Abstract
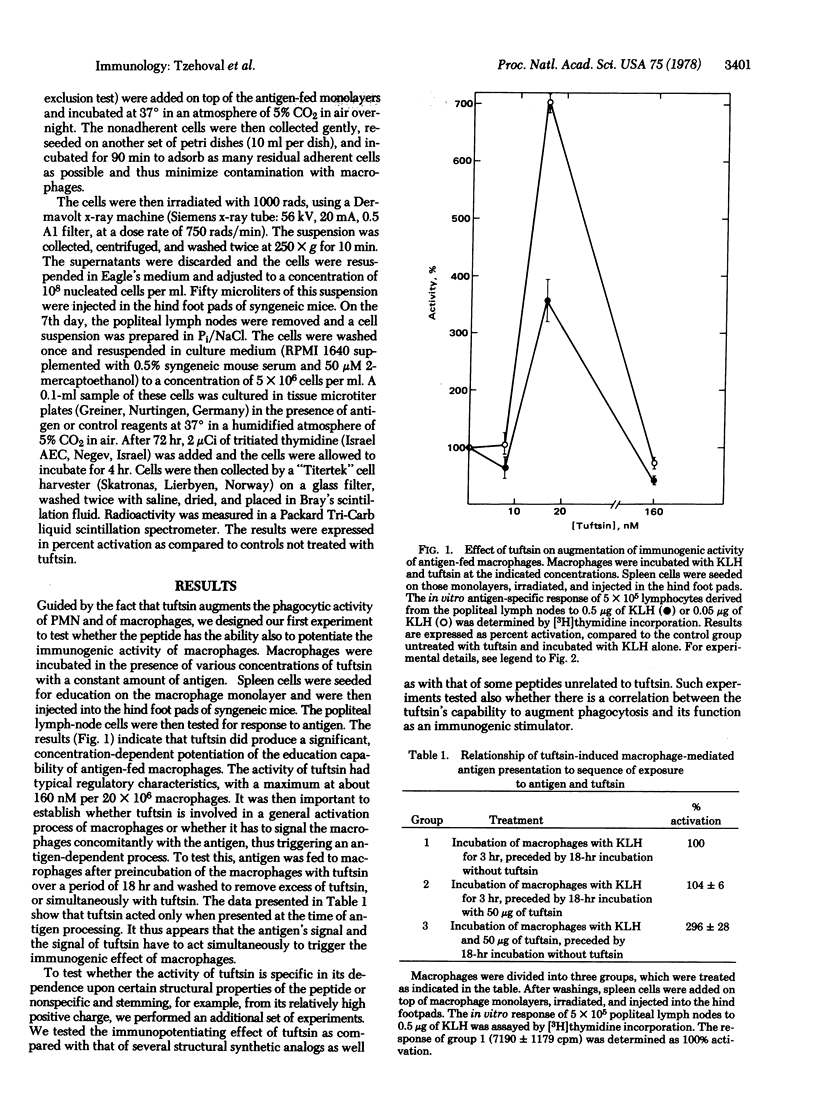
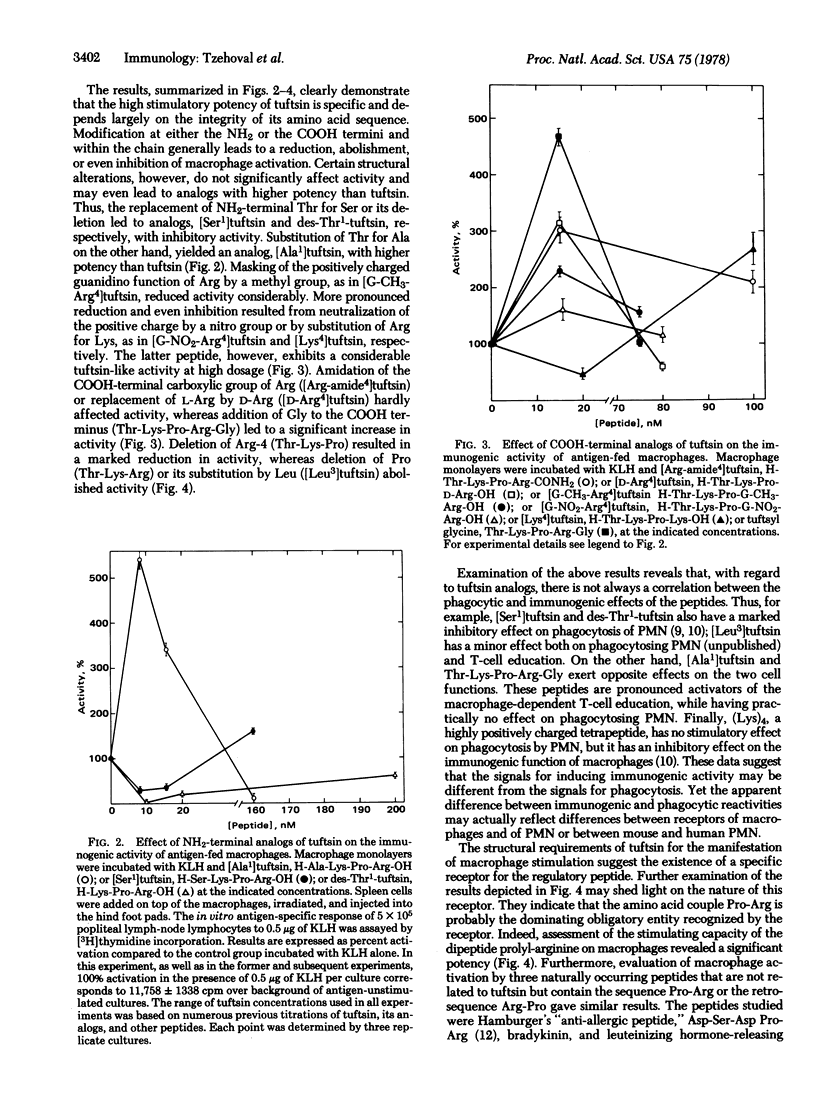
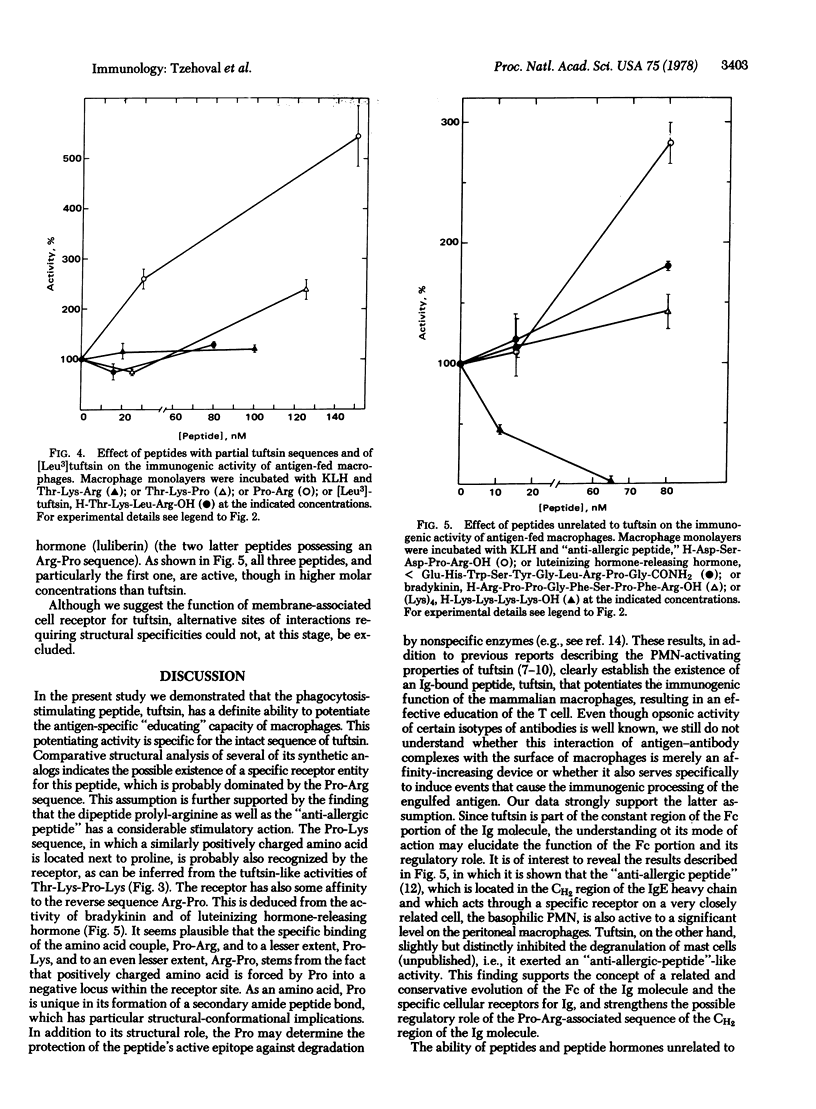
The immunoglobulin heavy-chain-associated tetrapeptide, tuftsin (Thr-Lys-Pro-Arg), known for its phagocytosis-stimulating activity, was found to augment the antigen-specific, macrophage-dependent education of T lymphocytes. The investigation of stereospecific characteristics of the tetrapeptide, by use of structural analogs with different modifications, revealed strict structural requirements for eliciting the immunogenic activity of macrophages. It was found that the most important moiety for its activity is the dipeptide Pro-Arg. This finding is of interest in view of the appearance of this particular dipeptide in other bioregulatory peptides, including many of the peptide hormones. The significance of the appearance of a common structure in such molecules, which may act through specific receptors on different target cells, is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun T., Hechter O., Rudinger J. "Insulin-like" action of oxytocin: evidence for separate oxytocin-sensitive and insulin-sensitive systems in fat cells. Endocrinology. 1969 Dec;85(6):1092–1096. doi: 10.1210/endo-85-6-1092. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Regulation of cellular and humoral immune responses by T-cell subclasses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):23–32. doi: 10.1101/sqb.1977.041.01.006. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854–6861. [PubMed] [Google Scholar]
- Chang D., Greibrokk T., Knudsen R., Howard G., Humphries J., Folkers K., Bowers C. Y. Differentiation of factor C-LHIH and the synthetic contraceptive polypeptide, H-Thr-Pro-Arg-Lys-OH. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1208–1213. doi: 10.1016/s0006-291x(75)80358-5. [DOI] [PubMed] [Google Scholar]
- Fridkin M., Patchornik A., Katchalski E. Use of polymers as chemical reagents. II. Synthesis of bradykinin. J Am Chem Soc. 1968 May 22;90(11):2953–2957. doi: 10.1021/ja01013a037. [DOI] [PubMed] [Google Scholar]
- Fridkin M., Stabinsky Y., Zakuth V., Spirer Z. Tuftsin and some analogs: synthesis and interaction with human polymorphonuclear leukocytes. Biochim Biophys Acta. 1977 Jan 24;496(1):203–211. doi: 10.1016/0304-4165(77)90129-5. [DOI] [PubMed] [Google Scholar]
- Hamburger R. N. Peptide inhibition of the Prausnitz-Küstner reaction. Science. 1975 Aug 1;189(4200):389–390. doi: 10.1126/science.1145208. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Wingender W., Finn F. M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Adachi T. Generation of specific helper cells and suppressor cells in vitro for the IgE and IgG antibody responses. J Immunol. 1976 Jul;117(1):40–47. [PubMed] [Google Scholar]
- Levy D. Preparation of photo-affinity probes for the insulin receptor site in adipose and liver cell membranes. Biochim Biophys Acta. 1973 Oct 18;322(2):329–336. doi: 10.1016/0005-2795(73)90308-5. [DOI] [PubMed] [Google Scholar]
- Najjar V. A., Nishioka K. "Tuftsin": a natural phagocytosis stimulating peptide. Nature. 1970 Nov 14;228(5272):672–673. doi: 10.1038/228672a0. [DOI] [PubMed] [Google Scholar]
- Nishioka K., Constantopoulos A., Satoh P. S., Najjar V. A. The characteristics, isolation and synthesis of the phagocytosis stimulating peptide tuftsin. Biochem Biophys Res Commun. 1972 Apr 14;47(1):172–179. doi: 10.1016/s0006-291x(72)80025-1. [DOI] [PubMed] [Google Scholar]
- Seelig S., Sayers G., Schwyzer R., Schiller P. Isolated adrenal cells: ACTH(11-24), a competitive antagonist of ACTH(1-39) and ACTH(1-10). FEBS Lett. 1971 Dec 15;19(3):232–234. doi: 10.1016/0014-5793(71)80521-5. [DOI] [PubMed] [Google Scholar]
- Steinman L., Tzehoval E., Cohen I. R., Segal S., Glickman E. Sequential interaction of macrophages, initiator T lymphocytes and recruited T lymphocytes in a cell-mediated immune response to soluble antigen. Eur J Immunol. 1978 Jan;8(1):29–34. doi: 10.1002/eji.1830080107. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes. I. Analysis with an in vitro primary response to soluble protein antigens. J Exp Med. 1976 Nov 2;144(5):1263–1273. doi: 10.1084/jem.144.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes: specific sensitization by antigens associated with allogeneic macrophages. Proc Natl Acad Sci U S A. 1977 May;74(5):2104–2108. doi: 10.1073/pnas.74.5.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A. Persistence of immunogenicity of antigen after uptake by macrophages. J Exp Med. 1968 May 1;127(5):915–926. doi: 10.1084/jem.127.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]


