Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by pronounced inflammation and leucocyte infiltration in affected joints. Despite significant therapeutic advances, a new targeted approach is needed. Our objective in this work was to investigate the anti-inflammatory effects of the Ras inhibitor farnesylthiosalicylic acid (FTS) on adjuvant-induced arthritis (AIA) in rats, an experimental model for RA. Following AIA induction in Lewis rats by intradermal injection of heat-killed Mycobacterium tuberculosis, rats were treated with either FTS or dexamethasone and assessed daily for paw swelling. Joints were imaged by magnetic resonance imaging and computerized tomography and analysed histologically. The anti-inflammatory effect of FTS was assessed by serum assay of multiple cytokines. After adjuvant injection rats demonstrated paw swelling, leucocyte infiltration, cytokine secretion and activation of Ras-effector pathways. Upon FTS treatment these changes reverted almost to normal. Histopathological analysis revealed that the synovial hyperplasia and leucocyte infiltration observed in the arthritic rats were alleviated by FTS. Periarticular bony erosions were averted. Efficacy of FTS treatment was also demonstrated by inhibition of CD4+ and CD8+ T cell proliferation and of interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-17 release. The Ras effectors PI3K, protein kinase B (AKT), p38, and extracellular-regulated kinase (ERK) were significantly attenuated and forkhead box protein 3 (FoxP3) transcription factor, a marker of regulatory T cells, was significantly increased. Thus, FTS possesses significant anti-inflammatory and anti-arthritic properties and accordingly shows promise as a potential therapeutic agent for RA. Its effects are apparently mediated, at least in part, by a decrease in proinflammatory cytokines.
Keywords: FTS, ras, rheumatoid arthritis
Introduction
Farnesylthiosalicylic acid (FTS) is a potent non-toxic anti-cancer drug that targets oncogenic and pathologically activated Ras 1. It has been evaluated in Phase II clinical trials of patients with pancreatic and non-small-cell lung cancer, with successful results 2. Because Ras proteins – important players in cancer – are known to activate the immune system, we hypothesized that FTS-induced inhibition of Ras might be beneficial in autoimmune and inflammatory conditions 3.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disorder principally affecting synovial joints. The Ras-signalling pathway participates in the pathogenesis of RA, and the activated Ras phenotype has been documented in both humans and animals with inflammatory arthritis 4. These observations suggest strongly that targeting of Ras might be an effective therapeutic approach for this condition. Over-production of inflammatory cytokines is a pathogenic feature underlying articular destruction in RA. Treatment options have increased with the recent advent of biologicals. Other options include the use of agents that neutralize the cytokine tumour necrosis factor (TNF)-α 5. Although some of these treatments have yielded significant improvements in RA management, their drawbacks include partial efficacy and susceptibility of users to infection. New strategies with better clinical efficacy and safety profiles are therefore needed urgently.
Here we investigated the therapeutic effects of FTS on inflammation in rats with adjuvant-induced arthritis (AIA), a polyarticular erosive type of arthritis induced by injection of complete Freund's adjuvant (CFA) 6. This model exhibits many features of human RA and is used widely to study RA pathogenesis and in the search for new drugs to treat inflammatory arthritis 7. Using this model we examined the ability of FTS to ameliorate synovitis, a prominent feature of AIA.
Methods
Arthritis induction
The study was approved by the Institutional Ethics Committee at the Tel-Aviv University, Tel-Aviv, Israel (Approval L-12-005). Male 8-week-old Lewis rats obtained from Harlan Biotech (Rehovot, Israel) were fed a regular diet. Rats (n = 60) were injected intradermally at the base of the tail with 100 μl CFA prepared by suspending heat-killed Mycobacterium tuberculosis (Difco, Franklin Lakes, NJ, USA) in mineral oil at 10 mg/ml. Each paw was virtually divided into four parts and each part was independently scored daily for AIA as 0 (no abnormality) or 1 (redness and swelling of a joint or part of a joint). The total score for all four paws, with a maximal attainable score of 16, represented the rat's clinical arthritis index 8.
Drug administration
Immediately after AIA induction the rats were divided randomly into three groups (n = 20 each). From day 3 after AIA induction, rats were treated daily with FTS or vehicle control (carboxymethyl cellulose, CMC) or with oral dexamethasone (positive control), as described in Fig. 1. In some experiments, a fourth group of naive rats, where disease was not induced, was included.
Figure 1.

Treatment with farnesylthiosalicylic acid (FTS) suppresses clinical and radiographic signs of adjuvant-induced arthritis (AIA). (a) AIA was induced in Lewis rats by injection of complete Freund's adjuvant (CFA). Rats were treated daily, starting from day 3, with either oral FTS (80 mg/kg) or with 0·5% carboxy methyl cellulose (CMC) as a vehicle control (n = 20 per group). A third group of AIA-treated rats was treated with dexamethasone (0·2 mg/kg) on days 1, 2, 3, 5, 8 and 11 (positive control). AIA severity was graded on a histological scale of 0–2, and on a clinical index with a maximal achievable score of 16 (see Materials and methods). The graph shows the mean clinical scores per group daily, from days 11 to 18. **P < 0·05; ***P < 0·001, Student's t-test. (b) Photographs of the front and hind paws of AIA-induced rats (and naive rats where disease was not induced), 16 days after disease induction. (c) On day 16, front and hind paws of AIA and naive rats were scanned (n = 8 per group) by magnetic resonance imaging (MRI) using a 7T/30 spectrometer (Bruker Biospin, Ettlingen). T2-weighted images (TR = 3222 and TE = 67·2 ms) were sequenced. Fourteen slices, 1·5 mm thick, were acquired. Final image resolution was 0·156 × 0·156 mm3. Representative images are presented (upper panel). Statistical analysis of the results is presented as means ± standard error of the mean (lower panel). ***P < 0·001 compared with vehicle-treated controls; Student's t-test.
Magnetic resonance imaging (MRI) of rats with AIA
On day 16 (all days specified are days after AIA induction), rats were anaesthetized by inhalation of 2% isoflurane (Nicholas Piramal, Mumbai, India) in 98% oxygen. Inflammatory infiltrates in their front and back paws were assessed by MRI. Infiltrate volumes were determined using matlab (Mathworks, Natick, MA, USA).
Computed tomography (CT) scans of rats with AIA
CT images of the synovial joints of the front paws of rats were acquired 25 days post-immunization. Briefly, rats anaesthetized by intraperitoneal ketamine (100 mg/kg) and xylazine (20 mg/kg) were placed into a TomoScope® Synergy micro-CT scanner. For cortical bone mass assessment, the thickness of cortical parts of the ulna and the humerus was measured using 3D-Doctor software, version 4·0 (Able Software, Lexington, MA, USA). Cortical and trabecular bone losses were quantified with Image-Pro Plus, version 5·1 (Media Cybernetics, Rockville, MD, USA).
Tissue preparation and analysis
Animals were killed on days 18–25 after AIA induction and fresh synovial tissues were excised. Joints were promptly removed, trimmed, fixed in 10% paraformaldehyde and decalcified in hydrochloric acid (Calci-Clear Rapid; National Diagnostics, Charlotte, NC, USA) for 48 h. The tissue was embedded in paraffin and microtomed into 5-μm slices for haematoxylin and eosin (H&E) staining. One blinded pathologist assessed all sections. Four joints from each rat were each evaluated and scored separately. Histological changes were classified as synovial proliferation, fibrin deposition, presence of acute and chronic inflammatory infiltrates, angiogenesis, oedema, pannus formation, focal loss of cartilage, bone erosion and presence of bursitis and of extra-articular inflammation. Each of these categories was scored as 0 (no positive findings), 1 (focal to mild findings) or 2 (pronounced findings). Histological changes were listed as separate categories (Table 1); each scored as 0, 1 or 2, as defined above.
Table 1.
Histological analysis of ankle joints.
| Histological finding | Vehicle-treated controls | FTS | P-value |
|---|---|---|---|
| Synovial proliferation | 1·6 | 1 | 0·03 |
| Fibrin deposition | 0·6 | 0 | 0·03 |
| Acute inflammation | 0·6 | 0 | 0·03 |
| Chronic inflammation | 1·2 | 0·25 | 0·009 |
| Angiogenesis | 0·6 | 0 | 0·03 |
| Tissue oedema | 0·6 | 0 | 0·03 |
| Pannus formation | 1 | 0 | 0·04 |
| Cartilage loss | 0·8 | 0 | 0·004 |
| Bursitis | 1 | 0 | 0·04 |
| Extra-articular infiltrate | 0·8 | 0 | 0·05 |
Average score (0–2); n = 10; Student's t-test. FTS: farnesylthiosalicylic acid.
Flow cytometry
On day 18, rat splenocytes and inguinal lymph nodes (ILNs) (n = 10 per group) were harvested and stained with fluorescein isothiocyanate (FITC)-labelled anti-CD4 (RPA-T4) monoclonal antibodies (mAbs) and phycoerythrin (PE)-labelled anti-CD8 (OX8) mAbs. Forkhead box protein 3 (FoxP3) was labelled using an anti-FoxP3 staining kit (eBioscience, San Diego, CA, USA), according to the manufacturer's instructions. FACScalibur flow cytometry and CBA software (BD Biosciences, San Jose, CA, USA) were used for data collection and analysis.
Cytokine measurement
Serum levels of the cytokines interferon (IFN)-γ, TNF-α, interleukin (IL)-4, IL-6, IL-10 and IL-17 and transforming growth factor (TGF)-β were determined by enzyme-linked immunosorbent assay (ELISA) on day 18 (Bender MedSystems, Vienna, Austria).
Western blotting
On day 18, rat splenocytes and ILNs were homogenized. The resulting lysates were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting, as described in Fig. 3. Protein bands were visualized using an enhanced chemiluminescence kit (ECL; Amersham, GE Healthcare Life Sciences, Pittsburgh, PA, USA) and quantified by densitometry with Image EZQuant-Gel software. Ras-GTP in the lysates was measured by the Ras-binding domain GST pull-down assay (RBD-GST), followed by Western blotting with anti-pan-Ras antibody 9. The data were normalized to the expression levels of the housekeeping protein tubulin, total Ras, total protein kinase B (AKT) and total extracellular-regulated kinase (ERK).
Figure 3.
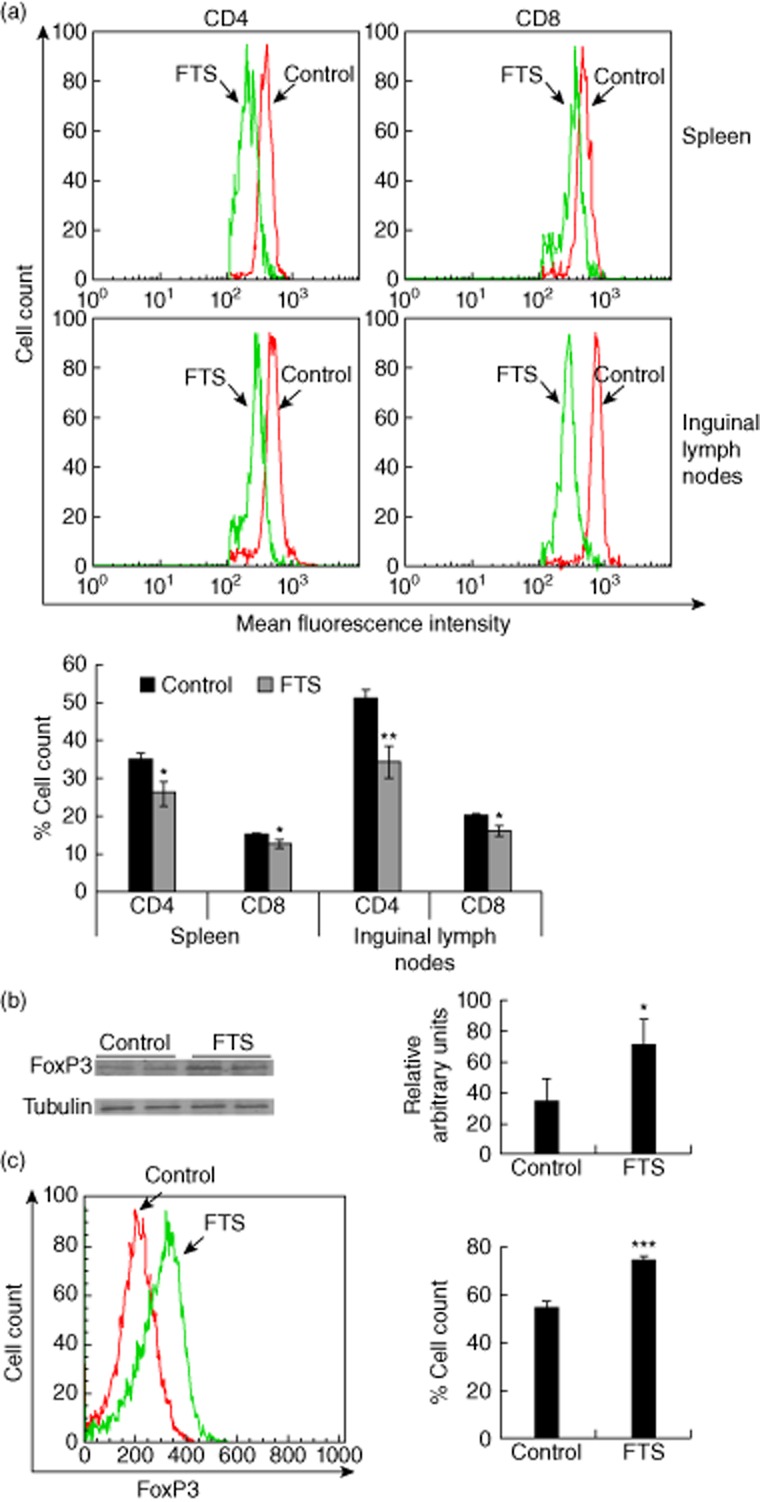
Effects of farnesylthiosalicylic acid (FTS) on T lymphocyte subsets in adjuvant-induced arthritis (AIA). Spleens, inguinal lymph nodes (ILNs) and sera of FTS-treated and vehicle-treated rats with AIA were excised on day 16 after disease induction. a. Spleens (upper panel) and ILNs (middle panel) were assayed for CD4+ and CD8+ T cells by flow cytometry. Statistical analysis of the flow cytometry results (n = 10) is presented (lower panel). (b,c) ILNs were tested for forkhead box protein 3 (FoxP3) expression by Western blotting (b) and flow cytometry (c), as described in the Methods section. Representative blots and flow cytometry are shown in the left panels and the statistical analysis in the right panels. *P < 0·05; **P < 0·01; ***P < 0·001 compared to vehicle-treated controls, Student's t-test.
Human study
CD3+ T cells were purified from the peripheral blood of healthy human donors and from two patients with active inflammatory arthritis [disease activity score (DAS) > 3·2]. Cells were isolated by using whole blood CD3 microbeads (Miltenyi Biotec, San Diego, CA, USA). Human CD3+ T cells were incubated with FTS (50 μM) for 48 h, and then assessed for Ras-GTP by RBD-GST assay. The use of human specimens for this study was approved by the New York University Institutional Review Board (Protocol S12-00831).
Statistical analysis
Values are reported as means ± standard deviation (s.d.). Statistical significance was defined as a two-tailed P-value of less than 0·05, obtained by Student's t-test. All statistical analyses were performed using the spss program.
Results
Treatment with FTS suppresses clinical and radiographic signs of AIA
To determine whether FTS treatment would suppress the clinical signs of AIA, we started daily treatment of rats on day 3 after disease induction (Fig. 1). Dexamethasone-treated AIA rates served as a positive control group in this experiment. As shown in Fig. 1a, arthritis was significantly less severe in the FTS-treated group than in the vehicle-treated control. The clinical index in the vehicle-treated group was 13, whereas in the FTS-treated group it was 7. Differences appeared as early as day 11 after arthritis induction and were maintained until day 18, when the experiment was terminated.
In correlation with the clinical index, macroscopic evaluation of the front and back paws demonstrated less swelling in FTS-treated rats than in vehicle-treated controls (Fig. 1b). Although rats in the vehicle-treated group were less mobile than those in the FTS-treated group, all rats maintained the same body weight loss (∼10%) during the experiment. To quantify the inflammatory infiltrate burden objectively, we scanned the front and back paws to obtain T2-weighted MRI images (Fig. 1c, upper panel). Quantification of the MRI analysis (Fig. 1c, lower panel) revealed significantly smaller paw volumes in the FTS-treated rats than in the vehicle-treated group (38% decreased paw volume in the FTS-treated rats relative to the vehicle-treated group). We therefore concluded that FTS treatment inhibits both articular inflammatory infiltration into the paws and inflammatory clinical signs in rats with AIA.
Effects of FTS on joint and bone morphology in AIA
To study the effects of FTS on the joints of rats with AIA, we examined H&E-stained tissue sections from naive rats (where disease was not induced), FTS-treated AIA-induced rats and vehicle-treated AIA-induced rats (Fig. 2a). Relative to naive rats, all treated rats showed destruction of articular cartilage and replacement of spaces in the joints with mononuclear cells. Sections from FTS-treated rats, however, showed significantly less proliferation and infiltration of mononuclear cells, restricted pannus formation and less destruction of cartilage than the vehicle-treated controls. Comparison of the quantified histological changes revealed that the arthritic findings with the most pronounced inhibition in the FTS-treated rats were cartilage loss (P < 0·004) and chronic inflammatory infiltrates (P < 0·009; Table 1). FTS treatment also resulted in significantly less damage to almost all the histological parameters (Table 1). Thus, FTS markedly ameliorated joint injuries induced in rats by AIA.
Figure 2.
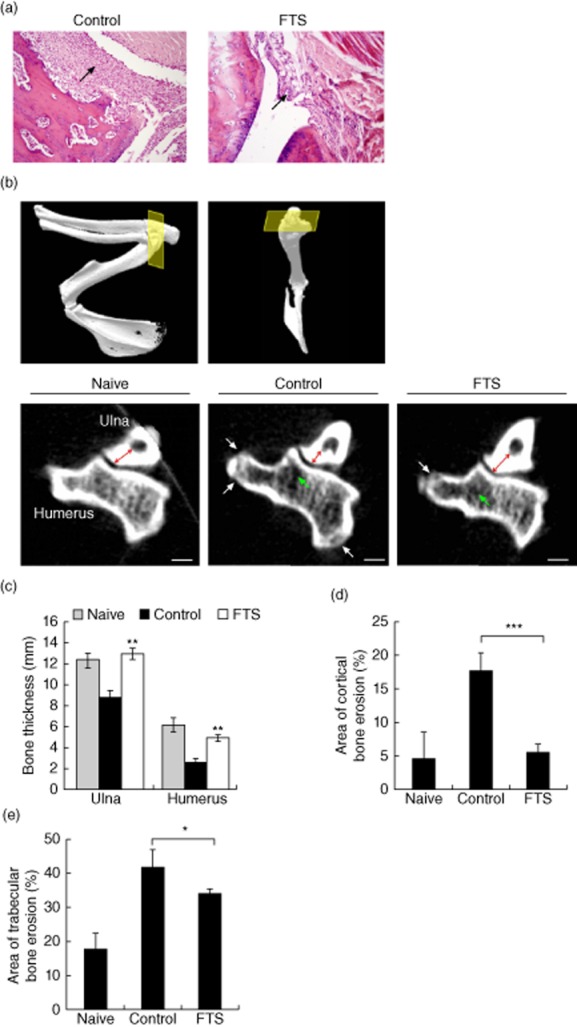
Effect of farnesylthiosalicylic acid (FTS) on joint morphology in rats with adjuvant-induced arthritis (AIA). (a) Representative joint tissues were stained with haematoxylin and eosin (H&E) on day 16. Tissue from a vehicle-treated control rat is shown in the left panel, and tissue from an FTS-treated rat is shown on the right (×100 magnification). Arrows indicate lymphocyte infiltration. (b) Naive rats (where disease was not induced) and AIA-Lewis male rats were either treated with FTS (80 mg/kg) or vehicle [0·5% carboxy methyl cellulose (CMC)], and on day 25 rats were anaesthetized and scanned by micro-computerized tomography (CT), as described in Materials and methods. Three-dimensional reconstructions of the frontal right limb of naive rat is shown in (b) from different angles. The yellow square depicts the plane of interest of the synovial joint on which the analysis was performed. Images of coronal sections of ulna and humerus bones from representative naive (left), control (middle) and FTS-treated rats (right) are shown in the lower panel. Cortical bone thicknesses of ulna (red arrows) and humerus, as well as bone erosion of the cortical (white arrows) and trabecular (green arrows) parts of the humerus, were assessed. (c) Quantification of bone thicknesses of ulna and humerus. (d,f) Cortical (d) and trabecular (e) bone erosion of the humerus, calculated as percentages of the total area occupied by bone. *P < 0·05; **P < 0·01; ***P < 0·001 compared to vehicle-treated control. Values are means ± standard error of the mean (n = 5).
To determine the effect of FTS on the degree of bone damage we scanned the front paws of rats on day 25 after disease induction. Analysis of the CT scans revealed that AIA rats treated with FTS demonstrated increased bone thickness (144% increase in the ulna thickness and 166% increase in the humerus thickness) relative to the vehicle-treated rats (Fig. 2b,c) and significantly smaller cortical and trabecular erosions (67% decrease in the volume of the cortical erosions and 20% decrease in the volume of the trabecular erosions) relative to vehicle-treated rats (Fig. 2d,e). Thus, both bone mass and erosions were conserved in rats treated with FTS.
Effects of FTS on T lymphocyte subsets in AIA
Our next tasks were to identify the inflammatory cellular components of arthritis induction in our model and to determine whether those components are modified by FTS treatment. We used flow cytometry to study the distribution of both CD4+ and CD8+ T cells harvested from spleens and ILNs 18 days after arthritis induction. As shown in the upper and middle panels of Fig. 3a (and quantified in the lower panel of the same figure), treatment with FTS was followed by a decrease in both CD4+ and CD8+ T cell counts compared to those in the vehicle-treated group (decrease of 22 and 15% in the levels of CD4+ and CD8+ T cells in the spleens, respectively, and decrease of 34 and 25% in levels of CD4+ and CD8+ T cells in ILNs, respectively). Differences were more significant in the CD4+ T cell subpopulation, pointing to marked inhibition in the recruitment of these cells to the secondary lymphoid organs. No significant differences were recorded between CD4+ or CD8+ T cells originating from the spleens and from the ILNs.
Previous work from our laboratory showed that FTS treatment resulted in induction of regulatory T cells 10. To determine the amounts of these cells in rats with AIA, we conducted biochemical measurements of FoxP3, the master regulator of regulatory cells. As shown in Fig. 3b, FoxP3 levels were significantly higher in lysates from the FTS-treated group than from the vehicle-treated controls (233% increase over the vehicle-treated group), strongly suggesting an active role for FoxP3, and by implication for regulatory T cells, in amelioration of the inflammatory response. Fluorescence activated cell sorter (FACS) analysis of the same cells supported the induction of intracellular FoxP3 expression in the FTS-treated rats (Fig. 3c).
FTS treatment increases production of anti-inflammatory cytokines and decreases the levels of proinflammatory cytokines
To further characterize the function(s) of these cells, we assayed the serum levels of different T cell-derived cytokines that play central roles in the pathogenesis of RA. We found that vehicle-treated rats exposed to heat-killed M. tuberculosis showed an increase in serum IFN-γ and TNF-α, both T helper type 1 (Th1) response-related cytokines and important mediators of cellular host defence against pathogens 11. In contrast, after rats that were similarly exposed to M. tuberculosis were treated with FTS, their IFN-γ and TNF-α levels were attenuated significantly (decrease of 80% in IFN-γ levels and 98% lower levels of TNF-α compared to the vehicle-treated rats; Fig. 4a,b). IL-6 is a proinflammatory cytokine associated with Th2 responses and is an important participant in the efferent arm of RA 12,13. Intriguingly, serum IL-6 was significantly lower in the FTS-treated group than in the controls (34% decrease in level compared to vehicle-treated rats; Fig. 4c). Unlike IL-6, the Th2-mediated cytokine IL-4 exerts a protective role in RA and has the ability to suppress synoviocyte proliferation and activation 14–16. IL-4 was increased significantly in FTS-treated rats compared to vehicle-treated controls (by 1223%; Fig. 4d). IL-17, the key cytokine released from Th17 cells, is a major participant in the pathogenesis of RA and other autoimmune conditions 17. Treatment with FTS yielded lower serum levels of this cytokine than in vehicle-treated controls (66% decrease; Fig. 4e). Finally, serum levels of the anti-inflammatory cytokines IL-10 and TGF-β were significantly higher in FTS-treated rats than in the vehicle-treated controls (increase of 209% in the levels of IL-10 and 123% in levels of TGF-β; Fig. 4f,g) 18. Thus, the inhibited recruitment of CD4+ cells (Fig. 3), the marked decline in serum IFN-γ and TNF-α and the increase in serum IL-4, IL-10 and TGF-β all seem to confirm the anti-inflammatory therapeutic effects of FTS.
Figure 4.

Analysis of serum cytokines. Sera of naive, adjuvant-induced arthritis (AIA)rats treated with farnesylthiosalicylic acid (FTS) and AIA rats treated with vehicle were tested for interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4, IL-6, IL-10, IL-17 and transforming growth factor (TGF)-β. Cytokine levels are expressed as pg/ml. *P < 0·05; **P < 0·01; ***P < 0·001 compared to vehicle-treated control group, Student's t-test.
FTS reduces activation of Ras effector pathways in AIA and RA lymphocytes
Next we characterized the effects of FTS on several Ras-effector pathways in our experimental model. GTP-loaded Ras was assessed by GST-RBD assays of lysates of splenocytes and ILNs prepared from FTS and vehicle-treated rats with AIA (Fig. 5). Activated Ras levels were significantly lower in all lysates from FTS-treated than from vehicle-treated rats (29% decrease in GTP-Ras levels; Fig. 5a), as were the levels of the Ras effector PI3K (49% decrease relative to the vehicle-treated group; Fig. 5b). To characterize related distal signalling events, we also examined the lysates for three phosphorylated kinases. As shown (and quantified) in Fig. 5c, phosphorylated AKT, p38 and ERK were significantly lower in lysates from FTS-treated rats than from vehicle-treated controls (decrease of 50, 60 and 62% in levels of P-AKT, P-p38 and P-ERK levels, respectively).
Figure 5.
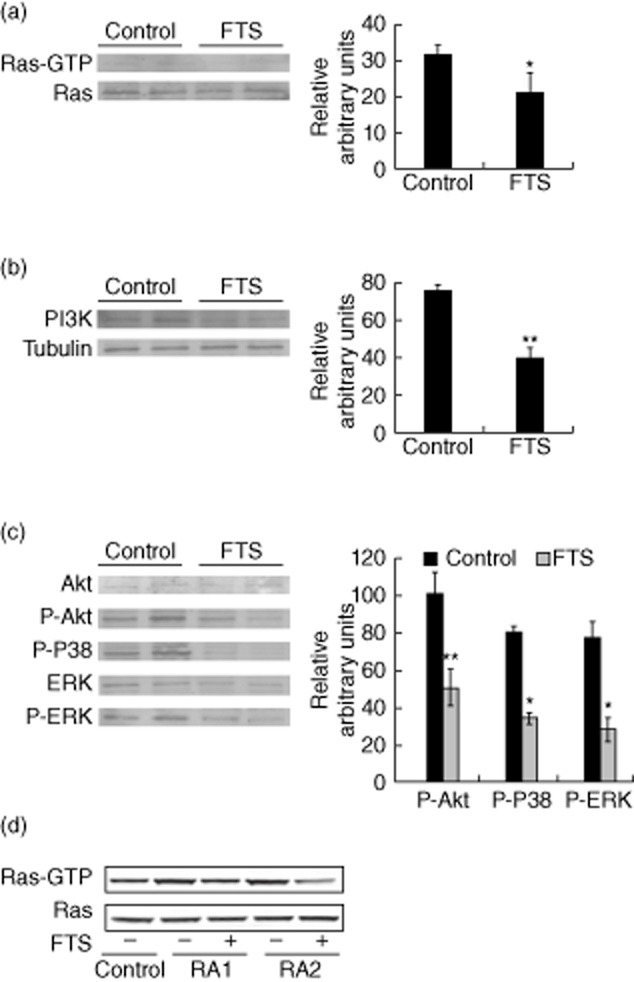
Farnesylthiosalicylic acid (FTS) reduces activation of protein kinase B (AKT), p38 and extracellular-regulated kinase (ERK) signalling and increases PI3K and forkhead box protein 3 (FoxP3) expression in rats with adjuvant-induced arthritis (AIA). (a,b,c). Inguinal lymph nodes (ILNs) of FTS-treated and control rats with AIA were tested for (a) guanosine triphosphate (GTP)-loaded Ras by glutathione S-transferase-rho binding domain (GST-RBD) pull-down assays, (b) PI3K expression, (c) phospho-AKT, phospho-p38 and phospho-ERK. Right panels show the results of densitometric analysis. (d) CD3+ lymphocytes from two rheumatoid arthritis (RA) patients were treated with FTS (50 μM) for 48 h and GTP-loaded Ras was studied by GST-RBD pull-down assay. *P < 0·05; **P < 0·01 compared to vehicle-treated control; Student's t-test. The following antibodies were used: anti-pan-Ras (Ab-3; Calbiochem), anti-PI3K (Millipore), anti-Akt, anti-phospho-Akt, anti-p38, anti-phospho-p38 (all from Cell Signaling), anti-ERK (Santa Cruz Biotechnology), anti-phospho ERK (Sigma-Aldrich), anti-tubulin and anti-FoxP3 (eBioScience).
Finally, to gain a first impression of the clinical significance of our findings, we studied the effect of FTS on Ras activation in lymphocytes from RA patients. We found that Ras-GTP levels were higher in lymphocytes from patients with active RA than in those from healthy volunteers (Fig. 5d). Treatment of these lymphocytes with FTS decreased the levels of Ras-GTP significantly, indicating that – as in our rat model – FTS inhibits Ras activation in lymphocytes of patients with RA.
Discussion
Transformation by Ras proteins is critically dependent upon their membrane association, which is mediated by lipid–protein interactions. These highly specific interactions are critical for Ras signalling. FTS, by interfering with such interactions, dislodges Ras from the cell membrane, thereby inhibiting its signalling and cell transformation. The beneficial effects of FTS have been demonstrated convincingly in Phase II clinical trials of patients with pancreatic and non-small-cell lung cancer 2.
Ras proteins play an important role in cancer and in other biological phenomena, especially in activation of the immune system 9,19. In lymphocytes, Ras proteins regulate signalling downstream of the antigen receptor, as well as the secretion, adhesion and proliferation of cytokines 9. Mice transgenic for activated Ras present autoimmune phenotypes due to unregulated immune responses 20. Activated Ras thus appears to be an important target for treating inflammatory conditions, and FTS and its analogues may serve as ‘silver bullets’ against this target.
Because Ras is required for the activation and proliferation of lymphocytes, key events in the pathogenesis of autoimmunity, Ras inhibition might serve as an immunosuppressive therapy for patients with systemic lupus erythematosus (SLE), a prototypic autoimmune disease. In a previous study of the effects of FTS on Murphy-Roth large (MRL)/lpr mice, an animal model for SLE, our group showed that FTS treatment of affected mice results in a 50% decrease in morbidity, indicating that FTS might be useful for treating autoimmune diseases 21. Our group also demonstrated beneficial effects of FTS in other animal models of autoimmune diseases such as anti-phospholipid syndrome, type I diabetes, multiple sclerosis and Crohn's disease 22–24.
Patients with RA exhibit an inflammatory response of the synovia, which is secondary to hyperplasia of synovial cells and heavy infiltration by lymphocytes, and results in pannus formation 25. The pathology leads to destruction of articular cartilage and ankylosis of the joints. Autoimmunity plays a pivotal role in both its chronicity and its progression. Autoantibodies, immune complexes and T cell-mediated antigen-specific responses are evidently major players in the pathogenesis. These factors lead to overburdening of the immune response and therefore require regulation by immunomodulation. Various treatments are available 26. Analgesia and anti-inflammatory drugs, including steroids, are used to suppress the symptoms, while disease-modifying anti-rheumatic drugs (DMARDs) inhibit or halt the underlying immune process and prevent long-term damage to the joints. Inflammatory cytokines contribute to perpetuation of the disease. IL-1 and TNF-α, important mediators of the inflammatory response, promote induction of adhesion molecules and proteinase gene expression, and increase production of additional inflammatory cytokines such as IL-6 27. More recent treatment options mainly employ cytokine-neutralizing agents to neutralize TNF-α. Despite significant improvement, the response is often incomplete and new therapeutics are needed.
Autoantigens that cross-react with mycobacteria have been implicated in the pathogenesis of AIA, a model of experimental arthritis induced in rats by intradermal injection of CFA 6. In both RA and AIA, proinflammatory cytokines contribute to the progression of joint destruction and synovial hyperplasia. These similarities between AIA and RA support the established usage of AIA as a tool in screening new drugs for RA.
In this study we examined the therapeutic effect of oral FTS on inflammation in rats with AIA. Our results showed clearly that such treatment improved the outcome in rats with AIA. We showed that the anti-inflammatory effects of FTS were mediated by inhibiting the recruitment of lymphocytes to lymphoid organs. We also demonstrated a significant FTS-related decline in the proinflammatory cytokines IFN-γ, TNF-α, IL-6 and IL-17, and an increase in the anti-inflammatory cytokines IL-4, IL-10 and TGF-β. We were also able to demonstrate the induction of the transcription factor FoxP3. Histopathologically, FTS treatment resulted in a decline in cartilage damage, inflammatory infiltrate and pannus formation (Table 1). Importantly, imaging studies confirmed the ability of FTS to prevent bony erosions and cortical bone loss in treated rats (Fig. 2). As a proof-of-concept, we were able to show that FTS specifically inhibits Ras activation in lymphocytes harvested from patients with active RA (Fig. 5d).
Based on our studies both in vitro and in vivo, we propose the following model to explain the anti-inflammatory effects of FTS on rats with AIA (Fig. 6). Ras regulates the PI3K, p38 and mitogen-activated protein kinase (MAPK) signalling pathways, and inhibition of Ras by FTS results in reduced downstream signalling by these pathways 28. As a result, the proinflammatory cytokines IL-6, IL-17, IFN-γ and TNF-α are reduced, leading to attenuated cartilage damage and lymphocyte infiltration (Fig. 6). Because Ras also negatively regulates expression of FoxP3, inhibition of Ras by FTS leads to higher FoxP3 expression which, in turn, contributes to enhanced translation and release of IL-10 and TGF-β 10.
Figure 6.
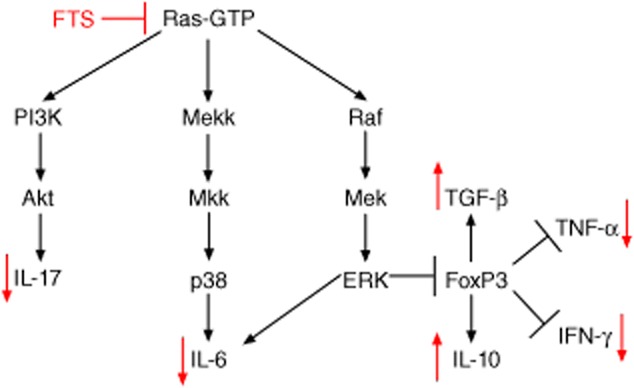
Proposed model illustrating the anti-inflammatory effects of farnesylthiosalicylic acid (FTS) in rats with adjuvant-induced arthritis (AIA).
Our study is not free of weaknesses. One major limitation is the non-contributory genetic background of the rats which, unlike collagen-induced arthritis models, do not carry genetically susceptible alleles. In summary, our results suggest that oral administration of FTS effectively suppresses synovitis in rats with AIA, and might therefore be helpful in the treatment of RA. Further studies are currently under way to narrow down the possible molecular mechanisms of FTS action in this disease.
Acknowledgments
This work was supported in part by The Israel Science Foundation 912/06 (Y. K.) and by the Prajs-Drimmer Institute for The Development of Anti-degenerative Drugs (Y. K). Y. K. is the incumbent of the Jack H. Skirball Chair in Applied Neurobiology at Tel Aviv University. The authors thank Ms Shirley Smith for scientific editing of the manuscript.
Disclosures
None.
References
- Blum R, Cox AD, Kloog Y. Inhibitors of chronically active Ras: potential for treatment of human malignancies. Recent Pat Anticancer Drug Discov. 2008;3:31–47. doi: 10.2174/157489208783478702. [DOI] [PubMed] [Google Scholar]
- Bustinza-Linares E, Kurzrock R, Tsimberidou AM. Salirasib in the treatment of pancreatic cancer. Future Oncol. 2010;6:885–891. doi: 10.2217/fon.10.71. [DOI] [PubMed] [Google Scholar]
- Mor A, Philips MR, Pillinger MH. The role of Ras signaling in lupus T lymphocytes: biology and pathogenesis. Clin Immunol. 2007;125:215–223. doi: 10.1016/j.clim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Pap T, Nawrath M, Heinrich J, et al. Cooperation of Ras- and c-Myc-dependent pathways in regulating the growth and invasiveness of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2004;50:2794–2802. doi: 10.1002/art.20461. [DOI] [PubMed] [Google Scholar]
- Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol. 2011;25:549–567. doi: 10.1016/j.berh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hegen M, Keith JC, Jr, Collins M, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis. 2008;67:1505–1515. doi: 10.1136/ard.2007.076430. [DOI] [PubMed] [Google Scholar]
- Bolon B, Stolina M, King C, et al. Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J Biomed Biotechnol. 2011;2011:569068. doi: 10.1155/2011/569068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZF, Jiao XL, Wang T, et al. Norisoboldine alleviates joint destruction in rats with adjuvant-induced arthritis by reducing RANKL, IL-6, PGE(2), and MMP-13 expression. Acta Pharmacol Sin. 34:403–413. doi: 10.1038/aps.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Campi G, Du G, et al. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol 2007. 2013;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- Mor A, Keren G, Kloog Y, George J. N-Ras or K-Ras inhibition increases the number and enhances the function of Foxp3 regulatory T cells. Eur J Immunol. 2008;38:1493–1502. doi: 10.1002/eji.200838292. [DOI] [PubMed] [Google Scholar]
- Meyer O. Interferons and autoimmune disorders. Joint Bone Spine. 2009;76:464–473. doi: 10.1016/j.jbspin.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlin T. Tocilizumab for the treatment of systemic juvenile idiopathic arthritis. Expert Rev Clin Immunol. 2012;8:517–525. doi: 10.1586/eci.12.49. [DOI] [PubMed] [Google Scholar]
- Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor α, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan A, Grusby MJ, Kaplan CD, et al. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:3345–3352. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Briolay J, Rissoan MC, et al. IL-4 inhibits growth factor-stimulated rheumatoid synoviocyte proliferation by blocking the early phases of the cell cycle. J Immunol. 1993;151:4908–4917. [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Rutz S, Ouyang W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol. 2011;23:605–612. doi: 10.1016/j.coi.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Mor A, Ben-Moshe O, Mekori YA, Kloog Y. Inhibitory effect of farnesylthiosalicylic acid on mediators release by mast cells: preferential inhibition of prostaglandin D2 and tumor necrosis factor-α release. Inflammation. 2011;34:314–318. doi: 10.1007/s10753-010-9236-x. [DOI] [PubMed] [Google Scholar]
- Katzav A, Kloog Y, Korczyn AD, et al. Inhibition of Ras by farnesylthiosalicylate significantly reduces the levels of autoantibodies in two animal models of the antiphospholipid syndrome. Immunobiology. 2003;207:47–50. doi: 10.1078/0171-2985-00208. [DOI] [PubMed] [Google Scholar]
- Katzav A, Kloog Y, Korczyn AD, et al. Treatment of MRL/lpr mice, a genetic autoimmune model, with the Ras inhibitor, farnesylthiosalicylate (FTS) Clin Exp Immunol. 2001;126:570–577. doi: 10.1046/j.1365-2249.2001.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Aizman E, George J, Kloog Y. Ras inhibition induces insulin sensitivity and glucose uptake. PLOS ONE. 2011;6:e21712. doi: 10.1371/journal.pone.0021712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman E, Mor A, Chapman J, Assaf Y, Kloog Y. The combined treatment of Copaxone and Salirasib attenuates experimental autoimmune encephalomyelitis (EAE) in mice. J Neuroimmunol. 2010;229:192–203. doi: 10.1016/j.jneuroim.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Oron T, Elad-Sfadia G, Haklai R, et al. Prevention of induced colitis in mice by the ras antagonist farnesylthiosalicylic acid. Dig Dis Sci. 2012;57:320–326. doi: 10.1007/s10620-011-1880-y. [DOI] [PubMed] [Google Scholar]
- Viatte S, Plant D, Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol. 9:141–153. doi: 10.1038/nrrheum.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt E, Crofford L. Rheumatoid arthritis: new treatments, better outcomes. Nurse Pract 2012. 2013;37:16–22. doi: 10.1097/01.NPR.0000421427.01505.0e. [DOI] [PubMed] [Google Scholar]
- Li J, Hsu HC, Mountz JD. Managing macrophages in rheumatoid arthritis by reform or removal. Curr Rheumatol Rep. 2012;14:445–454. doi: 10.1007/s11926-012-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- Oelzner P, Fleissner-Richter S, Bräuer R, Hein G, Wolf G, Neumann T. Combination therapy with dexamethasone and osteoprotegerin protects against arthritis-induced bone alterations in antigen-induced arthritis of the rat. Inflamm Res. 2010;59:731–741. doi: 10.1007/s00011-010-0184-6. [DOI] [PubMed] [Google Scholar]


