Abstract
Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) is a recently defined inflammatory central nervous system (CNS) disorder, prominently involving the brainstem and in particular the pons. The condition features a combination of clinical symptoms essentially referable to brainstem pathology and a characteristic magnetic resonance imaging (MRI) appearance with punctate and curvilinear gadolinium enhancement ‘peppering’ the pons. The radiological distribution is focused in the pons and adjacent rhombencephalic structures such as the cerebellar peduncles, cerebellum, medulla and the midbrain. While the lesion burden with a perivascular pattern is typically most dense in these pontine and peripontine regions, enhancing lesions may additionally extend into the spinal cord and supratentorial structures such as the thalamus, basal ganglia, capsula interna, corpus callosum and the cerebral white matter. Another core feature is clinical and radiological responsiveness to glucocorticosteroid (GCS)-based immunosuppression. As withdrawal of GCS treatment results commonly in disease exacerbation, long-term immunosuppressive therapy appears to be mandatory for sustained improvement. Diagnosis of CLIPPERS is challenging, and requires careful exclusion of alternative diagnoses. A specific serum or cerebrospinal fluid (CSF) biomarker for the disorder is currently not known. Pathogenesis of CLIPPERS remains poorly understood, and the nosological position of CLIPPERS has still to be established. Whether CLIPPERS represents an independent, actual new disorder or a syndrome that includes aetiologically heterogeneous diseases and/or their prestages remains a debated and not finally clarified issue. Clinicians and radiologists should be aware of this condition and its differential diagnoses, given that CLIPPERS constitutes a treatable condition and that patients may benefit from an early introduction of GCS ensued by long-term immunosuppression. Based on previous reports in literature – currently encompassing more than 50 reported cases of CLIPPERS – this review addresses clinical features, diagnostic criteria, differential diagnoses and therapeutic management of this peculiar disorder.
Keywords: brainstem, CLIPPERS, glucocorticosteroids, neuroinflammation, perivascular infiltration
Other Articles Published in this Series.
Paraneoplastic neurological syndromes. Clinical and Experimental Immunology 2014, 175: 336–48.
Diagnosis, pathogenesis and treatment of myositis: recent advances. Clinical and Experimental Immunology 2014, 175: 349–58.
Disease-modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies. Clinical and Experimental Immunology 2014, 175: 359–72.
Monoclonal antibodies in treatment of multiple sclerosis. Clinical and Experimental Immunology 2014, 175: 373–84.
Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clinical and Experimental Immunology 2014, 175: 397–407.
Myasthenia gravis: an update for the clinician. Clinical and Experimental Immunology 2014, 175: 408–18.
Cerebral vasculitis in adults: what are the steps in order to establish the diagnosis? Red flags and pitfalls. Clinical and Experimental Immunology 2014, 175: 419–24.
Multiple sclerosis treatment and infectious issues: update 2013. Clinical and Experimental Immunology 2014, 175: 425–38.
Introduction
Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) represents a rare central nervous system (CNS) inflammatory disorder involving predominantly the pons. The disorder was first described in 2010 by Pittock and colleagues as a distinct form of brainstem encephalitis centred on the pons, which is characterized by a predominant T cell pathology, and responsive to immunosuppression with glucocorticosteroids (GCS) [1]. All eight patients of the authors' series had clinical symptoms related to brainstem involvement, particularly gait ataxia and diplopia. Other accompanying symptoms included dysarthria, altered sensation and paraesthesias of the face, dizziness, nystagmus, spastic paraparesis, sensory loss and pseudobulbar affect. More striking was a characteristic pattern of magnetic resonance imaging (MRI) changes, consisting of ‘punctate and curvilinear’ perivascular gadolinium enhancement ‘peppering’ the pons as well as additional parts of the rhombencephalon, such as the brachium pontis, the medulla, the midbrain and/or the cerebellum. In some patients a subtler radiating pattern of similar enhancing lesions was noted beyond the rhombencephalon, extending variably into the spinal cord or supratentorial CNS structures (e.g. into the basal ganglia and the corpus callosum). Cerebrospinal fluid (CSF) analysis revealed inconsistant patterns, including mild pleocytosis, mildly elevated protein and/or (in part transient) CSF oligoclonal bands. CSF cytology was negative for malignant cells in all eight patients. Pittock et al. [1] and subsequently also other study groups carried out extensive laboratory, CSF, imaging and pathological surveys to carefully exclude an abundance of alternative causes for the condition. Evidence of specific inflammatory, demyelinating, infectious, neoplastic, paraneoplastic or vasculitic disorders could not be noted. Importantly, four patients underwent brain biopsy, which demonstrated predominantly T cell infiltration in the hindbrain white matter, largely but not wholly perivascular in distribution, accompanied by a moderate number of histiocytes and activated microglia. All patients had a favourable initial clinical response to high-dose GCS administration, reflected by clinical and concomitant radiological improvement. However, the patients routinely worsened following GCS taper and required chronic GCS or other immunosuppressive treatment as maintenance therapy. In consideration of the T cell-predominant, perivascular inflammatory pathology in affected CNS tissue, the clinico-radiological response to immunosuppressive therapies, and no explicit evidence for other underlying disease aetiologies, CLIPPERS has been proposed to be an immune-mediated, inflammatory process of unknown aetiology. Reports of similar cases and case–series have followed quickly since the first description of CLIPPERS in 2010 (Table 1), which recapitulated key features of the initial observations, but also accounted to extend the phenotype of the new defined entity. In this review, we will overview the clinical, radiological and pathological features of CLIPPERS and emphasize diagnostic and therapeutic aspects.
Table 1.
Reported cases of CLIPPERS*.
| Author/source | Country | Cases | Sex |
Biopsies | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Pittock et al. [1] | United States and Belgium | 8 | 3 | 5 | 4 |
| Duprez and Sindic [20] | Belgium | 1 | – | 1 | – |
| Gabilondo et al. [19] | Spain | 1 | – | 1 | – |
| Jones et al. [21] | United Kingdom | 1 | – | 1 | 1 |
| Kastrup et al. [10] | Germany | 3 | 3 | – | 3 |
| List et al. [17] | Germany | 1 | – | 1 | – |
| Huhn and Obermann [13] | Germany | 1 | 1 | – | 1 |
| Taieb et al. [18] | France | 1 | 1 | – | 1 |
| Biotti et al. [22] | France | 1 | 1 | – | – |
| Lefaucheur et al. [23] | France | 1 | 1 | – | – |
| Simon et al. [7] | Australia | 5 | 4 | 1 | 5 |
| Taieb et al. [2] | France | 12 | 9 | 3 | 7 |
| Sczesni et al. [12] | Germany | 1 | 1 | – | – |
| Humbroich et al. [25] | Germany | 1 | 1 | – | – |
| Hillesheim et al. [9] | United States | 1 | 1 | – | 1 |
| Tohge et al. [11] | Japan | 1 | – | 1 | – |
| Sempere et al. [24] | Spain | 1 | 1 | – | – |
| Wetter et al. [26] | Germany | 1 | 1 | – | 1 |
| Wijntjes et al. [27] | Netherlands | 1 | 1 | – | – |
| Sonoda et al. [48] | Japan | 1 | 1 | – | – |
| Safdieh et al. [28] | United States | 5 | 4 | 1 | 5 |
| Maddison et al. [49] | United Kingdom | 1 | n.r. | n.r. | – |
| Egelseer et al. [8] | Germany | 1 | 1 | – | – |
| Mann et al. [40] | Germany | 1 | 1 | – | – |
| Hidarilak et al. [39] | United States | 1 | – | 1 | – |
| Al-Mufti et al. [50] | United States | 1 | – | 1 | – |
| Tarabishy et al. [51] | United States | 1 | n.r. | n.r. | – |
| Pesaresi et al. [52] | Italy | 1 | 1 | – | – |
| Total | 56 | 37 (+2)† | 17 (+2)† | 29 | |
Reports of extraordinary and mimicking cases of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) were not included in this table. In addition to some extraordinary cases of CLIPPERS (Ortega et al. [29], Ferreira et al. [34], Buttmann et al. [32]), several presumptive cases of CLIPPERS were reported, in which other discrete diseases were finally diagnosed (Limousin et al. [30], De Graaff et al. [31], Jones et al. [21]).
In two cases sex of the affected patient was not identifiable; n.r.: not reported.
Pathogenesis
The pathogenesis of CLIPPERS is poorly understood and ultimately unknown. The perivascular and T cell-predominant inflammatory cell infiltrates in affected CNS lesions, patterns of CSF changes and typical gadolinium enhancement together with the clinico-radiological response to GCS-based immunosuppressive therapies suggest an (auto-)immune-mediated or other inflammatory pathogenesis [1]. Characteristic imaging patterns of punctate enhancement involving white and grey matter and neuropathology with prevailing perivascular inflammatory infiltrates favour an inflammatory disorder with a vascular or perivascular tropism [2]. An intriguing and as-yet unexplained feature is why the perivascular inflammation involves predominantly the pontine and adjacent hindbrain areas. To explain this particular feature of CLIPPERS, Pittock et al. [1] and other authors discussed a specific immune-mediated process directed against an (auto-)antigenic epitope in perivascular regions. These immunological targets may be microstructures localized preferentially in the pons and peripontine regions, which are not characterized to date. Considering the anatomical arrangement of small intra-axial veins of the CNS, the predominant involvement of brainstem structures might also be related to a primary venous inflammatory CNS disorder [2]. Furthermore, the brainstem seems particularly susceptible to immune attack, as illustrated by the entities of Bickerstaff encephalitis and neuro-Behçet's disease [3,4]. The separate but no less interesting phenomenon of paraneoplastic brainstem encephalitis adds further illustration of this anatomical–immunological diathesis [5,6]. Effectors of the inflammation process seem to be T lymphocytes, with a predominance of CD4 cells, as seen in brain biopsy specimens [7] and CSF samples in a study by Taieb and coworkers [2]. The preponderance of CD4 T cells noted in CLIPPERS pathology might suggest an immune response in context of the major histocompatibility complex (MHC) class II-restricted antigen presentation. CD4 T cells recognize mainly peptides derived from exogenous proteins and presented by MHC II class molecules, whereas activation of CD8 T cells depends usually on endogenous cytosol-derived peptides supplied by MHC class I molecules. Hence, a T cell-mediated response to exogenous antigens (or environmental allergens) might be assumed rather than a response to intracellular pathogens (e.g. intracellular infections, abnormal proteins in cancerous cells). Potential predispositions or triggering events of the postulated immune mediated/inflammatory process are unclear. In individual cases, preceding vaccinations may act as a trigger [8,9]. It can also be speculated that an allergic trigger factor may contribute to the evolution of the perivascular inflammation process. Elevated serum immunoglobulin (Ig)E levels, which were noted in several cases [10–13], may be seen in this context. Atopic disease and in particular atopic myelitis [14,15], pathological conditions presenting with elevated IgE levels in response to various environmental antigens, may offer clues to the link of elevated IgE and distinct forms of neuroinflammatory diseases such as CLIPPERS [11].
Collectively, pathogenetic concepts in CLIPPERS are still based principally on assumptions and speculations. Further systematic studies should focus on defining more clearly the hallmark immunopathological, radiological and serological features that set apart the disorder from other inflammatory CNS disorders. More in-depth investigations of immunopathological findings and cerebrospinal fluid assays on cytokines and chemokines may provide an insight into the nature of inflammation in this condition. The identification of a novel antibody association – in analogy to the aquaporin-4 autoantibody in erstwhile similarly cryptic neuromyelitis optica [16] – would surely secure CLIPPERS as a new disease in the spectrum of immune-mediated disorders of the CNS.
Clinical characteristics
CLIPPERS manifests characteristically in a subacute manner and presents usually with a varying symptomatology related to brainstem, cranial nerve and/or cerebellar involvement, frequently including gait ataxia, dysarthria, diplopia and/or altered facial sensation. An overview on clinical characteristics described in CLIPPERS patients is shown in Table 2. Clinical manifestations may be heterogeneous, multi-faceted and variable in individual cases, but comprise essentially the following.
Commonly prominent symptoms related to multilocular brainstem including cranial nerve and cerebellar involvement, which may present in various combinations or rarely in isolation (e.g. ataxia, dysarthria, oculomotor abnormalities, tingling of the face, vertigo) [1,7,9–13,17–27]; and
Possible additional features such as: (i) symptoms referable to long tract affections and/or a spinal cord syndrome (e.g. pyramidal tract signs, spasticity, para-/tetraparesis, altered limb superficial and deep sensation, sphincteric dysfunction) [1,7,8,11,20,22,27]; (ii) pseudobulbar affect (pathological crying and laugther) [1,7,17]; (iii) cognitive dysfunctions (e.g. mnestic deficits, dysexecutive syndrome) [7,10,13]; presumably also (iv) headaches [7,28] and abnormal fatigue [1,7,9,17,28].
Table 2.
Clinical features of CLIPPERS*.
| Symptoms/signs referable to brainstem-, cranial nerve-and/or cerebellar dysfunctions |
|
| Symptoms/signs referable to long tract affections and/or spinal cord syndrome |
|
| Cognitive dysfunction |
|
| Possible additional features |
|
| Generally absent symptoms/signs |
|
Symptoms/signs marked in italic type are encountered prevalently in CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) patients.
Meningism, alterations of quantitative consciousness, significant systemic symptoms (such as fever, night sweating, weight loss, lymphadenopathy) and symptoms related to connective tissue diseases, rheumatic disorders or Behçet's disease (such as arthritis, uveitis, sicca syndrome, oral and/or genital ulcera, pathergia) are generally not a feature of CLIPPERS patients.
There are obviously considerable differences regarding the age at onset of CLIPPERS, ranging from 13 to 86 years. In larger series, the mean age at onset was 52·4 years (range 16–86 years) [1], 43·4 years (range 20–65 years) [7] and 46·5 years (range 13–64 years) [2], respectively. The disorder affects both genders. A minor male preponderance may be assumed considering the hitherto reported cases (Table 1).
Initial symptoms usually evolve subacutely over several weeks. The clinical course without specific treatment seems to be relapsing–remitting in nature [2]. Progressive clinical worsening is seen during relapses, which may leave residual neurological sequelae.
Neuroimaging
MRI findings in CLIPPERS show a very striking and characteristic lesion pattern and a high degree of similarity among affected individuals. Because the typical radiological picture is readily identifiable and represents a core criterion of CLIPPERS, brain and spinal cord MRI examinations play a crucial role in the diagnostic process. The hallmark feature on MRI is that of multiple ‘punctate’ (‘speckled’; ‘patchy spot-like’) and/or ‘curvilinear’ gadolinium enhancement ‘peppering’ the pons with or without spread into the cerebellar peduncles and the cerebellum (Fig. 1). The lesions may extend into adjacent CNS structures, caudally to the medulla oblongata or the cervicothoracal spinal cord, rostrally to the midbrain, and even involve supratentorial regions such as the thalami, capsula interna, basal ganglia, corpus callosum and cerebral white matter [1,10,11,18–20]. It seems to be typical of CLIPPERS that visualization of lesions is somewhat faint on T2-and fluid-attenuated inversion recovery (FLAIR)-weighted images and becomes clearly apparent on the enhanced scans, disclosing the patchy spot-like gadolinium enhancement in a ‘salt-and-pepper like appearance’ [10]. The enhancement corresponds to the lymphocytic perivascular inflammatory pattern seen histologically and described later in this review. Lesions are typically less numerous and smaller as distance from the pons increases [1]. They usually cause no mass effect and minimal or no vasogenic oedema. In some exceptional cases brainstem mass effect in the form of pons or middle cerebellar peduncle swelling was observed during relapses [2]. Magnetic resonance as well as digital subtraction angiography of intracranial and neck vessels do not display specific abnormalities, in particular no changes seen in vasculitic disorders [1,7,10,12,24,29]. Gadolinium enhancement decreases as the patient responds to immunosuppressive therapy [1,7,10]. Pontocerebellar/cerebellar, spinal cord and cerebral atrophy may be observed in the course of the disease [2,7], the latter particularly in patients with cognitive impairment [7].
Figure 1.
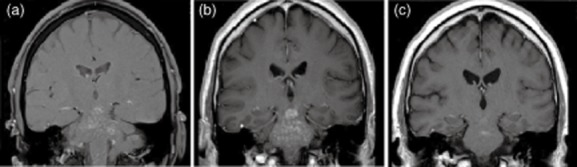
Brain magnetic resonance imaging (MRI) of a patient with CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) (coronar post-contrast T1-weighted images). (a) Initial MRI shows foci of gadolinium enhancement with a punctate and curvilinear pattern predominantly in the pons, the cerebellar peduncles and the mesencephalon. (b) Progression of gadolinium-enhancing lesions 6 weeks later. (c) Following oral glucocorticosteroids (GCS) and additive methotrexate therapy, a follow-up MRI performed 5 months later shows marked reduction in the extent of gadolinium enhancing lesions. Figures reproduced from [25], with kind permission of Springer Science + Business Media.
Diagnosis
Diagnosis of CLIPPERS is based on clinical, radiological, laboratory and CSF investigations and, if necessary, brain biopsy. Extensive investigations are mandatory to exclude alternative conditions that may mimic CLIPPERS syndrome. To date, validated diagnostic criteria for CLIPPERS are not available. Due to the varied clinical presentation and the potential for diagnostic confusion, Simon et al. [7] has highlighted core features of CLIPPERS including clinical (I), radiological (II), GCS response (III) and histopathological (IV) criteria (Table 3). Evidence of an inflammatory CNS disorder matching the core features I–III and exclusion of alternative causes by extensive non-invasive diagnostic procedures may allow a highly probable diagnosis of CLIPPERS. In this constellation, avoidance of brain biopsy – which would enable evaluation of the histopathological criteria (IV) – may be justified. Such a non-invasive approach in ‘typical cases’ was already suggested by Pittock et al. [1], who treated 50% of patients without biopsy. In some cases, however, which clinically and radiologically seemed to be compatible with CLIPPERS, other underlying conditions were detected only by biopsy [e.g. primary CNS lymphoma (PCNSL) [30], lymphomatoid granulomatosis that evolved into fatal B cell lymphoma of the CNS [31]; low-grade glioma [21], potentially primary angiitis of CNS (PACNS) [32]]. Performance of a biopsy, usually a brainstem or cerebellar lesion biopsy from areas that are specifically involved radiologically, should therefore be recommended in cases which exhibit one or more of the following attributes: (i) alternative aetiologies remain a distinct possibility despite rigorous investigations, (ii) uncommon, atypical clinical or MRI-findings are noticed (e.g. signs of systemic disease; MRI disclosing dominant brainstem mass effects or necroses) and (iii) resistance to GCS treatment is evident. In these cases, brain biopsy may finally add the definitive support for a similar pattern of CNS perivascular lymphocytic inflammation as in the originally described CLIPPERS patients and exclude other diagnoses.
Table 3.
Core features of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) (adapted from Simon et al. [7]).
| I. Clinical |
|
| II. Radiological |
|
| III. Glucocorticosteroid responsiveness |
|
| IV. Histopathological |
|
| Differential diagnoses should be excluded |
| e.g. neurosarcoidosis, Sjögren's syndrome, neuro-Behçet's disease, MS, ADEM, NMO, Bickerstaff encephalitis, other autoimmune encephalitides, CNS vasculitis, CNS infections, histiocytosis, lymphoma, glioma, paraneoplastic syndromes |
MS: multiple sclerosis; ADEM: acute disseminated encephalomyelitis; NMO: neuromyelitis optica; CNS: central nervous system.
Relevant differential diagnoses of CLIPPERS are depicted in Table 4 and include neurosarcoidosis, neuro-Behçet's disease, Sjögren's syndrome, inflammatory demyelinating CNS diseases such as multiple sclerosis (MS) and acute disseminated encephalomyelitis (ADEM), neuromyelitis optica (NMO), Bickerstaff brainstem encephalitis and other autoimmune encephalitides, CNS vasculitides, including PACNS, CNS infections, CNS histiocytosis, lymphomatoid granulomatosis, CNS lymphoma, glioma and paraneoplastic disorders [1,7,9,10,17,21,29–35]. The following sections summarize the auxiliary diagnostic procedures and findings in hitherto reported cases, accomplished to complete the essential clinical and MRI examinations.
Table 4.
Relevant differential diagnoses of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids).
| Disease entity | Diseases |
|---|---|
| Dominant inflammatory |
|
| Infectious |
|
| Paraneoplastic |
|
| Dominant neoplastic (and/or clonal proliferative) |
|
CNS: central nervous system; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA: N-methyl-D-aspartate; GABA: gamma-aminobutyric acid; GAD: glutamic acid decarboxylase; VGKC: voltage-gated potassium channel.
Laboratory investigations
Basically, seminal abnormalities were not detected in thorough laboratory and serological studies. Extensive work-ups were performed by virtually all study groups (e.g. [[1],[[2],[[7],[[10],[[12],[[17],[[22],[[25],[[27]), and included assessments for:
Complete blood count, routine blood chemistry, C-reactive protein, erythrocyte sedimentation rate
Autoantibodies and markers of vasculitis: anti-nuclear antibodies (ANA), extractable nuclear antigens (ENA), p-/c-anti-neutrophil cytoplasmic antibodies (ANCA), dsDNA antibodies, anti-phospholipid antibodies, aquaporin-4 antibodies, anti-ganglioside antibodies (e.g. anti-GQ1b antibodies), rheumatoid factor, circulating immune complexes, cryoglobulins
Onconeuronal antibodies/paraneoplastic antibodies (targeting intracellular antigens), e.g. anti-Hu-,-Yo,-Ri,-Ma/Ta,-collapsin response mediator protein 5 (CRMP5),-amphiphysin,-Purkinje cell cytoplasmic antibody type 2 (PCA2),-anti-neuronal nuclear antibody 3 (ANNA3) antibodies; facultative paraneoplastic antibodies (targeting surface neuronal antigens), e.g. anti-voltage-gated potassium channel (VGKC),-voltage-dependent calcium channel (VGCC),-N-methyl-D-aspartate (NMDA) antibodies; and anti-glutamic acid decarboxylase (GAD) antibodies
Tumour markers; paraproteins
Angiotensin-converting enzyme, soluble interleukin (IL)-2 receptor level
Examinations for infectious agents (by appropriate serological testing methods in serum and CSF samples, bacterial/fungal cultures from blood and CSF and CSF PCR studies) revealed no association that would point to an infectious process. These surveys were performed for viral [e.g. herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus (HHV) 6 and 8, John Cunningham virus (JCV) and BK virus (BKV), parvovirus B19, adenoviridae, enteroviridae, flaviviridae, hepatitis C virus (HCV), hepatitis B virus (HBV), human immunodeficiency virus (HIV), human T cell lymphotrophic virus (HTLV)], bacterial (e.g. Treponema pallidum, Borrelia burgdorferi, Tropheryma whipplei, Brucella spp., Coxiella spp., Chlamydia spp., Mycoplasma spp., Listeria monocytogenes, Mycobacterium tuberculosis complex), fungal (e.g. cryptococcus spp.) and parasitic (e.g. Toxoplasma spp., Echinococcus spp.) agents.
Some peculiar findings in single cases deserve a special mention, as follows.
Slightly elevated autoimmune antibodies (elevated ANA, dsDNA or SSB/SSA antibodies): in several cases somewhat elevated anti-nuclear [1,7,17,26], double-stranded DNA [17] and Sjögren's syndrome antigen A (SSA) [7] or B (SSB) [1] antibodies were observed. These findings – without a constant pattern and without identification of a distinct clinical correlate of a familiar autoimmune disorder – were determined to be of doubtful pathogenic significance in the majority of these cases. Similar findings have also been observed in patients with other immune-mediated CNS diseases [e.g. multiple sclerosis (MS), neuromyelitis optica (NMO)] and may thus be considered an unspecific phenomenon associated with these immune-mediated disease processes.
Raised level of angiotension converting enzyme (ACE): in one of seven patients tested in the series of Pittock and colleagues an elevated value of ACE was detected [1]. In other series [2,7,10] and case reports no increased ACE levels could be found.
Hypercalcaemia with high 1,25-dihydroxy vitamin D3 level: in one of five cases in Simon et al.'s series mildly elevated serum corrected calcium and elevated 1,25-dihydroxycholecalciferol prior to any treatment was found, which normalized with GCS therapy. The patient had low serum ACE level, normal thoracic CT (with no evidence of hilar lymphadenopathy) and whole-body gallium scans [7].
Moderate panlymphocytopenia (moderate CD4 and CD8 lymphocytopenia): in a case described by List et al. [17], laboratory work-up revealed a moderate CD4 (420 μl; normal range 500–1200 μl) and CD8 (100 μl, normal range 300–800 μl) lymphocytopenia, while the remaining blood count was unremarkable. The significance of this finding remained unclear.
Elevation of serum IgE-levels: in several cases, markedly and persistent elevated serum IgE levels were noted (in two of three patients [10] and in three further cases [11–13]). The study groups of Kastrup and Sczesni found neither an accompanying hypereosinophilia nor evidence for systemic internal, dermal or allergological conditions in their patients, which had no history of asthma, allergies or a Churg–Strauss syndrome [10,12]. To date, the significance of elevated IgE levels in the context of CLIPPERS is unclear. It was speculated that an allergic trigger factor may contribute to the evolution of the perivascular inflammation process in CLIPPERS [10,12]. Similarities to atopic disease/dermatitis were highlighted by Tohge et al. [11].
CSF analyses
CSF analysis in CLIPPERS may reveal either normal findings or mildly to moderately elevated protein levels (usually up to 1 g/l) and/or mild pleocytosis (leucocyte count usually between 5 and 50/μl, with a predominant lymphocytic or lymphomonocytic pattern) [1,2,7,10,12,17]. An increased CSF T cell ratio of CD4 : CD8 was detected in three of four patients analysed in the study by Taieb et al. [2]. Monoclonal lymphocytes or malignant cells must be excluded by cytomorphology and flow cytometry immunophenotyping. Infectious agents were not found in any case of CLIPPERS. Interestingly, some authors reported CSF-restricted oligoclonal bands which, when serially assessed, were often observed as a transient phenomenon [1,2,17]. The non-persistent occurrence of oligoclonal bands recalls other immunological, non-MS pathologies such as neurosarcoidosis, acute disseminated encephalomyelitis (ADEM) and primary Sjögren's syndrome, whereas resolution of CSF oligoclonal bands is felt to be at least highly atypical or even exclusionary of MS.
Other technical investigations including non-MR imaging
Next to MRI of the brain and the spinal cord, various additional imaging investigations have been performed in CLIPPERS patients and usually revealed no seminal findings. The examinations included chest X-rays, computerized tomography (CT) scans of the chest, abdomen and pelvis [1,10,12,21,26,27,29], whole-body positron emission tomography (PET) and fluorodeoxyglucose (FDG)-PET/CT scans [1,7,10,18,19,22,26,27], and brain PET scans [7,19]. In some patients biopsies of tissues apart from the CNS (e.g. parotid/labial salivary glands [7,18,22], conjunctiva [1,7], lung [1], upper jejunum [26], bone marrow [7,12] and skin [7]) have been performed and generally showed no relevant pathology.
CNS biopsies and neuropathology
The pathological phenotype of CLIPPERS is not specific. Biopsy of an affected brain structure will primarily help to rule out important differential diagnoses. Although not specific, particular neuropathological findings may be found which are consistent with CLIPPERS and add final support to the diagnosis. Sites chosen for brain biopsy are usually the pons or the cerebellum, but also other affected regions in the brainstem or in supratentorial locations such as the basal ganglia. Notwithstanding the delicate site of disorder, brainstem biopsies are seen increasingly as safer than often previously considered [36,37].
Pathology of biopsy specimens from CLIPPERS patients reveals a striking perivascular, CD4-dominated T cell or lymphohistiocytic infiltrate [1,2,7,32]. The inflammatory infiltration, largely perivascular, but also more diffuse parenchymal in distribution, involves basically the white matter, but also the grey matter and scantily leptomeningeal tissue. The perivascular distribution affects both small arteries and veins. Single vessels may reveal a transmural lymphocytic infiltrate (without granulomas within the vessel wall), split walls and occasionally inflammatory vessel occlusion, although the characteristic histological features of vasculitis (in terms of destruction of the vessel wall with fibrinoid necrosis, leucocytoclasia, fibrin thrombi) could not be identified [7,32]. The infiltrate comprises lymphocytes and is usually accompanied by a variable number of histiocytes and activated microglia. B cells and plasma cells are found in lower numbers; neutrophils and eosinophils are normally absent. Immunohistochemical stains demonstrate that the cellular infiltrate is composed predominantly of CD4-positive T cells and histiocytes; analysis of T cells reveals commonly a high CD4 : CD8 ratio [1,2,7,32]. The severity of inflammation may be variable among individual cases, and ranges from large nodules and sheets of inflammation to scattered foci of inflammatory cells [7]. Accompanying reactive gliosis may be noted except in areas of intense perivascular inflammation, where fragmentation and beading of astrocytes can occur [7,32]. Biopsies in Simon et al.'s series also demonstrated neuro-axonal injury, possibly explaining atrophy and disability that may develop over time [7]. Simon and co-workers noted that varying degrees of myelin loss in small areas may accompany the most intense inflammatory infiltrate (considered as a secondary phenomenon), but focal demyelination in isolation was not seen. Focal neuronophagia has been observed in some cases [7].
Differential diagnoses
CLIPPERS syndrome has a broad spectrum of differential diagnoses, which are listed in Table 4. Patients whose clinico-radiological constellations initially implied the diagnosis of ‘CLIPPERS/presumptive CLIPPERS’ later turned out to suffer from the following conditions:
Biopsy-confirmed lymphomatoid granulomatosis that evolved into fatal B cell lymphoma of the central nervous system [31]
Primary central nervous system lymphoma (PCNSL) [30]
Low-grade glioma [21]
Potential primary angiitis of the CNS: this case presented with a number of clinical and paraclinical features described as typical for CLIPPERS, but additionally showed symptoms and findings compatible with PACNS [32]
Furthermore, two cases of ‘superimposed’ CLIPPERS in patients with pre-existing MS were described [29,34]. Features of CLIPPERS developed in one of these MS patients shortly after withdrawal of natalizumab [34]. The authors of this case report hypothesized that CLIPPERS resulted from an immune reconstitution inflammatory syndrome (IRIS) after natalizumab discontinuation.
In their reply to the original paper, Taieb et al. [38] doubted immune reconstitution pathophysiological mechanism, as IRIS in CNS is characterized histologically by a pronounced inflammatory infiltrate with a predominance of CD8 T cells, not consistent with the findings in the reported case with a cellular infiltrate composed predominantly of CD4 T cells (CD4 : CD8 ratio of 2:1).
‘Red flags’
Analysis of the above-mentioned and other cases yielded some important ‘red flags’ that should alert physicians to an incorrect diagnosis and raise concern regarding illness other than CLIPPERS:
No response to treatment with GCS at the beginning or during follow-up. GCS therapy failure seems to be a very strong indicator for an alternative diagnosis and raises the possibility of a tumour or neuronal degeneration [21,30,31]
Unusual clinical findings such as fever, marked B symptoms, extracerebral organ manifestations (such as arthritis, uveitis, sicca syndrome, lymphadenopathy, etc.) and meningism should lead to increased alertness. Conversely, some findings such as dysarthria and ataxia are so common in CLIPPERS that their absence should be considered a hint that the disorder might be something else
MRI findings: although they may be subtle, abnormalities of brainstem are so common in CLIPPERS that their absence is worth noting. Pontine lesions with necrosis may point to a PCNSL [30] and marked mass effects to CNS tumours in general
CSF findings: marked pleocytosis (> 100/μl) or malignant cells should prompt reevaluation of the diagnosis
Treatment
So far, relatively few patients with CLIPPERS have been reported and it is difficult to recommend general therapeutic proceedings. CLIPPERS, as the term already implies, represents a typically steroid-responsive disorder: both clinical and radiological responses can be noted following GCS therapy. With the use of GCS, patients usually show early and marked clinical improvement within days, although in many cases the restitution may remain incomplete. Simultaneously, a reduction or even a clear disappearance of enhancing lesions can be noted as the patients responds to immunosuppressive therapy [1,2,7]. Despite relatively prompt improvement of the imaging correlates of CLIPPERS, patients may develop brainstem, cerebellar, spinal cord and even cortical atrophy in follow-up MRI studies [2,7].
The initial treatment of choice seems to be a relatively short course of high-dose intravenous methylprednisolone (e.g. methylprednisolone 1 g over 5 days), followed by oral GCS. This regimen should also be applied and started as early as possible in case of relapses [2,7]. Attempts to withdraw or taper GCS below a particular lower dose limit usually provoke the recurrence of inflammation, accompanied by a relapse of clinical symptoms as well as MRI activity signs. The high risk of relapse during reduction of GCS [1,10] and the chronicity of the disorder account for the reason to establish a maintenance immunosuppressive therapy, usually consisting of an oral GCS combined with a GCS-sparing immunosuppressant. This long-term therapy with immunosuppressive agents seems to be necessary to maintain remission [1,2,10,19,20]. The following therapeutic substances were used in long-term therapy.
Chronic glucocorticosteroid therapy
Chronic GCS therapy seems to be necessary, as attempts to taper oral GCS below a daily dose of 10–20 mg (prednisone equivalent) [2,12,19] leads almost inevitably to neurological relapse. As chronic GCS therapy is limited by GCS side effects, additional GCS-sparing agents were commonly used to reduce the daily glucocorticosteroid dose in long-term therapy. It seems noteworthy, however, that some immunosuppressive agents, given alone without sustained GCS therapy, are obviously not capable of maintaining remission and therefore cannot replace GCS completely.
GCS sparing immunosuppressants
Various immunosuppressive agents, given as monotherapy alone or combined with sustained oral GCS, have been used to maintain clinical improvements and prevent further relapses. So far, an independent benefit of GCS-sparing therapies could not be substantiated reliably. After complete GCS withdrawal, only methotrexate [1,17,24] and potentially rituximab [2] were described to be effective in a few patients.
The following immunmodulatory or immunosuppressive agents have been used for long-term therapy in hitherto published cases, either as monotherapy or as add-on therapy. Although at least partial benefits were reported, their GCS-independent efficacy could not been proved so far:
azathioprine (as add-on therapy [12,19,22,27,39]; as monotherapy in one patient not controlling recurrence [1])
methotrexate, weekly oral administration (as add-on therapy [1,9,10,25–27]; as monotherapy [1,10,24])
cyclophosphamide, intravenous pulses (as add-on therapy [2,10,13,40] and as monotherapy [7]: mainly reported to improve the clinical syndrome although presumably not protecting against subsequent relapse)
rituximab (anti-CD20 monoclonal antibody treatment) (as add-on therapy [18] or monotherapy [2] possibly beneficial) [41–43]
There is some evidence that intravenous immunoglobulin (IVIG) therapy [19] and oral hydroxychloroquine [1] are not effective. Considering the histiocytic components found in CLIPPERS pathology, it was suggested that the use of tumour necrosis factor-inhibiting drugs such as infliximab should be explored in the treatment of CLIPPERS [7]. To the best of our knowledge, this therapeutic approach has not been tried so far.
It appears that prompt recognition of the disease and early and vigorous pulse GCS treatment with an ensuing maintenance immunosuppression result in the best long-term functional outcome. Prompt pulse GCS treatment of relapses may limit clinical worsening during relapses and permanent neurological sequelae [2,7]. Regarding maintenance therapy, independent beneficial effects of specific immunosuppressive/modulating agents alone or as add-on treatment need to be proved in further studies. Moreover, follow-up studies are necessary to determine the duration of a prolonged GCS-based maintenance therapy.
Concluding remarks
CLIPPERS is a newly described pontine-centric inflammatory disorder with distinct clinical and radiological features. The cardinal feature of the condition is a punctate and/or curvilinear gadolinium enhancement, ‘peppering’ the pons and adjacent hindbrain structures on MRI. Since 2010, the unique MRI features of this condition have attracted the attention of many clinicians, neuroradiologists and pathologists, leading to an increasing number of case reports and small case-series. To date, these comprise more than 50 cases (Table 1) and are accounted to extend the clinical, neuroimaging and pathological phenotypes of the disorder. However, the pathogenesis of CLIPPERS is still unknown, and its nosological position is still to be established. A specific biomarker of the disorder is lacking. Although the neuropathological findings in CLIPPERS described to date are distinct from MS, sarcoidosis, neuro-Behçet's disease, glioma and lymphoma, they are by no means specific. The marked perivascular T cell inflammation, perivascular gadolinium enhancement pattern on MRI and steroid-responsiveness seems to be consistent with an (auto-)immune mediated nature of this condition. So far, extensive and multi-modal investigations could not identify other well-recognized (immune-mediated) systemic diseases or lymphoproliferative disorders as exclusive causes of CLIPPERS. However, some authors have raised concerns that radiologically compatible CLIPPERS may conceal a number of different pathologies. It still remains ultimately unclear if CLIPPERS represents a separate and independent new disease entity or a syndrome with heterogeneous aetiologies [7,44,45]. Moreover, the question remains open as to why a disorder with such a striking and highly characteristic MRI appearance has only been described so recently. Has it been unrecognized for many years, or does it represent a ‘truly novel’ disease? Before the condition was described originally and termed ‘CLIPPERS’ in 2010 [1], ‘real’ cases of CLIPPERS may have resulted in being diagnosed as diseases that fitted the given clinico-radiologial constellations most clearly (e.g. MS, Bickerstaff encephalitis, PACNS, etc.). Indeed, a few cases in the previous literature have been reported as ‘Bickerstaff encephalitis’ that appear, from the clinical features and images presented, at least partially reminiscent of CLIPPERS [46,47]. An increasing level of awareness since the publication of the seminal paper on CLIPPERS [1], retrospective reviews of unusual or unclassifiable MRI scans in selected patient cohorts and the meanwhile almost ubiquitous availability of MR scanning may, arguably, have contributed to an increased probability of identifying cases of CLIPPERS in recent years. Another explanatory approach is that CLIPPERS may, in fact, be a newly emerged, ‘truly novel’ disease, speculatively reflecting the immune response to a novel environmental factor. Notwithstanding, physicians should be aware of this condition and relevant differential diagnoses so that the benefits of early diagnosis and GCS-based therapy are not lost. Further studies to determine the exact nosological position of the disorder, potential biomarkers, reliable diagnostic criterias as well as the optimal form and duration of treatment are necessary.
Disclosure
The authors disclose no conflict of interest.
References
- 1.Pittock SJ, Debruyne J, Krecke KN, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2010;133:2626–2634. doi: 10.1093/brain/awq164. [DOI] [PubMed] [Google Scholar]
- 2.Taieb G, Duflos C, Renard D, et al. Long-term outcomes of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) in a consecutive series of 12 patients. Arch Neurol. 2012;69:847–855. doi: 10.1001/archneurol.2012.122. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Kuwabara S, Odaka M, et al. Bickerstaff's brainstem encephalitis and Fisher syndrome form a continuous spectrum. J Neurol. 2008;255:674–682. doi: 10.1007/s00415-008-0775-0. [DOI] [PubMed] [Google Scholar]
- 4.Al-Araji A, Kidd DP. Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurol. 2009;8:192–204. doi: 10.1016/S1474-4422(09)70015-8. [DOI] [PubMed] [Google Scholar]
- 5.Blaes F. Paraneoplastic brain stem encephalitis. Curr Treat Options Neurol. 2013;15:201–209. doi: 10.1007/s11940-013-0221-1. [DOI] [PubMed] [Google Scholar]
- 6.Jubelt B, Mihai C, Li TM, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep. 2011;11:543–552. doi: 10.1007/s11910-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 7.Simon NG, Parratt JD, Barnett MH, et al. Expanding the clinical, radiological and neuropathological phenotype of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) J Neurol Neurosurg Psychiatry. 2012;83:15–22. doi: 10.1136/jnnp-2011-301054. [DOI] [PubMed] [Google Scholar]
- 8.Egelseer S, Dietrich W, Böhm C, Erbguth FJ. 2011. CLIPPERS – eine MRT-Blickdiagnose? [‘CLIPPERS – a visual diagnosis by MRI?'] (Meeting abstract, 84. Kongress der Deutschen Gesellschaft für Neurologie mit Fortbildungsakademie,) [in German]. Available at: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=135264&XNSPRACHE_ID=1&XNKONGRESS_ID=143&XNMASKEN_ID=900 (accessed 21 July 2013)
- 9.Hillesheim PB, Parker JR, Parker JC, Jr, Escott E, Berger JR. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids following influenza vaccination. Arch Pathol Lab Med. 2012;136:681–685. doi: 10.5858/arpa.2011-0428-CR. [DOI] [PubMed] [Google Scholar]
- 10.Kastrup O, van deNes J, Gasser T, Keyvani K. Three cases of CLIPPERS: a serial clinical, laboratory and MRI follow-up study. J Neurol. 2011;258:2140–2146. doi: 10.1007/s00415-011-6071-4. [DOI] [PubMed] [Google Scholar]
- 11.Tohge R, Nagao M, Yagishita A, Matsubara S. A case of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) in East Asia. Intern Med. 2012;51:1115–1119. doi: 10.2169/internalmedicine.51.7040. [DOI] [PubMed] [Google Scholar]
- 12.Sczesni KC, Alekseyev A, Schlegel U, Skodda S. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Nervenarzt. 2012;83:775–781. doi: 10.1007/s00115-011-3430-4. [in German] [DOI] [PubMed] [Google Scholar]
- 13.Huhn JI, Obermann M. Neuro-quiz. Akt Neurol. 2011;38:481–482. [in German] [Google Scholar]
- 14.Kira J, Horiuchi I, Suzuki J, et al. Myelitis associated with atopic disorders in Japan: a retrospective clinical study of the past 20 years. Intern Med. 2001;40:613–619. doi: 10.2169/internalmedicine.40.613. [DOI] [PubMed] [Google Scholar]
- 15.Osoegawa M, Ochi H, Minohara M, et al. Myelitis with atopic diathesis: a nationwide survey of 79 cases in Japan. J Neurol Sci. 2003;209:5–11. doi: 10.1016/s0022-510x(02)00441-0. [DOI] [PubMed] [Google Scholar]
- 16.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenic relevance. Nat Rev Neurol. 2010;6:383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- 17.List J, Lesemann A, Wiener E, et al. A new case of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Brain. 2011;134:e185. doi: 10.1093/brain/awr035. author reply e186. [DOI] [PubMed] [Google Scholar]
- 18.Taieb G, Wacongne A, Renard D, Figarella-Branger D, Castelnovo G, Labauge P. A new case of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids with initial normal magnetic resonance imaging. Brain. 2011;134:e182. doi: 10.1093/brain/awq390. author reply e183. [DOI] [PubMed] [Google Scholar]
- 19.Gabilondo I, Saiz A, Graus F, Villoslada P. Response to immunotherapy in CLIPPERS syndrome. J Neurol. 2011;258:2090–2092. doi: 10.1007/s00415-011-6068-z. [DOI] [PubMed] [Google Scholar]
- 20.Duprez TP, Sindic C. Contrast-enhanced magnetic resonance imaging and perfusion-weighted imaging for monitoring features in severe CLIPPERS. Brain. 2011;134:e184. doi: 10.1093/brain/awr034. author reply e186. [DOI] [PubMed] [Google Scholar]
- 21.Jones JL, Dean AF, Antoun N, Scoffings DJ, Burnet NG, Coles AJ. ‘Radiologically compatible CLIPPERS’ may conceal a number of pathologies. Brain. 2011;134:e187. doi: 10.1093/brain/awr134. [DOI] [PubMed] [Google Scholar]
- 22.Biotti D, Deschamps R, Shotar E, et al. CLIPPERS: chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Pract Neurol. 2011;11:349–351. doi: 10.1136/practneurol-2011-000043. [DOI] [PubMed] [Google Scholar]
- 23.Lefaucheur R, Bouwyn JP, Ahtoy P, Gérardin E, Derrey S, Maltête D. Teaching neuroimages: punctuate and curvilinear enhancement peppering the pons responsive to steroids. Neurology. 2011;77:e57–58. doi: 10.1212/WNL.0b013e31822cfa4a. [DOI] [PubMed] [Google Scholar]
- 24.Sempere AP, Mola S, Martin-Medina P, Bernabeu A, Khabbaz E, Lopez-Celada S. Response to immunotherapy in CLIPPERS: clinical, MRI, and MRS follow-up. J Neuroimaging. 2013;23:254–255. doi: 10.1111/j.1552-6569.2011.00631.x. [DOI] [PubMed] [Google Scholar]
- 25.Humbroich K, Schimrigk S. CLIPPERS: an increasingly diagnosed syndrome. Nervenarzt. 2012;83:782–784. doi: 10.1007/s00115-012-3481-1. [in German] [DOI] [PubMed] [Google Scholar]
- 26.Wetter A, Küpeli G, Meila D, Brassel F, Nacimiento W. CLIPPERS syndrome in a patient with a fluctuating cerebellar syndrome. Neurographics. 2013;3:11–13. [Google Scholar]
- 27.Wijntjes J, Wouda EJ, Siegert CE, Karas GB, Vlaar AM. Need for prolonged immunosupressive therapy in CLIPPERS – a case report. BMC Neurol. 2013;13:49. doi: 10.1186/1471-2377-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safdieh JE. CLIPPERS: is it for real? Neurology Alert. 2012;30:45. Available at: http://connection.ebscohost.com/c/articles/71856811/clippers-real (accessed 21 July 2013) [Google Scholar]
- 29.Ortega MR, Usmani N, Parra-Herran C, Adams DJ, Steingo B, Rammohan KW. CLIPPERS complicating multiple sclerosis causing concerns of CNS lymphoma. Neurology. 2012;79:715–716. doi: 10.1212/WNL.0b013e3182648b77. [DOI] [PubMed] [Google Scholar]
- 30.Limousin N, Praline J, Motica O, et al. Brain biopsy is required in steroid-resistant patients with chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) J Neurooncol. 2012;107:223–224. doi: 10.1007/s11060-011-0724-0. [DOI] [PubMed] [Google Scholar]
- 31.De Graaff HJ, Wattjes MP, Rozemuller-Kwakkel AJ, Petzold A, Killestein J. Fatal B-cell lymphoma following chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. JAMA Neurol. 2013;70:915–918. doi: 10.1001/jamaneurol.2013.2016. [DOI] [PubMed] [Google Scholar]
- 32.Buttmann M, Metz I, Brecht I, Brück W, Warmuth-Metz M. Atypical chronic lymphocytic inflammation with pontocerebellar perivascular enhancement responsive to steroids (CLIPPERS), primary angiitis of the CNS mimicking CLIPPERS or overlap syndrome? A case report. J Neurol Sci. 2012;324:183–186. doi: 10.1016/j.jns.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Jerca O, Manouchehrinia A, Tanasescu R. A new defined entity among the spectrum of central nervous system inflammation: chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Rom J Neurol Psychiatry. 2011;X:77–81. [Google Scholar]
- 34.Ferreira RM, Machado G, Souza AS, Lin K, Corrêa-Neto Y. CLIPPERS-like MRI findings in a patient with multiple sclerosis. J Neurol Sci. 2013;327:61–62. doi: 10.1016/j.jns.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Keegan BM, Pittock SJ. Cutting-edge questions about CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) Arch Neurol. 2012;69:819–820. doi: 10.1001/archneurol.2012.1015. [DOI] [PubMed] [Google Scholar]
- 36.Rachinger W, Grau S, Holtmannspotter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80:1134–1139. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 37.Rice CM, Gilkes CE, Teare E, Hardie RJ, Scolding NJ, Edwards RJ. Brain biopsy in cryptogenic neurological disease. Br J Neurosurg. 2011;25:614–620. doi: 10.3109/02688697.2010.551677. [DOI] [PubMed] [Google Scholar]
- 38.Taieb G, Renard D, Vincent T, Uro-Coste E, Labauge P, et al. Pathophysiology of CLIPPERS still unclear. Neurology. 2012;79:715–716. Reply to Ortega. Available at: http://www.neurology.org/content/79/7/715/reply (published 24 August 2012; accessed 21 July 2013) [Google Scholar]
- 39.Hidarilak N, Traylor A. A mysterious case: chronic lymphocytic inflammation with pontocerebellar perivascular enhancement responsive to steroids (CLIPPERS) syndrome (Meeting abstract 364, American Federation for Medical Research and Participating Societies Regional Meetings: Southern Regional Program Abstracts, 2013) J Investig Med. 2013;61:476. [Google Scholar]
- 40.Mann S, Karmon A, Schwarze M, Salbeck R, Schelling P, Glahn J. 2012. Fallbericht: mal was anderes: CLIPPERS [‘Case report: something different: CLIPPERS'] (Meeting abstract, 85. Kongress der Deutschen Gesellschaft für Neurologie mit Fortbildungsakademie) Available at: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=154317&XNSPRACHE_ID=1&XNKONGRESS_ID=168&XNMASKEN_ID=900 (accessed 21 July 2013)
- 41.Rommer PS, Zettl UK, Kieseier B, et al. Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clin Exp Immunol. 2014;175:397–407. doi: 10.1111/cei.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rommer PS, Dudesek A, Stüve O, Zettl UK. Monoclonal antibodies in treatment of multiple sclerosis. Clin Exp Immunol. 2014;175:373–384. doi: 10.1111/cei.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melzer N, Meuth SG. Disease-modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies. Clin Exp Immunol. 2014;175:359–372. doi: 10.1111/cei.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scolding N. The other BSE. Brain. 2011;134:2194–2196. doi: 10.1093/brain/awr181. [DOI] [PubMed] [Google Scholar]
- 45.Kira J. The expanding phenotype of CLIPPERS: is it a disease or a syndrome? J Neurol Neurosurg Psychiatry. 2012;83:2–3. doi: 10.1136/jnnp-2011-301626. [DOI] [PubMed] [Google Scholar]
- 46.Weidauer S, Ziemann U, Thomalske C, Gaa J, Lanfermann H, Zanella FE. Vasogenic edema in Bickerstaff's brainstem encephalitis: a serial MRI study. Neurology. 2003;61:836–838. doi: 10.1212/01.wnl.0000085864.70923.5d. [DOI] [PubMed] [Google Scholar]
- 47.Roos RP, Soliven B, Goldenberg F, Badruddin A, Baron JM. An elderly patient with Bickerstaff brainstem encephalitis and transient episodes of brainstem dysfunction. Arch Neurol. 2008;65:821–824. doi: 10.1001/archneur.65.6.821. [DOI] [PubMed] [Google Scholar]
- 48.Sonoda K, Yamasaki R, Matsushita T, Yoshimura T, Murai H, Kira J. Case of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids showing features common to multiple sclerosis. Clin Exp Neuroimmunol. 2013;4:104–106. [Google Scholar]
- 49.Maddison P, Gozzard P, Jaspan T. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS): no evidence for antibodies to neuronal surface antigens (Meeting abstract 0918, Annual Meeting of the Association of British Neurologists, 2011) J Neurol Neurosurg Psychiatry. 2012;83:e1. [Google Scholar]
- 50.Al-Mufti F, DuMonde M, Nuoman R, Coyle P. CLIPPERS: rare disease or tip of the iceberg? A case report and literature review (Meeting abstract P03.190, 65th Annual meeting of the American Academy of Neurology, 2013) Neurology. 2013;80:P03.190. [Google Scholar]
- 51.Tarabishy B, Aho T, Patel S. 2013. A case of CLIPPERS: a rare, recently described, inflammatory brainstem condition with a review of the differential diagnosis (Meeting abstract E309, Annual meeting of the American Roentgen Ray Society). Available at: http://www.arrsmeeting.org/abstracts/electronicexhibits/index.cfm?fid=E309 (accessed 21 July 2013)
- 52.Pesaresi I, Sabato M, Desideri I, Puglioli M, Moretti P, Cosottini M. 3.0T MR investigation of CLIPPERS: role of susceptibility weighted and perfusion weighted imaging. Magn Reson Imaging. 2013;31:1640–1642. doi: 10.1016/j.mri.2013.06.012. [DOI] [PubMed] [Google Scholar]


