. [DOI] [PubMed] [Google Scholar]
- 32.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Shimosato D, Shiki M, Niwa H. Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells. BMC Dev. Biol. 2007;7:80. doi: 10.1186/1471-213X-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rugg-Gunn PJ, et al. Cell-surface proteomics identifies lineage-specific markers of embryo-derived stem cells. Dev. Cell. 2012;22:887–901. doi: 10.1016/j.devcel.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu XB, Pan J, Zhang C, Huang SY. Sox17 facilitates the differentiation of mouse embryonic stem cells into primitive and definitive endoderm in vitro. Dev. Growth Differ. 2008;50:585–593. doi: 10.1111/j.1440-169x.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 37.Shimoda M, et al. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J. Cell Sci. 2007;120:3859–3869. doi: 10.1242/jcs.007856. [DOI] [PubMed] [Google Scholar]
- 38.Aksoy I, et al. Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J. 2013;32:938–953. doi: 10.1038/emboj.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felgner PL, et al. Lipofection: a highly efficient, lipid-mediated DNAtransfection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Smedt SC, Demeester J, Hennink WE. Cationic polymer-based gene delivery systems. Pharm. Res. 2000;17:113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 41.Friend DS, Papahadjopoulos D, Debs RJ. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim. Biophys. Acta. 1996;1278:41–50. doi: 10.1016/0005-2736(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 42.Gao X, Huang L. Cationic liposome-mediated gene transfer. Gene Ther. 1995;2:710–722. [PubMed] [Google Scholar]
- 43.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew. Chem. Int. Ed. Engl. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 45.Doetschman T, et al. Targeted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- 46.Andreason GL, Evans GA. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques. 1988;6:650–660. [PubMed] [Google Scholar]
- 47.Shigekawa K, Dower WJ. Electroporation of eukaryotes and prokaryotes: a general approach to the introduction of macromolecules into cells. Biotechniques. 1988;6:742–751. [PubMed] [Google Scholar]
- 48.Cherng JY, et al. Effect of DNA topology on the transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid complexes. J. Control. Release. 1999;60:343–353. doi: 10.1016/s0168-3659(99)00089-9. [DOI] [PubMed] [Google Scholar]
- 49.McNally MA, Lebkowski JS, Okarma TB, Lerch LB. Optimizing electroporation parameters for a variety of human hematopoietic cell lines. Biotechniques. 1988;6:882–886. [PubMed] [Google Scholar]
- 50.Stuchbury G, Munch G. Optimizing the generation of stable neuronal cell lines via pre-transfection restriction enzyme digestion of plasmid DNA. Cytotechnology. 2010;62:189–194. doi: 10.1007/s10616-010-9273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung S, et al. Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells. 2002;20:139–145. doi: 10.1634/stemcells.20-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 53.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev. Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 54.Iacovino M, et al. Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells. 2011;29:1580–1588. doi: 10.1002/stem.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ting DT, Kyba M, Daley GQ. Inducible transgene expression in mouse stem cells. Methods Mol. Med. 2005;105:23–46. doi: 10.1385/1-59259-826-9:023. [DOI] [PubMed] [Google Scholar]
- 56.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- 58.Macfarlan TS, et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- 60.Yasunaga M, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat. Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 61.Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505. doi: 10.1242/dev.02413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.