Abstract
There is an urgent need to identify ways of enhancing the mucosal immune response to oral vaccines. Rotavirus vaccine protection is much lower in Africa and Asia than in industrialized countries, and no oral vaccine has efficacy approaching the best systemic vaccines. All-trans retinoic acid (ATRA) up-regulates expression of α4β7 integrin and CCR9 on lymphocytes in laboratory animals, increasing their gut tropism. The aim of this study was to establish the feasibility of using ATRA as an oral adjuvant for oral typhoid vaccination. In order to establish that standard doses of oral ATRA can achieve serum concentrations greater than 10 nmol/l, we measured ATRA, 9-cis and 13-cis retinoic acid in serum of 14 male volunteers before and 3 h after 10 mg ATRA. We then evaluated the effect of 10 mg ATRA given 1 h before, and for 7 days following, oral typhoid vaccine in eight men, and in 24 men given various control interventions. We measured immunoglobulin (Ig)A directed against lipopolysaccharide (LPS)and protein preparations of vaccine antigens in whole gut lavage fluid (WGLF) and both IgA and IgG in serum, 1 day prior to vaccination and on day 14. Median [interquartile range (IQR)] Cmax was 26·2 (11·7–39·5) nmol/l, with no evidence of cumulation over 8 days. No adverse events were observed. Specific IgA responses to LPS (P = 0·02) and protein (P = 0·04) were enhanced in WGLF, but no effect was seen on IgA or IgG in serum. ATRA was well absorbed, well tolerated and may be a promising candidate oral adjuvant.
Keywords: IgA, oral vaccines, retinoic acid, retinoid pharmacokinetics, typhoid vaccine
Introduction
The global burden of intestinal infectious disease represents a major obstacle to the realization of health goals for many low- and middle-income countries 1,2. Diarrhoea remains a major cause of death in children in the tropics, and this is compounded by malnutrition. While many infectious diseases are controlled by immunization, this has not proved possible for diarrhoeal diseases, and only now is immunization against rotavirus becoming a reality for many children who would benefit from it 3. Immunization against diarrhoea-causing pathogens remains a challenge not only because of the large (and expanding) number of potential pathogens 4, but also because oral vaccines are intrinsically less effective than the best systemic vaccines, such as yellow fever or hepatitis B vaccines. Even those vaccines that are most effective, such as rotavirus vaccine, are substantially less effective in tropical than in temperate regions 3; for example, while the pentavalent oral rotavirus vaccine is 98% effective against severe rotavirus diarrhoea in the United States and Finland and other relatively affluent countries 5, it is only 48% effective in Bangladesh and Vietnam 6. Similarly, the single strain rotavirus vaccine was 90% effective against severe diarrhoea in Europe 7, but was only 49% effective in Malawi 8. An adjuvant that could enhance the effect of oral vaccines could have a dramatic impact on programmes for controlling diarrhoeal disease, with major benefits for child health in developing countries. Several strategies for adjuvanticity are currently being explored 9, including AB5 enterotoxins and their derivatives, ligands at innate immune receptors, mucoadhesives, particulates, saponins, oil emulsions and cytokines.
Retinoic acid is the oxidation product of retinol and comprises four isoforms: all-trans, 9-cis, 11-cis and 13-cis retinoic acids. All-trans retinoic acid (ATRA) binds to the heterodimeric retinoic acid receptor (RAR/RXR), whereas 9-cis RA binds to the RXR homodimer 10; the ligand-receptor unit then binds other co-regulatory molecules, and these complexes then bind to specific retinoic acid response elements (RAREs) in the regulatory regions of specific genes 11,12. Recent work has elucidated a complex role for ATRA in immune regulation. First, ATRA up-regulates expression of the gut-homing phenotype markers α4β7 integrin and CCR9 through the RAR/RXR heterodimer 13. Secondly, ATRA up-regulates expression of the polymeric immunoglobulin (Ig)A receptor, which exports secretory IgA into the gut lumen 14. Thirdly, ATRA is implicated in T cell subset allocation following antigen presentation 15. Fourthly, ATRA is implicated in lineage commitment of forkhead box protein 3 (FoxP3+) regulatory T cells 15,16, and with the tissue tropism of RORγt+ Th17 cells 17. Fifthly, ATRA acts in a positive feedback loop to encourage DCs to promote gut homing 18. Sixthly, vitamin A is required for development of oral tolerance 19. For the purpose of this work, the first two of these are of considerable interest as these are highly desirable characteristics of an orally active adjuvant. Recent work in mice has demonstrated that ATRA can induce mucosal immune responses to antigens injected by the subcutaneous 20 or intramuscular 21 routes.
In clinical oncology, ATRA (tretinoin, marketed as Vesanoid; Roche, Welwyn Garden City, UK) has been used for 20 years to induce myelocytic differentiation in patients with promyelocytic leukaemia 22. In this condition, but at high doses (45 mg/m2/day for up to 3 months), ATRA has a considerable number of adverse effects 23. The concentration of ATRA required to produce the immune effects described above 13 was approximately 10 nmol/l (which is equivalent to 3 ng/ml), several orders of magnitude lower than the Cmax generated by the very high doses used in oncological practice 24. One other adverse effect of ATRA, teratogenicity 25, is of greater relevance as it occurs at concentrations much closer to those found in healthy post-prandial adults 26. The purpose of this study was to determine the dose required to produce concentrations into the sort of range used in vitro 13, then to determine if ATRA has adjuvant properties when given alongside oral vaccines to adult male volunteers in a population where this adjuvant potential is most needed.
Materials and methods
Fifty adult men were recruited from a cohort of adults in Misisi compound, a high-density township in Lusaka, Zambia, in which we have carried out several studies on intestinal infection and immunity 27,28. In consecutive studies, volunteers participated in pharmacokinetic assessments, then in an assessment of the impact of ATRA on responses to oral typhoid vaccine. Volunteers were interviewed and examined including nutritional assessment [body mass index (BMI) and body composition determined using a Tanita BC-418 and grip strength using a Takeda dynamometer], screened for helminths by examination of a single stool sample using the Kato-Katz technique, and offered HIV testing. Participation was deferred if participants gave any history of diarrhoea within 1 month, taking antibiotics within 2 weeks or vaccination within 6 months.
Ethical approval
Approval for this study was obtained from the Biomedical Research Ethics Committee of the University of Zambia (reference 020-06-10) and the Pharmaceutical Regulatory Authority of the Ministry of Health of the Government of the Republic of Zambia (DMS/7/9/22/TROPGAN003).
Measurement of retinoic acid isoforms in blood
To confirm the dose of tretinoin required to achieve Cmax concentrations of 10 nmol/l or more, eight men were given ATRA (Vesanoid; Roche) 10 mg daily for 8 days. On days 1 and 8, volunteers attended while fasting and a sample of blood was collected at 08:00 h and 3 h post-dose, as 3 h is a good estimate of Tmax for retinoic acids 24,29–31. The pre- and post-dose samples on days 1 and 8 were designated t1, t2, t3 and t4, respectively; t1 and t3 were thus measures of C0 and t2 and t4 were measures of Cmax. A further six men were given 60 mg retinyl palmitate (Accucaps Industries Ltd, Windsor, ON, Canada; supplied by the World Health Organization) as a single dose on day 1 and only one sample was collected on day 8, giving time-points t1, t2 and t3; t1 was thus a measure of C0, t2 was Cmax and t3 was a late post-dose measure.
All blood samples were collected into clot activator Vacutainer tubes (Becton Dickinson, San Jose, CA, USA) and kept in the dark, refrigerated for 20 min, before being centrifuged at 461 g and serum stored at −80°C. Aliquots of serum were shipped to Craft Laboratories (Wilson, NC, USA) for analysis by high-performance liquid chromatography (HPLC).
Vaccine administration
Volunteers (n = 27), screened for the absence of helminths, were randomized to receive one of two vaccines, Vivotif or Dukoral, with or without ATRA given as 10 mg daily for 8 days. Volunteers were not given any vaccine, which they had received previously. A further five volunteers were evaluated, but no vaccine or ATRA was given. These vaccine and control groups are summarized in Table 1. As this was a Phase I study, no attempt was made at concealment of treatment allocation and no placebo was used, but allocation was concealed from laboratory staff. Two vaccines were used, one to evaluate responses and one as a control to exclude non-specific responses. Vivotif (Ty21a; Berna Biotech, Bern, Switzerland) is the only licensed oral typhoid vaccine; it also confers immunity against Salmonella paratyphi. Vivotif induces anti-lipopolysaccharide (LPS) systemic IgG and IgA, antibody-secreting cells in peripheral blood and cell-mediated immunity (CMI). CMI to Vivotif is associated with interferon (IFN)-γ secretion characteristic of a Th1 response. Immunity conferred by Ty21a lasts up to 7 years, but uniquely requires three to four doses given only 1–2 days apart. Safety has been established in more than 200 million vaccines 32. The control vaccine, Dukoral (Berna Biotech), is administered as a liquid preparation on a single occasion. This vaccine has been shown to be safe and protective in several trials conducted in settings where cholera is endemic. Of relevance to the setting of this study, Dukoral administered in a mass immunization campaign in Mozambique was associated with 78% protection against cholera 33.
Table 1.
Demographic and nutritional characteristics of study participants
| Vaccine study | ||||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P | |
| n | 8 | 6 | 7 | 6 | 5 | |
| Vaccine | Vivotif | Vivotif | Dukoral | Dukoral | None | |
| ATRA | + | − | + | − | − | |
| Age (years) | 35 (28–45) | 38 (29–40) | 31 (28–40) | 39 (37–40) | 21 (19–22) | 0·07 |
| HIV-positive (n) | 2 | 2 | 3 | 4 | 0 | |
| BMI (kg/m2) | 21 (18–24) | 22 (19–23) | 20 (18–24) | 19 (18–19) | 19 (18–20) | 0·47 |
| Fat (%) | 10 (6–19) | 15 (10–20) | 14 (9–25) | 13 (10–13) | 9 (6–12) | 0·29 |
| Grip strength (kg) | 40 (29–43) | 35 (32–39) | 39 (35–42) | 36 (33–40) | 40 (35–41) | 0·87 |
All participants were men. All participants in the PharmacoKinetics study subsequently participated in the vaccine study, so these are shown broken down by study group. Continuous variables are shown as median and interquartile range (IQR). Fat is expressed as percentage body weight, measured by impedance. ATRA: all-trans retinoic acid; BMI: body mass index.
Dukoral was given as a single dose; Vivotif was given on days 1, 3 and 5 (or days 1, 4 and 6 if a weekend made this more convenient, so that all doses of vaccine could be administered under supervision). ATRA was administered 1 h before the first dose of vaccine; this was supervised on day 1 and unsupervised on days 2–8.
Measurement of mucosal IgA responses using whole gut lavage
Immunological assessment was carried out on days 0 and 14. Following an overnight fast, volunteers underwent whole gut lavage as described previously 34, and a 10-ml blood sample was collected. Briefly, each volunteer drank a sufficient quantity (usually 2–4 litres) of a gut lavage solution (Klean-Prep; Norgine, Uxbridge, UK) to yield a clear fluid per rectum. Once the rectal output had almost cleared of visible particulates a 50 ml aliquot was collected and protease inhibitors added: ethylenediamine tetraacetic acid (EDTA) (Sigma, Poole, UK) was added to a final concentration of 10 mmol/l, and one tablet of Sigmafast protease inhibitor cocktail (Sigma) was dissolved in the fluid. This contains 4-(2-aminoethyl) benzenesulphonylfluoride hydrochloride, arginine, pepstatin A, bestatin, pancreatic trypsin inhibitor, acetyl-Leu-Leu-arginal, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, phosphoramidon and 1S-octyl-β-D-thioglucopyranoside. The tube was centrifuged in a refrigerated centrifuge at 4°C at 2626 g for 20 min and aliquots of supernatant stored at −80°C.
Analysis of IgA responses to oral vaccination
Three different vaccine antigen preparations were made: a suspension of whole bacteria (2 × 107 in each well), a protein extract (30 ng in each well) and a preparation of LPS made using a modified phenol extraction technique 35 (1 μg in each well). Briefly, vaccine bacteria were heated in distilled water at 67°C, mixed with an equal volume of preheated 90% aqueous phenol (pH 8·0) and incubated for 15 min, then centrifuged at 16 000 g for 5 min. Plates were coated with antigen in phosphate-buffered saline (PBS) overnight at 4°C, and casein in PBS (Perbio Science, Cramlington, UK) was used as blocking buffer for 1 h before adding 100 μl of serum (dilution 1:100) or 100 μl whole gut lavage fluid (WGLF; undiluted) and incubating for 2 h at room temperature (22°C). Washing was carried out six times using PBS with 0·05% Tween20. Secondary antibody, goat anti-human IgA or anti-human IgG conjugated to alkaline phosphatase (Cambridge Bioscience, Cambridge, UK) was incubated for 1 h at room temperature and washed six times. Following 20 min incubation with p-nitrophenyl phosphate (Sigma) the plate was read at 405 nm in anEL800 plate reader (BioTek, Potton, UK). All work was carried out with pyrogen-free pipette tips (Starlab, Milton Keynes, UK). Secretory IgA in WGLF was assayed by ELISA (Immunodiagnostik AG, Bensheim, Germany).
Data analysis
Pharmacokinetic data are expressed as median and interquartile range (IQR); values at individual time-points were compared in different groups using the Kruskal–Wallis test. Graphic representation was performed using polynomial (Epanechnikov) smoothing of the time-points at 0 and 3 h in order to demonstrate individual data points and summary data with 95% confidence intervals. Serum and WGLF responses to vaccine antigens are expressed as optical density (OD) values, but total IgA is expressed in ng/ml; values are expressed as median and IQR and the Kruskal–Wallis test was used.
Results
Of 50 men recruited, 18 had intestinal helminth infections and were excluded, leaving 32 men aged between 18 and 50 years (mean age 31) who participated in the study. Of these, 11 men (34%) were HIV-seropositive, which is consistent with previous studies in this community 28. Mean BMI was 20·4 kg/m2, mean percentage body fat was 13·2% and mean grip strength was 36·8 kg; no significant difference in these parameters was apparent using the Kruskal–Wallis or t-tests in volunteers assigned to different study groups (Table 1).
Pharmacokinetics
Predose concentrations of ATRA, 9-cis and 13-cis RA were similar in men allocated to retinyl palmitate or to tretinoin, but post-dose ATRA and 13-cis RA were much higher following administration of tretinoin (Fig. 1). Post-dose concentrations exceeded 10 nmol/l on all but two occasions. These were two HIV-seropositive men whose Cmax on day 1 failed to rise above 10 nmol/l, one rising to 9·6 and the other to 6·7 nmol/l; in the second case the Cmax on day 8 was also low at 7·8 nmol/l. Cmax values in HIV-seropositive men (median 13·8 nmol/l, IQR 6·7–24·8) appeared to be lower than in HIV-seronegative men (median 38·9, IQR 27·6–40), but the difference was not significant (P = 0·10). There was a suggestion that Cmax on day 8 was lower than on day 1, which would indicate induction of glucuronidation and oxidation enzymes, but this was not significant in this small group. No evidence of accumulation of any of the RA isoforms over the 8 days was observed, as trough concentrations remained low on day 8. No adverse events were reported by these men during the 8 days of treatment.
Figure 1.
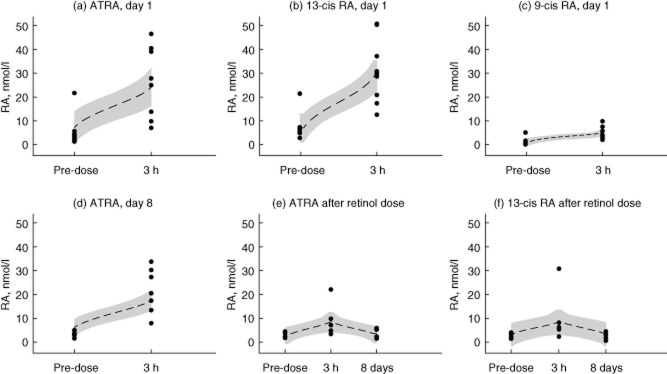
Pharmacokinetics. (a–c) Serum concentrations of (a) all-trans retinoic acid (ATRA), (b) 13-cis retinoic acid (RA) and (c) 9-cis RA just before or 3 h after an oral dose 10 mg ATRA on day 1. Although pure ATRA was given, immediate isomerization occurs and the peak concentration of 13-cis RA exceeds that of ATRA. (d) Serum concentration of ATRA before and after 10 mg ATRA on the eighth day; there is no evidence of accumulation, but Cmax appears to be reduced compared with day 1, probably reflecting induction of degradative enzymes. (e,f) Serum concentrations of (e) ATRA and (F) 13-cis RA before and 3 h after a single dose of 60 mg retinyl palmitate, and then on day 8 at the same time as the baseline sample was taken for the volunteers shown in (d). Dotted lines show the polynomial smoothed curve, and the shaded band the 95% confidence limits.
Effect of tretinoin on responses to oral vaccines
Specific IgA in whole gut lavage fluid (WGLF) against LPS and protein extract was increased in vaccine recipients who were given ATRA 10 mg daily for 8 days compared with those who were not given ATRA, or those who were given Dukoral with or without ATRA (Fig. 2). There was no difference in changes in total WGLF IgA concentrations between ATRA recipients and controls (P = 0·18; Fig. 3). There were three individuals whose total IgA in WGLF increased, but two of these showed no rise in specific IgA in WGLF, and of the four participants with a clear rise in specific IgA in WGLF, only one had a rise in total IgA. No effect was seen on serum IgA or IgG responses to either of the vaccine antigens evaluated (Fig. 5).
Figure 2.
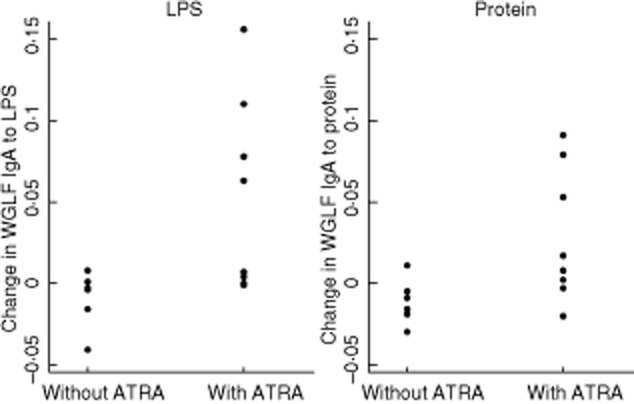
Summary of changes in specific immunoglobulin (Ig)A in Vivotif recipients. Responses are shown measured as change in optical density (OD) values at 405 nm, in response to vaccination with or without 10 mg all-trans retinoic acid (ATRA) daily for 8 days (P = 0·02 for lipopolysaccharide (LPS), P = 0·04 for protein extract).
Figure 3.
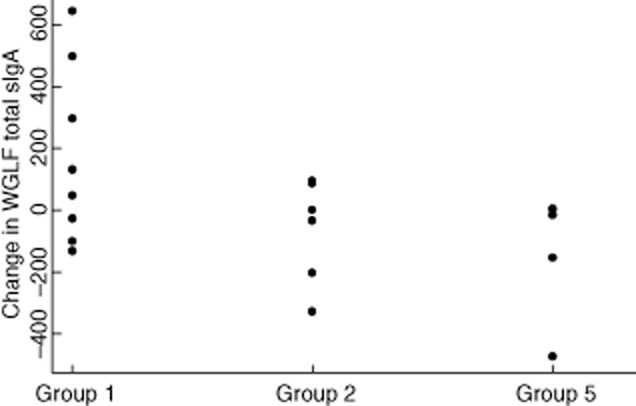
Secretory immunoglobulin (Ig)A concentrations in whole gut lavage fluid. Total IgA (ng/ml) in men in different groups was not statistically significant (P = 0·07).
Figure 5.
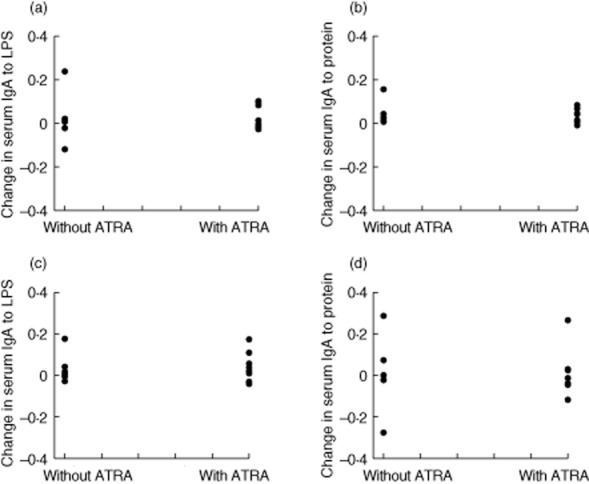
Changes in serum immunoglobulin (Ig)A and IgG in Vivotif recipients. No difference in serum responses to the vaccine antigens was seen in the vaccinees given 10 mg all-trans retinoic acid (ATRA)daily for 8 days alongside vaccination.
Correlations between serum and WGLF responses
We explored correlations between gut and serum IgA responses to LPS and protein extracts and in relation to total IgA in WGLF (Fig. 4). Correlations between responses to LPS and protein were strong in both WGLF and serum (Fig. 4a,b). There was a suggestion of a threshold effect of total IgA in WGLF such that only WGLF samples with total IgA concentrations of 200 ng/ml showed detectable specific IgA in WGLF (Fig. 4c,d). Correlations between specific IgA responses in WGLF and serum were less strong than within compartments (Fig. 4e,f).
Figure 4.
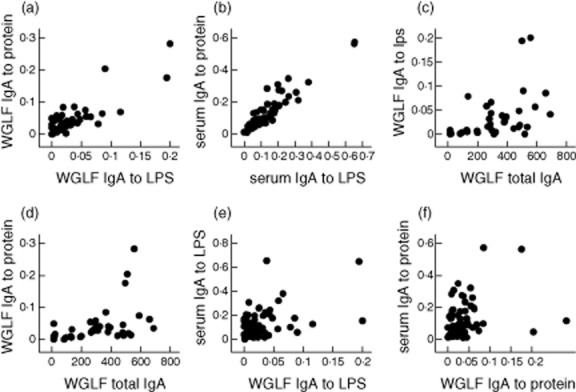
Correlations between whole gut lavage fluid (WGLF) and serum specific and total immunoglobulin (Ig)A responses. (a) Specific IgA responses to lipopolysaccharide (LPS) and protein extract in WGLF were closely correlated (ρ = 0·71; P < 0·0001). (b) Specific IgA responses to LPS and protein extract in serum were very closely correlated (ρ = 0·91; P < 0·0001). (c) Specific IgA to LPS in WGLF against total IgA in WGLF were correlated (ρ = 0·58; P = 0·0001). (d) Specific IgA to protein extract in WGLF against total IgA in WGLF were correlated (ρ = 0·60; P = 0·0001). (e) WGLF and serum-specific IgA to LPS were correlated moderately (ρ = 0·34; P = 0·006). (f) WGLF and serum-specific IgA to LPS were correlated moderately (ρ = 0·28; P = 0·03). Specific IgA concentrations given as optical density (OD) values at 1:200 dilution, and total IgA in WGLF as ng/ml.
We also explored the extent to which these results might be influenced by HIV status. Of the participants who were given Vivotif, two of eight who were also given ATRA were HIV-seropositive, and two of six who were not given ATRA were HIV-seropositive. The increases in specific IgA in WGLF from days 0 to 14 were −0·02 and 0·091 in the two HIV-seropositive participants compared with median (IQR) increases of 0·0125 (0·002, 0·053) in the six HIV-seronegative participants. While these numbers are small, we found no evidence of an inability to generate ATRA-augmented vaccine responses in HIV infection. While the strong correlations between specific IgA responses to LPS and protein within compartments (serum or WGLF) were unaffected by HIV status, the correlations between specific IgA in WGLF and total IgA in WGLF was restricted to HIV-uninfected (seronegative) participants (for example, ρ = 0·72 and P < 0·0001 for responses to protein in HIV-negative compared with ρ = 0·21 and P = 0·61 in HIV-seropositive participants).
Discussion
In view of the global burden of diarrhoeal disease and the reduced efficacy of oral vaccines in tropical populations, the need for effective oral adjuvants is urgent 36, and new strategies are needed for vaccination against diarrhoeal diseases. We have explored the possibility that all-trans retinoic acid could be utilized as an oral adjuvant in a population with a high burden of HIV. We have demonstrated that ATRA can be given safely in a simple commercially available oral capsule and that rapid rises in serum concentration are predictable. The great majority of adults in this population demonstrated a Cmax higher than the concentration (10 nmol/l), which was associated with changes in lymphocyte homing markers in vitro 13. We went on to examine gut and serum responses to Vivotif antigens (crude extracts of LPS and protein from vaccine bacteria) in vaccinees given Vivotif and in controls given an irrelevant vaccine, Dukoral. Our results suggest that ATRA could play an adjuvant role when given alongside Vivotif, an oral typhoid vaccine. Our results need to be confirmed in other populations and with other vaccines, but suggest that immunological effects of ATRA demonstrable in vitro and in vivo 13,20 could potentially be translated into an important strategy for enhancing responses to easily administered vaccines.
The dose of tretinoin used in this study (10 mg/day, about 6–7 mg/m2) is approximately one-eighth of that used in oncological practice and generated serum Cmax concentrations of 6·7–42 nmol/l. We extrapolated from in-vitro data that a concentration of 10 nmol/l would be required to be sure of an effect on homing receptor expression in lymphocytes, although the concentration in intestinal epithelial cells would, of course, be higher. These values of Cmax, while satisfactory for this purpose, are lower than might be expected based on data from adults 24,29 or children 30. To set this in context, 45 mg/m2 in adults generated Cmax concentrations of 508 nmol/l in adults 29. Even at much lower doses (25 mg/m2) in children, Cmax varied between 48·5 and 513 nmol/l 30. We surmise that the low Cmax we observed in some participants (and high values in none) reflects the impact of environmental enteropathy 28,37, and in those individuals with HIV infection Cmax was lower still. A great deal of work has been conducted on vitamin A (retinol and carotenoid) absorption, but many questions remain unanswered 38,39, and mechanisms of absorption of retinoic acids and their derivatives remain largely unexplored. Nevertheless, these values provide a starting-point for future work directed at immunological manipulation rather than oncological treatment.
The effect of vitamin A supplementation (VAS) on vaccine efficacy has been the focus of a modest number of studies that have generated disparate results 40. There is also some evidence that the effects of VAS on responses to vaccination (specific and non-specific) may be sex-dependent, with a greater benefit in boys than in girls 41, and this may apply particularly to diphtheria–tetanus–pertussis (DTP) vaccination 41. It is apparent from our pharmacokinetic data that oral administration of retinoic acid generates a much sharper rise in serum concentration of ATRA than does oral dosing with retinyl palmitate, and thus the immunological effects may be different, or at least clearer.
The adjuvanticity of ATRA we observed in this study applied only to the mucosal compartment; no effect was observed on serum IgA or IgG responses. Further work will be required to ascertain whether ATRA may increase IgA transport into the gut, through up-regulation or enhanced function of the polymeric immunoglobulin receptor (pIgR). However those individuals with the clearest increases in specific IgA to LPS and protein extracts in WGLF were not those in whom we found evidence of increased total IgA, which would be expected if the principal effect were on pIgR. The correlations between specific IgA to LPS and both serum-specific IgA and total WGLF IgA suggest to us that gut antibody responses to this vaccine are dependent upon both enhanced induction of the immune response and increased antibody secretion, but this remains to be established.
In order to avoid having to reject volunteers who are HIV-seropositive, which could lead to stigmatization in this close-knit community, we included men who are HIV-infected alongside men without HIV infection. This opportunity for comparison yielded data that suggest that absorption of ATRA is reduced in HIV-infected men compared with uninfected men. Despite this apparent impairment, there was no evidence of impairment of immune induction in the gut, and it would appear that HIV-infected men were as able to recognize the vaccine alloantigens as uninfected men. The quantity and quality of vaccine responses in HIV infection is impaired for some (for example with hepatitis B vaccine 42), but not all, vaccines (human papilloma virus vaccine is effective 43), but we found no evidence in this early study that mucosal immune responses are impaired due to HIV.
While we believe that these results are promising, we must point out that retinoic acids are embryotoxic 25, with no clearly safe dose, and ATRA could not be used for vaccination programmes for women of childbearing age. ATRA appears to be safe in men at the doses we used in this study, and teratogenicity is not a concern in children at the ages at which vaccines are given. Nevertheless, the programmatic implications of this safety issue would need to be considered carefully. If the potential adjuvanticity of ATRA can be confirmed with a range of other vaccines, it might help to overcome the impaired vaccine efficacy in populations with the greatest need of vaccination against diarrhoea pathogens.
Acknowledgments
We are grateful to John Mbewe, Coillard Kaunga, Rose Soko, Themba Banda, Stayner Banda and Vera Yambayamba for work on data collection, and to the Craft Laboratories for analysis of retinoic acid isoforms. Funding was granted by the Bill & Melinda Gates Foundation through a Grand Challenges Explorations grant (OPP1018115).
Disclosures
None.
Author contributions
P. K. originated the study and is guarantor. M. M. L., M. C. K. and P. K. designed the study and the outcome measures. R. B., E. S., V. K. and P. K. supervised clinical data collection and S. S. designed and carried out the parasitological evaluations. P. K. wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript, including the authorship list.
References
- Petri WA, Miller M, Binder HJ, et al. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- Grimwood K, Lambert SB, Milne RJ. Rotavirus infections and vaccines. Pediatr Drugs. 2010;12:235–256. doi: 10.2165/11537200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–148. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. New Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis in the first two years of life in European infants: randomised double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- Mahdi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- Tang X-H, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol-Mech. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Takenouchi-Ohkubo N, Asano M, Chihaya H, et al. Retinoic acid enhances the gene expression of human polymeric immunoglobulin receptor (pIgR) by TNF-alpha. Clin Exp Immunol. 2004;135:448–454. doi: 10.1111/j.1365-2249.2004.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Coombes JL, Siddiqui KR, et al. A functionally specialised population of mucosal CD103+ DCs induces Foxp3 regulatory T cells via a TGFβ and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina TN, Mikheeva T, Brusko TM, et al. Retinoic acid and rifamycins differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLOS ONE. 2011;6:e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang SG, Hogen Esch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca E, Wang S, DeCalisto J, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–185. doi: 10.1053/j.gastro.2011.04.010. epub before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani B, Villablanca EJ, Quintana FJ, et al. Gut tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral tolerance in mice. Gastroenterology. 2011;141:2109–2118. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Friedrischsen M, Boelter J, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol. 2011;85:8316–8327. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukaemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- Rogers JE, Yang D. Differentiation syndrome in patients with acute promyelocytic leukemia. J Oncol Pharm Pract. 2011;18:109–114. doi: 10.1177/1078155211399163. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Takitani K, Koh M, Inoue A, Kawakami C, Kuno T, Tamai H. Pharmacokinetics of all-trans retinoic acid in adults and children with acute promyelocytic leukemia. Am J Hematol. 2006;81:720–721. doi: 10.1002/ajh.20717. [DOI] [PubMed] [Google Scholar]
- Adams J. The neurobehavioral teratology of retinoids: a 50-year history. Birth Defects Res (Part A) 2010;88:895–905. doi: 10.1002/bdra.20721. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Brors O, Bock J, et al. Exposure to retinyl esters, retinol, and retinoic acids in non-pregnant women following increasing single and repeated doses of vitamin A. Ann Nutr Metab. 2005;49:155–164. doi: 10.1159/000086879. [DOI] [PubMed] [Google Scholar]
- Kapulu MC, Simuyandi M, Sianongo S, et al. Differential changes in expression of intestinal antimicrobial peptide genes during Ascaris lumbricoides infection in Zambian adults do not respond to helminth eradication. J Infect Dis. 2011;203:1464–1473. doi: 10.1093/infdis/jir035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–419. [PubMed] [Google Scholar]
- Ozpolat B, Lopez-Berestein G, Adamson P, Fu CJ, Williams AH. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci. 2003;6:292–301. [PubMed] [Google Scholar]
- Lanvers C, Reinhardt D, Dubbers A, et al. Pharmacology of all-trans retinoic acid in children with acute promyelocytic leukemia. Med Pediatr Oncol. 2003;40:293–301. doi: 10.1002/mpo.10257. [DOI] [PubMed] [Google Scholar]
- McNamara PJ, Jewell RC, Jensen BK, Brindley CJ. Food increases the bioavailability of acitretin. J Clin Pharmacol. 1988;28:1051–1055. doi: 10.1002/j.1552-4604.1988.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret J-F. Experience with registered mucosal vaccines. Vaccine. 2003;21:678–683. doi: 10.1016/s0264-410x(02)00579-0. [DOI] [PubMed] [Google Scholar]
- Lucas MES, Deen JL, von Seidlein L, Wang X-Y, Ampuero J, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. New Engl J Med. 2005;352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- Daley AC, Randall R, Darsley M, et al. Genetically modified enterotoxigenic Escherichia coli vaccines induce mucosal immune responses without inflammation. Gut. 2007;56:1550–1556. doi: 10.1136/gut.2006.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderitz O, Risse HJ, Schulte-Holthausen H, et al. Biochemical studies of the smooth–rough mutation in Salmonella minnesota. J Bacteriol. 1965;89:343–354. doi: 10.1128/jb.89.2.343-354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendram B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Prendergast A, Kelly P. Enteropathies in the developing world; neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–763. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin carotenoids. Biochim Biophys Acta. 2012;1821:70–77. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:61–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savy M, Edmond K, Fine PE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139:2154S–2218. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- Benn CS. Combining vitamin A and vaccines: convenience or conflict? Dan Med J. 2012;59:B4378. [PubMed] [Google Scholar]
- Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS. 2009;20:595–600. doi: 10.1258/ijsa.2009.009126. [DOI] [PubMed] [Google Scholar]
- Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;15:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]


