Abstract
As part of our clinical tests on bone regeneration using cultured periosteal sheets, here, we prepared cultured periosteal sheets in two types of stem-cell culture media, STK1 and STK3. Human periosteum was expanded either in 1% human serum–supplemented STK1 for 28 days, in 1% human serum–supplemented STK1 for 14 days followed by 1% human serum–supplemented STK3 for 14 days (1% human serum–supplemented STK1+3), or in 10% fetal bovine serum–supplemented Medium 199 for 28 days (control). Cultured periosteal sheet diameter and DNA content were significantly higher, and the multilayer structure was prominent in 1% human serum–supplemented STK1 and 1% human serum–supplemented STK1+3. The messenger RNA of osteoblastic markers was significantly upregulated in 1% human serum–supplemented STK1+3. Osteopontin-immunopositive staining and mineralization were evident across a wide area of the cultured periosteal sheet in 1% human serum–supplemented STK1+3. Subcutaneous implantation in nude mice following expansion in 1% human serum–supplemented STK1+3 produced the highest cultured periosteal sheet osteogenic activity. Expansion in 1% human serum–supplemented STK1+3 successfully induced cultured periosteal sheet growth while retaining osteogenic potential, and subsequent osteoblastic induction promoted the production of homogeneous cell material.
Keywords: Periosteum, stem-cell culture medium, bone regeneration
Introduction
Jawbone defects can occur due to a variety of causes, such as periodontitis, external injuries, and neoplastic lesions, and impairment of oral cavity function as a result of large-scale jawbone defects is extremely difficult to alleviate. Autogenous bone grafting is the gold standard for the treatment of jawbone defects. However, bone graft harvest is a highly invasive surgical procedure and is thus a considerable burden on patients. Several bone-regeneration therapies, such as those using growth factors1 and bone substitute materials,2 and cell-based therapy3 have already been tested for reducing the burden of bone harvesting. Of them, cell-based therapy is of particular interest because it can directly supply cells responsible for bone regeneration and utilize transplanted cells as producers of growth factors that exert paracrine actions, thereby facilitating bone regeneration with low invasiveness.
Cell-based therapies using bone marrow, the periodontal ligament, and the periosteum as cell sources have been reported in many bone-regeneration studies. Although the bone marrow is not collected from oral tissue, it is rich in mesenchymal stromal cells (MSCs) that sufficiently differentiate into osteoblasts and is thus widely used.4,5 Periodontal ligament cells differentiate into not only osteoblasts but also various types of constituent cells of the periodontium, but therapeutic indications are more restricted since tooth extraction is required for cell harvesting.6,7 On the other hand, cell-based therapy using periosteal cells is indicated for most patients because the periosteum can be recovered from within or outside the oral cavity regardless of age.8 Furthermore, the periosteum contains osteogenic progenitor cells and MSCs, especially in the layers near the bone surface, as well as its main constituent, fibroblasts, and thus offers high bone-forming capacity.9,10
We are interested in the clinical use of the periosteum and have been conducting basic and clinical studies using a cultured periosteal sheet (CPS) prepared from periosteum explant cultures. Although cell growth rates are slow in the form of a CPS, the use of CPSs enables expansion of a subset of cells with osteogenic potency without performing enzymatic dissociation and osteoblastic induction.11,12 A recent clinical study showed that autologous grafting of a cultured CPS in the treatment of alveolar bone atrophy induced rapid bone formation and recruitment of osteoclasts.8 Taken together, it is suggested that cell-based bone-regeneration therapy induces bone metabolism in the grafts, thereby facilitating physiological and functional bone regeneration. However, several disadvantages and risks are associated with this approach. For example, CPS preparation requires a period of 6 weeks, and this lengthy culturing is associated with a high risk of contamination, neoplastic cell transformation, and undesirable cell differentiation. In addition, because fetal bovine serum (FBS) is contained in the culture media, interpreparation variations in the media can be large, and the risk of infection attributed to this animal product is not completely eliminated. Furthermore, there are considerable differences in the cell growth rate and bone-forming capacity among individuals. In our clinical study, 20%–40% of particulate autogenous bone in the graft material was still necessary for stable bone formation.8 Thus, a fast and safe method for autogenous explant culture, which enables preparation of grafts with high bone-forming capacity without relying on the scaffold, is necessary for the clinical use of CPSs. Indeed, with concerns growing over the efficacy and safety of cell-based therapy in recent years, it is necessary to address issues related to the safety of the culture methods and the quality control of cell products.13,14
Generally speaking, serum-free medium contains a known concentration of supplements, such as growth factors. The serum-free STK series media are unique because they offer three different media formulated specifically for the maintenance, expansion, and osteogenic induction of stem cells. STK1 and STK2 are formulated for the selective and efficient expansion of MSC, and STK3 is formulated to promote rapid osteogenic induction of MSC after expansion. Thus, proliferation and differentiation can be optimally controlled by managing the combination of STK media.
In this study, we examined whether the use of STK1 and STK3 media reduces the time required for CPS preparation and investigated the effect of the combination of STK1 and STK3 on the expansion and differentiation of MSC and osteoblast progenitor cells in the periosteum. Providing that a combination of stem-cell STK media is effective, good manufacturing practice-grade graft materials can be prepared from a wide variety of cell sources for cell-based bone-regeneration therapy in the future.
Materials and methods
Isolation and culture of human periosteal sheets
After obtaining informed consent, human periosteum tissue segments were aseptically dissected from the periodontal tissue of the buccal side of the retromolar region in the mandible of volunteers.11 Small pieces of periosteum were placed on six-well plates and subjected to explant culture in humidified 5% CO2, 95% air at 37°C by the following three culture methods: STK1™ (DS Pharma, Osaka, Japan) supplemented with 1% human serum and antibiotics for 28 days (1% HS-STK1 group), STK1 supplemented with 1% human serum and antibiotics for 14 days followed by STK3™ (DS Pharma) supplemented with 1% human serum and antibiotics for 14 days (1% HS-STK1+3 group), and Medium 199 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen), 25 µg/mL ascorbic acid 2-phosphate, and antibiotics for 28 days (control group). The culture medium was refreshed twice a week.
All subjects enrolled in this study gave informed consent. The study protocol was approved on 22 June 2006 by the Ethics Committee for Human Subject Use at Niigata University Medical and Dental Hospital in accordance with the Declaration of Helsinki 1975 as revised in 2008.
Flow-cytometric analysis
CPS cells expanded by the three culture methods were subjected to flow-cytometric analysis for the following MSC marker surface antigens: anti-CD73-PE (IgG1), anti-CD90-fluorescein isothiocyanate (FITC; IgG1), anti-CD105-FITC (IgG2a), anti-CD34-FITC (IgG1), and anti-CD45-FITC (IgG1). Details of the analysis procedure are described in our previous article.15
Determination of cellular DNA content
CPSs were rinsed twice with phosphate buffered saline (PBS), detached using a cell scraper, and transferred to sample tubes. After centrifugation, CPSs that were precipitated were lysed in cell lysis buffer (Invitrogen). DNA content was determined using the Qubit® dsDNA BR Assay Kit (Invitrogen).
Histological and immunohistochemical examination
CPSs cultured on plastic plates were harvested, fixed, and embedded in paraffin, as described previously.11 CPSs implanted in nude mice were retrieved with surrounding tissue, fixed, and decalcified with 0.5 M ethylenediaminetetraacetic acid (EDTA) and embedded in paraffin. The blocks were sectioned to a thickness of 6 µm for staining with hematoxylin and eosin (HE) or von Kossa, and the CPS thickness of the HE sections was measured.
For immunohistochemical staining, sections were subjected to antigen-retrieval, blocking of endogenous peroxidase, and blocking of nonspecific binding with 2.5% normal horse serum (Vector Labs, Burlingame, CA, USA). Sections were probed with the following primary antibodies diluted with Immuno Shot Mild (Cosmobio, Tokyo, Japan): mouse monoclonal anti-proliferating cell nuclear antigen (PCNA; 1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rabbit polyclonal anti-osteopontin (OPN; 1:200; Abcam, Cambridge, MA, USA). Sections were then probed with the secondary antibody, anti-rabbit IgG (Invitrogen) diluted with Immuno Shot Mild (Cosmobio), or ImmPRESS® anti-mouse IgG (Vector Labs) for 30 min at room temperature. Target proteins were visualized using diaminobenzidine solution (KPL Inc., Gaithersburg, MD, USA), and the sections were faintly counterstained with HE. Alternatively, the sections were examined by immunofluorescence staining as follows. Sections were probed overnight at 4°C with the following primary antibodies diluted with blocking buffer: goat polyclonal anti-receptor activator of nuclear factor kappa-B ligand (RANKL; 1:100; Santa Cruz Biotechnology, Inc.), goat polyclonal anti-bone morphogenetic protein (BMP)-2/4 (1:100; R&D Systems Inc., Minneapolis, MN, USA), rabbit polyclonal anti-runt-related transcription factor 2 (RUNX2; 1:200; Abcam), or mouse monoclonal anti-vascular endothelial growth factor (VEGF) antibody (Abcam). The primary antibodies were probed with DyLight 488–conjugated anti-goat IgG (1:100; Rockland, Gilbertsville, PA, USA), DyLight 549–conjugated anti-mouse IgG (1:100; Rockland), or Alexa Flour 488–conjugated anti-rabbit IgG (1:100; Invitrogen) diluted with Immuno Shot Mild (Cosmobio) for 30 min at room temperature. After DNA staining with 4′,6-diamidino-2-phenylindole (DAPI, 0.5 µg/mL; Dojin, Kumamoto, Japan), the sections were examined under a fluorescence microscope (ECLIPSE 80i; Nikon, Tokyo, Japan).
For direct staining, cultured CPSs were fixed in 10% neutralized formalin on dishes and stained using an alkaline phosphatase (ALP)-staining kit (Muto Chemicals, Tokyo, Japan) or with 40 mM Alizarin red-S (Wako Pure Chemicals, Osaka, Japan) for calcium deposits.
Complementary DNA synthesis and quantitative real-time polymerase chain reaction
The total RNA was extracted from CPSs using an RNeasy mini purification kit (Qiagen, Germantown, MD, USA) as described previously,16 then reverse transcribed using the SuperScript VILO™ complementary DNA (cDNA) Synthesis Kit (Invitrogen). Quantitative real-time polymerase chain reaction (PCR) was performed using a Smart Cycler System (Cepheid, Sunnyvale, CA, USA). Real-time monitoring of the following genes was performed using TaqMan probes according to the manufacturer’s standard protocol (TaqMan Gene Expression Assays and TaqMan Universal PCR Master Mix; Applied Biosystems, Carlsbad, CA, USA): RUNX2 (Hs00231692_m1), ALP (Hs00758162_m1), collagen, type I, alpha 1 (COL1A1; Hs01076775_g1), secreted phosphoprotein 1 (SPP1; Hs00959010_m1), BMP-2 (Hs00154192_m1), tumor necrosis factor ligand superfamily member 11 (TNSF11; Hs00243522_m1), VEGF-A (Hs00900055_m1), fibroblast growth factor receptor 1 (FGFR1; Hs00241111_m1), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh; Hs99999905_m1).
Micro-computed tomography
To evaluate the volume of mineral deposits formed in the CPSs, micro-computed tomography imaging was performed using an SMX-100CT scanner (Shimadzu, Kyoto, Japan) as described previously.11 The X-ray tube voltage, current, and slice thickness were 58 kV, 58 µA, and 1.1 × 10−2 mm, respectively. The field of view was 4.24 mm with a 512 × 512 matrix; therefore, each pixel was 8.3 × 10−3 mm. Images were reconstructed using three-dimensional software (VGStudio Max 2; Volume Graphics GmbH, Heidelberg, Germany).
Animal implantation study
Balb/c-nu/nu mice (male; age: 5 weeks; weight: 15–20 g) were obtained from Charles River Laboratories (Yokohama, Japan) and housed at the Brain Research Institute Center for Bioresource-based Research, Niigata University, for at least 1 week prior to the experiment. After rinsing with PBS, CPSs were harvested with a cell scraper and implanted into the subcutaneous tissue of nude mice. Surgical procedures were performed aseptically as described previously.15 The care and use of animals was in accordance with the Guiding Principles for the Care and Use of Animals, as approved by Niigata University.
Statistical analysis
Significant differences between the groups were analyzed using Student’s t-test. p values < 0.05 were considered significant.
Results
Expansion of periosteal sheets and formation of a multilayer structure
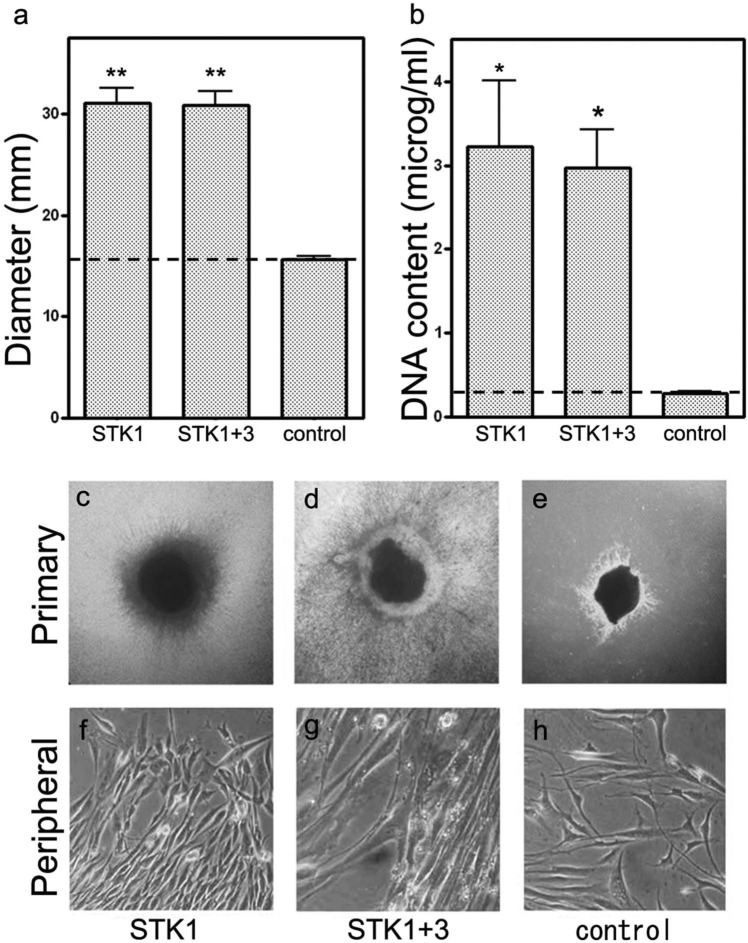
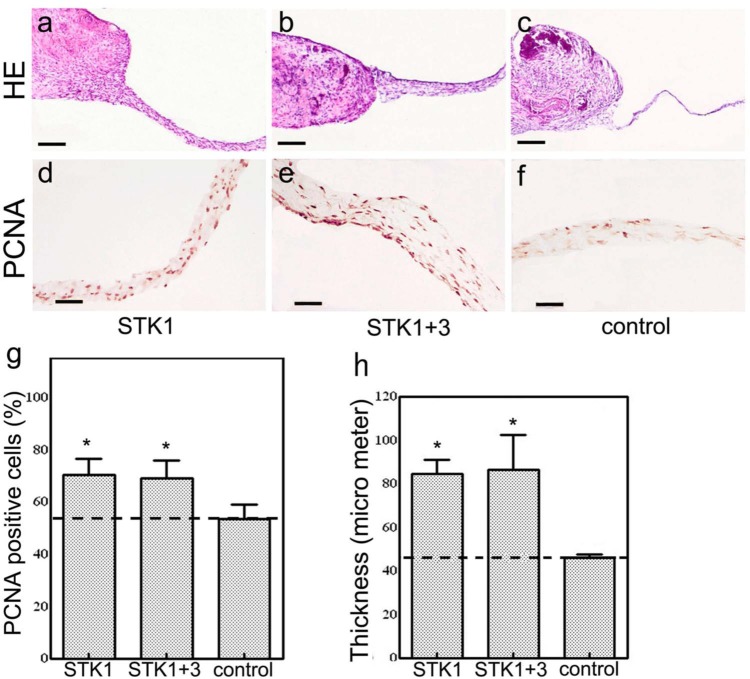
Periosteal cells were developed as outgrowths from periosteal tissue fragments to form CPSs on culture plates regardless of the type of culture media used. Cell migration was observed earlier in the 1% HS-STK1 and 1% HS-STK1+3 groups than in the control group: the diameter of the CPSs and total DNA content recovered from the CPSs were significantly higher in the 1% HS-STK1 and 1% HS-STK1+3 groups than in the controls (p < 0.05; Figure 1(a) and (b)). The formation of a multilayer structure was observed only in the area adjacent to the primary periosteal segment, and the peripheral outgrowth presented a single-layer structure with a sparse cell distribution in the control group (Figure 1(e) and (h)). In contrast, the formation of a dense extracellular matrix and a multilayer structure was characteristic in the peripheral region in the 1% HS-STK1 and 1% HS-STK1+3 groups (Figure 1(c), (d), (f), and (g)). The thickness of the CPSs in the peripheral region was approximately 50 µm in the control group and approximately 90 µm in both the 1% HS-STK1 and 1% HS-STK1+3 groups (Figure 2(a)–(c) and (h)). In good agreement with the increase in CPS thickness, the percentage of PCNA-positive cells was also significantly higher in stem-cell culture media than in the control medium (p < 0.05; Figure 2(d)–(g)). No significant differences were noted in the expansion and formation of the multilayer structure between the 1% HS-STK1 and 1% HS-STK1+3 groups.
Figure 1.
(a) Diameter and (b) DNA content of CPSs. CPSs expanded with 1% HS-STK1 and 1% HS-STK1+3 showing (a) a significantly increased diameter as well as (b) increased DNA content. (c–h) Phase contrast light microscope findings of CPSs. (c, d, f and g) Dense and multilayered peripheral outgrowths are visible in the CPSs expanded with 1% HS-STK1 and 1% HS-STK1+3. (e and h) CPSs expanded with 10% FBS-M199 show sparse outgrowths, in which the multilayered region was limited around the primary periosteal fragment. Primary: region of the primary periosteal fragment and peripheral: area of the peripheral outgrowth of the periosteal cells.
HS: human serum supplemented; FBS: fetal bovine serum; CPSs: cultured periosteal sheets.
n = 3, *p < 0.05, **p < 0.01, compared with the control.
Figure 2.
(a–c) Histology of CPSs (hematoxylin and eosin staining). CPSs expanded with 1% HS-STK1 and 1% HS-STK1+3 exhibiting thicker outgrowth of the peripheral region formed by extracellular matrix–rich multilayered cells than 10% FBS-M199. (d–f) Immunohistochemical observations of PCNA-positive cells. (g) PCNA-positive cells were notably increased at the peripheral region of the CPSs expanded with 1% HS-STK1 and 1% HS-STK1+3. (h) Measurement of CPS thickness. (a–c) bar, 200 µm and (d–f) bar, 50 µm.
CPS: cultured periosteal sheet; HS: human serum supplemented; FBS: fetal bovine serum; PCNA: proliferating cell nuclear antigen.
n = 3, *p < 0.05, **p < 0.01, compared with controls.
Osteogenic potential of CPSs
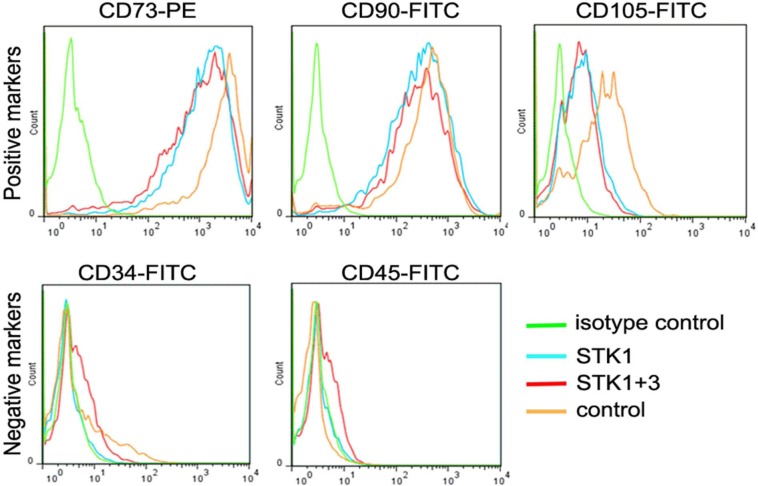
Flow cytometry for cell surface antigens revealed that the CPSs contained cells expressing positive markers of the MSC, namely, CD73, CD90, and CD105,17 regardless of the type of culture media (Figure 3(a)–(c)). The STK1 medium was designed specifically for the maintenance of undifferentiated primary stem cells, and indeed, no selective expansion of the mesenchymal stem-cell subsets was observed. However, the fraction of the CD105-positive subset appeared to be slightly smaller in the 1% HS-STK1 and 1% HS-STK1+3 groups than in the controls (Figure 3(c)). Cells expressing negative markers of MSC (CD34 and CD45)17 were negligible in all groups (Figure 3(d) and (e)).
Figure 3.
Flow-cytometric analysis of cell surface antigens expressed in CPSs for (a) CD73, (b) CD90, (c) CD105, (d) CD34 and (e) CD45.
CPS: cultured periosteal sheet; HS: human serum supplemented; FBS: fetal bovine serum; FITC: fluorescein isothiocyanate; green line: isotype control (periosteal sheets cultured in 10% FBS-M199); blue line: 1% HS-STK1; red line: 1% HS-STK1+3; orange line: 10%FBS-M199.
Characterization of osteoblastic differentiation of CPSs
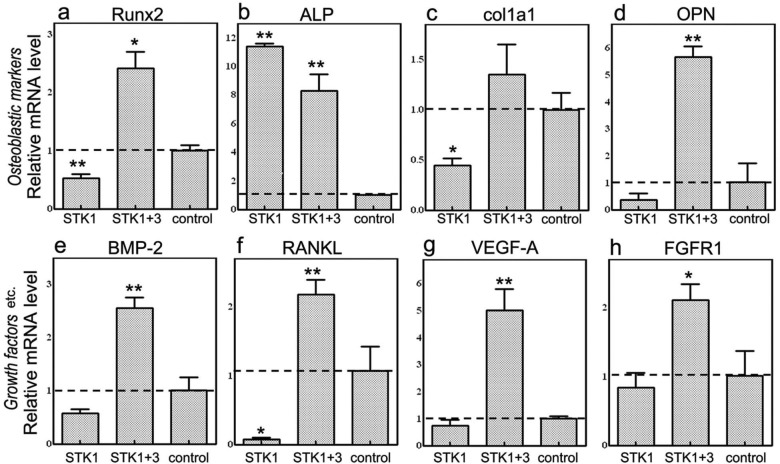
In good agreement with our previous findings,16 expression of mRNA encoding osteoblastic markers (Runx2, ALP, col1a1, and OPN) was observed in CPSs cultured in the 10% FBS-M199 medium without specific osteoblastic induction (Figure 4(a)–(d)). When the mRNA levels in the 1% HS-STK1 group were compared with those in the control group, the ALP mRNA level was upregulated, while the OPN mRNA (a late-stage differentiation marker) was downregulated, suggesting suppression of final differentiation into osteoblasts in the 1% HS-STK1 group (Figure 4(a)–(d)). On the other hand, the mRNA levels of all osteoblastic markers were upregulated in the 1% HS-STK1+3 group (Figure 4(a)–(d)). This suggests that switching the medium from the STK1 medium designed for primary stem-cell culture to the STK3 medium designed for differentiation of progenitors into osteoblasts at the midpoint of the 28-day culture resulted in the production of CPSs expressing high levels of osteoblastic markers. The 1% HS-STK1+3 group also exhibited remarkably high mRNA expression of local bone-regeneration factors BMP-2, RANKL, and VEGF-A, which mediate cell differentiation into osteoblasts, recruitment of osteoclasts, and angiogenesis, respectively (Figure 4(e)–(g)).
Figure 4.
Gene expression analysis of osteoblastic markers and bone-related growth factors of periosteal sheets expanded with 1% HS-STK1, 1% HS-STK1+3, and 10% FBS-M199 by qRT-PCR. (a) Runx2, (b) ALP, (c) col1a1, (d) OPN, (e) BMP-2, (f) RANKL, (g) VEGF-A, and (h) FGFR1.
HS: human serum supplemented; FBS: fetal bovine serum; Runx2: runt-related transcription factor 2; qRT-PCR: quantitative reverse transcriptase polymerase chain reaction; ALP: alkaline phosphatase; OPN: osteopontin; RANKL: receptor activator of nuclear factor kappa-B ligand; col1a1: collagen, type I, alpha 1; BMP: bone morphogenetic protein; VEGF-A: vascular endothelial growth factor-A; FGFR1: fibroblast growth factor receptor 1.
n = 4, *p < 0.05, **p < 0.01, compared with controls.
Histochemistry showed that no ALP activity in the CPSs was detected in the 1% HS-STK1 group (Figure 5(a) and (d)), while it was detected within the primary periosteal segment and in the limited adjacent area in the control group (Figure 5(c) and (f)). In contrast, even ALP activity was widely detected across the CPSs, including the primary periosteal segment and the peripheral region in the 1% HS-STK1+3 group (Figure 5(b) and (e)). Similar to the ALP staining results, OPN was immunopositive only within the primary periosteal segments in the controls and in the 1% HS-STK1 group (Figure 5(g), (i), (j) and (l)), while OPN was positive over a wide area, including the primary segment and peripheral region in the 1% HS-STK1+3 group (Figure 5(h) and (k)). ALP activity staining and immunostaining for OPN were particularly strong in the primary periosteal segments and in the highly stratified peripheral region. By the same token, immunofluorescence demonstrated the same expression patterns of the other osteoblast-related marker and growth factors (i.e. Runx2, BMP-2/4, RANKL, and VEGF) as those of mRNA (Figure 5(m)–(x)).
Figure 5.
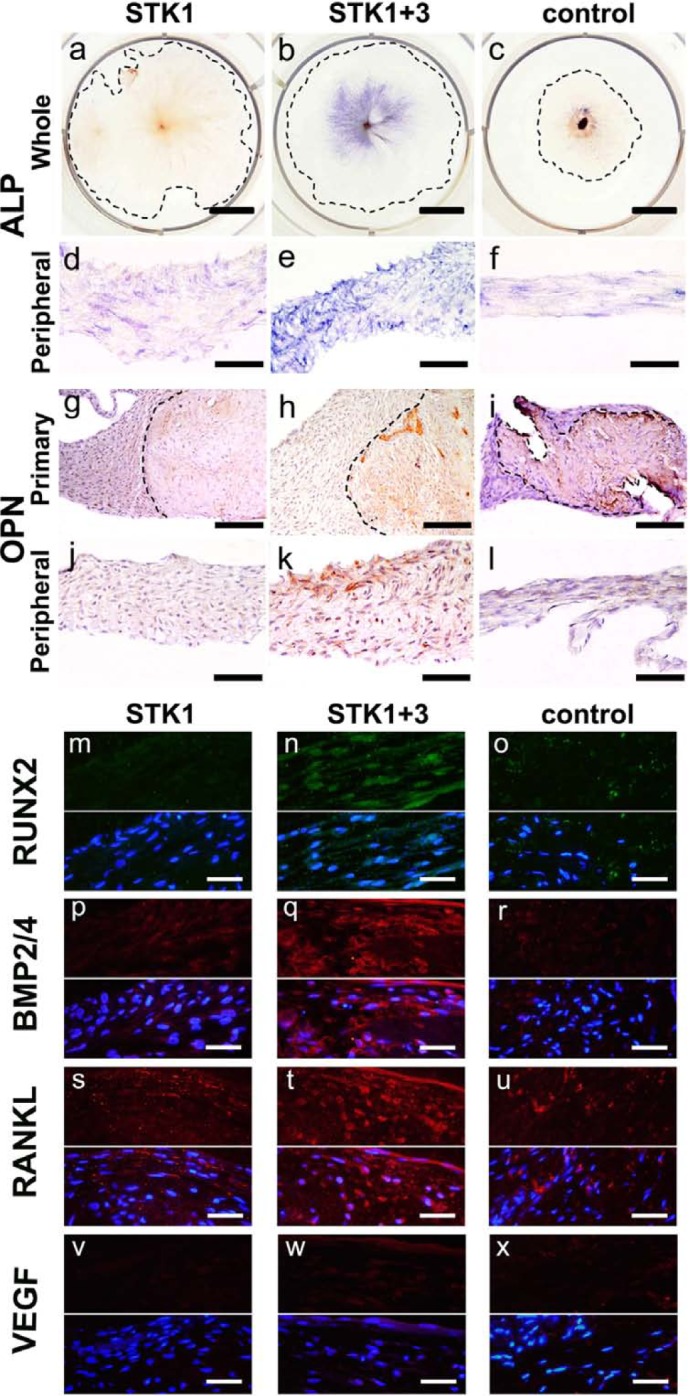
(a–f) ALP activity and (g–i) immunoreactivity against OPN. (b and e) ALP activity was observed in the central part of CPSs expanded with 1% HS-STK1+3, (a and d) while the peripheral region of the 1% HS-STK1 sheets did not exhibit positive enzymatic activity. (c and f) The primary periosteal fragment region of the 10% FBS-M199 showed focal ALP activity. (h and k) OPN was consistently observed in the central part of the CPSs expanded with 1% HS-STK1+3, (g and j) while CPSs of 1% HS-STK1 did not exhibit any positive staining. (i) Focal positive staining of OPN was found in primary periosteal fragments of 10% FBS-M199. (m–x) IF of the other osteoblastic marker and growth factors (i.e. Runx2, BMP-2/4, RANKL, and VEGF) showed the strongest expression in CPSs of STK1+3, which is consistent with the results of mRNA expression by quantitative real-time PCR (upper half: IF; lower half: IF plus nuclear staining with DAPI). (a–c). Primary: region of the primary periosteal fragment and peripheral: area of the peripheral outgrowth of periosteal cells. Bar, 10 mm; (d–f and j–l) bar, 100 µm; (g–i) bar, 200 µm; and (m–x) bar, 40 µm.
IF: immunofluorescence; ALP: alkaline phosphatase; CPS: cultured periosteal sheet; HS: human serum supplemented; Runx2: runt-related transcription factor 2; FBS: fetal bovine serum; OPN: osteopontin; RANKL: receptor activator of nuclear factor kappa-B ligand; BMP: bone morphogenetic protein; VEGF: vascular endothelial growth factor; mRNA: messenger RNA; PCR: polymerase chain reaction; DAPI: 4′,6-diamidino-2-phenylindole.
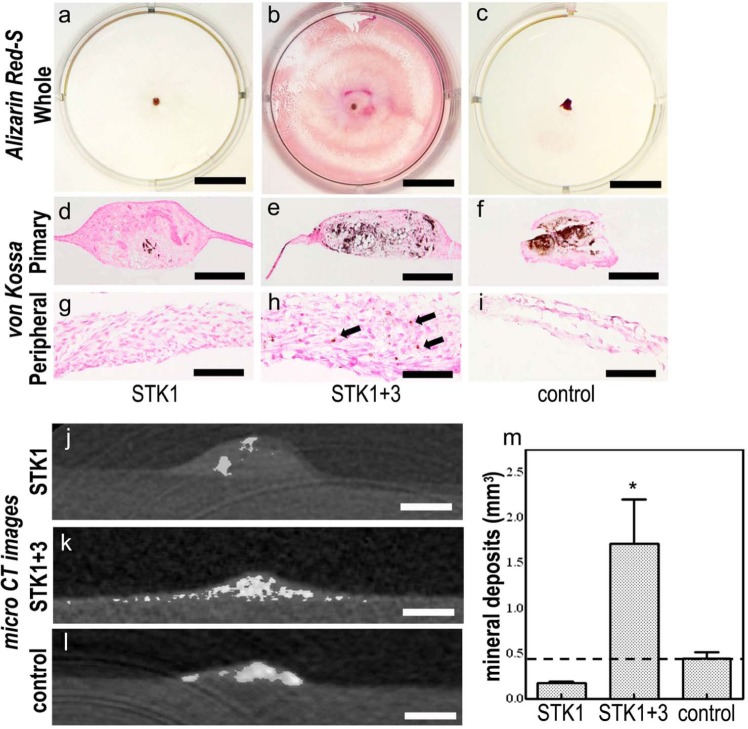
Alizarin red-S staining was negative in the 1% HS-STK1 group (Figure 6(a)) and positive within the primary segment of the 10% FBS-M199 group (Figure 6(c)). On the other hand, alizarin red-S staining was positive over a wide area of the CPSs in the 1% HS-STK1+3 group (Figure 6(b)). Von Kossa staining clearly exhibited mineralization within the primary periosteal segments in the controls and in the 1% HS-STK1+3 group (Figure 6(e) and (f)), while the peripheral region in the 1% HS-STK1+3 group showed partially positive staining (Figure 6(h), arrows). Micro-computed tomography revealed minor mineralization within the primary segment of the CPS in the 1% HS-STK1 group (Figure 6(j)). Considerable mineralization was observed in the primary segment of the CPS in the 1% HS-STK1+3 and control groups (Figure 6(k) and (l)), while mineralization in the peripheral region was observed only in the 1% HS-STK1+3 group (Figure 6(k)). The volume of the mineralized area was significantly larger in the 1% HS-STK1+3 group than in the 1% HS-STK1 and control groups (p < 0.05; Figure 6(m)).
Figure 6.
(a–i) Alizarin red-S and von Kossa staining of CPSs. (a–f) Under all conditions, the dye-affinity of Alizarin red-S and von Kossa was confirmed in the primary periosteal fragment, (b and h: arrows), while CPSs expanded with 1% HS-STK1+3 exhibited only enlargement of satiability extending to the peripheral region. (j–l) Micro CT images in the CPSs. (j and l) In CPSs of 1% HS-STK1 and 10% FBS-M199, mineral deposition was limited to primary periosteal fragments. (k and m) A CPS of 1% HS-STK1+3 exhibited mineral deposition occurring over a wide range of CPSs and a remarkable increase in total mineralized volume. (a–c). Primary: region of the primary periosteal fragment and peripheral: area of the peripheral outgrowth of the periosteal cells Bar, 10 mm; (d–f) bar 400 µm; and (g–i) bar, 100 µm.
CPS: cultured periosteal sheet; HS: human serum supplemented; CT: computed tomography; FBS: fetal bovine serum.
n = 3, *p < 0.05, compared with the controls.
Animal implantation study
CPSs implanted into subcutaneous tissue of a nude mouse showed no bone formation 28 days after implantation in the 1% HS-STK1 group (Figure 7(a)). On the other hand, mice that received a CPS of the 1% HS-STK1+3 or control group showed bone formation (Figure 7(b) and (c)). Additional formation of osteoid with a line of cuboidal osteoblasts was a characteristic finding of bone formed in CPSs from the 1% HS-STK1+3 group, indicating high bone formation ability (Figure 7(b)). Although the CPSs of the control medium were used for our clinical studies, they did not clearly show the above characteristics (Figure 7(c)), suggesting relatively low bone formation ability.
Figure 7.
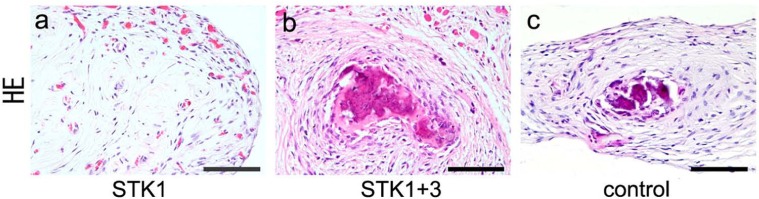
Histology of CPSs after 4 weeks of subcutaneous implantation into the back of nude mice. CPSs expanded with (a) 1% HS-STK1, (b) 1% HS-STK1+3, and (c) 10% FBS-M199, respectively. (a–c) Bar, 100 µm.
CPS: cultured periosteal sheet; HS: human serum supplemented; FBS: fetal bovine serum.
Discussion
Establishment of CPS expansion conditions with STK media
We initially aimed to establish CPS expansion conditions using the STK series for stem-cell culture without serum supplementation. However, a preliminary study showed poor cell migration and adhesion, and consequently, poor cell expansion was observed in the serum-free condition. We found that these issues can be addressed to obtain stable CPS expansion by means of supplementation with 1% human serum. For clinical use, only 10 cm3 of blood or less is required to make the necessary amount of STK medium supplemented with 1% autologous serum, and thus, the associated invasiveness was considered to be low enough.
Expansion and stratification of periosteal sheets induced by the STK media
We found that explant cultures developed a prominent multilayer structure after 28 days of expansion in the 1% HS-STK1 and 1% HS-STK1+3 group, and their diameter and total DNA content were roughly twofold higher than in the controls (Figures 1 and 2). We have been using the control medium (10% FBS-M199) for clinical studies, but it takes 6 weeks to obtain CPSs, of which the multilayer structure is only within the primary segment. The method using STK media induces expansion of the CPS and formation of the multilayer structure, suggesting a reduction in the time required for CPS preparation and a consequent reduction in cost and contamination risk.
We previously tested another stem-cell culture medium (MesenPRO, Invitrogen) and observed that the formation of the multilayer structure was attributed partly to the accumulation of the extracellular matrix.15 Multilayered CPSs in the 1% HS-STK1+3 group showed upregulation of ALP activity and OPN, Runx2, BMP-2/4, RANKL, and VEGF expression concurrently with an increase in the number of the PCNA-positive cells (Figures 2 and 5), suggesting induction of both differentiation into osteoblasts and cell proliferation. Considering that the periosteum is responsible for the maintenance and supply of osteogenic progenitor cells in bone tissue,18 the formation of a multilayer structure induced in the stem-cell culture media may provide favorable conditions for osteogenic progenitor cells to maintain the ability to proliferate and differentiate.
Direct binding between the extracellular matrix and growth factors, such as transforming growth factor (TGF)-β,19 the BMP family,20 FGF,21 and VEGF,22 regulates their localization and activity. These growth factors regulate the growth and differentiation of various subsets of cells, such as osteoblast and osteoclast progenitors, and vascular endothelial cells supplied by the neighboring tissue and blood stream, and thereby play pivotal roles in bone formation. The high expression levels of growth factors and considerable extracellular matrix deposition observed in the CPSs of the 1% HS-STK1+3 group suggest that these cell sheets serve as a rich source of osteoprogenitors and also as a delivery system of growth factors.8,23
The multilayer structure provides mechanical strength to the CPSs, allowing grafting without dissociation of cells leading to loss of intercellular or cell–extracellular matrix attachment. This reduces the burden on cells during the implantation procedure and contributes to the maintenance of cell phenotype,24 cell survival, and engraftment in host tissue. In contrast to the control group, expansion by the 1% HS-STK1 and 1% HS-STK1+3 protocols provided a sufficient number of cells as well as sufficient thickness of the CPSs.
Homogeneous bone formation activity of the CPSs expanded by the STK media
Our clinical studies, wherein 10% FBS-M199 has been used for cell expansion, identified interindividual differences in the bone formation activity of CPSs, which were likely attributed to differences in biopsy procedures9 or age-dependent differences in the number of osteogenic progenitor cells and their potency in the periosteum samples.
Our flow-cytometry findings show that the CPSs of all protocol groups exhibited subsets of cells expressing MSC-specific surface markers and comparable expression profiles of these markers17 (Figure 3). Analysis of the mRNA expression of osteoblastic markers suggested suppression of final differentiation into osteoblasts in the 1% HS-STK1 group. On the other hand, upregulation of the mRNAs of osteoblastic markers and bone-related growth factors, as well as ALP activity; expression of OPN, Runx2, BMP-2/4, RANKL, and VEGF; and mineralization over a wide range of CPSs was detected in the 1% HS-STK1+3 group (Figures 4 –6). Taken together, it is suggested that periosteum explants consisting of a heterogeneous cell population were expanded to CPSs formed by more homogeneous osteogenic cells under the condition used in the 1% HS-STK1+3 protocol. In other words, it is likely that the STK1 medium designed for primary stem-cell culture is effective for maintaining and expanding osteogenic progenitor cells derived from the periosteum and consequently elevating the bone-forming potential of CPSs.
Although the composition of STK series media has not been disclosed, growth factors such as FGF2, platelet-derived growth factor, and TGF-β are generally necessary for maintenance, differentiation, and expansion of stem cells.25,26 Since FGF2 is involved in the maintenance and expansion of undifferentiated cells,27,28 it is commonly included in serum-free media for maintenance and expansion of embryonic stem cells.29 A previous study using a cell culture system reported that pretreatment of periosteal cells with FGF2 resulted in selective expansion of osteogenic progenitor cells and increased sensitivity to the subsequent BMP-2 stimulation that causes osteogenic induction.30 An in vivo study also demonstrated that sustained release of FGF2 simultaneously promotes the proliferation and differentiation of osteogenic progenitor cells, which consequently induces active proliferation of periosteal cells and bone formation.31 These findings are in accordance with the results of this study, which show that cell expansion in the STK media designed for stem-cell culture produces homogeneous CPSs with high osteogenic potential.
Conclusion
The use of the serum-free stem-cell culture medium successfully reduces the time required for CPS preparation while reducing the serum content in the medium to 1%. In addition to keeping the invasiveness of the blood collection low, which contributes to making the chemically defined culture medium the source of necessary growth factors, smaller variations in growth rate and differentiation profiles are expected. Our findings demonstrate that the stem-cell culture media effectively expand the osteogenic progenitor cells derived from the periosteum without losing their potential and that these cells form homogeneous and high-quality osteogenic graft materials upon subsequent differentiation stimuli. Since various cell sources are expected to be devised in the future, development of practical culture methods is of particular interest to clinical applications of cell-based bone-regeneration therapy. Detailed conditions of cell expansion remain to be defined.
Footnotes
Declaration of conflicting interests: There are no potential conflicts of interest.
Funding: This project was funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Sports, Science and Technology, Japan (grant number: 23592985).
References
- 1. Yun YR, Jang JH, Jeon E, et al. Administration of growth factors for bone regeneration. Regen Med 2012; 7(3): 369–385 [DOI] [PubMed] [Google Scholar]
- 2. Lichte P, Pape HC, Pufe T, et al. Scaffolds for bone healing: concepts, materials and evidence. Injury 2011; 42(6): 569–573 [DOI] [PubMed] [Google Scholar]
- 3. Pagni G, Kaigler D, Rasperini G, et al. Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev 2012; 64(12): 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz Z, Doukarsky-Marx T, Nasatzky E, et al. Differential effects of bone graft substitutes on regeneration of bone marrow. Clin Oral Implants Res 2008; 19(12): 1233–1245 [DOI] [PubMed] [Google Scholar]
- 5. Rickert D, Sauerbier S, Nagursky H, et al. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 2011; 22(3): 251–258 [DOI] [PubMed] [Google Scholar]
- 6. Tsumanuma Y, Iwata T, Washio K, et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011; 32(25): 5819–5825 [DOI] [PubMed] [Google Scholar]
- 7. Chen FM, Sun HH, Lu H, et al. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012; 33(27): 6320–6344 [DOI] [PubMed] [Google Scholar]
- 8. Nagata M, Hoshina H, Li M, et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone 2012; 50(5): 1123–1129 [DOI] [PubMed] [Google Scholar]
- 9. Simon TM, Van Sickle DC, Kunishima DH, et al. Cambium cell stimulation from surgical release of the periosteum. J Orthop Res 2003; 21(3): 470–480 [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro FV, Suaid FF, Ruiz KG, et al. Periosteum-derived cells as an alternative to bone marrow cells for bone tissue engineering around dental implants. A histomorphometric study in beagle dogs. J Periodontol 2010; 81(6): 907–916 [DOI] [PubMed] [Google Scholar]
- 11. Kawase T, Okuda K, Kogami H, et al. Characterization of human cultured periosteal sheets expressing bone-forming potential: in vitro and in vivo animal studies. J Tissue Eng Regen Med 2009; 3(3): 218–229 [DOI] [PubMed] [Google Scholar]
- 12. Kawase T, Okuda K, Kogami H, et al. Human periosteum-derived cells combined with superporous hydroxyapatite blocks used as an osteogenic bone substitute for periodontal regenerative therapy: an animal implantation study using nude mice. J Periodontol 2010; 81(3): 420–427 [DOI] [PubMed] [Google Scholar]
- 13. Müller I, Kordowich S, Holzwarth C, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 2006; 8(5): 437–444 [DOI] [PubMed] [Google Scholar]
- 14. Tannenbaum SE, Turetsky TT, Singer O, et al. Derivation of xeno-free and GMP-grade human embryonic stem cells—platforms for future clinical applications. PLoS One 2012; 7(6): e35325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uematsu K, Kawase T, Nagata M, et al. Tissue culture of human alveolar periosteal sheets using a stem-cell culture medium (MesenPRO-RS™): in vitro expansion of CD146-positive cells and concomitant upregulation of osteogenic potential in vivo. Stem Cell Res 2013; 10(1): 1–19 [DOI] [PubMed] [Google Scholar]
- 16. Kawase T, Kogami H, Nagata M, et al. Manual cryopreservation of human alveolar periosteal tissue segments: effects of pre-culture on recovery rate. Cryobiology 2011; 62(3): 202–209 [DOI] [PubMed] [Google Scholar]
- 17. Dominici M, Le Blanc K, Müller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8(4): 315–317 [DOI] [PubMed] [Google Scholar]
- 18. Mahajan A. Periosteum: a highly underrated tool in dentistry. Int J Dent 2012; 2012: Article id 717816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saharinen J, Hyytiäinen M, Taipale J, et al. Latent transforming growth factor-beta binding proteins (LTBPs)—structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev 1999; 10(2): 99–117 [DOI] [PubMed] [Google Scholar]
- 20. Sengle G, Charbonneau NL, Ono RN, et al. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem 2008; 283(20): 13874–13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs. Curr Opin Struct Biol 2005; 15(5): 506–516 [DOI] [PubMed] [Google Scholar]
- 22. Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell 2010; 21(5): 687–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasso R, Gaetani M, Molino E, et al. The role of bFGF on the ability of MSC to activate endogenous regenerative mechanisms in an ectopic bone formation model. Biomaterials 2012; 33(7): 2086–2096 [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Yamato M, Nishida K, et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J Controlled Release 2006; 116(2): 193–203 [DOI] [PubMed] [Google Scholar]
- 25. Dvorak P, Dvorakova D, Koskova S, et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells 2005; 23(8): 1200–1211 [DOI] [PubMed] [Google Scholar]
- 26. Ng F, Boucher S, Koh S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008; 112(2): 295–307 [DOI] [PubMed] [Google Scholar]
- 27. Chaudhary LR, Hofmeister AM, Hruska KA. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 2004; 34(3): 402–411 [DOI] [PubMed] [Google Scholar]
- 28. Lee JH, Um S, Jang JH, et al. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res 2012; 348(3): 475–484 [DOI] [PubMed] [Google Scholar]
- 29. Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells 2005; 23(3): 315–323 [DOI] [PubMed] [Google Scholar]
- 30. Agata H, Asahina I, Yamazaki Y, et al. Effective bone engineering with periosteum-derived cells. J Dent Res 2007; 86(1): 79–83 [DOI] [PubMed] [Google Scholar]
- 31. Kodama N, Nagata M, Tabata Y, et al. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone 2009; 44(4): 699–707 [DOI] [PubMed] [Google Scholar]