Abstract
Viral respiratory infections can have a profound effect on many aspects of asthma including its inception, exacerbations, and, possibly, severity. Of the many viral respiratory infections that influence asthma, the common cold virus, rhinovirus, has emerged as the most frequent illness associated with exacerbations and other aspects of asthma. The mechanisms by which rhinovirus influences asthma are not fully established, but current evidence indicates that the immune response to this virus is critical in this process. Many airway cell types are involved in the immune response to rhinovirus, but most important are respiratory epithelial cells and possibly macrophages. Infection of epithelial cells generates a variety of proinflammatory mediators to attract inflammatory cells to the airway with a subsequent worsening of underlying disease. Furthermore, there is evidence that the epithelial airway antiviral response to rhinovirus may be defective in asthma. Therefore, understanding the immune response to rhinovirus is a key step in defining mechanisms of asthma, exacerbations, and, perhaps most importantly, improved treatment.
Keywords: Asthma, exacerbation, rhinovirus, immune response to virus, pathogenesis
The role of respiratory infections in asthma has always been of clinical interest and importance, but their overall contribution to this disease has, until recently, been underappreciated. With the use of highly sensitive and specific molecular diagnostic and detection methods, the identification of infectious organisms has increased, and with these findings has come a greater and clearer understanding of the contributions of specific respiratory infections to asthma. From a wide variety of studies, the common cold virus, rhinovirus, has emerged as a key and perhaps central microorganism in many aspects of asthma. For example, recent studies have pointed to the importance and relevance of symptomatic infections with rhinovirus early in life as a major factor in recurrent wheezing and possibly even as a sentinel event in the pathogenesis of asthma.1,2 Equally important, and better characterized, is the demonstration that rhinovirus infections are the major cause of asthma exacerbations, accounting for 50% to 80% of all such attacks in both children and adults.3–5 Finally, recent observations have raised the possibility that the presence of rhinovirus in the lower airway may be a contributor to disease persistence and severity.6
To understand more fully how this apparently innocuous respiratory virus may lead to pathogenic events in asthma, and to develop more specific treatments, it is instructive to define, as well as possible, the immune response to rhinovirus and then translate this information in terms of alterations in airway inflammation and asthma control. Furthermore, it is helpful to determine whether factors in the immune responses to rhinovirus are attenuated in some individuals with asthma and to ascertain whether these abnormalities increase their susceptibility to untoward effects of the virus.
In this review, we focus on what is known about the immune response to rhinovirus with in vitro models and observations from in vivo studies, and then discuss how these findings may relate to asthma exacerbations and possibly disease pathogenesis. In addition, we explore the possibilities that some patients with asthma may have abnormal antiviral responses to rhinovirus, and we consider how such deficiencies may play a role in the ability of this virus to exacerbate asthma. Finally, we discuss current treatment in terms of further understanding the immune response to virus, then speculate on the potential for novel interventions to regulate specifically and possibly more effectively the immune response to rhinovirus to thus prevent a loss of asthma control.
RHINOVIRUS
Rhinovirus is a genus of positive, single-stranded RNA viruses of the family Picornaviridae. Thus far, there are >100 rhinovirus serotypes identified, with serotype defined on the ability of a given serum to neutralize growth of a given strain of virus in cell culture.7 The rhinovirus capsid, which protects the central RNA core, is composed of 60 copies of each of 4 structural proteins. Virus protein 1 (VP1), VP2, and VP3 are located on the capsid surface and are responsible for its antigenic diversity.8 The fourth protein, VP4, is located inside the virus and anchors the RNA core to the viral capsid.9 The VP1 protein is the most surface-exposed of the rhinovirus capsid proteins, and it contains a number of major epitopes recognized by neutralizing antibodies.10 Analysis of genetic sequence variations in the VP1 gene has shown close concordance with the known serotypes.10 Serotypes have classically been divided on the basis of susceptibility to antiviral agents into 2 groups—human rhinovirus (HRV)-A (susceptible) and HRV-B (not susceptible)—which have been found to correspond with phylogenetic analysis of the VP4/VP2 sequences.11,12 With the use of increasingly sophisticated techniques, a novel genetic cluster, HRV-C, has been identified in infants hospitalized with respiratory illness.12,13 The role and contribution of this new rhinovirus class to respiratory illnesses and asthma awaits further work.
Rhinovirus infections typically cause upper respiratory symptoms in the common cold, including rhinorrhea, sore throat, nasal congestion, sneezing, cough, and headache. In addition, rhinovirus is also the pathogen most commonly recovered in acute exacerbations of asthma. Moreover, it has been long recognized that hospital admissions for asthma correlate with the seasonal peaks of rhinovirus.3 In addition, rhinovirus is the most frequently identified virus in children hospitalized for wheezing episodes outside respiratory syncytial virus (RSV) season and especially after age 3 years.14
Rhinovirus was previously thought to infect primarily upper airway epithelium because optimal replication occurs between 33°C and 35°C, below the core temperature of the conducting airways and lung parenchyma.15 However, temperatures found throughout the lower airway lumen will also allow rhinovirus to replicate effectively.16,17 Rhinovirus also replicates equally well ex vivo in cultured epithelial cells (ECs) derived either from the upper or lower airway, and rhinovirus replication has been detected in lower airway ECs and secretions after experimental inoculation with rhinovirus.18,19 Finally, rhinovirus has been identified by in situ hybridization in the lower respiratory tract ECs in 45% of infants with recurrent respiratory symptoms when using a probe for just a single rhinovirus species.20 Thus, infections from rhinovirus can occur throughout the airway and in the parenchyma as well.
The intercellular adhesion molecule 1 (ICAM-1) is the major group rhinovirus receptor because it serves >90% of rhinovirus serotypes, whereas some of the remaining minor group rhinoviruses bind members of the low-density lipoprotein receptor family.21,22 ICAM-1 is a cell surface glycoprotein that normally regulates leukocyte trafficking and accumulation at sites of inflammation via engagement of lymphocyte function-associated antigen (LFA)-1 and macrophage-1 antigen (Mac-1) on the cell surface.23 Rhinovirus attachment to ICAM-1 initiates entry into the host cell with insertion of the virus genome and thus infects the cell.
TARGETS FOR INFECTION AND IMMUNE RESPONSES
Epithelium
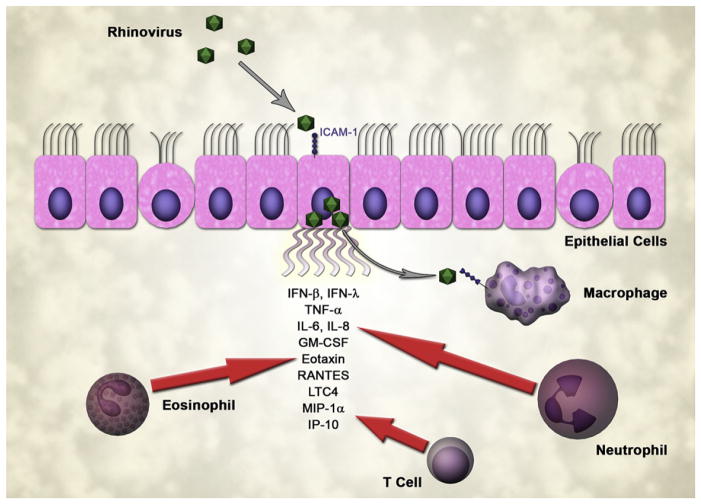
Rhinovirus, like most respiratory viruses, replicates primarily in airway ECs. In addition to attaching ICAM-1 to gain entry to the cell, rhinovirus infection induces expression of ICAM-1 to further the availability of receptors for rhinovirus to bind to and infect the cell.24 Experimental infection of bronchial ECs with rhinovirus results in little cellular damage, a pattern of response that suggests that rhinovirus-induced asthma exacerbations occur through mechanisms other than direct cellular injury.18 The induction of gene expression in rhinovirus-infected ECs appears to involve a double-stranded RNA–mediated pathway, suggesting that active rhinovirus replication triggers production of cytokines and chemokines that are necessary for the recruitment of inflammatory cells as well as being part of the host antiviral response.25 Rhinovirus infection of bronchial ECs induces the secretion of a wide variety of inflammatory cytokines and chemokines including IL-1, IL-6, IL-8, GM-CSF, eotaxins, and regulated upon activation, normal T-cell expressed and secreted (RANTES).18,26–28 Thus, the epithelium not only serves as a target and possible reservoir for the infecting virus but also is the site and source of initial inflammatory response (Fig 1).
FIG 1.
EC response to rhinovirus infection. ECs secrete cytokines that attract neutrophils, eosinophils, and lymphocytes. Macrophages may be infected in part by rhinovirus released from the epithelium.
INNATE IMMUNE CELL RESPONSES TO RHINOVIRUS
Macrophages
Macrophages are the most numerous cell type found in the airway lumen. Rhinovirus can attach to airway macrophages but may have limited replication in this cell.29,30 More importantly, the interaction between rhinovirus and macrophages stimulates secretion of proinflammatory cytokines such as IL-1, IL-8, TNF-α, IFN-γ, and macrophage inflammatory protein (MIP)–1α.29–33 TNF-α induction of EC expression of ICAM-1 allows for leukocyte trafficking to areas of infection but also increases the major rhinovirus receptor availability on the cells of primary rhinovirus replication.34 IL-8 is a potent chemokine for neutrophil recruitment. Rhinovirus infection also induces release of type I IFNs, including IFN-α, from airway macrophages, a process that may limit virus spread by inducing an antiviral state in ECs.35
Neutrophils
Neutrophils are the predominant cell type recovered from sputum during acute asthma exacerbations.36 In experimental rhinovirus infections of subjects with asthma or allergic rhinitis, IL-8 and granulocyte colony-stimulating factor (G-CSF) levels increase rapidly in nasal lavage fluid and a day or 2 later in sputum samples. The generation of these particular cytokines parallels the increase in neutrophils, suggesting these factors are involved in recruitment of neutrophils to areas of rhinovirus infection.37 In an experimental rhinovirus infection, peripheral blood neutrophils, but not total white blood cell counts, increase within 48 hours of inoculation. These findings correspond to changes in nasal lavage fluid G-CSF and IL-8 concentrations.38 In adults presenting with naturally occurring virus-induced asthma exacerbations, sputum neutrophils are increased as well as levels of neutrophil elastase, a marker of neutrophil degranulation.39 Neutrophil activation and degranulation likely contribute to airway obstruction and lower respiratory tract symptoms in asthma exacerbations or, at least in part, through neutrophil protease-induced mucus secretion by airway gland serous cells.40
Eosinophils
Rhinovirus infection may also induce eosinophil infiltration and activation within the airway. Experimental rhinovirus infection increases bronchial eosinophilic infiltration in biopsies taken at the height of cold symptoms in both asthma and control subjects.41 However, only in asthma subjects does the eosinophilic infiltrate persist 6 to 8 weeks after infection.
Rhinovirus-associated eosinophil activation correlates with changes in airway hyperresponsiveness and is reflected by an increase in eosinophilic cationic protein in the sputum supernatants.42 RANTES, a potent chemotactic cytokine and activator of eosinophils, is produced by ECs in response to rhinovirus infection18 and can be found in higher concentrations in nasal washings from children with wheezing compared with individuals with cold symptoms alone.43 Collectively, these findings imply that RANTES is an important factor in the activation of eosinophils in rhinovirus-induced asthma exacerbations.
Properties of the eosinophil also suggest an innate antiviral role. Eosinophils produce and secrete ribonucleases, including eosinophil-derived neurotoxin and eosinophilic cationic protein, which have antiviral properties.44 In a murine model using RSV, another single-stranded RNA respiratory virus, increased numbers of eosinophils improve viral clearance and reduce airway dysfunction.45 In experimental rhinovirus infection after nasal allergen challenge, elevated percentages of eosinophils in nasal lavage before inoculation correlated with a delayed onset and reduced severity of cold symptoms.46 These outcomes suggest eosinophil products have antiviral effects in rhinovirus infections. In contrast, elevated sputum eosinophil counts predict the likelihood for an asthma exacerbation, a finding that implies the presence of eosinophils may enhance susceptibility for a rhinovirus infection.47
Eosinophils may also act as antigen-presenting cells in human viral respiratory infections. Murine models have demonstrated that eosinophils serve as antigen-presenting cells under conditions of allergic inflammation as well as parasitic infection.48,49 As further support for this concept, human eosinophils express surface molecules necessary for activation of naive CD4+ T cells—that is, MHC class II proteins—when stimulated with cytokines including IFN-γ, GM-CSF, IL-3, IL-4, and IL-5.50–52 In addition, eosinophils expressing MHC II proteins are present in sputum from subjects with asthma as well as in bronchoalveolar lavage fluid after allergen challenge.53,54 CD40, a costimulatory surface molecule sufficient for activation of CD4+ T cells, is also expressed on eosinophils.55 When eosinophils are pretreated with GM-CSF to express ICAM-1, they can bind rhinovirus and present viral antigen to rhinovirus-specific T cells in an MHC-restricted manner, resulting in T-cell proliferation and IFN-γ secretion.56 However, compared with macrophages, eosinophils are capable of but less efficient at inducing CD4+ T-cell proliferation and IFN-γ production in the presence of bacterial superantigens, and appear unable to present efficiently antigens that require intracellular processing57 (Fig 2).
FIG 2.
Immune cell response to rhinovirus. Innate immune response from macrophages includes secretion of cytokines, which recruit additional innate and adaptive immune cell responses. ECP, Eosinophilic cationic protein; EDN, eosinophil-derived neurotoxin.
ADAPTIVE IMMUNE CELL RESPONSES TO RHINOVIRUS
T cells
Bronchial biopsies of subjects with asthma and control subjects with an experimental rhinovirus infection show T-cell infiltration of the airway epithelium and submucosa.41 Peripheral blood lymphopenia also occurs during a rhinovirus infection, possibly reflecting vigorous T-cell recruitment to the lung.58 Both airway lymphocyte infiltration and peripheral blood lymphopenia correlate inversely with changes in bronchial hyperresponsiveness during rhinovirus infection and revert to baseline levels during convalescence (unlike airway eosinophilia in subjects with asthma). These findings suggest that T cells may also contribute to lower respiratory tract symptoms during rhinovirus infection.41
The recruitment of T cells may also contribute to the clearance of virus through production of TH1 cytokines including IFN-γ and IL-2. Rhinovirus-infected ECs secrete RANTES and IFN-γ–inducible protein (IP)–10, which promote T-cell chemotaxis.59 IP-10 induces chemotaxis through engagement of a highly expressed receptor on activated CD4+, CD8+, and natural killer T cells, chemokine receptor (CXCR) 3.60 Expression of IP-10 appears to require active rhinovirus replication, possibly in response to double-stranded RNA.61 IP-10 expression is increased in asthma exacerbations because of rhinovirus and other viruses, and the level of expression can discriminate between virus-induced and nonvirus-induced asthma exacerbations.59 A vigorous TH1 response to viral infection may be hampered in asthma by the presence of large numbers of airway eosinophils. IFN-γ induces indoleamine 2,3-dioxygenase production by eosinophils, which in turn induces TH1-cell–specific apoptosis.62
B cells
After virus inoculation, B-cell responses to infection can be detected in the generation of mucosal IgA by day 3, followed by IgM, and finally IgG after 7 to 8 days.63 Rapid induction of specific preformed neutralizing IgG to rhinovirus serves to prevent or limit the extent of reinfection. Elevated serum titers of serotype-specific IgG neutralizing antibody correlate with attenuated cold symptoms and reduced viral shedding.64 In RSV infection in infants, RSV-specific IgE titers in nasopharyngeal secretions were significantly higher in subjects with wheezing and correlated with the degree of hypoxia.65 Experimental rhinovirus infection of subjects with allergic rhinitis induced a rapid increase in serum IgE without evidence of elevation in antigen-specific IgE.66 Whether IgE or rhinovirus-specific IgE plays a role in lower airway responses to rhinovirus infection remains to be demonstrated.
CONSEQUENCE OF RHINOVIRUS INFECTIONS ON AIRWAY INFLAMMATION
What are the products generated?
Products generated in response to rhinovirus infection by structural and immune cells have both effector and regulatory functions. The chemotactic cytokines, IL-8, RANTES, and MIP-1α, are increased with rhinovirus infection and correlate with the severity of cold symptoms.18,26,67–69 IL-8 is produced by ECs and macrophages and serves primarily as a chemoattractant for neutrophils. RANTES attracts eosinophils and can induce eosinophil degranulation.69 MIP-1α is a chemokine for lymphocytes including both T cells and B cells.70 Acute inflammatory cytokines such as IL-6 are also elevated during rhinovirus infection and likely reflect an acute-phase inflammatory response.
Inducible elevations in leukotrienes have also been demonstrated in rhinovirus infection and may contribute to airway dysfunction and cell attraction. For example, leukotriene (LT) C4 levels are elevated in nasal lavage fluid during rhinovirus infection.71 In addition, rhinovirus infection in bronchial ECs induces expression of proteins in the biosynthetic pathway for LTB4 and LTC4.72 The possible importance of the leukotrienes is further noted in therapeutic trials to be discussed later in the section on leukotriene receptor antagonists.
Cell recruitment, cellular inflammation
The acute neutrophilic response with a rhinovirus infection correlates with the severity of cold symptoms. What role neutrophils play in the clearance of rhinovirus is not fully known but may be dualistic. Neutrophils can aid in clearance of cellular debris through phagocytosis, although the inflammation associated with rhinovirus infections has limited cytopathic effects. Conversely, activated airway neutrophils can contribute to the pathophysiology of asthma exacerbations. For example, neutrophil proteases induce airway edema, trigger airway smooth muscle bronchoconstriction, and induce mucus gland secretion, all cardinal features of asthma exacerbations. However, because both asthma and control subjects respond to rhinovirus infection with sputum neutrophilia, it appears that factors other than, or in addition to, neutrophilic inflammation are necessary for asthma exacerbation.69
Eosinophil recruitment also occurs with rhinovirus infection,41 although this is not uniformly noted. One study of patients hospitalized for asthma exacerbation found a suppression of sputum eosinophils during the acute exacerbation from rhinovirus, with sputum eosinophils returning with recovery from exacerbation.69 In this particular study, IL-10 emerged as a key factor in eosinophil regulation because its detection varied inversely with sputum eosinophil numbers. Leukotrienes are produced by activated eosinophils and can contribute to features commonly found in asthma exacerbations, including airway edema, plasma leakage, chemotaxis of inflammatory cells, and airway smooth muscle contraction.
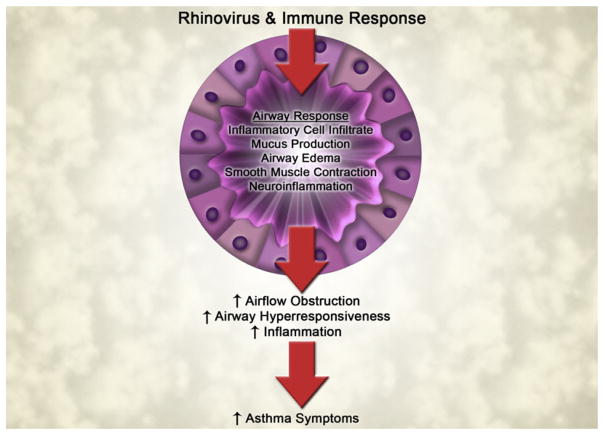
As discussed, T lymphocytes are recruited to the lung in rhinovirus infections and likely participate in many phases of the response to infection—inflammation, antiviral activity, and regulation of the immune responses to rhinovirus. The end result, inflammation versus antiviral activity, is likely highly variable among individuals with asthma and likely determines whether the patient wheezes or has a rapid resolution of the viral illness (Fig 3).
FIG 3.
Consequences of immune response to rhinovirus and altered airway responses in asthma.
Why are some patients with asthma more susceptible to rhinovirus lower respiratory tract infection?
Not all patients with asthma have an exacerbation with a cold. To explain this observation, investigators have begun to evaluate the antiviral response to rhinovirus in at-risk groups. An impaired innate antiviral response, particularly a reduction in IFN production, has been observed in some subjects with asthma. The clinical impact and relevance of these defects have been explored during experimental rhinovirus infections where the degree of deficiencies in IFN production contributes to the occurrence and severity of lower respiratory tract symptoms. Compared with bronchial ECs cultured ex vivo from normal subjects, samples from asthma subjects have a significantly impaired production of IFN-β, a type I IFN. This abnormality is also reflected in a reduction of EC apoptosis in response to rhinovirus infection, an impairment of innate antiviral activity that results in increased rhinovirus replication in bronchial ECs from subjects with asthma. These findings provide an explanation for why patients with asthma are susceptible to lower respiratory tract rhinovirus infection and have a more severe illness.73 The induction of IFN-λ, a type III IFN also involved in innate antiviral responses, is compromised in response to rhinovirus infection in primary bronchial EC cultures from subjects with asthma.74 Collectively, these asthma-associated defects will lead to impaired rhinovirus clearance, more severe infections, and a greater persistence of this virus in the lower airway.
A consequence of these defective antiviral responses can lead to an enhanced likelihood of rhinovirus replication in the airway during acute infection, causing greater airway inflammation. Among children evaluated for an asthma exacerbation, rhinovirus RNA was present in 82% of nasal aspirates at the initial visit and persisted in greater than 44% of the subjects 6 weeks after the initial visit without new symptoms of a cold.75 Naturally occurring rhinovirus infections lead to decrements in peak expiratory flow as well as both more severe and longer-lasting lower respiratory tract symptoms in asthma compared with controls.76 A reduction of the IFN-γ/IL-5 mRNA ratio, presumably representing a shift toward TH2 activity, in airway cells of subjects with asthma correlates with higher symptom scores as well as greater detection of rhinovirus RNA. Conversely, those subjects with higher IFN-γ/IL-5 mRNA ratios presumably shifted more toward a TH1 profile, had lower symptom scores, and had less detectable virus RNA at convalescence.37 These observations suggest that an imbalance of TH1 versus TH2 cytokine responses to rhinovirus infection may explain the persistence of rhinovirus in the airway and level of symptom scores. Support for this concept also comes from a study of rhinovirus-stimulated PBMCs in which atopic subjects with asthma had reduced production of IFN-γ and IL-12, with increased IL-4 and IL-10 compared with control subjects.77
Last, new evidence has shown the presence of rhinovirus in subjects with asthma without cold symptoms or an exacerbation. Rhinovirus antigen was identified in bronchial biopsies of some subjects with asthma and control subjects without exacerbation in the previous 2 weeks or cold symptoms in the previous 3 weeks.6 The detection of rhinovirus correlated with increased peripheral blood neutrophil counts and a greater degree of airflow obstruction in all subjects, as well as increased blood eosinophil counts in subjects with asthma. Rhinovirus was found significantly more often in subjects with asthma compared with controls, and subjects with asthma with rhinovirus present had lower FEV1 values. Whether rhinovirus infection can become persistent and contribute to asthma severity has yet to be established, however.
Does allergic sensitization play a role in rhinovirus-associated asthma exacerbations?
Atopy is a common condition in asthma, with allergic rhinitis occurring in as many as 80% of patients with asthma.78 The impact allergic sensitization may have in the asthmatic airway response to viral infection has generated much interest and research. Elevated IgE antibodies to airborne allergens have been suggested as a marker for patients at increased risk for lower respiratory tract symptoms with viral infections such as rhinovirus. For example, in children presenting for emergency care because of wheezing, the presence of airborne allergen specific IgE and high total IgE appeared to be a risk factor for wheezing with viral infections.79,80 In addition, a post hoc analysis of experimental rhinovirus infections in adult subjects with mild asthma revealed a subset of subjects with total IgE >300 who experienced significantly increased upper and lower respiratory tract symptoms compared with controls, although no asthma exacerbations occurred.81
Allergic sensitization may increase the susceptibility to rhinovirus infection. For example, IL-13 and other TH2 cytokines have been shown to increase expression of ICAM-1 on an EC line in vitro, which resulted in increased viral titers after experimental rhinovirus infection.82 Moreover, ECs recovered from nasal brushings of atopic subjects express significantly higher ICAM-1 levels than those from healthy controls, and increased ICAM-1 expression with allergen exposure was inducible only in the ECs from atopic subjects.83 Thus, allergic sensitization with allergen exposure may increase the likelihood of infection by rhinovirus by increasing ICAM-1 expression on ECs. This is supported clinically in a case-controlled study of children admitted for asthma exacerbations—the risk factor combination of detectable virus, allergic sensitization, and high allergen exposure was significantly higher in the hospitalized asthma group versus children with stable asthma.84 These results should be interpreted with some caution because the exacerbation group had significantly fewer subjects regularly using inhaled corticosteroid (ICS) compared with the stable asthma group. Further evidence for atopy increasing susceptibility to rhinovirus comes from a study of experimental rhinovirus infection in atopic and nonatopic subjects. Although there were no differences found in cold symptom scores or viral shedding between the groups, a subset of subjects in this study developed virus-specific neutralizing antibodies between screening and inoculation with the experimental rhinovirus.85 The atopic subjects in this subset developed severe colds, whereas those without atopy did not have colds.
In contrast with these finding, experimental rhinovirus cold symptoms were significantly delayed in onset and of shorter duration in subjects with allergic rhinitis after intranasal allergen challenge compared with those with placebo challenge. In addition, there was no difference between the 2 groups in rhinovirus titer recovered from postinoculation nasal lavage.46 Moreover, in a study of subjects with mild atopic asthma, allergen challenge before experimental rhinovirus infection did not alter cold symptom scores, asthma symptom scores, peak expiratory flow, FEV1, airway hyperresponsiveness, or exhaled nitric oxide despite increases sputum eosinophils, neutrophil elastase, and IL-8 levels86 (Fig 4).
FIG 4.
Antiviral response to rhinovirus in a healthy subject versus a subject with asthma.
What are the effects of treatment on virus-provoked asthma, and what may their effect tell us about immune response to the virus?
Corticosteroids
Corticosteroids are the backbone of therapy for persistent asthma and are also efficacious in treatment of childhood wheezing episodes. ICSs reduce asthma exacerbations among subjects with persistent asthma but do not eliminate these events completely.87 In a recent cohort of adults with asthma, the absence of ICS treatment was identified as a risk factor for exacerbations related to rhinovirus infection.88 However, although ICS therapy before experimental rhinovirus infection improved airway hyperresponsiveness and reduced bronchial eosinophil numbers, this treatment did not inhibit the recruitment of other inflammatory cells, including T cells.89
In selected situations, corticosteroid therapy in early childhood has been shown to reduce the frequency of recurrent wheezing. For example, treatment of an initial wheezing episode in childhood with oral corticosteroids reduces recurrent wheezing events for as long as 1 year.90 In children with an initial rhinovirus-associated wheezing, the reduction in recurrent wheezing was greatest in the first 2 months. In children with asthma, oral corticosteroid bursts given early in viral respiratory infections limit the severity and duration of asthma exacerbations but do not alter cold symptoms.91
Corticosteroids suppress inflammation through multiple mechanisms, with their greatest effect on eosinophil-associated processes. They suppress gene expression of proinflammatory cytokines through binding to the elements of the gene promoter region. For example, pretreatment with corticosteroids reduces rhinovirus-induced IL-6 production by bronchial ECs.92 The suppression of rhinovirus-induced IL-6 requires the presence of a negative glucocorticoid response element in the promoter sequence of the IL-6 gene. Corticosteroids also suppress inflammation through the production of anti-inflammatory molecules, the formation of transcription factor inhibitors, and the inhibition of intracellular signaling pathways.93–95 The production of IL-8 by ECs in response to IL-1β requires intact nuclear factor-κB.96 Corticosteroids suppress IL-1β–induced IL-8 production; this suppression does not require an intact glucocorticoid response element in the IL-8 promoter region, indicating the inhibition occurs before transcription.96 Other cytokines appear to be suppressed through transrepression, a process in which activated glucocorticoid receptors interact with transcription factors, including nuclear factor-κB and activator protein-1, to prevent translocation to the nucleus and binding to DNA, thereby preventing transcription of inflammatory products.93 Therefore, although corticosteroids are effective in asthma and diminish the frequency of wheezing with a cold, the effects are not uniform. These clinical observations suggest that although components of the immune response to viruses that promote asthma are sensitive to the corticosteroid effects, other aspects of the response to viruses are not modulated by this treatment.
Leukotriene receptor antagonists
In young children, the use of montelukast over the course of a year reduced the number of exacerbations by ~30% but did not reduce the use of oral corticosteroids presumably prescribed for severe exacerbations.97 In a cohort of predominantly school-age children, the addition of montelukast to usual therapy during the “September asthma epidemic” (presumably the time of rhinovirus-induced asthma exacerbations) significantly reduced the number of days of increased asthma symptoms. Finally, in children with a history of virus-induced asthma symptoms, short courses of montelukast initiated at the first indications of a cold or asthma symptoms significantly lowered the use of health care units and symptom scores, but did not change need for oral corticosteroids or rescue inhaler medication.98 Taken together, these studies indicate that leukotriene receptor antagonists taken prophylactically or therapeutically may mildly ameliorate, but do not eliminate, asthma exacerbations in children, including those induced by rhinovirus infection. Collectively, these findings also suggest that respiratory viral provocation of asthma involves the generation of leukotriene products, which, when blocked, can reduce the increase in asthma symptoms with a cold.
Antiviral agents
Given the ubiquity of the common cold, the optimal choice for the prevention of a rhinovirus infection and related asthma exacerbations would be the development of a vaccine. However, because of the antigenic diversity of the >100 serotypes of rhinovirus, a vaccine is not likely to be immediately forthcoming. The antiviral agent research involving rhinovirus to date has focused on the prophylaxis and treatment of upper respiratory infections but not asthma exacerbations per se. Still, what is known about the efficacy and limitations of these approaches provides insight on potential therapies for asthma exacerbations.
IFNs reduce host cell susceptibility to viral infections and have been studied as therapeutic agents for rhinovirus infections. Intranasal IFN-α2 has been effective in reducing transmission of rhinovirus-related colds among family members.99 It has also been shown to be fully effective compared with placebo in the prevention of rhinovirus-related colds among office workers when given during the fall rhinovirus season.100 However, symptoms of nasal obstruction, discomfort, and nasal bleeding limit its usefulness for long-term prophylaxis. Under in vitro conditions, IFN-β serine has antiviral activity to rhinovirus similar to that of IFN-α2.101 Although intranasal IFN-β serine appears to be better tolerated than IFN-α2, attempts at prophylaxis of natural rhinovirus colds using intranasal IFN-β serine have been ineffective.102
Pleconaril is an orally absorbed viral capsid-function inhibitor that blocks replication of ~90% of rhinovirus serotypes.103 Clinical trials have demonstrated a modest benefit in reducing the severity and duration of colds caused by picornaviruses when pleconaril was started within 24 hours of the onset of cold symptoms.104 However, the US Food and Drug Administration has not approved pleconaril because of concerns of emergence of viral resistance and the reduced effectiveness of oral contraceptives for women using pleconaril through its rapid induction of cytochrome P450 3A4.105
Imiquimod is an immune modulator capable of activating macrophages and other PBMCs, inducing production of IFN-α, TNF-α, and IL-12.106 It is used topically for the treatment of human papilloma virus infections including genital warts and molluscum contagiosum. Intranasal application in primates induces rapid production of IFN-α and TNF-α, suggesting it may provide local antiviral response to rhinovirus.107 In a rat model of respiratory infection with Sendai virus, treatment with imiquimod suppresses viral titers and reduces neutrophil and eosinophil numbers in the bronchoalveolar lavage fluid.108 There have been no published studies to date involving imiquimod and the treatment of or prophylaxis for human rhinovirus infection.
Attempts at limiting rhinovirus cell entry through blockade of its cell receptor have met with some experimental success. Because ICAM-1 serves as the receptor for >90% of rhinovirus serotypes, it is a logical therapeutic target. The use of monoclonal anti–ICAM-1 antibodies neither modified rhinovirus infection rates nor altered the clinical course of experimental infections of healthy subjects.109 However, use of multivalent anti–ICAM-1 antibodies blocks rhinovirus infection of EC cell lines in vitro.110 In addition, intranasal tremacamra, a recombinant soluble ICAM-1 molecule, reduces symptom scores, IL-8 levels, and viral titers in experimental rhinovirus infections of subjects without allergy.111 Although no adverse effects were observed in the trials with tremacamra, the need for frequent dosing (5–6 times per day) will likely limit tremacamra development.112
Another target for inhibition of viral replication is the rhinovirus 3C protease. After insertion into the host cell, the rhinovirus RNA undergoes translation into a precursor polyprotein, which requires 3C protease for cleavage into structural and enzymatic proteins essential for viral replication.113,114 Sequencing of the 3C protease from different rhinovirus serotypes reveals a high degree of homology within the coding region, which makes it a sensible target for therapy. Rupintrivir (formerly AG7088) is a 3C protease inhibitor that shows an in vitro ability to reduce viral titers as well as production of IL-6 and IL-8 in ECs infected with rhinovirus.115 In a phase II clinical trial of intranasal rupintrivir, the treatment group demonstrated reduced viral titers and lower symptom severity scores versus placebo, even though prophylactic administration before inoculation did not affect infection frequency.116 Side effects include blood-tinged mucus and nasal irritation.116
Given the strong link between symptom severity and neutrophilic recruitment, IL-8 is another logical target for therapy. Limiting neutrophil infiltration in this manner may ameliorate many of the central features of asthma exacerbations. There are no candidate anti–IL-8 compounds available currently. In addition, inhibition of the proinflammatory signal transduction cascade may prove fruitful. This proposed inhibition would optimally be specific enough to preserve IFN responses, for example, to allow rapid clearance of viral titers (Fig 5).
FIG 5.
Interface and interactions between antirhinovirus therapeutic agents and regulation of the immune response to rhinovirus. Experimental agents in boldface; proposed therapies in italics.
Summary
Defining the immune response to rhinovirus has provided insight to our understanding of the unique features of the inflammation associated with colds. Furthermore, the discovery of an increased susceptibility of patients with asthma to the adverse effects of a respiratory infection, which may stem from deficient antiviral responses to rhinovirus infection, has helped to define better the immune response to viruses, and to rhinovirus in particular. From these observations has emerged a clearer picture of the immune response and which components are beneficial and which are detrimental. We now have a better understanding of the inflammatory cytokine response to rhinovirus infection as well as the profile of cellular infiltrates involved, including neutrophils, eosinophils, and lymphocytes. What remains to be explained is how cellular recruitment and activation result in the symptoms of asthma exacerbations. Investigation into the next steps to identify specific cellular responses as intervenable targets for therapy will be key in ameliorating, and perhaps preventing, the most common cause of a loss of asthma control. It still remains unknown what factors contribute to early-onset asthma or whether this is simply a marker of susceptibility to rhinovirus. Finally, the evidence of persistent rhinovirus antigen within the lower airway during periods of asthma stability and its relation to chronic inflammation further solidify the intertwining relationship of rhinovirus and asthma as a clinical disease entity.
Acknowledgments
Supported by National Institutes of Health–National Heart, Lung, and Blood Institute grant no. HL069116 and National Institutes of Health–National Institute of Allergy and Infectious Diseases grant no. T32 AI007635.
Abbreviations used
- EC
Epithelial cell
- ICAM-1
Intercellular adhesion molecule 1
- ICS
Inhaled corticosteroid
- IP
Inducible protein
- LT
Leukotriene
- MIP
Macrophage inflammatory protein
- RANTES
Regulated upon activation, normal T-cell expressed and secreted
- RSV
Respiratory syncytial virus
GLOSSARY
- ELASTASE
A component of neutrophil azurophilic granules, elastase is a serine protease that degrades outer membrane proteins on Gram-negative bacteria and prevents the escape of Shigella from the phagosome. Mutations in the elastase-2 gene are responsiblefor autosomal-dominant cyclic neutropenia and congenital neutropenia. α-1-Antitrypsin inhibits elastase, and this may be a mechanism for loss of inflammation control and pulmonary damage in patients with α-1-antitrypsin deficiency
- GM-CSF, EOTAXIN, REGULATED UPON ACTIVATION, NORMAL T-CELL EXPRESSED AND SECRETED (RANTES)
All are factors that promote survival, proliferation, and chemotaxis of eosinophils. Eotaxins and IL-5 work together to promote eosinophil activation and degranulation. GM-CSF promotes chemotaxis and survival of mast cells. Eotaxin-1 and eotaxin-2 are important in eosinophil recruitment to the lung; eotaxin-3 promotes esophageal eosinophil accumulation
- IN SITU HYBRIDIZATION
The technique whereby a labeled probe is incubated with a tissue sample for direct visualization of the target RNA or DNA; this is used on chromosomes in a fluorescent in situ hybridization assay
- IFN-α, IFN-β, IFN-λ, IFN-γ
IFNs were originally named for their ability to interfere with viral function. IFN-α and IFN-β have similar functions, are produced by macrophages, inhibit viral replication, and increase MHC I expression. IFN-γ is made by T cells, is stimulated by IL-12, and increases the production of IL-1 and TNF-α. INF-λ (also known as IL-29) is part of the IL-10 superfamily, is induced by viral infections, and can downregulate IL-13. IFNs have clinical uses including the treatment of hepatitis and hypereosinophilic syndrome (IFN-α), multiple sclerosis (IFN-β), and chronic granulomatous disease (IFN-γ)
- IL-1, IL-6, IL-8
ILs are involved in the inflammatory response, especially a neutrophilic response. IL-1 is produced by neutrophils, epithelial cells, and endothelial cells following stimulation by endotoxin, bacteria, and viruses. IL-1 increases ICAM-1 (increased rhinovirus binding) and TNF-α expression, IL-1 (positive feedback), IL-6, IL-8, and GM-CSF (neutrophil survival) production. IL-6 stimulates the production of acute-phase reactants and class-switch to IgG1, whereas IL-8 induces neutrophil migration into target tissues. Glucocorticoids, IL-4, and IL-13 inhibit IL-6; IL-8 inhibits IL-4–induced IgE production
- IL-3, IL-4, IL-5
IL-3 and IL-5 promote the survival, activation, and chemotaxis of mast cells and eosinophils. IL-4 increases the production of IL-5 and IgE while decreasing IL-1, IL-6, and IL-8. IL-5 and IL-3 share a common β-chain in their receptors, whereas IL-4 and IL-13 bind a common α-chain on their receptors. IL-4 increases expression of vascular cell adhesion molecule 1 on endothelial cells, allowing trafficking of eosinophils
- IL-10
Associated with dampening immune responses, for example, after successful immunotherapy. IL-10 can be produced by CD25-positive regulatory T cells, and levels can be elevated in viral infections such as rhinovirus, respiratory syncytial virus, enterovirus, and influenza. IL-10 suppresses eosinophilia by inhibiting IL-5 and GM-CSF
- LEUKOTRIENE (LT) C4, B4
Products of inflammatory cells such as eosinophils and mast cells, LTC4 is a cysteinyl leukotrienes (along with LTD4 and E4), whereas LTB4 is produced by LTA4 hydrolase and binds to the BLT1,2 receptors rather than the cysteinyl leukotriene (cysLT) receptors. Both are potent bronchoconstricting agents, especially LTB4, which also acts as a chemoattractant for neutrophils. The variability in clinical response to cysLT receptor antagonists is a result, in part, of differences in promoters in genes such as 5-lipoxygenase (pharmacogenomics)
- NEGATIVE GLUCOCORTICOID RESPONSE ELEMENT
Glucocorticoid receptors are nuclear steroid receptors that dimerize and bind to the glucocorticoid response element to activate or repress transcription. Glucocorticoid receptor–mediated transcriptional repression can use a number of mechanisms, including (1) direct binding to negative glucocorticoid response element consisting of a consensus sequence ATYACnnTnTGATCn, (2) direct interference with binding of other transcription factors because of overlapping DNA binding sites, or (3) DNA-binding independent repression caused by competition for mutual coactivators or direct binding to other transcription factors
- PHYLOGENETICS
Phylogenetics is the study of evolutionary relationship among organisms, such as plants and viruses
- RESPIRATORY SYNCYTIAL VIRUS (RSV)
The most common cause of pneumonia and bronchiolitis in infants; repeated RSV infections commonly occur over a lifetime. RSV is a single-stranded, negative sense RNA paramyxovirus. Although RSV has been thought to be associated with the development of asthma, recent studies show that rhinovirus may be a more robust predictor of asthma in children than RSV. Premature infants with chronic lung disease are treated with palivizumab, a humanized mAb to RSV, during the appropriate season
References
- 1.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Lee KK, Hegele RG, Manfreda J, Wooldrage K, Becker AB, Ferguson AC, et al. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42:290–7. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khetsuriani N, Kazerouni NN, Erdman DD, Lu XY, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008;177:1082–9. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapparel C, Junier T, Gerlach D, Cordey S, Van Belle S, Perrin L, et al. New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics. 2007;8:224. doi: 10.1186/1471-2164-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledford RM, Patel NR, Demenczuk TM, Watanyar A, Herbertz T, Collett MS, et al. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J Virol. 2004;78:3663–74. doi: 10.1128/JVI.78.7.3663-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg SB. Respiratory consequences of rhinovirus infection. Arch Intern Med. 2003;163:278–84. doi: 10.1001/archinte.163.3.278. [DOI] [PubMed] [Google Scholar]
- 10.Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73:1941–8. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andries K, Dewindt B, Snoeks J, Wouters L, Moereels H, Lewi PJ, et al. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J Virol. 1990;64:1117–23. doi: 10.1128/jvi.64.3.1117-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–64. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:1–11. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol. 2002;2:132–8. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFadden ER, Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, et al. Thermal mapping of the airways in humans. J Appl Physiol. 1985;58:564–70. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–8. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 19.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–61. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 20.Malmstrom K, Pitkaranta A, Carpen O, Pelkonen A, Malmberg LP, Turpeinen M, et al. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol. 2006;118:591–6. doi: 10.1016/j.jaci.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–47. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 22.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A. 1994;91:1839–42. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–17. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–20. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, et al. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–6. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Z, Tang W, Gwaltney JM, Jr, Wu Y, Elias JA. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol. 1997;273:L814–24. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 28.Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, et al. Rhi-novirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1beta. Am J Physiol. 1997;273:L749–59. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- 29.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, et al. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–7. [PubMed] [Google Scholar]
- 30.Laza-Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–58. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston SL, Papi A, Monick MM, Hunninghake GW. Rhinoviruses induce inter-leukin-8 mRNA and protein production in human monocytes. J Infect Dis. 1997;175:323–9. doi: 10.1093/infdis/175.2.323. [DOI] [PubMed] [Google Scholar]
- 32.Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus-induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol. 2000;105:692–8. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 33.Wolpe SD, Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–73. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 34.Bloemen PG, van den Tweel MC, Henricks PA, Engels F, Wagenaar SS, Rutten AA, et al. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1993;9:586–93. doi: 10.1165/ajrcmb/9.6.586. [DOI] [PubMed] [Google Scholar]
- 35.Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus: induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–57. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–52. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 37.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–31. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 38.Jarjour NN, Gern JE, Kelly EA, Swenson CA, Dick CR, Busse WW. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105:1169–77. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 39.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 40.Schuster A, Fahy JV, Ueki I, Nadel JA. Cystic fibrosis sputum induces a secretory response from airway gland serous cells that can be prevented by neutrophil protease inhibitors. Eur Respir J. 1995;8:10–4. doi: 10.1183/09031936.95.08010010. [DOI] [PubMed] [Google Scholar]
- 41.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–86. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 42.Grunberg K, Smits HH, Timmers MC, de Klerk EP, Dolhain RJ, Dick EC, et al. Experimental rhinovirus 16 infection: effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–16. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 43.Pacifico L, Iacobini M, Viola F, Werner B, Mancuso G, Chiesa C. Chemokine concentrations in nasal washings of infants with rhinovirus illnesses. Clin Infect Dis. 2000;31:834–8. doi: 10.1086/314049. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–8. [PubMed] [Google Scholar]
- 45.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 46.Avila PC, Abisheganaden JA, Wong H, Liu J, Yagi S, Schnurr D, et al. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. J Allergy Clin Immunol. 2000;105:923–32. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 47.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 48.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–92. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–8. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A. 1989;86:1348–51. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils: HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993;150:2554–62. [PubMed] [Google Scholar]
- 52.Guida L, O’Hehir RE, Hawrylowicz CM. Synergy between dexamethasone and interleukin-5 for the induction of major histocompatibility complex class II expression by human peripheral blood eosinophils. Blood. 1994;84:2733–40. [PubMed] [Google Scholar]
- 53.Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PL, Virchow JC, Jr, et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–7. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–8. [PubMed] [Google Scholar]
- 55.Ohkawara Y, Lim KG, Xing Z, Glibetic M, Nakano K, Dolovich J, et al. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996;97:1761–6. doi: 10.1172/JCI118603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handzel ZT, Busse WW, Sedgwick JB, Vrtis R, Lee WM, Kelly EA, et al. Eosin-ophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–84. [PubMed] [Google Scholar]
- 57.Mawhorter SD, Kazura JW, Boom WH. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology. 1994;81:584–91. [PMC free article] [PubMed] [Google Scholar]
- 58.Levandowski RA, Ou DW, Jackson GG. Acute-phase decrease of T lymphocyte subsets in rhinovirus infection. J Infect Dis. 1986;153:743–8. doi: 10.1093/infdis/153.4.743. [DOI] [PubMed] [Google Scholar]
- 59.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–93. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 61.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 62.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–13. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 63.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Respir J. 2001;18:1013–25. doi: 10.1183/09031936.01.00228701. [DOI] [PubMed] [Google Scholar]
- 64.Alper CM, Doyle WJ, Skoner DP, Buchman CA, Seroky JT, Gwaltney JM, et al. Prechallenge antibodies: moderators of infection rate, signs, and symptoms in adults experimentally challenged with rhinovirus type 39. Laryngoscope. 1996;106:1298–305. doi: 10.1097/00005537-199610000-00025. [DOI] [PubMed] [Google Scholar]
- 65.Welliver RC, Wong DT, Sun M, Middleton EJ, Vaughan RS, Ogra PL. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–6. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 66.Skoner DP, Doyle WJ, Tanner EP, Kiss J, Fireman P. Effect of rhinovirus 39 (RV-39) infection on immune and inflammatory parameters in allergic and non-allergic subjects. Clin Exp Allergy. 1995;25:561–7. doi: 10.1111/j.1365-2222.1995.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 67.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13:386–93. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 68.van Benten IJ, KleinJan A, Neijens HJ, Osterhaus AD, Fokkens WJ. Prolonged nasal eosinophilia in allergic patients after common cold. Allergy. 2001;56:949–56. doi: 10.1034/j.1398-9995.2001.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, et al. In-terleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–9. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 70.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol. 2003;91:270–4. doi: 10.1016/S1081-1206(10)63529-6. [DOI] [PubMed] [Google Scholar]
- 72.Jame AJ, Lackie PM, Cazaly AM, Sayers I, Penrose JF, Holgate ST, et al. Human bronchial epithelial cells express an active and inducible biosynthetic pathway for leukotrienes B4 and C4. Clin Exp Allergy. 2007;37:880–92. doi: 10.1111/j.1365-2222.2007.02733.x. [DOI] [PubMed] [Google Scholar]
- 73.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 75.Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, et al. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy. 2005;35:672–8. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 76.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–4. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 77.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–32. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 79.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, et al. Risk factors for acute wheezing in infants and children: viruses, passive smoke and IgE antibodies to inhalant allergens. Pediatrics. 1993;92:535–40. [PubMed] [Google Scholar]
- 80.Heymann PW, Rakes GP, Hogan AD, Ingram JM, Hoober GE, Plansmills TAE. Assessment of eosinophils, viruses and IgE antibody in wheezing infants and children. Int Arch Allergy Immunol. 1995;107:380–2. doi: 10.1159/000237043. [DOI] [PubMed] [Google Scholar]
- 81.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TAE, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 82.Bianco A, Sethi SK, Allen JT, Knight RA, Spiteri MA. Th2 cytokines exert a dominant influence on epithelial cell expression of the major group human rhinovirus receptor, ICAM-1. Eur Respir J. 1998;12:619–26. doi: 10.1183/09031936.98.12030619. [DOI] [PubMed] [Google Scholar]
- 83.Bianco A, Whiteman SC, Sethi SK, Allen JT, Knight RA, Spiteri MA. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin Exp Immunol. 2000;121:339–45. doi: 10.1046/j.1365-2249.2000.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bardin PG, Fraenkel DJ, Sanderson G, Dorward M, Lau LCK, Johnston SL, et al. Amplified rhinovirus colds in atopic subjects. Clin Exp Allergy. 1994;24:457–64. doi: 10.1111/j.1365-2222.1994.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kluijver JC, Evertse CE, Sont JK, Schrumpf JA, Zeijlvan der Ham JG, Dick CR, et al. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med. 2003;168:1174–80. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- 87.Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 88.Venarske DL, Busse WW, Griffin MR, Gebretsadik T, Shintani AK, Minton PA, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–43. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grunberg K, Sharon RF, Sont JK, In ‘t Veen JC, Van Schadewijk WA, De Klerk EP, et al. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164:1816–22. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 90.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–5. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brunette MG, Lands L, Thibodeau LP. Childhood asthma prevention of attacks with short-term corticosteroid treatment of upper respiratory tract infection. Pediatrics. 1988;81:624–9. [PubMed] [Google Scholar]
- 92.Edwards MR, Haas J, Panettieri RA, Jr, Johnson M, Johnston SL. Corticosteroids and beta2 agonists differentially regulate rhinovirus-induced interleukin-6 via distinct Cis-acting elements. J Biol Chem. 2007;282:15366–75. doi: 10.1074/jbc.M701325200. [DOI] [PubMed] [Google Scholar]
- 93.Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sci. 2003;72:1549–61. doi: 10.1016/s0024-3205(02)02446-3. [DOI] [PubMed] [Google Scholar]
- 94.Pelaia G, Cuda G, Vatrella A, Grembiale RD, De Sarro G, Maselli R, et al. Effects of glucocorticoids on activation of c-jun N-terminal, extracellular signal-regulated, and p38 MAP kinases in human pulmonary endothelial cells. Biochem Pharmacol. 2001;62:1719–24. doi: 10.1016/s0006-2952(01)00791-2. [DOI] [PubMed] [Google Scholar]
- 95.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 96.Edwards MR, Mukaida N, Johnson M, Johnston SL. IL-1beta induces IL-8 in bronchial cells via NF-kappaB and NF-IL6 transcription factors and can be suppressed by glucocorticoids. Pulm Pharmacol Ther. 2005;18:337–45. doi: 10.1016/j.pupt.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 97.Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 98.Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, et al. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007;175:323–9. doi: 10.1164/rccm.200510-1546OC. [DOI] [PubMed] [Google Scholar]
- 99.Hayden FG, Albrecht JK, Kaiser DL, Gwaltney JM., Jr Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N Engl J Med. 1986;314:71–5. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- 100.Farr BM, Gwaltney JM, Jr, Adams KF, Hayden FG. Intranasal interferon-alpha 2 for prevention of natural rhinovirus colds. Antimicrob Agents Chemother. 1984;26:31–4. doi: 10.1128/aac.26.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sperber SJ, Hayden FG. Comparative susceptibility of respiratory viruses to recombinant interferons-alpha 2b and -beta. J Interferon Res. 1989;9:285–93. doi: 10.1089/jir.1989.9.285. [DOI] [PubMed] [Google Scholar]
- 102.Sperber SJ, Levine PA, Sorrentino JV, Riker DK, Hayden FG. Ineffectiveness of recombinant interferon-beta serine nasal drops for prophylaxis of natural colds. J Infect Dis. 1989;160:700–5. doi: 10.1093/infdis/160.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pevear DC, Hayden FG, Demenczuk TM, Barone LR, McKinlay M, Collett M. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinovirus. Antimicrob Agents Chemother. 2005;49:4492–9. doi: 10.1128/AAC.49.11.4492-4499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36:1523–32. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fleischer R, Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin Infect Dis. 2003;37:1722. doi: 10.1086/379830. [DOI] [PubMed] [Google Scholar]
- 106.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 107.Clejan S, Mandrea E, Pandrea IV, Dufour J, Japa S, Veazey RS. Immune responses induced by intranasal imiquimod and implications for therapeutics in rhinovirus infections. J Cell Mol Med. 2005;9:457–61. doi: 10.1111/j.1582-4934.2005.tb00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stokes JR, Sorkness RL, Kaplan MR, Castleman WL, Tomai MA, Miller RL, et al. Attenuation of virus-induced airway dysfunction in rats treated with imiquimod. Eur Respir J. 1998;11:324–9. doi: 10.1183/09031936.98.11020324. [DOI] [PubMed] [Google Scholar]
- 109.Hayden FG, Gwaltney JM, Jr, Colonno RJ. Modification of experimental rhinovirus colds by receptor blockade. Antiviral Res. 1988;9:233–47. doi: 10.1016/0166-3542(88)90055-1. [DOI] [PubMed] [Google Scholar]
- 110.Charles CH, Luo GX, Kohlstaedt LA, Morantte IG, Gorfain E, Cao L, et al. Prevention of human rhinovirus infection by multivalent fab molecules directed against ICAM-1. Antimicrob Agents Chemother. 2003;47:1503–8. doi: 10.1128/AAC.47.5.1503-1508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turner RB, Wecker MT, Pohl G, Witek TJ, McNally E, St George R, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 112.Fendrick AM. Viral respiratory infections due to rhinoviruses: current knowledge, new developments. Am J Ther. 2003;10:193–202. doi: 10.1097/00045391-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Rotbart HA. Antiviral therapy for enteroviruses and rhinoviruses. Antivir Chem Chemother. 2000;11:261–71. doi: 10.1177/095632020001100402. [DOI] [PubMed] [Google Scholar]
- 114.Maugeri C, Alisi MA, Apicella C, Cellai L, Dragone P, Fioravanzo E, et al. New anti-viral drugs for the treatment of the common cold. Bioorg Med Chem. 2008;16:3091–107. doi: 10.1016/j.bmc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 115.Zalman LS, Brothers MA, Dragovich PS, Zhou R, Prins TJ, Worland ST, et al. Inhibition of human rhinovirus-induced cytokine production by AG7088, a human rhinovirus 3C protease inhibitor. Antimicrob Agents Chemother. 2000;44:1236–41. doi: 10.1128/aac.44.5.1236-1241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hayden FG, Turner RB, Gwaltney JM, Chi-Burris K, Gersten M, Hsyu P, et al. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother. 2003;47:3907–16. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]