Abstract
To determine whether inhaled immunostimulatory DNA sequence oligonucleotides containing CpG motifs mitigate the pathophysiologic manifestation of the asthmatic phenotype (airways hyperresponsiveness and airways remodeling), rhesus monkeys with experimentally induced allergic airways disease were treated seven times with inhaled immunostimulatory oligonucleotides (or sham) periodically for 33 weeks. Airways hyperresponsiveness was reduced twofold in immunostimulatory DNA sequence–treated compared with sham-treated monkeys. Airways from immunostimulatory oligonucleotide-treated monkeys had thinner reticular basement membranes, fewer mucous cells, fewer eosinophils, and fewer mast cells than sham-treated allergic monkeys. We conclude that inhaled immunostimulatory oligonucleotides can attenuate the magnitude of airway hyperreactivity and airways remodeling produced in nonhuman primates with experimentally induced allergic airways disease.
Keywords: airway wall alterations, allergic asthma, immunostimulatory DNA sequence oligonucleotides, nonhuman primate
Immunostimulatory DNA sequences (ISS) contain a CpG dinuncleotide (CpG motif) that is characteristic of bacterial DNA but relatively rare in vertebrate DNA. Vertebrate innate immune systems can rapidly detect infection using pattern recognition receptors (that include the toll-like receptors on dendritic cells, macrophages, monocytes, and neutrophils. Once bound, these receptors activate the immune system to respond specifically to infection (1–4). ISS treatment (by systemic or mucosal delivery) has been shown to inhibit airways remodeling and airways hyperresponsiveness in murine models (5–10). We have previously defined a nonhuman primate model of allergic asthma using aerosolized house dust mite allergen (HDMA) (Dermatophagoides farinae), a known human allergen (11). This model exhibits the hallmarks of allergic asthma defined by the 1997 National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program, including positive skin tests, bronchial hyperresponsiveness, airway inflammation, and airways remodeling. To determine whether inhaled ISS mitigates the asthmatic phenotype (airways hyperresponsiveness and airways remodeling), rhesus monkeys with experimentally induced allergic airways disease were treated seven times with inhaled ISS (or sham) periodically for 33 weeks. Airways hypperresponsivenes was reduced in ISS-treated compared with sham-treated monkeys. Airways from ISS-treated monkeys had thinner basement membranes, fewer mucous cells, fewer eosinophils, and fewer mast cells than sham-treated monkeys. We conclude that inhaled ISS can attenuate the occurrence of airways hyperresponsiveness and airways remodeling in nonhuman primates with allergic disease. Some of the results of these studies have been previously reported in the form of abstracts (12, 13).
METHODS
Animal Protocol
Eight young adult (3–5 years old) rhesus macaques were sensitized and exposed to HDMA (D. farninae) for 11 weeks, as previously described (11). All eight monkeys demonstrated positive skin tests to HDMA. Differences between groups were analyzed by t test (17). After the HDMA sensitization, each monkey was exposed to HDMA aerosol once every 2 weeks for 20 weeks of exposure. Once all eight monkeys were determined to exhibit clinical and immunologic signs of allergic airways disease (confirmed by pulmonary function testing and bronchoalveolar lavage), the monkeys were sedated with ketamine and four of the eight monkeys were given three inhalation exposures (via facemask) of 12.5 mg ISS in 5 ml of sterile phosphate-buffered saline (PBS) over 6 weeks. Each ISS exposure occurred 24 hours before the HDMA exposure. The oligodeoxynucleotide sequence 5′-TGACTGTGAACGTTCGAGATGA-3′ (10, 18) was used for this study; this sequence has been previously shown to be an effective immune stimulus for both mice and humans (10, 14). The remaining four monkeys received only sterile PBS (sham treatment). Biweekly HDMA exposures continued after these three ISS treatments for 12 weeks, during which time clinical signs of allergic airways disease were monitored by pulmonary function testing and bronchoalveolar lavage. An additional four inhalation exposures of 1.25 mg ISS in 5 ml of sterile PBS were delivered as previously described.
Pulmonary Mechanics and Airway Responsiveness Testing
Pulmonary mechanics and airway responsiveness to histamine aerosol were measured as previously described (11).
Bronchoalveolar Lavage and Differential Cell Counts
To evaluate the inflammatory response to allergen, bronchoalveolar lavage samples from sedated monkeys were obtained by bronchoscopy with PBS (10 ml) after sensitization (Week 21), after the first three ISS or sham treatments (Week 27), and at necropsy. Lavage leukocyte counts and differentials were determined as previously described (11).
Evaluation of Airways Remodeling
Two weeks after the last ISS treatment, monkeys were killed, and lungs were inflation-fixed as previously described (11). Reticular basement membrane thickness was measured as previously described (15). Volume per surface area of basement membrane of Alcian blue/periodic acid-Schiff positive mucous cells was calculated for each monkey using standard stereologic methods (see the online supplement for details) made on at least five fields from trachea and used to calculate the mean and standard deviation for each group (16). Determination of significance was on the basis of a t test of p < 0.05 (SigmaStat; SPSS Science, Chicago, IL) (17). Mast cells were identified with mast cell tryptase monoclonal antibody AA1 (Novacastra Laboratories, Newcastle upon Tyne, UK) and nova red peroxidase substrate (Vector Labs, Burlingame, CA). Total mast cells, smooth muscle–associated mast cells, and glandular-associated mast cells were ranked by a blinded observer, with one indicating the fewest mast cells and eight indicating the most mast cells. Determination of significance was on the basis of the Mann Whitney test (p < 0.05, SigmaStat; SPSS Science) (17).
Quantitation of Immune Cells
The volume per surface area of basement membrane of eosinophils within airway epithelium and interstitium was determined by immunofluorescence staining of cryosections using mouse anti-human major basic protein monoclonal antibody (BIODESIGN, Saco, ME) and ALEXA 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR), as previously described (18).
RESULTS
Clinical Signs of Atopy and Allergic Asthma
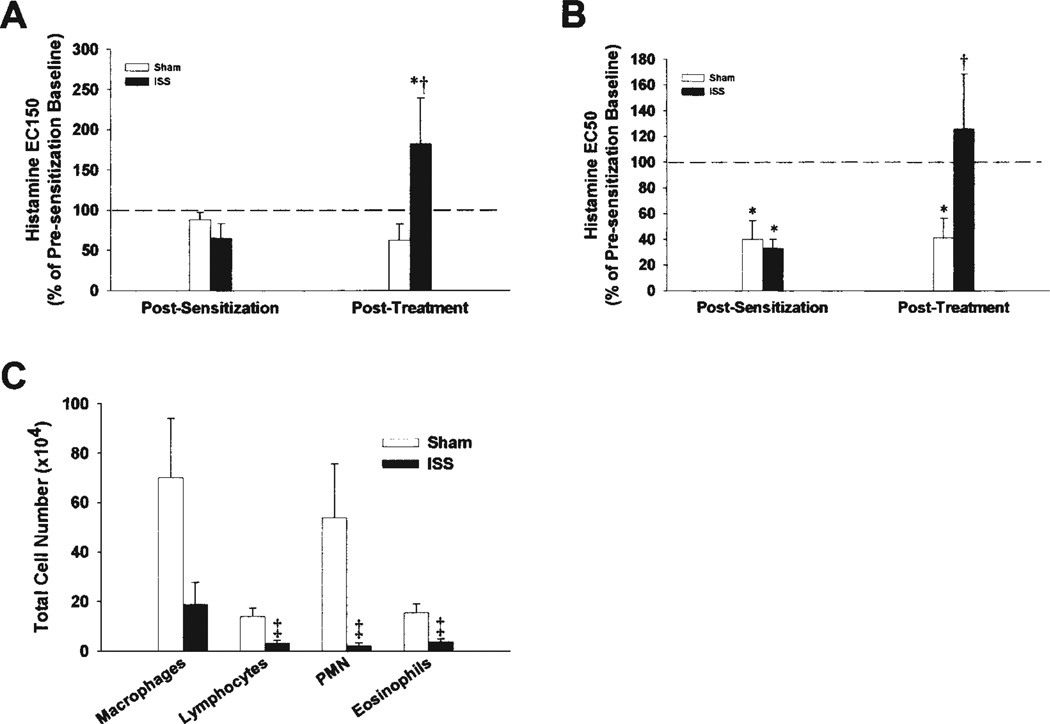
Inhaled ISS exposure altered clinical signs of atopy and allergic asthma in our HDMA-sensitized nonhuman primates. All eight monkeys in this study mounted a positive IgE response to a skin prick test for HDMA after 21 weeks of episodic HDMA challenge and before the start of ISS administration (Table 1). After three ISS treatments over 6 weeks, only one of four ISS-treated animals retained a positive skin-prick test to HDMA, whereas four of four sham-treated animals retained a positive skin-prick test to HDMA, indicating a loss of systemic IgE response in the ISS-treated monkeys. There were no significant changes in the levels of circulating eosinophils or systemic HDMA-specific IgE between sham- and ISS-treated asthmatic monkeys. Pulmonary function measurements were also altered after ISS treatment. Airway responsiveness was measured as the effective concentration of histamine required to produce a 150% increase in airways resistance (EC150) or a 50% decrease in dynamic lung compliance (EC50). Treatment with ISS resulted in a significant decrease in airways hyperresponsiveness to non-specific stimuli as measured by EC150 (Figure 1A) and EC50 (Figure 1B) values as compared with sham-treated monkeys.
TABLE 1.
COMPARISON OF SKIN REACTIVITY TO HOUSE DUST MITE ALLERGEN IN SENSITIZED RHESUS MONKEYS BEFORE AND AFTER IMMUNOSTIMULATORY DNA SEQUENCE OR SHAM TREATMENT
| Before ISS Treatment | After ISS Treatment | |||||
|---|---|---|---|---|---|---|
| Wheal Size (mm) | Wheal Size (mm) | |||||
| Group | Histamine | Diluent | HDMA | Histamine | Diluent | HDMA |
| Sham | 16 | 10 | 20 | 15 | 7 | 12 |
| Sham | 17 | 9 | 16 | 16 | 8 | 14 |
| Sham | 15 | 10 | 15 | 16 | 9 | 16 |
| Sham | 14 | 7 | 14 | 14 | 8 | 11 |
| Mean ± SD | 16 ± 1.3* | 9 ± 1.4 | 16 ± 2.6* | 15 ± 1.0* | 8 ± 0.8 | 13 ± 2.2* |
| ISS | 14 | 10 | 13 | 15 | 11 | 11 |
| ISS | 18 | 8 | 16 | 16 | 8 | 9 |
| ISS | 20 | 9 | 20 | 13 | 5 | 14 |
| ISS | 19 | 12 | 18 | 15 | 10 | 9 |
| Mean ± SD | 18 ± 2.6* | 10 ± 1.7 | 17 ± 3.0* | 15 ± 1.2* | 8 ± 2.6 | 11 ± 2.4† |
Definition of abbreviations: HDMA = house dust mite allergen; ISS = immunostimulatory DNA sequences.
Significantly greater than diluent control, p < 0.01.
Significantly different from histamine positive control, p = 0.024.
Figure 1.
Airway hyperresponsiveness to histamine and decreased lung compliance in house dust mite allergen (HDMA)–sensitized monkeys is reversed by immunostimulatory DNA sequences (ISS) (A, B). Data are expressed as the effective concentration of histamine required to produce a 150% increase in pulmonary flow resistance (EC150) (A), or a 50% decrease in dynamic lung compliance (EC50) (B). Data are expressed as mean ± standard error. *Significantly different than presensitization baseline (p < 0.05); †significantly different than sham-treated monkeys (p < 0.10). Inflammatory cells in lavage fluid in HDMA-sensitized monkeys is decreased by ISS (C). Data are expressed as mean ± SE of individual cell types. ‡Significantly different from sham-treated monkeys (p < 0.025). PMN = polymorphonuclear leukocytes.
To assess the acute inflammation in response to an allergen challenge, we compared airway immune cell infiltrate 24 hours after HDMA aerosol challenge by bronchoalveolar lavage after 21 weeks of episodic allergen challenge (but before the start of the sham/ISS treatment). There was no significant difference in the magnitude or distribution of immune cells from the bronchoalveolar lavage in the eight monkeys, indicating that, although our sample size was small, our population was homogenous. The pre-sham/pre-ISS treatment lavage samples consisted of a majority of macrophages followed in number by eosinophils, lymphocytes, and neutrophils (data not shown). After three ISS/sham treatments (Week 27), total airway leukocyte numbers after HDMA aerosol challenge were significantly reduced in ISS monkeys as compared with sham-treated monkeys (Figure 1C). In sham-treated monkeys, macrophages and neutrophils were the most abundant cell type in the lavage, whereas in ISS-treated animals the majority of cells were macrophages (Figure 1C). ISS treatment significantly decreased the number of lavage eosinophils and lymphocytes.
Airways Remodeling
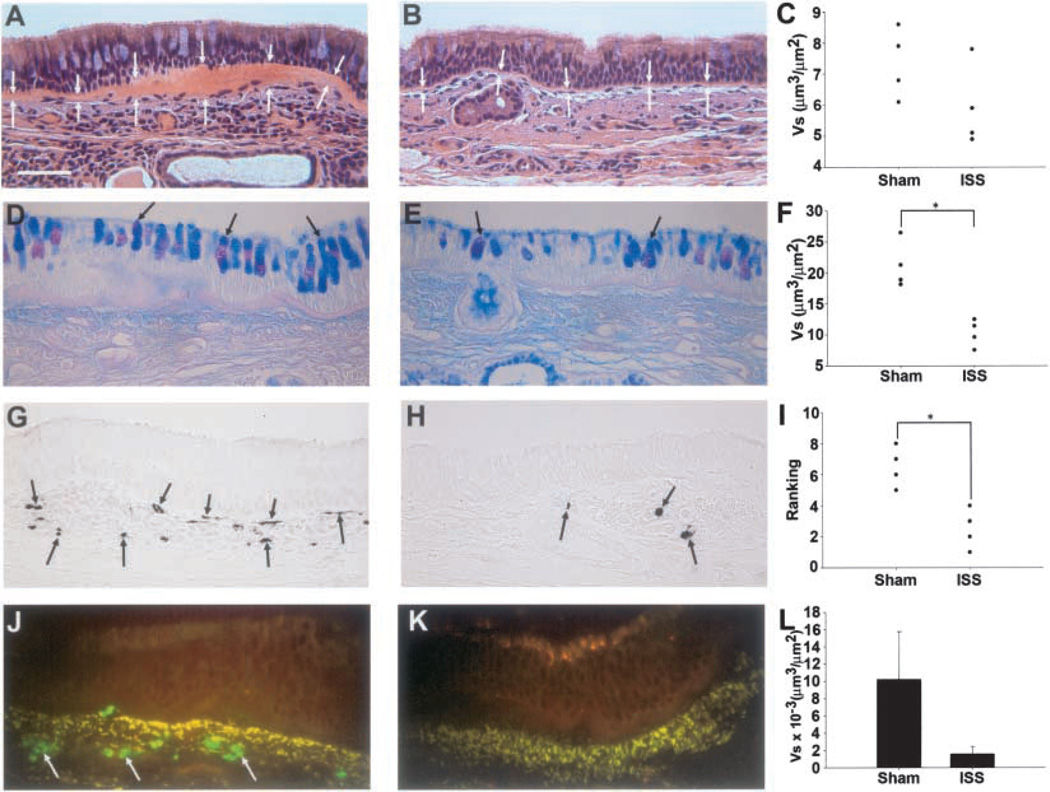
The reticular basement membrane of all monkeys was variable in thickness. However, there was a trend for the reticular basement membrane of the ISS-treated monkeys to be thinner than that of the sham-treated monkeys (Figures 2A–2C). The mass of surface mucous goblet cells was significantly less in ISS-treated monkeys than in sham-treated monkeys (Figures 2D–2F). In addition, ISS treatment significantly decreased the number of interstitial mast cells compared with sham-treated monkeys (Figures 2G–2I). No significant differences were found in the mast cells associated with smooth muscle or glands (data not shown). The mass of tissue eosinophils was less in ISS-treated monkeys compared with sham-treated monkeys, although not statistically significant (Figures 2J–2L).
Figure 2.
Thickened basement membrane zone in HDMA sensitized monkeys is mitigated by ISS. Hematoxylin and eosin-stained sections of proximal airway epithelium from sham-treated (A) and ISS-treated (B) HDMA-exposed monkeys; white arrows indicate the epithelial and interstitial borders of the basement membrane zone. Quantitative assessment of basement membrane thickness (Vs) (C). Mucous cell hyperplasia in HDMA-sensitized monkeys is mitigated by ISS. Alcian blue/periodic acid-Schiff-stained sections of distal airway epithelium from sham-treated (D) and ISS-treated (E) HDMA-exposed monkeys; large arrows indicate goblet mucous cells in terminal bronchioles. Quantitative assessment of surface epithelial mucous cell volume per surface area of basement membrane (F). *Significantly different than sham-treated monkeys (p < 0.05). Mast cell infiltration in HDMA-sensitized monkeys is diminished by ISS. Mast cell tryptase-stained sections of proximal airway epithelium from sham-treated (G) and ISS-treated (H) HDMA-exposed monkeys; arrows indicate interstitial tryptase-positive mast cells. Qualitative assessment of surface epithelial tryptase-positive mast cells (F). *Significantly different than sham-treated monkeys (p < 0.05). Eosinophil infiltration in HDMA-sensitized monkeys is diminished by ISS. Major basic protein labeled sections of proximal airway epithelium from sham-treated (J) and ISS-treated (K) HDMA-exposed monkeys; arrows indicate interstitial major basic protein positive eosinophils. Quantitative assessment of eosinophil volume per surface area of basement membrane × 10−3 (L).
DISCUSSION
Using HDMA-sensitized and challenged rhesus monkeys, we tested the hypothesis that ISS containing CpG motifs can attenuate airway hyperresponsiveness and airways remodeling, and diminish immediate-type hypersensitivity responses to allergen. Conceptually, the airways can be thought of as epithelial–mesenchymal trophic units comprised of epithelium lining the airway lumen attached to a basement membrane that is surrounded by layers of attenuated fibroblasts, extracellular matrix, and smooth muscle. The immune and nervous systems are interspersed throughout these components. The maintenance of normal communication between the epithelium and the mesenchymal components of the trophic unit, via the controlled release and production of signaling molecules, is critical to the preservation of normal structure and organization (19). Alterations of the airway trophic unit in asthma are reported as airways remodeling including (1) mucous cell hyperplasia; (2) reticular basement membrane thickening; (3) peribronchiolar or subbasement membrane fibrosis; and (4) smooth muscle hypertrophy (20–23). The pulmonary mucosa, airway wall structure, and systemic immune system of the adult rhesus monkey are similar to that of humans (24–27), and the same hallmarks of human allergic asthma are evident in the HDMA rhesus monkey model (11). Mouse models have provided much insight into the immunologic mechanisms of allergic asthma and the potential for ISS to alleviate them. In mice, both systemic (7, 9, 10, 28, 29) and mucosal (8) CpG oligonucleotide treatments have been shown to reduce clinical symptoms, such as airways hyperresponsiveness and airway eosinophilia, after inhaled ovalbumin administration. Evaluations of the ability of CpG motifs to attenuate airways remodeling in mice have not been as complete. Both mucosal and systemic ISS have been reported to reduce/prevent mucous cell metaplasia in mice exposed to inhaled ovalbumin (8, 9, 28, 29). However, this aspect of airways remodeling in mice does not accurately reflect the manifestation in humans. Normal bronchial epithelium in humans consists of ~ 25% mucous (goblet) cells, whereas bronchial epithelium in mice consists of only 1% mucous (goblet) cells (reviewed in [27]). This means that an increase in mucous cells in human airways during an inflammatory response is most likely due to proliferation and hyperplasia of existing cells, whereas an increase in mucous cells in mice is most likely due to a metaplastic change. Systemic CpG oligonucleotide treatment in mice has also been shown to reduce peribronchiolar or subbasement membrane fibrosis (9, 28) and peribronchiolar smooth muscle thickness or hypertrophy (28) associated with allergic asthma. We did not observe clearly detectable differences in smooth muscle mass or bundle orientation between sham- or ISS-treated monkeys with asthma. This may be due in part to the relatively short-term nature of the ISS treatment in this study. Another reason may be the relative lack of plasticity of the smooth muscle in an adult animal. No information on reticular basement membrane thickening, an integral aspect of airways remodeling in asthma, is available from murine models of asthma or from treatment with CpG motifs. The reticular basement membrane is very thin in the relatively small airways of the mouse and difficult to study at the light microscopic level. In our nonhuman primate model of allergic asthma, however, we have shown that mucosal administration of ISS mitigates mucous cell hyperplasia (bronchial epithelium of rhesus monkeys has baseline levels of mucous [goblet] cells) and reticular basement membrane thickening, in addition to mitigating mast cell and eosinophilic infiltration. Differences in the gross and subgross anatomy of the lungs (25), architecture of the tracheobronchial trees (26), epithelial composition and distribution (24, 27), and secretory glycoconjugate composition (30) between primates and rodents make extrapolation of murine ovalbumin models to humans difficult. The present study shows that biweekly administration of inhaled ISS can mitigate mast cell and eosinophil infiltration, mucus-cell hyperplasia, reticular basement membrane thickening, and airway hyperresponsiveness associated with allergic airways disease in an animal model that closely reflects the pathophysiology of human asthma.
Supplementary Material
Acknowledgment
The authors thank Sarah Davis and Brian Tarkington, as well as the animal care staff of the California National Primate Research Center, for all of their help and support for this project, and acknowledge Dynavax Technologies Corporation, Berkeley, CA for the gift of ISS and the editorial expertise of Dr. Suzette Smiley-Jewell.
Supported by National Institutes of Health grants ES00628, ES05707, RR00169, and AI40628.
Footnotes
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Conflict of Interest Statement: M.V.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; E.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; G.L.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; R.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; E.R. is a founder of Dynavax, has 300,000 shares of stock in this company, and his lab was sponsored by this company from 1998–2003 at $500,000 per year; D.M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.G.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. The University of California, San Diego is the owner of the patent related to the use of ISS for asthma.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 3.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med. 2001;29(Suppl 7):S2–S6. doi: 10.1097/00003246-200107001-00002. discussion S6–S7. [DOI] [PubMed] [Google Scholar]
- 5.Mutwiri G, Pontarollo R, Babiuk S, Griebel P, van Drunen Littel-van den Hurk S, Mena A, Tsang C, Alcon V, Nichani A, Ioannou X, et al. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet Immunol Immunopathol. 2003;91:89–103. doi: 10.1016/s0165-2427(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 6.Santeliz JV, Van Nest G, Traquina P, Larsen E, Wills-Karp M. Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperresponsiveness in a murine model of asthma. J Allergy Clin Immunol. 2002;109:455–462. doi: 10.1067/mai.2002.122156. [DOI] [PubMed] [Google Scholar]
- 7.Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002;283:L170–L179. doi: 10.1152/ajplung.00402.2001. [DOI] [PubMed] [Google Scholar]
- 8.Jain VV, Businga TR, Kitagaki K, George CL, O’Shaughnessy PT, Kline JN. Mucosal immunotherapy with CpG oligodeoxynucleotides reverses a murine model of chronic asthma induced by repeated antigen exposure. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1137–L1146. doi: 10.1152/ajplung.00073.2003. [DOI] [PubMed] [Google Scholar]
- 9.Jain VV, Kitagaki K, Businga T, Hussain I, George C, O’Shaughnessy P, Kline JN. CpG-oligodeoxynucleotides inhibit airway remodeling in a murine model of chronic asthma. J Allergy Clin Immunol. 2002;110:867–872. doi: 10.1067/mai.2002.129371. [DOI] [PubMed] [Google Scholar]
- 10.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 11.Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, et al. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae) Am J Pathol. 2001;158:333–341. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanucchi M, Evans M, Baker G, Miller L, Hyde D, Schelegle E, Raz E, Plopper C. Airways remodeling in young adult rhesus monkeys is inhibited by immunostimulatory DNA sequences (ISS) [abstract] Keystone Symposia: Regulation of Mucosal Inflammation/Hygiene, Allergy and Asthma. A108. 2003 [Google Scholar]
- 13.Fanucchi M, Evans M, Baker G, Miller L, Hyde D, Schelegle E, Raz E, Plopper C. Immunostimulatory DNA sequences (ISS) inhibition of airways remodeling in young adult rhesus monkeys [abstract] Am J Respir Crit Care Med. 2003;167:A7654. [Google Scholar]
- 14.Horner AA, Van Uden JH, Zubeldia JM, Broide D, Raz E. DNA-based immunotherapeutics for the treatment of allergic disease. Immunol Rev. 2001;179:102–118. doi: 10.1034/j.1600-065x.2001.790111.x. [DOI] [PubMed] [Google Scholar]
- 15.Evans MJ, Van Winkle LS, Fanucchi MV, Baker GL, Murphy AE, Nishio SJ, Schelegle ES, Gershwin LJ, Sannes PL, Plopper CG. Fibroblast growth factor-2 in remodeling of the developing basement membrane zone in the trachea of infant rhesus monkeys sensitized and challenged with allergen. Lab Invest. 2002;82:1747–1754. doi: 10.1097/01.lab.0000043911.94235.f3. [DOI] [PubMed] [Google Scholar]
- 16.Hyde DM, Magliano DJ, Plopper CG. Morphometric assessment of pulmonary toxicity in the rodent lung. Toxicol Pathol. 1991;19:428–446. doi: 10.1177/0192623391019004-112. [DOI] [PubMed] [Google Scholar]
- 17.Glantz SA. Primer of Biostatistics. 3rd ed. New York: McGraw-Hill, Inc; 1992. [Google Scholar]
- 18.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery PK. The development of large and small airways. Am J Respir Crit Care Med. 1998;157:S174–S180. doi: 10.1164/ajrccm.157.5.rsaa-1. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman P, Calhoun W, Panettieri RA, Jr, Peters S, Rennard S. Airway remodeling in asthma: do histogical changes and functional changes correlate? Medical Crossfire. 2002;4(5):34–47. 2003. [Google Scholar]
- 21.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123(Suppl 3):417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 22.Elias JA. Airway remodeling in asthma: unanswered questions. Am J Respir Crit Care Med. 2000;161:S168–S171. doi: 10.1164/ajrccm.161.supplement_2.a1q4-4. [DOI] [PubMed] [Google Scholar]
- 23.Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999;160:1035–1042. doi: 10.1164/ajrccm.160.3.9902064. [DOI] [PubMed] [Google Scholar]
- 24.Plopper CG, Hyde DM. Epithelial cells of bronchioles. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 85–92. [Google Scholar]
- 25.Tyler WS, Julian MD. Gross and subgross anatomy of the lungs, pleura, connective tissue septa, distal airways, and structural units. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 37–48. [Google Scholar]
- 26.McCamish LE. Architecture of the tracheobronchial tree. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 49–62. [Google Scholar]
- 27.Mariassy A. Epithelial cells of the trachea and bronchi. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 63–76. [Google Scholar]
- 28.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Rodriguez M, Lee SY, McElwain K, McElwain S, Raz E, et al. Immunostimulatory DNA inhibits TGF-β expression and airway remodeling. Am J Respir Cell Mol Biol. 2004;30:651–661. doi: 10.1165/rcmb.2003-0066OC. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda RK, Nayar J, Cho JY, Miller M, Rodriguez M, Raz E, Broide DH. Resolution of airway inflammation following ovalbumin inhalation: comparison of ISS DNA and corticosteroids. Am J Respir Cell Mol Biol. 2003;28:655–663. doi: 10.1165/rcmb.4853. [DOI] [PubMed] [Google Scholar]
- 30.St. George JA, Wang S. Secretory glycoconjugates of trachea and bronchi. In: Parent RA, editor. Comparative biology of the normal lung. Boca Raton, FL: CRC Press; 1992. pp. 77–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.