Summary
Literature reports describe kiwi fruit as a food with significant effects on human health, including anti-oxidant and anti-inflammatory activity. Fresh fruit or raw kiwi fruit extracts have been used so far to investigate these effects, but the molecule(s) responsible for these health-promoting activities have not yet been identified. Kissper is a kiwi fruit peptide displaying pore-forming activity in synthetic lipid bilayers, the composition of which is similar to that found in intestinal cells. The objective of this study was to investigate the kissper influence on intestinal inflammation using cultured cells and ex-vivo tissues from healthy subjects and Crohn's disease (CD) patients. The anti-oxidant and anti-inflammatory properties of kissper were tested on Caco-2 cells and on the colonic mucosa from 23 patients with CD, by challenging with the lipopolysaccharide from Escherichia coli (EC-LPS) and monitoring the appropriate markers by Western blot and immunofluorescence. EC-LPS challenge determined an increase in the intracellular concentration of calcium and reactive oxygen species (ROS). The peptide kissper was highly effective in preventing the increase of LPS-induced ROS levels in both the Caco-2 cells and CD colonic mucosa. Moreover, it controls the calcium increase, p65-nuclear factor (NF)-kB induction and transglutaminase 2 (TG2) activation inflammatory response in Caco-2 cells and CD colonic mucosa. Kissper efficiently counteracts the oxidative stress and inflammatory response in valuable model systems consisting of intestinal cells and CD colonic mucosa. This study reports the first evidence supporting a possible correlation between some beneficial effects of kiwi fruit and a specific protein molecule rather than generic nutrients.
Keywords: inflammation, lipopolysaccharide, reactive oxygen species
Introduction
Kiwi fruit is widely reported as a functional food and a nutraceutical source. Green kiwi fruit (Actinidia deliciosa) is the most well-known species in the genus Actinidia; the spread of other species, such as A. chinensis (gold kiwi fruit) and A. arguta, is now gradually increasing. Among the effects associated with the consumption of green kiwi fruit, laxation activity is probably the most popular. Human trial data support the public belief that this fruit is a safe and effective dietary intervention for the treatment of constipation 1,2. Kiwi fruit had already been used in ancient times in some Asian geographical areas where Chinese traditional medicine succeeded in treating different types of cancers, especially cancers of the digestive system and of the mammary gland, by kiwi therapy 3. More recently, many studies have been performed on different species of kiwi fruit and have suggested that this food is endowed with some additional health-promoting properties that may influence human wellness. For instance, anti-allergic activity in ovalbumin-sensitized murine models has been reported for extracts obtained from A. arguta 4,5. Results obtained by Abe and co-workers 6 suggest that green kiwi fruit may exert beneficial effects against diabetes via its ability to regulate adipocyte differentiation and function. High levels of in-vitro anti-oxidant activity and protection against oxidative DNA damage or oxidative stress have also been described for green and gold kiwi fruit 7–9. In addition, the anti-inflammatory properties of extracts from gold and green kiwi fruit in in-vitro models comprising lipopolysaccharide (LPS)-stimulated macrophages or intestinal epithelial cells have been reported recently 9.
Generally, the health-promoting effects of kiwi fruit have been investigated using the whole fruit, and very little attention has been paid so far to the small peptides naturally occurring in kiwi fruit and to their possible biological effects on humans.
Kissper is a 4 kDa peptide found in variable amounts in green kiwi fruit 10,11. It derives from the proteolytic cleavage of the precursor kiwellin, one of the most abundant protein components of this fruit 12. Kiwellin is a 20 kDa protein that may undergo in-vivo proteolytic processing originating kissper and KiTH, corresponding to the N- and C-terminal regions of the protein, respectively 13. Actinidin, a thiol protease present in very high amounts in green kiwi fruit, is responsible for the processing of kiwellin. It was reported that kiwellin is an allergenic molecule. However, immunoglobulin (Ig)E binding activity was observed for KiTH, the C-terminal domain, and for the entire molecule 13,14; no IgE binding activity of the peptide kissper has been observed so far. The functional characterization demonstrated that kissper is endowed with pH-dependent and voltage-gated pore-forming activity characterized by anion selectivity and channelling in model synthetic planar lipid membranes 10. The capacity of kissper to form channel-like pathways in a lipid bilayer similar to that found in intestinal cells suggests a possible influence of this food molecule on human gastrointestinal physiology.
The study described here is within the framework of a research programme focused on the analysis of possible effects of the kiwi fruit peptide kissper on human health. Our specific objective was the investigation of a possible involvement of kissper in the modulation of oxidative stress and inflammation of the gastrointestinal tract. The chronic intestinal inflammatory disease objective of the present investigation is Crohn's disease (CD), characterized by a chronic, segmental and recidivant inflammation of intestine with ulceration and fissuration 15.
Oxidative stress modulation plays an important role in the pathogenesis of inflammatory bowel diseases (IBD), including CD 16. High levels of reactive oxygen species (ROS) induce activation of the redox-sensitive nuclear transcription factor kappa-B (NF-κB) which, in turn, triggers the inflammatory mediators 17,18.
The experimental system chosen to test the kissper function included human intestinal epithelial cells and cultured biopsies of colonic mucosa from patients with CD.
Materials and methods
Kissper purification from green kiwi fruit
Kissper was purified following the already described procedure 10, with few modifications. Briefly, ripe kiwi fruits (A. deliciosa) were homogenized in water (1:1, w/v) by a household blender. After centrifugation, the supernatant was dialyzed against 10 mM Tris-HCl, pH 8·0, and loaded onto a DE52 (Whatman, Brentford, UK) column equilibrated in the same buffer. The column was eluted with 0·5 M NaCl in the equilibrating buffer, and aliquots of the collected fractions were analysed by reverse-phase–high-performance liquid chromatography (RP–HPLC). The fractions containing kissper were dialysed against 10 mM Tris-HCl, pH 8·0, and then loaded onto a Mono-Q HR 10/10 column, equilibrated in the same buffer. The column was eluted by a linear gradient from zero to 0·3 M NaCl. The fractions from Mono-Q were analysed by RP–HPLC, and those containing kissper were concentrated by ultrafiltration.
Chromatographic separation by RP–HPLC
Separation of the protein fractions by RP–HPLC was performed on a Vydac C8 column (0·21 × 25 cm) using a Beckman System Gold apparatus (Fullerton, CA, USA). Elution was accomplished by a linear gradient of eluent B (0·08% trifluoracetic acid in acetonitrile) in eluent A (0·1% trifluoracetic acid) at a flow rate of 1 ml/min. The eluate was monitored at 220 and 280 nm. The separated fractions were collected manually and analysed as needed.
Amino acid sequencing
Amino acid sequencing of the N-terminal region of the purified fraction of kissper was performed with an Applied Biosystems Procise 492 automatic sequencer (Applied Biosystems, Foster City, CA, USA), equipped with online detection of phenylthiohydantoin amino acids. Protein sequence analyses were performed using software available on the ExPASy Bioinformatics Resource Portal (http://www.expasy.org).
Kissper stability to the simulated gastric and intestinal digestion
In-vitro gastric digestion of the purified peptide was performed as described by Moreno et al. 19. Kissper was solubilized in simulated gastric fluid (SGF) (0·15 M NaCl adjusted to pH 2 with 1 M HCl) before the addition of pepsin (porcine pepsin; Roche Diagnostics GmbH, Mannheim, Germany) at the enzyme/substrate ratio of 1:20 (w/w). The digestion was performed at 37°C. Aliquots were taken at 0 and 120 min and analysed by RP–HPLC. Digestion was stopped by raising the pH to 7·4 by addition of 50 mM Na-phosphate buffer pH 7·4.
For intestinal digestion, porcine trypsin and bovine chymotrypsin (Roche Diagnostics GmbH) were used. Simulated intestinal fluid (SIF) was prepared as described in the United States Pharmacopeia 20, and consists of a mixture of trypsin and chymotrypsin in 0·05 M K-phosphate buffer pH 6·8. Digestions were performed at 37°C, at an enzyme/substrate ratio of 1:50 (w/w), both for trypsin and chymotrypsin. Aliquots of the digested samples were withdrawn at 0 and 120 min and then loaded onto a Vydac (Deerfield, IL, USA) C8 column for RP–HPLC analysis using a Beckman System Gold apparatus.
Cell lines
Caco-2 cells, a human colonic epithelial cell line, were cultured as recommended by the American Type Culture Collection (ATCC, Manassus, VA, USA). Experiments were initiated on days 14–15 after seeding and continued for 24–72 h, as the cells progressed through more mature stages of differentiation.
Cell cultures
Cells were seeded onto 12-well plates at a density of 2–3 × 105 cells/cm2, then pretreated with kissper (10 μg/ml) for 6 h and finally stimulated with Escherichia coli (EC-LPS, 1 μg/ml) for 24 h. The viability of the cells was estimated by examining their ability to exclude Trypan blue using Countess® Automated Cell Counter (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions.
Patients and ex-vivo organ cultures
After informed consent, biopsy specimens were taken from uninflamed colonic mucosal areas immediately next to inflamed tissues of 23 patients with CD (mean age of patients 25 years, range 16–41) some of whom also participated in a previous study 16. The primary site of involvement was ileocolonic in 13 patients and colonic in 10 patients. Normal controls (mean age 22·4 years, range 18–29) included mucosal samples, taken from six patients with uncomplicated diverticular disease and six patients with rectal bleeding due to haemorrhoids. Two to four specimens from each patient were used for histology; the other samples were cultured in vitro for 6 and 24 h with medium alone, EC-LPS (1 μg/ml E. coli serotype O127: B8 lipopolysaccharide; Sigma-Aldrich, Milan, Italy) in the presence or absence of kissper 10 μg/ml.
Immunoblot
Experiments were carried out as described by Luciani et al. 21. Briefly, cells were washed in ice-cold phosphate-buffered saline (PBS) and lysed with NP-40 lysis buffer (1% NP-40, 150 mmol/l NaCl, 50 mmol/l Tris, pH 7·4, 10 mmol/l NaMoO4) at 4°C for 30 min. Protease inhibitors were added to NP-40 lysis buffer to a final concentration of 1 μg/ml leupeptin, 2 μg/ml aprotinin, 50 μg/ml Pefabloc, 121 μg/ml benzamidine and 3·5 μg/ml E64. Cell lysates were centrifuged at maximal speed in an eppifuge at 4°C, and supernatants were collected. Cell lysates (50 μg) were loaded, separated onto 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. After blocking for 2 h with Tris-buffered saline (TBS)/Tween supplemented with 5% non-fat dry milk, blots were incubated with transglutaminase 2 (TG2) (clone CUB7402; NeoMarkers, Fremont, CA, USA) and actin (rabbit polyclonal IgG; Santa Cruz Biotechnology, Santa Cruz, CA, USA) The primary antibodies were counterstained using a horseradish peroxidase-conjugated anti-IgG antibody (Amersham, Little Chalfont, UK) for 60 min at room temperature. Proteins were visualized by chemiluminescence (ECL Plus; Amersham, GE Healthcare, Milan, Italy) and exposed to X-OMAT film (Eastman Kodak, Rochester, NY, USA). Protein concentrations were determined using a Bio-Rad (Hemel Hempstead, UK) protein assay to ensure equal protein loading prior to Western blot analysis.
Immunolocalization
Human tissue sections
Five-μm-thick cryostat sections were fixed in acetone for 10 min. The sections were incubated individually for 2 h at room temperature with the following antibodies: human TG2 (clone CUB7402; NeoMarkers, Fremont, CA, USA), phospho-p65(Ser536) (rabbit polyclonal; Cell Signaling Technology, Danvers, MA, USA), cyclooxygenase-1 (COX-2) (rabbit polyclonal; Cell Signaling Technology) and intercellular adhesion molecule 1 (ICAM-1) (rabbit polyclonal; Santa Cruz Biotechnology). Antigen expression and distribution was visualized using a donkey anti-rabbit IgG conjugated to Alexa Fluor 488 for 60 min at room temperature. Isotype control antibodies (IgG1 or IgG2), isotype-matched non-immune Igs or isotype-matched antibodies against inappropriate blood group antigens were used as specificity control. Data were analysed under fluorescence examination using a Leica fluorescence microscope. COX-2 or ICAM-1-positive mononuclear cells (MNC) were counted per mm2 of mucosa. Epithelial cells with nuclear p65 localization were counted per 100 epithelial cells. Data were examined in a blind fashion by two independent reviewers (I. R. and C. C.), completely unaware of the culture conditions for preventing any bias in their observation.
In-situ detection of TG2 enzymatic activity
TG2 activity was performed by preincubating live cells with biotin-MDC for 1 h at 37°C and then analysed by fluorescence examination using a Leica fluorescence microscope.
Calcium imaging
The cells were loaded with Fura-2 (30 min at 37°C) in calcium imaging buffer, in accordance with the manufacturer's instructions (Invitrogen, Molecular Probes, Monza, Italy) 22.
ROS detection
The cells or 5-μm cryostat sections were pulsed with 10 μmol/l 5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) (Invitrogen, Molecular Probes). CM-H2DCFDA, a ROS-sensitive probe, was used to track changes in the cellular redox state. The cells were analysed with a Wallac 1420 multi-label Counter (PerkinElmer, Waltham, MA, USA) and detected by Leica fluorescence microscopy.
Enzyme-linked immunosorbent assay (ELISA)
Tumour necrosis factor (TNF)-α secretion was measured using the BD OptEIATM ELISA kit II (BD Biosciences, San Jose, CA, USA), and transforming growth factor (TGF)-β1 was measured using the Boster Biological Technology (Abingdon, UK) ELISA kit in accordance with the manufacturer's instructions. Protein concentrations of whole-cell lysates were measured using the Bio-Rad Dc protein assay (Bio-Rad). TNF-α and TGF-β1 levels were normalized to standard protein concentrations 23.
Statistical analysis
All the experiments were performed in duplicate and were repeated at least three times. Group data from all experiments are presented as means ± standard error (s.e.). One-way analysis of variance (anova) was used for all the statistical analyses among multiple groups. In another data set, paired two-tailed Student's t-test was used for statistical analyses. Groups were compared by post-hoc Tukey–Kramer test. A P-value of <0·05 was considered significant.
Results
Kissper purification from green kiwi fruit
Several ripe kiwi fruit batches were analysed in order to assess the concentration of the peptide kissper in the protein extracts. Analysis of the kissper content in the kiwi fruit protein extracts was performed by RP–HPLC chromatographic separation. The fruits containing high amounts of peptide were selected and used as starting material for kissper purification, following the procedure described in the Materials and methods section. The purity of the preparation was checked by RP–HPLC analysis and N-terminal amino acid sequencing. The absence in the HPLC profile of peaks different from that of kissper suggested high purity of the peptide preparation, confirmed by N-terminal amino acid sequencing showing a single sequence.
Treatment of kissper with gastrointestinal proteases
Peptide kissper was treated with SGF and SIF. Analysis by RP–HPLC of all the aliquots taken at 0 and 120 min of incubation showed a single peak eluted at the retention time of the native peptide, thus indicating that kissper was resistant to the treatment with pepsin, trypsin and chymotrypsin. The absence of proteolytic cleavages was also confirmed by N-terminal amino acid sequencing analysis of the aliquots taken at 120 min of incubation, where only the N-terminal sequence of the native peptide was obtained.
Kissper effect on oxidative stress in Caco-2 cell lines and CD colonic mucosa
Caco-2 cells were pulsed with EC-LPS and 10 mmol/l CM-H2DCFDA in the presence or absence of kissper. ROS levels were monitored after 24 h of challenge (Fig. 1a). We observed a decrease of intracellular ROS levels in Caco-2 cells in the presence of kissper. Figure 1b shows that in Caco-2 cells calcium concentration was significantly different before and after EC-LPS treatment, indicating a possible mitochondrial damage. The addition of kissper modulates intracellular calcium increase in Caco-2 cells. We also evaluated the cell viability with or without kissper treatment. We observed a 72·3% of viability for the cells treated with medium only (7·2 × 105 total number of cells), 84% of viability for the cells treated with kissper and 75% of viability for cells treated with mock peptide (5·31 × 105 and 5·4 × 105 total number of cells, respectively). No significant difference was noted among the sets of experiments. Also, CD colonic mucosa before challenge showed higher ROS levels than controls. EC-LPS challenge led to an increase in ROS levels after 24 h of incubation in CD colonic mucosa, but not in controls (Fig. 2a). Moreover, treatment of CD colonic mucosa with kissper was highly effective in preventing EC-LPS-induced ROS levels (Fig. 2a).
Figure 1.
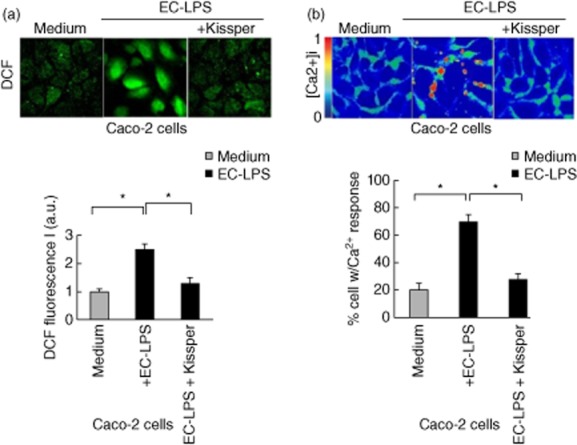
Kissper controls calcium release and reactive oxygen species (ROS) production in intestinal epithelial cells. (a) Immunofluorescence of intracellular ROS [chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA, top panel] or a Wallac 1420 multi-label counter (a, bottom line) DCF, 5-(and-6)CM-H2DCFDA). Values are means ± standard error (SE), n = 6. Asterisks indicate that means differ from samples cultured with medium. *P < 0·05. (b) Caco-2 cells were loaded with Fura-2AM. [Ca2+] imaging pseudocolour ratiometric images. Colours correspond to the scale of [Ca2+]i increase. Red, high [Ca2+]i contents. (b, bottom line) Quantification of results from three independent experiments is shown in the histogram. Values are means ± standard error (s.e.). Asterisks indicate that means differ from samples cultured with medium alone. *P < 0·05.
Figure 2.
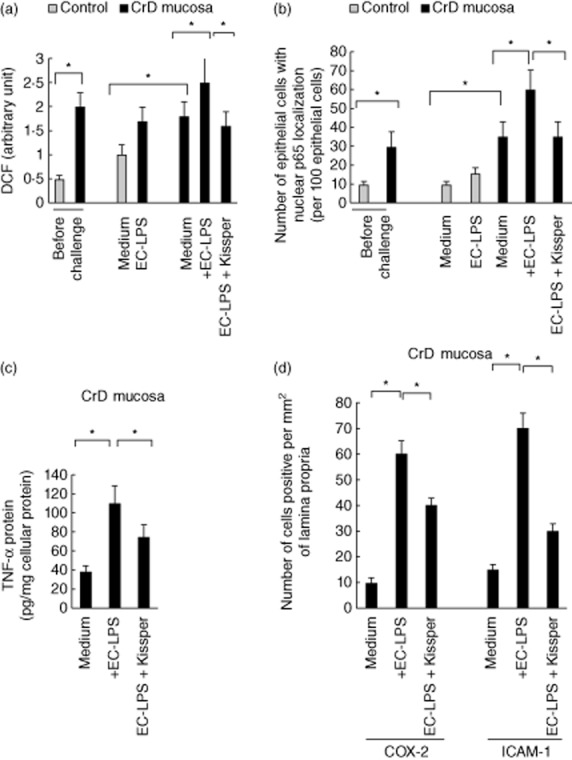
Effect of kissper in the control of mucosal inflammation. (a) Reactive oxygen species (ROS) levels in control (n = 12) and Crohn's disease (CD) colonic mucosa (n = 23) before challenge and after challenge with medium alone, medium with Escherichia coli-lipopolysaccharide (EC-LPS) or EC-LPS with kissper. Values are means ± standard deviation (s.d.). Asterisks indicate that means differ from control samples, *P < 0·05 versus control by analysis of variance (anova). (b) Number of epithelial cells with p65 nuclear localization per 100 epithelial cells in control (n = 12) and CD colonic mucosa (n = 23) before and after challenge with medium alone, medium with EC-LPS or EC-LPS and kissper following 24 h of incubation. Values are means ± s.d. *P < 0·05 versus control by anova. (c) Tumour necrosis factor (TNF)-α protein after challenge with medium alone, medium with EC-LPS or EC-LPS with kissper following 24 h of incubation. Values are means ± s.d. of three experiments. *P < 0·05 versus control by anova. (d) Immunofluorescence of cyclooxygenase 2 (COX-2) and intercellular adhesion molecule 1 (ICAM-1) and number of COX-2- and ICAM-1-positive lamina propria cells per mm2 of mucosa in CD colonic mucosa (n = 23 after challenge with medium alone, medium with EC-LPS or EC-LPS and kissper following 24 h of incubation. Scale bar, 10 μm. Values are means ± s.d. of three experiments. *P < 0·05 versus control by anova.
Kissper effect on ROS-mediated NF-kB activation in CD colonic mucosa upon challenge with EC-LPS
We investigated the effect of kissper in controlling NF-kB activation in CD colonic mucosa. Increased levels of p-65 nuclear localization were observed in EC-LPS-treated CD colonic mucosa. Pretreatment of cultured biopsies (Fig. 2b) with kissper was highly effective in controlling EC-LPS-induced p-65 nuclear localization. To determine whether or not kissper is able to switch off the mucosal inflammation in human EC-LPS-stimulated CD colonic mucosa, we also analysed the release of proinflammatory cytokines, such as TNF-α and COX-2 or ICAM-1 expression, by mucosal mononuclear cells. After 24 h of incubation, EC-LPS also induced an increase of ICAM-1 and COX-2 expression in CD colonic mucosa compared with samples cultured in medium alone. The proinflammatory cytokine TNF-α release and increased expression of ICAM-1, COX-2 following challenge with EC-LPS was controlled efficiently by kissper in CD colonic mucosa (Fig. 2c,d). Minimal TNF-α up-regulation and release, ICAM-1 and COX-2 were observed in controls after challenge with EC-LPS (data not shown).
Effect of kissper on TG2 expression in Caco-2 cell lines and CD mucosa
We evaluated the effect of kissper in controlling ROS-mediated TG2 activation. Immunofluorescence experiments carried out on CD colonic mucosa treated with EC-LPS show a decrease of TG2 protein expression. In fact, the increase of TG2 protein and activity in CD colonic mucosa was lowered by the addition of the kissper peptide (Fig. 3a). Also, Western blot analysis showed a decrease of TG2 protein expression in the presence of kissper, not observed in the control sample where the kiwi fruit peptide was absent (Fig. 3b). Moreover, we observed a decrease of TNF-α levels in Caco-2 cells (Fig 3c) and an increase of TGF-β1 levels in the supernatant of organ and cell cultures. In particular, our results demonstrate a 31% increase of TGF-β1 levels (pg/ml) in the supernatant of cells treated with kissper compared to that observed in cells treated with medium alone. The result was confirmed, although with a lower magnitude, in the organ culture, where we observed a 6% increase of TGF-β 1 levels in the supernatant of colonic biopsies treated with kissper compared with those not treated.
Figure 3.
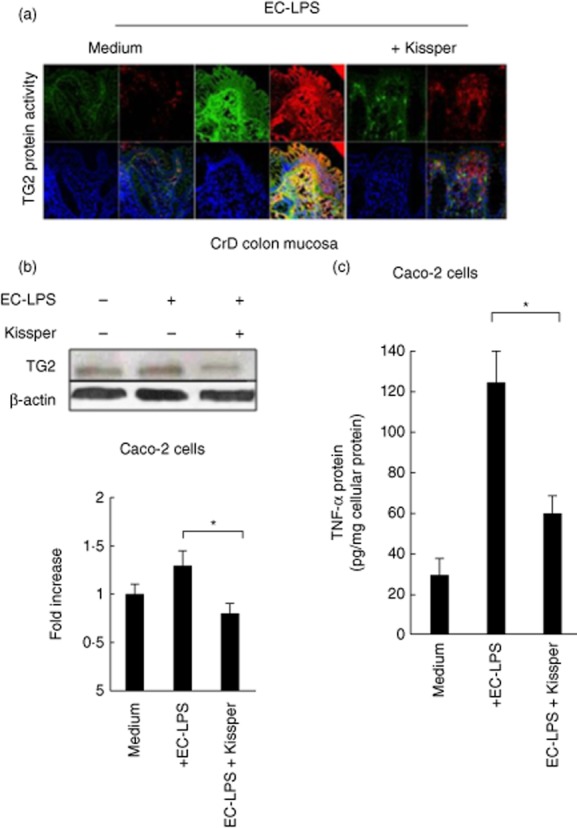
Kissper modulates transglutaminase 2 (TG2) expression in Crohn's disease (CD) colonic mucosa and Caco-2 cells. (a) Immunofluorescence analysis of TG2 protein (green) and activity (red) expression in CD colonic mucosa challenged for 6 h and other 24 h with Escherichia coli-lipopolysaccharide (EC-LPS) in the presence or absence of kissper. Incubation with kissper reduces the TG2 expression and activity in EC-LPS-challenged CD biopsies. (b) Immunoblot analysis of TG2 in Caco-2 cells after 24 h of challenge with EC-LPS. β-actin was used as loading control. (b, bottom line) Densitometric analysis of the band intensity. Values are means ± standard deviation (s.d.) of three experiments. (c) Tumour necrosis factor (TNF)-α protein after challenge with medium alone, medium with EC-LPS or EC-LPS with kissper following 24 h of incubation. Values are means ± s.d. of three experiments. *P < 0·05 versus control by analysis of variance (anova).
Discussion
The isolation and characterization of molecules associated with the specific biological effects of kiwi fruit appears to be an important goal, which might enlarge our knowledge about a possibly safe pharmacological use of naturally occurring drugs. Many studies indicate kiwi fruit as a health-promoting food and generally correlate the ascribed positive effects on human health to the high content of anti-oxidant compounds such as ascorbic acid, polyphenols and fibres 1,2,8,24. Recently, the anti-inflammatory properties of kiwi fruit extracts on LPS-stimulated macrophages or intestinal epithelial cells were reported 9. Raw kiwi fruit extracts were used for these experiments, therefore the molecule(s) responsible for the anti-inflammatory effect remain unknown.
To our knowledge, this study represents the first evidence supporting the hypothesis that some beneficial effects of kiwi fruit could be correlated with a specific protein molecule, rather than to nutrients such as vitamins or to generic classes of compounds, namely polyphenols and fibres. The strong resistance to gastric and intestinal proteolysis suggests that kissper could travel safely through the digestive system. This observation adds further support to the hypothesis that this molecule could affect human gastrointestinal physiology.
The inflammatory response is a complex event involving different cell types interacting within their natural environment. The ex-vivo colonic mucosa culture model is a useful tool to investigate inflammatory conditions and potential strategies to modulate the inflammatory response 25,26. The models used in this study represent a good approximation to in-vivo studies, as in cultured biopsy tissues all the anatomical connections are retained and all the cell types (epithelial, myeloid, lymphoid) interact in their natural environment.
At the molecular level, the inflammatory process includes up-regulation of the cellular concentration of ROS, calcium ions and specific molecules, such as TNF-α, TG2, COX-2 and ICAM-1, as well as activation of the transcription factor NF-κB by means of the phosphorylation of the p65 subunit. These molecules have been used as markers to monitor the capacity of the kiwi fruit peptide to counteract the EC-LPS-induced inflammatory response in Caco-2 cells and human colonic tissues.
The calcium and ROS signalling systems are integrated, such that calcium-dependent regulation of components of ROS homeostasis might influence intracellular redox status and vice versa 27–29. Transcription factor NF-κB is the most extensively studied intracellular pathway, being a target of ROS and oxidative stress 30. This factor is found in cytoplasm and is bound to IkB, which prevents it from entering the nuclei 31. When cells are stimulated, specific kinases phosphorylate IkB, causing its rapid degradation by proteasomes 31. The activation of NF-κB acts on genes for proinflammatory cytokines, chemokines, enzymes that generate mediators of inflammation, immune receptors and adhesion molecules that play a key part in the initial recruitment of leucocytes to the sites of inflammation. One of the results reported here shows that kissper can control the calcium content in EC-LPS stimulated Caco-2 cells, thus suggesting that this food molecule could play a role in the modulation of the pathway linking oxidative stress, activation of NF-kB and inflammation.
Kissper efficiently counteracted the ROS increase in a context of cell and biopsy model after EC-LPS challenge, used as trigger of ROS overproduction. Similarly, it was able to control the levels of TG2, the expression of which can be considered a link between oxidative stress and inflammation. TG2 plays an important role in mitochondrial physiology, and increased tissue levels of this protein have been described in a number of human diseases 32–35. The present study demonstrates that this kiwi fruit peptide determines a decrease of TG2 protein and activity expression in EC-LPS-stimulated Caco-2 cells and CD colonic mucosa. In addition, kissper reduces NF-kB p65 phosphorylation and release of proinflammatory cytokines, such as TNF-α and COX-2 or ICAM-1, by mucosal mononuclear cells, thus restoring the pattern observed in their controls after challenge with EC-LPS of CD colonic tissues. Similarly, pretreatment with the kiwi fruit peptide controls ROS-mediated NF-kB p65 activation and induces an increase of TGF-β1 levels in colonic mucosa and intestinal epithelial Caco-2 cells after challenge with EC-LPS.
In conclusion, the data reported here suggest a beneficial effect of this kiwi fruit peptide on the physiology of human intestine. It is important to underline that the present study provides convincing data concerning the non-toxicity and the protective effect of kissper, and in-vivo studies can be planned safely. Therefore, kissper appears to be a novel molecule that deserves attention and stimulates the implementation of further studies to define more clearly its anti-inflammatory effect on the physiological and pathological conditions of the gastrointestinal tract, as well as to evaluate a possible pharmaceutical use of this molecule in the therapy of intestinal inflammatory diseases.
Acknowledgments
Dr Ilaria Russo is a recipient of a fellowship from Regione Campania, Assessorato Sanità and from AIFA Farmacoviglianza Educational Project 2009/2010. This work was supported partially by the Italian Ministry of Economy and Finance (MEF) Project, CISIA-Conoscenze Integrate per la Sostenibilità e l'Innovazione del made in Italy Agroalimentare.
Disclosure
The authors have no conflicts of interest to disclose.
References
- Rush EC, Patel M, Plank LD, Ferguson LR. Kiwifruit promotes laxation in the elderly. Asia Pac J Clin Nutr. 2002;11:164–168. doi: 10.1046/j.1440-6047.2002.00287.x. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lin YT, Lu YT, Liu YS, Liu JF. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr. 2010;19:451–457. [PubMed] [Google Scholar]
- Motohashi N, Shirataki Y, Kawase M, et al. Cancer prevention and therapy with kiwifruit in Chinese folklore medicine: a study of kiwifruit extracts. J Ethnopharmacol. 2002;81:357–364. doi: 10.1016/s0378-8741(02)00125-3. [DOI] [PubMed] [Google Scholar]
- Park EJ, Kim B, Eo H, et al. Control of IgE and selective T(H)1 and T(H)2 cytokines by PG102 isolated from Actinidia arguta. J Allergy Clin Immunol. 2005;116:1151–1157. doi: 10.1016/j.jaci.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim SH, Park EJ, et al. Suppression of allergic diarrhea in murine ovalbumin-induced allergic diarrhea model by PG102, a water-soluble extract prepared from Actinidia arguta. Int Arch Allergy Immunol. 2009;150:164–171. doi: 10.1159/000218119. [DOI] [PubMed] [Google Scholar]
- Abe D, Saito T, Kubo Y, Nakamura Y, Sekiya K. A fraction of unripe kiwi fruit extract regulates adipocyte differentiation and function in 3T3-L1 cells. Biofactors. 2010;36:52–59. doi: 10.1002/biof.70. [DOI] [PubMed] [Google Scholar]
- Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003;24:511–515. doi: 10.1093/carcin/24.3.511. [DOI] [PubMed] [Google Scholar]
- Lee DE, Shin BJ, Hur HJ, et al. Quercetin, the active phenolic component in kiwifruit, prevents hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Br J Nutr. 2010;104:164–170. doi: 10.1017/S0007114510000346. [DOI] [PubMed] [Google Scholar]
- Edmunds SJ, Roy NC, Love DR, Laing WA. Kiwifruit extracts inhibit cytokine production by lipopolysaccharide-activated macrophages, and intestinal epithelial cells isolated from IL10 gene deficient mice. Cell Immunol. 2011;270:70–79. doi: 10.1016/j.cellimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ciardiello MA, Meleleo D, Saviano G, et al. Kissper, a kiwi fruit peptide with channel-like activity: structural and functional features. J Pept Sci. 2008;14:742–754. doi: 10.1002/psc.992. [DOI] [PubMed] [Google Scholar]
- Ciardiello MA, Giangrieco I, Tuppo L, et al. Influence of the natural ripening stage, cold storage, and ethylene treatment on the protein and IgE-binding profiles of green and gold kiwi fruit extracts. J Agric Food Chem. 2009;57:1565–1571. doi: 10.1021/jf802966n. [DOI] [PubMed] [Google Scholar]
- Tamburrini M, Cerasuolo I, Carratore V, et al. Kiwellin, a novel protein from kiwi fruit. Purification, biochemical characterization and identification as an allergen. Protein J. 2005;24:423–429. doi: 10.1007/s10930-005-7638-7. [DOI] [PubMed] [Google Scholar]
- Tuppo L, Giangrieco I, Palazzo P, et al. Kiwellin, a modular protein from green and gold kiwi fruits: evidence of in vivo and in vitro processing and IgE binding. J Agric Food Chem. 2008;56:3812–3817. doi: 10.1021/jf703620m. [DOI] [PubMed] [Google Scholar]
- Bernardi ML, Picone D, Tuppo L, et al. Physico-chemical features of the environment affect the protein conformation and the immunoglobulin E reactivity of kiwellin (Act d 5) Clin Exp Allergy. 2010;40:1819–1826. doi: 10.1111/j.1365-2222.2010.03603.x. [DOI] [PubMed] [Google Scholar]
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn's mucosa through modulation of antioxidant defense machinery. PLOS ONE. 2012;7:e32841. doi: 10.1371/journal.pone.0032841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease – radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- Moreno FJ, Mellon FA, Wickham MS, Bottrill AR, Mills EN. Stability of the major allergen Brazil nut 2S albumin (Ber e 1) to physiologically relevant in vitro gastrointestinal digestion. FEBS J. 2005;272:341–352. doi: 10.1111/j.1742-4658.2004.04472.x. [DOI] [PubMed] [Google Scholar]
- Stippler E, Kopp S, Dressman JB. Comparison of US pharmacopeia simulated intestinal fluid TS (without pancreatin) and phosphate standard buffer pH 6.8, TS of the international pharmacopoeia with respect to their use in in vitro dissolution testing. Dissolut Technol. 2004:6–10. [Google Scholar]
- Luciani A, Villella VR, Esposito S, et al. Cystic fibrosis: a disorder with defective autophagy. Autophagy. 2011;7:104–106. doi: 10.4161/auto.7.1.13987. [DOI] [PubMed] [Google Scholar]
- Barreto-Chang OL, Dolmetsch RE. Calcium imaging of cortical neurons using Fura-2. AM. J Vis Exp. 23:e1067. doi: 10.3791/1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani A, Villella VR, Vasaturo A, et al. SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J Immunol. 2009;183:2775–2784. doi: 10.4049/jimmunol.0900993. [DOI] [PubMed] [Google Scholar]
- Fiorentino A, D'Abrosca B, Pacifico S, Mastellone C, Scognamiglio M, Monaco P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J Agric Food Chem. 2009;57:4148–4155. doi: 10.1021/jf900210z. [DOI] [PubMed] [Google Scholar]
- Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- Tortora R, Russo I, De Palma GD, et al. In vitro gliadin challenge: diagnostic accuracy and utility for the difficult diagnosis of celiac disease. Am J Gastroenterol. 2012;107:111–117. doi: 10.1038/ajg.2011.311. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Holmes E, Khan F, et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- Malorni W, Farrace MG, Rodolfo C, Piacentini M. Type 2 transglutaminase in neurodegenerative diseases: the mitochondrial connection. Curr Pharm Des. 2008;14:278–288. [PubMed] [Google Scholar]
- Luciani A, Villella VR, Vasaturo A, et al. Lysosomal accumulation of gliadin p31-43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut. 2010;59:311–319. doi: 10.1136/gut.2009.183608. [DOI] [PubMed] [Google Scholar]
- Verma A, Wang H, Manavathi B, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- Maiuri L, Luciani A, Giardino I, et al. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARgamma down-regulation. J Immunol. 2008;180:7697–7705. doi: 10.4049/jimmunol.180.11.7697. [DOI] [PubMed] [Google Scholar]


