Abstract
Due to clinical efficacy and safety profile, extracorporeal photochemotherapy (ECP) is a commonly used cell treatment for patients with cutaneous T cell lymphoma (CTCL) and graft-versus-host disease (GVHD). The capacity of ECP to induce dendritic antigen-presenting cell (DC)-mediated selective immunization or immunosuppression suggests a novel mechanism involving pivotal cell signalling processes that have yet to be clearly identified as related to this procedure. In this study we employ two model systems of ECP to dissect the role of integrin signalling and adsorbed plasma proteins in monocyte-to-DC differentiation. We demonstrate that monocytes that were passed through protein-modified ECP plates adhered transiently to plasma proteins, including fibronectin, adsorbed to the plastic ECP plate and activated signalling pathways that initiate monocyte-to-DC conversion. Plasma protein adsorption facilitated 54·2 ± 4·7% differentiation, while fibronectin supported 29·8 ± 7·2% differentiation, as detected by DC phenotypic expression of membrane CD80 and CD86, as well as CD36, human leucocyte antigen D-related (HLA-DR) and cytoplasmic CD83. Further, we demonstrate the ability of fibronectin and other plasma proteins to act through cell adhesion via the ubiquitous arginine–glycine–aspartic (RGD) motif to drive monocyte-to-DC differentiation, with high-density RGD substrates supporting 54·1 ± 5·8% differentiation via αVβ3 and α5β1integrin signalling. Our results demonstrate that plasma protein binding integrins and plasma proteins operate through specific binding domains to induce monocyte-to-DC differentiation in ECP, providing a mechanism that can be harnessed to enhance ECP efficacy.
Keywords: DC, integrins, monocyte, transimmunization
Introduction
Extracorporeal photochemotherapy (ECP) has become a widely used cell therapy for cutaneous T cell lymphoma (CTCL) 1,2 and, more recently, graft-versus-host disease (GVHD), as in total face transplantation 1,3–8. As a treatment for autoimmune-associated diseases, ECP requires multiple cycles and extensive treatment to be most effective 7,8. Described here, a combination of understanding the mechanisms underlying the therapy and a potential method for maximizing those mechanisms would substantially optimize ECP therapeutic strategies.
In ECP, an infused cell preparation is produced over 2 h, through integration of dendritic antigen-presenting cell (DC) induction and lymphocyte apoptosis as simultaneous complementary influences. The leucapheresis preparation, containing blood plasma, leucocytes and lymphocytes, is passaged as a thin film through a chamber enabling contact between monocytes and two plastic surfaces (Fig. 1a). In the device, the mixture of monocytes and lymphocytes is exposed to the ultraviolet (UVA)-activated DNA cross-linking drug, 8-methoxypsorlen (8-MOP). Monocytes are relatively more resistant to 8-MOP UVA damage than lymphocytes, providing an abundance of apoptosed T cells for phagocytosis by antigen-presenting monocytes-turned-DC 9. DC phagocytose apoptotic T cells, presenting antigens on their surface to enhance anti-tumour responses in CTCL 9,10 and exhibit functionality in alloreactive diseases 11, both inhibiting the donor-mediated autoaggression related to GVHD 1 while also preventing recipient-mediated rejection of solid organs and composite tissue transplants 12,13. The capacity of DC to regulate T cell responses in both positive and negative directions 14 suggests that ECP's efficient generation of large numbers of DC may be central to its clinical efficacy.
Figure 1.
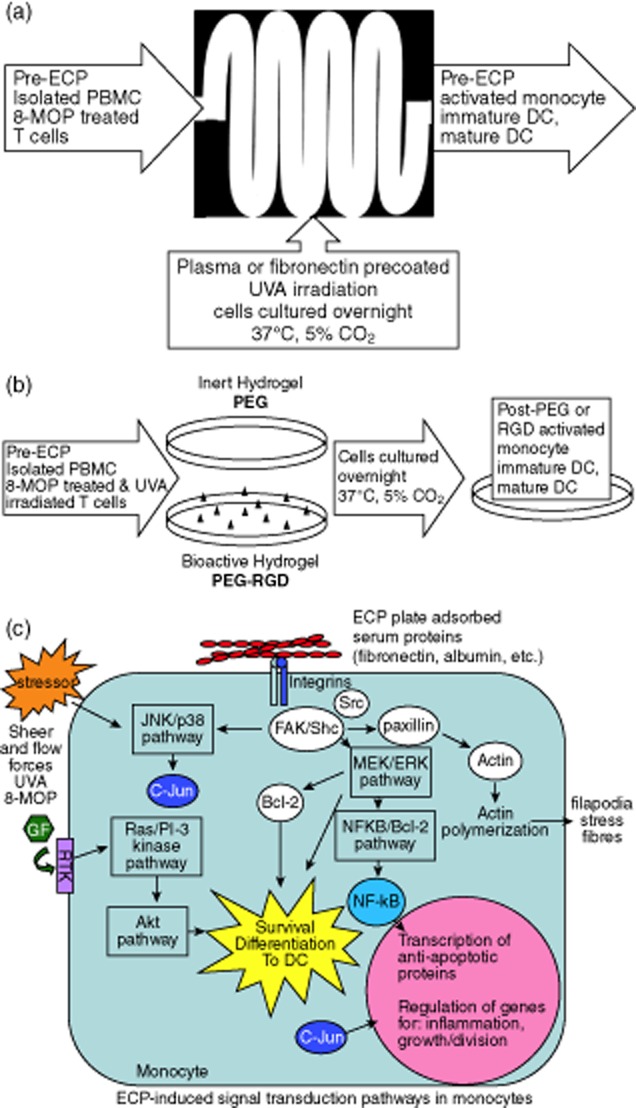
Extracorporeal photochemotherapy (ECP) model systems and potential signalling mechanism. (a) Benchtop mock ECP system using in hospital ECP plastic exposure plate (Therakos®), ultraviolet (UVA) source and peristaltic pump. (b) Integrin-specific induction of ECP-type monocyte to dendritic cell (DC) differentiation using polyethylene glycol based hydrogels with and without bioactive arginine–glycine–aspartic (RGD) sequence. (c) Hypothesis of ECP-induced signal transduction pathway, initiated through ECP plate adsorbed plasma protein binding to monocyte-presented integrins. Integrins associate with intracellular proteins to form focal adhesions, capable of signalling through mitogen-activated protein kinase/extracellular-regulated kinase (MEK/ERK) pathways to induce survival and differentiation.
We therefore examined the mechanism(s) by which ECP induces monocyte-to-DC differentiation, potentially reducing the number of treatments required for effective therapy. A potential mechanism is that plasma proteins that adsorb to plastic surfaces 15 modulate the inflammatory response to biomaterials and biodevices in vitro 16 and ECM components in vivo 17,18. Monocyte surface integrins are the principal cell adhesion receptors responsible for signalling of cell behaviour and differentiation after binding to the arginine–glycine–aspartic (RGD) tripeptide components of fibronectin and other plasma proteins 18,19. Because integrin signalling, in co-ordination with growth factor receptors, plays an important role in cell migration, survival, proliferation and differentiation (Fig. 1c) 17,20, we examined its potential relevance to ECP. In this paper we report that overnight, rather than 2-h, adhesion to plasma proteins adsorbed to the ECP plastic exposure plate activates signalling pathways that induce monocyte differentiation into functional DC. Further, we used a modified bioactive (poly)ethylene glycol (PEG) hydrogel to demonstrate that plasma protein cell binding domains may initiate integrin-dependent adhesion signalling pathways that result in the production of mature DC (Fig. 1b). An enhanced understanding of the role of plasma proteins in monocyte-to-DC differentiation will allow us to further tune the ECP system, enhancing its clinical efficacy.
Methods
Benchtop mock ECP and cell culture
Peripheral blood leucocytes from healthy subjects were obtained under the guidelines of the Yale Human Investigational Review Board. Lymphocytes were collected using a cocktail of T cell-specific magnetic beads, including CD4-, CD8- and CD3-specific microbeads (Miltenyl Biotec, Auburn, CA, USA) and separation columns (Miltenyl Biotec). The mononuclear cell fraction [peripheral blood mononuclear cell (PBMC)] was isolated by centrifugation over a Ficoll-Hypaque gradient and divided into pretreatment (pre-ECP) and overnight incubation fractions (post-ECP) (Fig. 1a). Cells were resuspended in normal saline and treated using the benchtop ECP apparatus incorporating a UVA light source, a plastic exposure plate (Therakos®, Exton, PA, USA) and a peristaltic pump (Cole Parmer Technology, Vernon Hills, IL, USA). 8-MOP (100 ng/ml)-treated T cells were exposed to UVA (1–2 Joules) and apoptosed T cells were co-incubated with isolated PBMCs during ECP treatment. Plastic exposure plates were precoated with 20 or 30 μg/ml fibronectin (Sigma, St Louis, MO, USA) at 4°C, or incubation at 25°C with 50% plasma before introducing the sample. ECP-treated samples were cultured overnight (37°C, 5% CO2) in a 1-litre platelet storage bag (PL-2410; Baxter, Deerfield, IL, USA) in RMPI-1640 medium (Gibco, Carlsbad, CA, USA); n = 6–14 for each experimental condition, unless noted otherwise. Microscopy was obtained on day 2 of ECP, providing images of differentiated DC.
Construction of polyethylene glycol hydrogels conjugated with RGD
PEG hydrogels were prepared as described previously 21,22. Briefly, PEG diacrylate (DA) was prepared by combining 0·1 mmol/ml dry PEG (10000 Da; Fluka, Sigma-Aldrich, St. Louis, MO, USA), 0·4 mmol/ml acryloyl chloride and 0·2 mmol/ml triethylamine in anhydrous dichloromethane and stored under argon overnight. The resulting PEG DA was then precipitated with ether, filtered and dried. PEG DA was dialyzed prior to use. Peptides were conjugated to PEG monoacrylate by reacting the peptide with acryloyl-PEG-N-hydroxysuccinimide (acryloyl-PEG-NHS, 3400 Da; JenKem Techonology, Allen, TX, USA) in sodium bicarbonate (pH 8·5) at a 1:1 molar ratio for 2 h. The coupled acryloyl-PEG-peptide was lyophilized and the conjugated PEG-peptide was dialyzed prior to use. All polymers and polymer conjugates were characterized by proton nuclear magnetic resonance (NMR) (Avance 400 Mhz; Bruker BioSpin, Billerica, MA, USA).
Hydrogels were prepared by combining 0·1 g/ml PEG diacrylate and 0·01 g/ml acryloyl-PEG-RGDS (American Peptide Company, Sunnyvale, CA, USA) in phosphate-buffered saline (PBS, pH 7·2) containing 0·1 g/l glucose. The solution was then sterilized by filtration (0·2 μm with 0·8 μm pre-filter; Pall Corporation, Port Washington, NY, USA); 4 μl/ml 2,2-dimethoxy-2-phenylacetophenone in n-vinylpyrrolidone (300 mg/ml) was added as the photoinitiator. The resulting solution was exposed to UV light (365 nm, 10 mW/cm2) for 30 s to convert the liquid polymer solution to a covalently cross-linked hydrogel. The polymerized gels were then incubated overnight in PBS to reach equilibrium swelling. PEG DA hydrogels without peptides were used as controls.
Magnetic bead enrichment of monocytes and culture on PEG hydrogels
Prior to seeding on PEG hydrogels, PBMC were lymphocyte-depleted using a cocktail of lymphocyte-specific magnetic microbeads (Miltenyi Biotech), yielding 60–80% monocyte enrichment. Monocytes were resuspended in RPMI-1640 medium and seeded on hydrogels 1–2 h before the addition of apoptotic lymphocytes prepared with 8-MOP. Briefly, 8-MOP was added to isolated lymphocytes at 100 ng/ml. UVA exposure was applied at 1–2 Joules. Isolated PBMC and apoptosed lymphocytes were then co-cultured overnight in RPMI-1640 medium supplemented with 7·5% antibody serum (Gemini Bio-Products, West Sacramento, CA, USA) and 7·5% autologous plasma (37°C, 5% CO2). Cells were recovered from hydrogels with Detachin™ and gentle scraping (Fig. 1b).
Immunophenotyping and integrin expression
Beckman Coulter (Brea, CA, USA) monoclonal antibodies against antigens expressed on monocytes and/or DC included: human leucocyte antigen D-related (HLA-DR), CD36, intracellular CD83 and CD80 and CD86.
Monoclonal antibodies for integrin subunits were obtained from R&D Systems (Minneapolis, MN, USA), and included CD29 (β1), CD49d (α4), CD49e (α5) and CD51 (αV). Background staining was established with isotype controls and immunofluorescence analysed using a FC500 flow cytometer (Beckman Coulter). Cell fixation and permeabilization for combined membrane and cytoplasmic staining was performed according to the manufacturer's instructions (Intraprep kit; Beckman Coulter).
Statistical analysis
Analysis of variance (anova) and Student's paired t-test were used to analyse data collected from various conditions, using a minimum of four human volunteers per study. Data represented are mean ± standard error (s.e.).
Results
Role of plastic ECP plate-adsorbed plasma protein in induction of DC differentiation
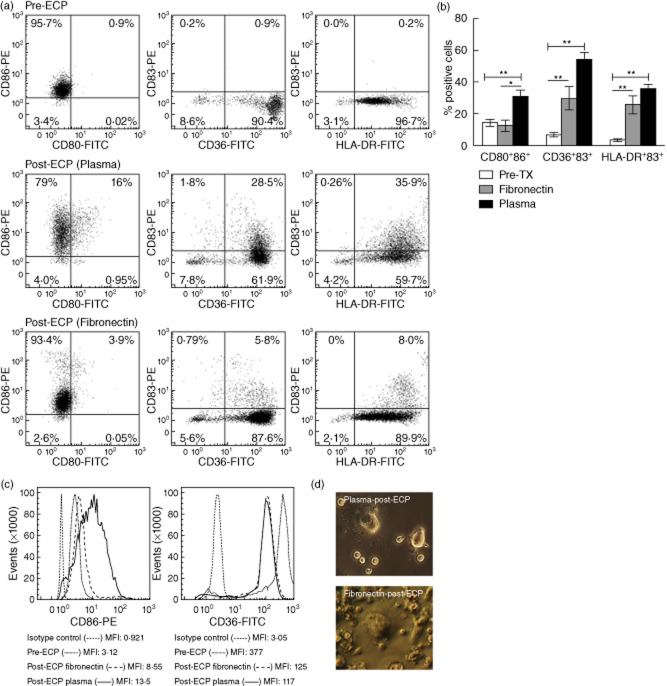
Adhesive substrates have been shown to elicit differential DC maturation and adaptive immune responses 16. Key to interactions with blood plasma, we examined the extent to which plasma proteins, including isolated fibronectin, contribute to DC differentiation. We compared the fraction of DC conversion obtained using either fibronectin-coated or plasma-coated UVA exposure plates and characterized the specific phenotype of DC generated from ECP-processed blood monocytes using flow cytometry to quantify indicators of monocyte activation or conversion. Monocyte-to-DC markers include: (i) up-regulation of co-stimulatory CD80 and CD86; (ii) acquisition of cytoplasmic CD83 (indicative of immature DC); (iii) up-regulation of HLA-DR; and (iv) down-regulation of CD36. When freshly isolated peripheral blood monocytes (pre-ECP) were examined, low levels (14·3 ± 2·4%) of co-stimulatory and DC differentiation markers (6·7 ± 1·5% CD36/CD83 and 3·3 ± 0·8% HLA-DR/CD83) were expressed (Fig. 2a). After passage through a fibronectin-coated plate, followed by overnight incubation (post-ECP), the percentage of CD36+CD83+ and HLA-DR+CD83+ cells (29·8 ± 7·2% and 25·9 ± 5·7%, respectively) in the activated monocyte/DC population increased significantly [P = 0·01 and P = 0·002, respectively, when compared to untreated PBMCs (pre-ECP)] (Fig. 2a,b). A larger increase in the percentage of CD36+CD83+ and HLA-DR+CD83+ cells (54·2 ± 4·7% and 36·1 ± 2·4%, respectively) was seen with plasma-coated plates (P = 0·008 and P = 0·003, respectively, compared with untreated plates) (Fig. 2a,b). While measurably lower, the percentages of neither CD36+CD83+ nor HLA-DR+CD83+ cells passaged through fibronectin-coated plates were significantly different from plasma-coated plate passaged cells.
Figure 2.
Plastic adherent plasma protein and fibronectin stimulated monocyte-to-dendritic cell (DC) conversion and characterization. Extracorporeal photochemotherapy (ECP)-treated cells were cultured overnight and analysed by flow cytometry gated on the monocyte/DC population. DC were identified by co-expression of membrane CD80 and CD86 and membrane staining for CD36 or human leucocyte antigen D-related (HLA-DR) combined with cytoplasmic reactivity with CD83. (a) Two-colour histograms from freshly isolated monocytes (pre-ECP) and overnight cultured ECP treated using plasma-coated [post-ECP (plasma)] or fibronectin-coated [post-ECP (fibronectin)] exposure plates. (b) Average fraction of DC obtained using plasma-coated [post ECP (plasma), mean ± standard error (s.e.)] or fibronectin-coated [post-ECP (fibronectin), mean ± s.e.] exposure plates compared to freshly isolated monocytes (pre-ECP, mean ± s.e.). *P < 0·05; **P < 0·01 as confirmed by analysis of variance (anova) and paired Student's t-test. (c) Monocyte-to-DC conversion was supported further by an increase in the mean fluorescence intensity (MFI) of the co-stimulatory molecule CD86 and a decrease in the MFI of CD36 post-ECP. Both trends are characteristic of DC maturation, signifying an increase in antigen-presenting capacity and decrease in the ability to phagocytose apoptotic cells. One-colour histograms were normalized to 100% of peak value. (d) Phase-contrast microscopy demonstrates appropriate dendrite formation on ECP differentiated DC on plasma- and fibronectin-coated plates.
In Fig. 2c, representative one-colour histograms illustrate up-regulation of CD86 and down-regulation of CD36 in ECP-treated cells. As demonstrated in Fig. 2a, samples circulated through plasma-coated plates yielded a higher fraction of cells expressing CD80 than samples circulated through fibronectin-coated plates. In both conditions, an increase in the mean fluorescence intensity (MFI) of HLA-DR, CD80 and CD86, and a concomitant decrease in the MFI of CD36 (receptor for monocytes) on plasma- and fibronectin-coated ECP plates, further supported DC maturation 23. However, the lower fraction of monocytes entering the DC pathway in response to fibronectin-coated plates suggests that fibronectin plays an important role, but is not the sole active component of plasma. As additional verification that monocyte-to-DC conversion in both plasma- and fibronectin-dependent ECP occurs, Fig. 2d demonstrates appropriate dendrite formation and extensions visible on differentiated DC.
Although cell viability was similar in both conditions (96%), we observed a substantial difference in the percentage of cells retrieved post-ECP: 48 ± 6% (n = 3) for plasma-coated versus 21 ± 9% (n = 4) for fibronectin-coated plates. When samples were passed through an uncoated plate, the yield was reduced further (8 ± 4%, n = 4). This difference indicates that cells are irretrievable from the plasma-free plates, due to either robust adhesion to plastic or fibronectin (i.e. more cells adhere tightly to the plastic or fibronectin and are not released) or suboptimal protection from a non-physiological environment. While monocyte interaction with plate-adsorbed fibronectin plays an important role in the early events that induce monocyte-to-DC conversion, other plasma components are necessary both for optimal cell recovery and for DC differentiation.
Integrin expression on ECP-processed monocytes/DC
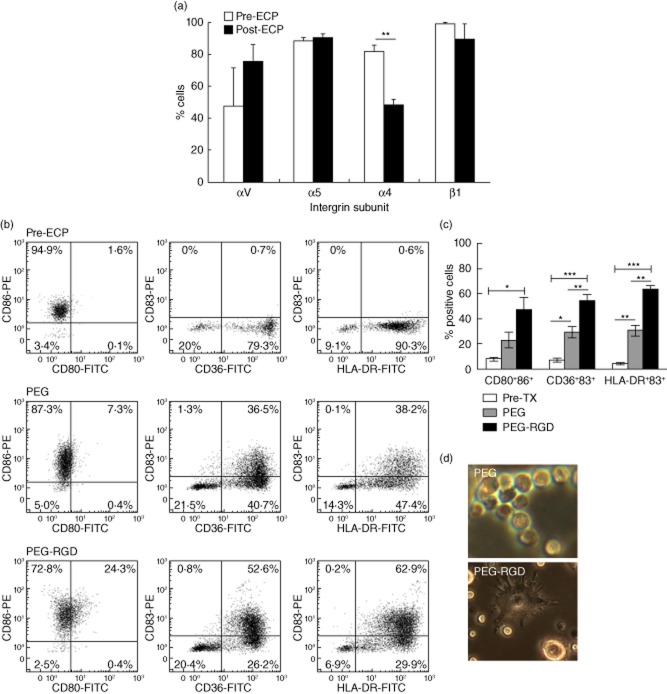
One mechanism by which cells adapt to and interact with their environment is through modulated expression of surface integrins, promoting cell attachment to surface integrin ligands, including fibronectin 15,19,24. We therefore investigated the expression profile of fibronectin-binding integrins on the monocyte/DC population throughout the ECP process.
Consistent with other reports 18, analysis by flow cytometry showed that freshly isolated peripheral blood monocytes strongly express β1 integrin subunits (99·4 ± 0·4%)(Fig. 3a). β integrin subunits may complex with α5 or αV (i.e. α5β1,αVβ1, αVβ3) to bind the RGD motif located within the central cell-binding domain of fibronectin 25. Alternatively, β1 subunits may complex with α4 subunits to form α4β1 integrins, which interact with the LDV motif in the IIICS or V region of fibronectin 25.
Figure 3.
Monocyte-to-dendritic cell (DC) conversion is stimulated by arginine–glycine–aspartic (RGD)-conjugated polyethylene glycol (PEG) hydrogels. (a) Modulation of integrin subunit expression after overnight incubation of extracorporeal photochemotherapy (ECP)-treated samples (post-ECP, n = 3) in comparison to peripheral blood monocytes (pre-ECP, n = 4). **P < 0·01 as confirmed by Student's t-test. (b) Purified monocytes were cultured overnight on PEG hydrogels conjugated with RGD (PEG–RGD) or unmodified PEG hydrogels (PEG). Dendritic cells were identified by co-expression of membrane CD80 and CD86 and membrane staining for CD36 or human leucocyte antigen D-related (HLA-DR) combined with cytoplasmic reactivity with CD83. Representative two-colour histograms from freshly isolated monocytes cultivated on PEG or PEG–RGD hydrogels. (c) Average fraction of DC obtained using PEG–RGD or PEG hydrogels. *P < 0·05; **P < 0·01; ***P < 0·0001 as confirmed by analysis of variance (anova) and paired Student's t-test. (d) Phase-contrast microscopy demonstrates poor dendrite formation on ECP differentiated DC on PEG with improved morphology on PEG–RGD.
Pre- and post-ECP treatment, expression of α5 and αV integrin subunits were similar (88·7 ± 1·6% versus 90·4 ± 2·4% and 47·6 ± 23·9 versus 75·9 ± 10·2%, respectively), and the expression of β1 was maintained at similar levels (89·4 ± 9·6% post-ECP) (Fig. 3a). Expression of α4 subunits after passage through the exposure plate and overnight incubation, however, was significantly lower (81·8 ± 3·8% versus 48·4 ± 2·4%, P < 0·01 when compared to pre-ECP samples) (Fig. 3a). These data correlate with a more than twofold decrease in previously reported α4 mRNA transcripts post-ECP 26.
Stimulation of monocyte-to-DC differentiation by RGD-conjugated hydrogels
We then evaluated the effect of αVβ3 and α5β1-mediated cell adhesion as a mechanism for monocyte-to-DC differentiation. PEG is regarded as an inert biomaterial due to its resistance to protein adsorption and hence low levels of non-specific cell adhesion 27. Unmodified PEG is therefore useful as a background surface for exploring functional activity related to individual moieties, such as the ubiquitously expressed, protein-derived cell adhesive sequence RGD 27. RGD is well represented within plasma proteins, and is a ligand known to specifically engage αVβ3 and α5β1 integrins 28–30. We utilized PEG hydrogels conjugated with RGD (PEG–RGD) to determine whether integrin-mediated adhesion would promote monocyte-to-DC differentiation. The mononuclear cell fraction was isolated from peripheral blood of healthy donors and was lymphocyte-depleted using a cocktail of lymphocyte-specific magnetic beads. The enriched monocytes were co-cultured overnight with 8-MOP-treated apoptotic lymphocytes, added to further approximate the conditions encountered in modified ECP or transimmunization therapies, on PEG–RGD or unmodified PEG hydrogels. Under static conditions, monocytes on PEG alone adhered at 11·3 ± 3·8%, and demonstrated increased adhesion (60·8 ± 13·8%, P < 0·001) on RGD-conjugated hydrogels.
Co-expression of co-stimulatory molecules and markers of DC maturation, as described previously, defined DC differentiation as an effect of integrin adhesion to RGD. Figure 3b,c shows that control (pretreatment) co-expression of CD80/CD86 was 7·9 ± 1·5%, while 6·7 ± 1·4% co-expressed CD36/CD83 and 4·2 ± 0·9% co-expressed CD83/HLA-DR. As illustrated in Fig. 3b,c, a substantial percentage of monocytes cultured on PEG–RGD hydrogels entered into the DC pathway, with 54·1 ± 5·8% co-expressing CD36/CD83 (P < 0·0001 when compared to pretreated cells), and 63·8 ± 2·9% co-expressing intracellular CD83 and surface HLA-DR (P < 0·0001 when compared to pretreated cells). Notably, a non-sense peptide RGE (Asp-Gly-Glu) was used as an additional non-adhesive control, eliciting a total of 25·9 ± 1·9% HLA-DR+CD83+ cells, indicating that adhesive peptides are required for the functional result observed. The fraction of monocyte-to-DC differentiation on PEG–RGD was at maximum 60–70% greater than on unmodified PEG hydrogels, as determined by the mean percentage of cells co-expressing each of the aforementioned markers (Fig. 3b,c). Of the PEG-cultured monocytes, 29·2 ± 4·7% were CD36+CD83+ (P = 0·01 when compared to pretreated cells), while only 30·6 ± 4·1% were HLA-DR+CD83+ (P = 0·003 when compared to pretreated cells). Both the percentages of CD36+CD83+ and HLA-DR+CD83+ cells were significantly higher in cells cultured on PEG–RGD compared to PEG hydrogels (P = 0·006 and P = 0·006, respectively). As additional verification that monocyte-to-DC conversion in RGD-dependent ECP occurs, Fig. 3d demonstrates appropriate dendrite formation and extensions visible on differentiated DC.
Discussion
Clarification of the mechanism(s) underlying ECP's apparent stimulation of therapeutically relevant immunological responses in immunocompetent CTCL patients and immunomodulatory responses in the transplantation setting is important for both clinical and scientific reasons. The study described here advances our understanding of ECP and modified ECP, known as transimmunization 31, by showing the influence of plasma proteins on monocyte-to-DC differentiation during the therapeutic process. Our findings should encourage modification of current transimmunization approaches by enhancing efficacy of the easily tunable ECP system.
UVA-activated 8-MOP contributes in two significant ways to the effects of ECP, in both clinical and experimental animal systems. First, because DNA cross-linking by the transiently activated drug can be so finely titrated, the 8-MOP causes a particularly gradual apoptosis of exposed leukemic lymphocytes. Phagocytosis, by the device-activated monocytes of these lethally damaged lymphocytes, further drives the monocytes to differentiate into mature, immunogenic dendritic cells (DCs). Secondly, while viability of monocytes is significantly less compromised by UVA-activated 8-MOP than that of lymphocytes, the drug truncates maturation of the most heavily exposed fraction of monocytes. Therefore, the DCs deriving from that subset of monocytes are comparatively less mature, and display lower densities of co-stimulatory molecules CD80 and CD86. These relatively immature DCs tend to tolerize T cells in an antigen-selective manner 11. Therefore, based on the findings reported in this paper, it has become possible to skew the induced DC population towards immunogenic function, simply by diminishing the amount of UVA exposure of the processed blood components.
We have reported previously that passage through the ECP exposure plate, followed by overnight incubation, induces a large fraction of processed human monocytes to acquire phenotypic markers typical of DC 20,26. In order to examine whether protein adsorption of plasma proteins, such as fibronectin, support monocyte-to-DC conversion through integrin-binding, we developed two experimental systems: (i) fibronectin-coated ECP exposure plates and (ii) PEG–RGD hydrogels. The first system allowed us to examine the contribution of fibronectin to DC differentiation in an environmental context that parallels ECP, while the second system allowed us to verify the integrin-specific mechanism of plasma protein-mediated differentiation.
ECP treatment of samples using fibronectin-coated exposure plates, followed by overnight incubation, generated between half to approximately 80% of the level of DC differentiation obtained with plasma-coated plates, according to the mean percentage of cells co-expressing the DC-related markers CD80+CD86+, CD36+CD83+ and HLA-DR+CD83+. Overall, however, there were no significant differences between the conversion and maturation rates dependent upon plasma and fibronectin proteins.
Fibronectin contains domains with functional binding activities for other ECM elements, such as fibrin and heparin. Therefore, it is possible that other plasma components potentiate fibronectin-mediated signalling or participate in distinct but co-ordinated signalling events important for DC differentiation in ECP. Additionally, other signals, such as synergistic growth factor signalling, may be required for fibronectin adhesion to stimulate maximal monocyte activation and entry into the DC pathway 17,20,32. Alternatively, other plastic-adherent plasma proteins may provide additional activating signals to adhering monocytes. Nevertheless, the present studies implicate fibronectin plate adsorption as a convincing candidate among plasma protein modifications that influence monocyte biology during ECP and that participate in the early events of monocyte-to-DC conversion.
The second model system employed in these studies, peptide-conjugated PEG hydrogels, was designed to investigate whether the RGD motif, located in the central cell-binding domain of fibronectin, can drive large-scale monocyte-to-DC conversion. The use of PEG as a non-specific and non-adhesive substrate provides a system to evaluate the effects of integrin-specific ligation in monocyte-to-DC differentiation. As suggested previously in studies conducted by Babensee et al., DC maturation may be influenced by non-specific adhesion to biomaterials 33,34, independent of integrin-mediated cell binding, accounting for the low, although significant, background levels of monocyte activation and DC maturation observed on PEG gels alone. However, the incorporation of RGD peptides into PEG hydrogels provided a large boost to the ability of the monocytes to differentiate and mature, demonstrating further that integrin-specific interactions with plasma proteins can facilitate down-stream signalling supportive of differentiation.
This study not only provides further support for the possibility that rapid, synchronized monocyte-to-DC differentiation is a fundamental principle behind ECP, but also contributes insight into the mechanism(s) that underlie the conversion of monocytes into DC. The capacity of fibronectin to contribute to this phenomenon further elucidates ECP's mechanism and raises practical and research possibilities. For example, as engagement of monocyte integrin receptors by fibronectin-modified biomaterials and complementary proteins does not involve the ultraviolet energy intrinsic to conventional ECP, it may be possible to improve the efficacy of the treatment by sparing monocytes exposure to activated 8-MOP. The availability of maturationally synchronized DC may be advantageous to other DC-based immunotherapies. Finally, the possibility that ECP's efficiency in stimulating rapid conversion of monocytes to DC reflects normal physiology may be of broad scientific import.
Acknowledgments
This work was supported by: NIH Training Grant 5 T32 AR07016-35 (J. R.); the NIH cancer center grant 3 P30 CA 16359-28S1 (R. L. E.); and the NY Cardiac Foundation (C. L. B., R. L. E.). The authors thank Martin Blanchard and Professor Joseph Madri for critical review of the manuscript.
Disclosures
Yale University owns patents deriving from the dendritic cell research of R. E. T. and R. L. E. Although no products have been derived from these laboratory studies it is possible that, along with their parent institution, these two authors could benefit personally from commercialization of these discoveries in the future.
Author contributions
A. L. G. created biomaterial systems for the experiments. A. L. G., C. B. and J. R. conducted the experiments. A. L. G., C. B., M. G., R. E. T. and R. L. E. were responsible for the experimental design. All authors contributed to the writing and editing of the manuscript.
References
- Edelson RL, Berger CL, Gasparro FP, et al. Treatment of leukemic cutaneous T-cell lymphoma with extracorporeally-photoactivated 8-methoxypsoralen. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- Di Renzo M, Sbano P, De Aloe G, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clin Exp Immunol. 2008;151:407–413. doi: 10.1111/j.1365-2249.2007.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcindor T, Gorgun G, Miller KB, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- Foss FM, Gorgun G, Miller KB. Extracorporeal photopheresis in chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:719–725. doi: 10.1038/sj.bmt.1703529. [DOI] [PubMed] [Google Scholar]
- Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- Couriel D, Hosing C, Saliba R, et al. Extracorporeal photopheresis for acute and chronic graft-versus-host disease: does it work? Biol Blood Marrow Transplant. 2006;12:37–40. doi: 10.1016/j.bbmt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Berger C, Xu A, Hanlon D, et al. Induction of human tumor-loaded dendritic cells. Int J Cancer. 2001;4:438–447. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1073>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Girardi M, Berger C, Wilson L, et al. Transimmunization for cutaneous T cell lymphoma: a phase I study. Leuk Lymph. 2006;47:1495–1503. doi: 10.1080/10428190600581419. [DOI] [PubMed] [Google Scholar]
- Edelson RL. Mechanistic insights into extracorporeal photochemotherapy: efficient induction of monocyte-to-dendritic cell maturation. Transfus Apher Sci. 2013 doi: 10.1016/j.transci.2013.07.031. PII: S1473-0502(13)00258-9, doi: 10.1016/j.transci.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinix H, Volc-Platzer B, Knobler R. Extracorporeal photochemotherapy in the treatment of severe graft-versus-host disease. Leuk Lymph. 2000;36:425–434. doi: 10.3109/10428190009148389. [DOI] [PubMed] [Google Scholar]
- Hivelin M, Siemionow M, Grimbert P, Lantieri L. Extracorporeal photopheresis: from solid organs to face transplantation. Transpl Immunol. 2009;21:117–128. doi: 10.1016/j.trim.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29:4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR, Chi-Rosso G, Bajardi A, Gotwals P, Green CD, Koteliansky VE. Global expression analysis of extracellular matrix–integrin interactions in monocytes. Immunity. 2000;13:749–758. doi: 10.1016/s1074-7613(00)00073-x. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A, Koteliansky V. Regulation of monocyte gene expression by the extracellular matrix and its functional implications. Immunol Rev. 2002;186:208–220. doi: 10.1034/j.1600-065x.2002.18617.x. [DOI] [PubMed] [Google Scholar]
- Kao W, Lee D, Schense J, Hubbell J. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55:79–88. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Truong H, Danen EHJ. Integrins: signaling, disease, and therapy. Int J Radiat Biol. 2007;83:743–751. doi: 10.1080/09553000701481808. [DOI] [PubMed] [Google Scholar]
- Gonzalez AL, El-Bjeirami W, West JL, McIntire LV, Smith CW. Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis. J Leukoc Biol. 2007;81:686–695. doi: 10.1189/jlb.0906553. [DOI] [PubMed] [Google Scholar]
- Gonzalez AL, Gobin AS, West JL, McIntire LV, Smith CW. Integrin interactions with immobilized peptides in polyethylene glycol diacrylate hydrogels. Tissue Eng. 2004;10:1775–1786. doi: 10.1089/ten.2004.10.1775. [DOI] [PubMed] [Google Scholar]
- Albert M, Pearce S, Francisco L, et al. Immature dendritic cells phagocytose apoptotic cells via alphaVbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakaran P, Radhika A, Jacob S. Monocyte macrophage differentiation in vitro: fibronectin-dependent upregulation of certain macrophage-specific activities. Glycoconj J. 2007;24:49–55. doi: 10.1007/s10719-006-9011-2. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Berger C, Hoffmann K, Vasquez JG, et al. Rapid generation of maturationally synchronized human dendritic cells: contribution to the clinical efficacy of extracorporeal photochemotherapy. Blood. 2010;116:4838–4847. doi: 10.1182/blood-2009-11-256040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt DR, Kao WJ. Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths. J Biomed Mater Res A. 2007;83A:617–625. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Katz B, Lafrenie R, Yamada K. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann NY Acad Sci. 1998;857:119–129. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- Park J, Babensee JE. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012;8:3606–3617. doi: 10.1016/j.actbio.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babensee JE. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20:101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]