Abstract
Importance
Federal legislation has led to a notable increase in pediatric studies submitted to the Food and Drug Administration (FDA), resulting in new pediatric information in product labeling. However, approximately 50% of drug labels still have insufficient information on safety, efficacy, or dosing in children. Neonatal information in labeling is even scarcer because neonates comprise a vulnerable subpopulation for which end point development is lagging and studies are more challenging.
Objective
To quantify progress made in neonatal studies and neonatal information in product labeling as result of recent legislation.
Design
1. Cohort of neonatal drug studies; and 2. Cohort of infants exposed to these drugs..
Setting
1. Neonatal drug studies: FDA website; 2. National review: infants admitted to a neonatal intensive care unit (NICU)
Participants
1) We identified drug studies between 1997 and 2010 that included neonates as a result of pediatric legislation using information available on the FDA website. We determined what studies were published in the medical literature, the legislation responsible for the studies, and the resulting neonatal labeling changes. 2) We then examined the use of these drugs in neonates admitted to 290 NICUs (the Pediatrix Data Warehouse) in the United States from 2005–2010.
Exposures
Infants exposed to a drug studied in neonates as identified by the FDA website
Main outcome measures
Number of drug studies with neonates and rate of exposure per 1000 admission among infants admitted to a NICU
Results
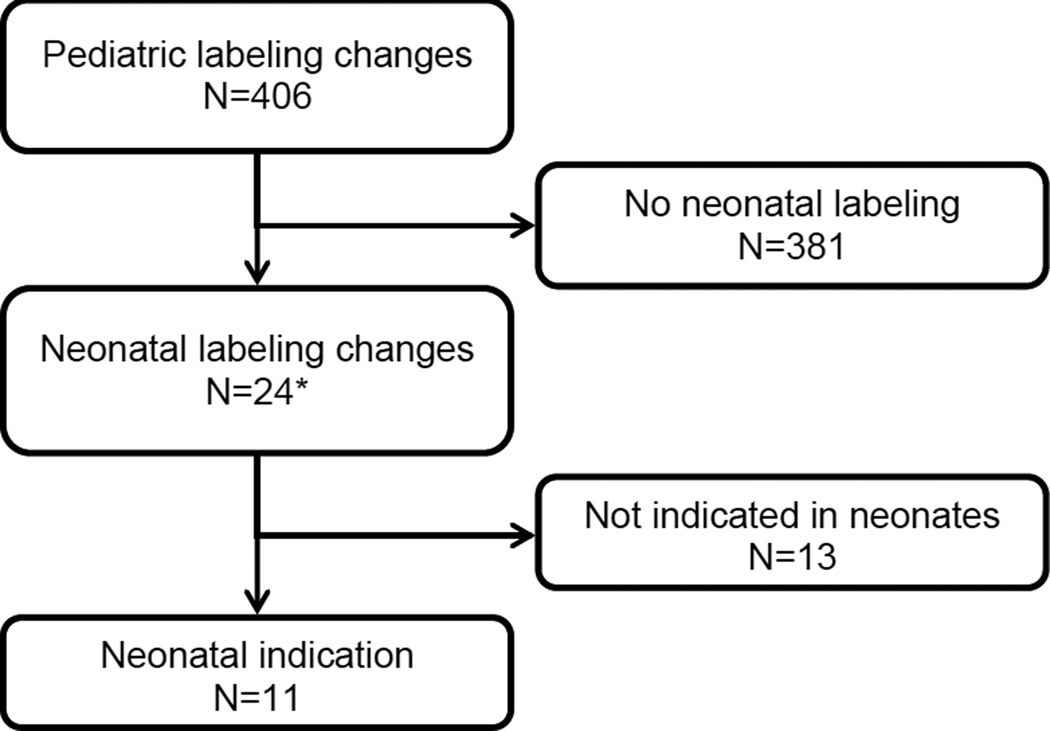
In a review of the FDA databases, we identified 28 drugs studied in neonates and 24 related labeling changes. Forty-one studies encompassed the 28 drugs, and 31 (76%) of these were published. Eleven (46%) of the 24 neonatal labeling changes established safety and effectiveness. In a review of a cohort of 446,335 hospitalized infants, we identified 399 drugs used and 1,525,739 drug exposures in the first 28 postnatal days. Thirteen (46%) of the 28 drugs studied in neonates were not used in NICUs; 8 (29%) were used in fewer than 60 neonates. Of the drugs studied, ranitidine was used most often (15,627 neonates, 35 exposures per 1000 admissions).
Conclusions and Relevance
Few drug labeling changes made under pediatric legislation include neonates. Most drugs studied are either not used or rarely used in U.S. NICUs.
Strategies to increase the study of safe and effective drugs for neonates are needed.
Keywords: neonate, labeling, FDA, medications, drugs
Neonates, typically defined as infants up to 28 days of age, are at high risk of catastrophic drug-related adverse events. Their unique physiology prevents successful extrapolation of pharmacokinetic data from older patients, and appropriate dosing for most therapeutic agents used in neonates is unknown.1 Despite neonatal medicine’s history of catastrophic adverse events resulting from inadequate study of drugs prior to their widespread use,2–5 the majority of drugs used in neonates have not undergone sufficient study to receive Food and Drug Administration (FDA) labeling that is safe and effective when applied to this population.6–10
Since 1997, a combination of pediatric incentives and requirements has significantly increased pediatric drug research and development and stimulated an increase in pediatric labeling.11–14 The legislation encompassing these initiatives was permanently reauthorized in 2012.15 A large number of pediatric labeling changes have resulted from these policies16; however, approximately 50% of drug product labeling has insufficient information on the safety, efficacy, or dosing appropriate for use in children.17
We analyzed the effect of recent pediatric initiatives on neonatal studies and labeling. We determined whether studies conducted under pediatric legislation included neonates, if there was a labeling change that included neonates, and the types of neonatal labeling changes made (e.g., if safety and effectiveness were established). We also identified the proportion of neonates in a large cohort of hospitalized infants that was exposed to the drugs studied in neonates.
METHODS
FDA REVIEW
We reviewed the pediatric resources on the FDA website for studies submitted between 1997 and 2010, including 1) the pediatric labeling changes database, 2) medical, statistical, and clinical pharmacology reviews, 3) pediatric medical and clinical pharmacology summaries, and 4) reviews posted at Drugs@FDA,16,18–20 to identify pediatric studies and labeling changes that included neonates. We defined a neonate as any infant ≤28 postnatal days of age. In cases where we could not identify the exact postnatal age, we included the review and labeling change if it referred to an infant <1 postnatal month, “0” for the lower limit of ages (e.g., 0 years of age), or “newborn.” We identified all drugs with pediatric studies that included neonates, the indication studied, the number of pediatric studies for each drug including neonates, the number of those studies published, the number of neonates studied if specified, the legislation responsible for the study, and whether the drug was approved for use in neonates for the indication studied. We compared the studies identified in FDA reviews to the medical literature (MEDLINE) to determine the proportion of studies identified in the reviews that were published.
NATIONAL REVIEW
We assembled a cohort of neonates (infants up to 28 days of age) from 2005–2010 using a de-identified dataset that included infants discharged from 290 neonatal intensive care units (NICUs). The data were obtained from an administrative database that prospectively captures information from daily progress notes generated by clinicians using a computer-assisted tool on all infants cared for by the Pediatrix Medical Group. From the daily notes, data were extracted, de-identified (in compliance with the Health Insurance Portability and Accountability Act of 1996), and consolidated into the Pediatrix BabySteps Clinical Data Warehouse. The “medications” and “demographics” tables were used for our analysis. We included all neonates. We used standard summary statistics to describe drug use. We searched the drug list for drugs that were identified in the FDA review to determine the exposure among neonates. The Duke University Institutional Review Board designated this study as exempt due to the de-identified nature of the data. The FDA’s institutional review board exempted the national review from Research Involving Human Subject Committee review.
RESULTS
We identified 28 drugs with 41 studies resulting in 24 labeling changes (including addition of pharmacokinetic information) that included neonates (Table). Of the 28 drugs, 16 had studies conducted under the incentive, 3 under the requirement, and 9 were conducted under both. Between 1997 and 2010, as a result of the pediatric initiatives, a total of 406 pediatric labeling changes were made.16 Twenty-four (6%) of the 406 pediatric labeling changes included new neonatal information (Table, Figure; note that linezolid received 2 labeling changes). Fourteen (50%) of the drugs were for infectious diseases, including 8 antiviral drugs, 4 topical ophthalmic antibiotics, 1 antifungal, and 1 antibiotic. The remaining drugs included 5 for gastroesophageal reflux disease (GERD), 3 anesthetics, 3 cardiovascular drugs, 1 analgesic/antipyretic, 1 pulmonary drug, and 1 volume replacement. The majority of the studies identified in the FDA review were published in the medical literature (31/41, 76%).
Table.
Drugs and Studies Including Neonates under Pediatric Legislation between 1997 and 2010
| FDA Review | National Review | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pediatric labeling change date |
Generic name or proper name (biologics) |
Trade name |
Indication studied | # Studies published/ total # studies |
# Neonates studied |
Indicated for use in neonates? |
Legislation | # Neonates exposed to drug |
Exposure/ 1000 neonates in 290 NICUs |
| N=31/41 | N=3215 | N=24 | N=25,384/ 446,335* |
||||||
| 12/27/2007 | 6% Hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection | Voluven | Plasma volume substitute for hypovolemia | 121/1 | ? (41) | Yes | Requirement | 0 | 0 |
| 11/2/2010 | Acetaminophen IV | Ofirmev injection | Pain management, reduction of fever | 2/3 | 47 | No. Neonatal PK information added to label. Safety and efficacy not established in children <2 years. | Requirement | 28 | 0 |
| None | Bivalirudin | Angiomax | Anticoagulant for procedures for congenital heart disease | 2/2 | 10 | No labeling change. | Incentive | 0 | 0 |
| 7/29/2008 | Caspofungin | Candidiasis | Empirical therapy for presumed fungal infections in febrile, neutropenic patients; candidemia and certain Candida infections; esophageal candidiasis; invasive aspergillosis in patients who are refractory to or intolerant of other therapies | 1/1 | 18 | No. Limited PK data in neonates and infants <3 months insufficient to establish a safe and effective dose of caspofungin in the treatment of neonatal candidiasis. Safety and efficacy have not been established in <3 months. | Incentive + requirement | 107 | 0.2 |
| None | Ciprofloxacin ophthalmic | Ciloxan | Bacterial conjunctivitis | 0/1 | ? | No labeling change. | Incentive | 9 | <0.1 |
| 4/1/2002 | Didanosine | Videx | HIV | 122/1 | 10 | Yes, in neonates ≥2 weeks. | Incentive | 0 | 0 |
| 12/22/2006 | Emtricitabine | Emtriva | HIV | 0/1 | 22 | No. Neonatal PK information added to labeling. Safety and efficacy have not been established <3 months. | Incentive | 0 | 0 |
| 6/18/2009 | Esomeprazole magnesium | Nexium | Gastroesophageal reflux disease | 123/1 | 26 | No. Neonatal PK information added to labeling. Safety and efficacy not established in <1 year. | Incentive + requirement | 0 | 0 |
| 6/6/2002 | Famotidine | Pepcid | Gastroesophageal reflux disease | 224,25/2 | 12 | Yes | Incentive | 1646 | 3.7 |
| 4/1/2004 | Fenoldopam | Corlopam | Reduction of blood pressure | 0/1 | 2 | Yes, in neonates >2 kg. | Incentive | 0 | 0 |
| None | Gatifloxacin ophthalmic solution | Zymar | Bacterial conjunctivitis | 0/1 | 171 | No | Incentive | 0 | 0 |
| 10/8/2002 | Lamivudine | Epivir | HIV | 226,27/2 | 36 | No. Neonatal PK information added to labeling. Safety and efficacy not established in <3 months. | Incentive | 8 | <0.1 |
| 10/28/2008 | Lansoprazole | Prevacid | Gastroesophageal reflux disease | 128,29/1 | 24 | No. Neonatal PK information added to labeling. Safety and efficacy not established <1 year. | Incentive + requirement | 2374 | 5.3 |
| 5/12/2005 and 12/19/2002 | Llinezolid | Zyvox | Infections due to susceptible organisms; CNS infections to susceptible organisms | 230,31/3 | At least 4315,16 | 1) Yes for infections due to susceptible organisms. 2) No for CNS infections to susceptible organisms. Neonatal PK information added to labeling. |
Incentive | ||
| 6/20/2008 | Lopinavir/ritonavir | Kaletra | HIV | 132/1 | 10 | Yes, in neonates ≥14 days. | Incentive + requirement | 0 | 0 |
| 4/15/2003 | Moxifloxacin | Vigamox | Bacterial conjunctivitis | 133/1 | 209 | No. No neonatal information in labeling. Safety and efficacy not established <1 year. | Incentive | 119 | 0.3 |
| 3/19/2004 | Nelfinavir | Viracept | HIV | 134/1 | 31 | No. Neonatal PK information added to labeling. Safety and efficacy not established <2 years. | Incentive + requirement | 0 | 0 |
| 6/24/2008 | Nevirapine | Viramune | HIV | 135/1 | ? (36) | Yes, in neonates ≥15 days. | Requirement | 21 | <0.1 |
| 12/21/2010 | Nitric oxide | INOmax | Prevention of bronchopulmonary dysplasia | 336–38/3 | 2180 | No. Information on neonatal clinical trials and adverse reactions was added to labeling. | Incentive | 4929 | 11 |
| None | Ofloxacin ophthalmic | Ocuflox | Bacterial conjunctivitis | 0/1 | ? | No labeling change. | Incentive | 4 | <0.1 |
| 11/12/2009 | Pantoprazole | Protonix | Gastroesophageal reflux disease | 139/1 | 68 | No. Neonatal PK information was added to labeling. Safety and efficacy not established <5 years. | Incentive + requirement | 39 | <0.1 |
| 10/22/1999 | Ranitidine | Zantac | Gastroesophageal reflux disease | 140/1 | 12 | No. Information on neonatal clinical trials was added to labeling. | Incentive | 15,627 | 35 |
| 3/8/2004 | Remifentanil | Ultiva | Maintenance of anesthesia | 141,42/1 | ? (60) | Yes | Incentive + requirement | 0 | 0 |
| 8/28/2008 | Rocuronium | Zemuron | Adjunct to general anesthesia | 0/2 | 28 | Yes | Incentive + requirement | 37 | <0.1 |
| 3/30/2001 | Sevoflurane | Ultane | General anesthesia | 143/1 | ? (180) | Yes | Incentive | 0 | 0 |
| 10/1/2001 | Sotalol | Betapace | Arrhythmia | 244–46/2 | 9 | No. Information on PK and PD in children 3 days to 12 years was added to labeling. Safety and efficacy not established in pediatrics. | Incentive | 58 | 0.1 |
| 3/29/2002 | Stavudine | Zerit | HIV | 247/3 | 223 | Yes | Incentive | 0 | 0 |
| 8/28/2009 | Valganciclovir | Valcyte | Treatment of congenital CMV | 148/1 | 24 | No. Information on PK, PD, and safety was added to labeling. | Incentive + requirement | 0 | 0 |
CNS = central nervous system; CMV = cytomegalovirus; PD = pharmacodynamic; PK = pharmacokinetic.
Notes: The number of neonates studied includes all neonates who participated in the study, including those on placebo or comparator. Six product reviews/summaries (shaded in gray) did not contain the precise number of neonates studied. The number of neonates studied ranged from 1 to over 200.
This is the number of neonates who received at least 1 drug.
represents that the number of neonates could not be determined.
is the total number of pediatric participants in the study.
Figure.
Neonatal labeling changes under legislation from 1997–2010 and exposure of neonates to drugs with a neonatal indication. *There are 24 neonatal labeling changes involving 23 drugs. Linezolid has 2 labeling changes.
Eleven (46%) of 24 labeling changes including new neonatal information also included an approval for use in neonates: 4 for HIV (didanosine, lopinavir/ritonavir, nevirapine, and stavudine), 3 for anesthesia (remifentanil, rocuronium, and sevoflurane), and 4 for other conditions (Table). Thirteen (54%) of the 24 neonatal labeling changes included the statement “safety and effectiveness have not been established”: acetaminophen IV, caspofungin, emtricitabine, esomeprazole magnesium, lamivudine, lansoprazole, linezolid, nelfinavir, nitric oxide, pantoprazole, ranitidine, sotalol, and valganciclovir (Table).
Five of the products studied in neonates did not obtain a neonatal labeling change. Prior to 2007, it was possible to enroll children in studies and not have any information from the study included in labeling. Moxifloxacin ophthalmic had a pediatric labeling change, but no neonatal information was added; no pediatric labeling change was made for bivalirudin, ciprofloxacin ophthalmic, gatifloxacin ophthalmic, or ofloxacin ophthalmic.
We found that 22/28 (79%) drugs clearly specified the number of neonatal participants in the review, summary, or labeling; 6/28 (21%) provided only the number of pediatric participants in a pediatric age range (e.g., 0–3 months) and ranged from 1 to over 200 neonates. The largest proportion of neonates was enrolled in 3 studies of inhaled nitric oxide for the prevention of bronchopulmonary dysplasia (2180/3215, 68%); this drug failed to prevent bronchopulmonary dysplasia.
PEDIATRIX DATABASE
We identified 399 drugs prescribed to 446,335 hospitalized neonates. Of the 28 drugs studied, the gastroesophageal reflux drugs were used most often (Table): ranitidine was the drug used most often (n=15,627 neonates; 35 exposures per 1000 neonates), lansoprazole was third (n=2374 neonates; 5.3 per 1000 neonates), and famotidine fourth (n=1646 neonates; 3.7 per 1000 neonates). Inhaled nitric oxide was the second most used drug overall, with 4929 neonates exposed (11 per 1000 neonates).
Thirteen of the 28 drugs studied in neonates were not used in the Pediatrix neonatal population (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection, bivalirudin, didanosine, emtricitabine, esomeprazole magnesium, fenoldopam, gatifloxacin ophthalmic solution, lopinavir/ritonavir, nelfinavir, remifentanil, sevoflurane, stavudine, and valganciclovir).
Eight of 28 were used in fewer than 60 neonates per drug (acetaminophen IV, ciprofloxacin ophthalmic, lamivudine, nevirapine, ofloxacin ophthalmic, pantoprazole, rocuronium, sotalol). Seven were used in more than 60 neonates per drug (caspofungin, famotidine, lansoprazole, linezolid, moxifloxacin, nitric oxide, ranitidine). Of the 11 drugs with a neonatal indication, 7 were never used in the Pediatrix neonatal population: 6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection, didanosine, fenoldopam, lopinavir/ritonavir, remifentanil, sevoflurane, and stavudine. The other 4 drugs were used infrequently: famotidine, 3.7 per 1000 neonates; linezolid, 0.9 per 1000 neonates; and nevirapine and rocuronium, both <0.1 per 1000 neonates (Table).
The majority of labeling changes occurred prior to 2008 (18/28 drugs, 64%). Ten (36%) of the drugs received labeling changes in the latter 3 years of the study period (2008–2010). Of these, 2 of the 10 were in the top 3 drugs used in the NICU (lansoprazole: n=2374 and inhaled nitric oxide: n=4929; both with no efficacy established).
DISCUSSION
Neonates are an understudied population. Several factors are considered before neonatal studies can be performed: there must be a public health benefit, and the studies must be feasible, ethical, and sufficient to support dosing, safety, and efficacy. Nearly 40% (11/28) of the drugs involving neonates pursuant to pediatric legislation between 1997 and 2010 were deemed safe and effective in neonates. Most of these were drugs used to treat infectious diseases (e.g., didanosine, lopinavir/ritonavir, and nevirapine), as well as anesthesia drugs (e.g., remifentanil and rocuronium). Of the infectious disease drugs, the majority were for HIV. Although neonatal HIV has been almost eradicated from the United States, it is critical to study HIV drugs because it remains a problem in developing countries.
Thirteen drugs studied received a labeling change stating that “safety and effectiveness have not been established” (Table). This is not surprising because neonates are a challenging population to study. There is considerable variability in drug metabolism and physiology within the neonatal population, which influences the pharmacokinetics, pharmacodynamics, safety, and efficacy of medications. Neonates are typically enrolled in pharmacokinetic/pharmacodynamic, safety, and dose-finding studies first; once an appropriate dose is established, safety and efficacy studies may be done. The sample size for most neonatal studies is very small due to the limitations inherent to these trials, including: low study consent rates for parents of vulnerable infants; limited blood volume available to conduct pharmacokinetic studies; lack of pediatric population pharmacokinetic/pharmacodynamic analysis expertise; difficulties associated with timing of blood samples in critically ill infants; lack of availability of sensitive drug concentration assays from very-small-volume specimens (e.g., dried blood spots); and lack of robust clinical end points.49
We found that 13 drugs studied in neonates were not used at all among over 400,000 hospitalized neonates. One reason may be that there is a disparity between the drugs being studied in neonates (particularly HIV drugs) and the conditions of patients in the Pediatrix database; 6 of the 13 drugs studied, but not used, were for HIV or HIV complications. Four drugs failed to receive a neonatal indication, including 1 to treat HIV, so it is possible that this is the reason that clinicians chose not to use these medications. The other products were for rare conditions that are not represented in the assessed population or would be used outside of the NICU: 2 were anesthetics not used in the NICU, 1 was for a rare cardiovascular condition, and 1 was for volume replacement (Table). It is possible that, because nearly one third of the labeling changes occurred in the last 3 years of the study period, we did not capture their use because of a delay in uptake by clinicians. However, only 3 of the 10 labeling changes during that time period had “safety and efficacy established,” so we would not expect the drug to be used, and 2 of the 3 were HIV drugs, a rare condition in the United States, making it unlikely that extending the national review would identify increasing use of these drugs. Many factors influence drug selection in neonates, including the current standard of care, availability of the drug in the hospital formulary, and the level of comfort using a given drug in neonates in light of the existing knowledge base.
Few hospitalized neonates were exposed to a drug approved for use in neonates as a result of federal legislation (<0.5% of all drug exposures in neonates). We found that most of the exposure to drugs was off-label for neonates; only a minority of neonates received a drug approved for use in neonates (Table). Drugs that were used most often were used off-label despite studies indicating they were not effective for the indication studied. For example, ranitidine, lansoprazole, and inhaled nitric oxide (for the prevention of bronchopulmonary dysplasia) were the top 3 drugs used in neonates (representing 91% of all drug exposures in neonates from the 28 drugs studied); however, none have FDA labeling for the indication studied because of lack of efficacy. Gastroesophageal reflux is difficult to diagnose and treat, particularly in neonates. Unfortunately, anti-reflux medications, such ranitidine and lansoprazole, are associated with serious side effects in neonates,50 and quality improvement efforts in the Pediatrix Medical Group have reduced exposure in this vulnerable population. Although inhaled nitric oxide use is approved in term and near-term infants for hypoxic respiratory failure, it failed to prevent bronchopulmonary dysplasia and is not recommended.51 Thus, many neonates are exposed to drugs that are not indicated in this population, exposing them to unnecessary adverse events without the possibility of clinical benefit.
There are several potential limitations to our study. Unfortunately, we could not identify the number of neonatal participants who were in 6 studies of the drugs with publicly available information from the FDA reviews and labeling. We could not determine the clinical indication for use of drugs in the Pediatrix data. For drugs that are most often used for surgery, such as remifentanil and rocuronium, we most likely underestimated the exposure because we did not capture drugs that were used in the operating room. Finally, we only include neonates who were hospitalized. For premature infants, this is most likely a representative sample. However, our database does not include outpatient neonates, such as neonates who were discharged well from the term nursery.
In conclusion, federal legislation encouraging the study of products used in the pediatric population has resulted in very few labeling changes that include new neonatal information. Studying drugs in neonates is critical; however, due to scientific and regulatory challenges, trials involving neonates are scant. The rapid physiological changes in the developing neonate affect study design and end points. Study designs and procedures that are appropriate in adults and older children may not be appropriate in neonates. Extrapolation of efficacy from adults or older pediatric populations—a tool that can sometimes be used to decrease the “trial burden” for the pediatric population—is less easily adapted to the neonate. The Pediatric Exclusivity program is voluntary. Therefore, sponsors are not obligated to perform the pediatric studies outlined in the FDA’s written request. Furthermore, appropriate formulations for use in neonates may not exist. Due to these challenges of performing clinical trials in infants, few labeling changes have included infant-specific information. Novel trial designs need to be developed, and appropriate study end points must be identified and validated. Education of parents and caregivers regarding the need for studies of drugs being given to neonates will also increase trial success. The scientific and clinical research community will need to work together with the FDA to conduct essential neonatal studies.
ACKNOWLEDGMENTS
The authors thank Dianne Murphy, MD, at the FDA for her advice, review, and editing of this paper.
Matthew Laughon states that he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study used CTSA biostatistical services through the Division of Pediatric Quantitative Sciences (NIH-5UL-1RR024128-01). Dr. Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin, under the Best Pharmaceuticals for Children Act) and from NICHD (1K23HL092225-01). Dr. Cohen-Wolkowiez receives support for research from the National Institutes of Health (1K23HD064814); he also receives support from the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org) and from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives salary support for research from the National Institutes of Health and the U.S. Department of Health and Human Services (NICHD 1K23HD060040-01, DHHS-1R18AE000028-01, and HHSN267200700051C); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
The funding bodies mentioned above played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors. No official endorsement by the U.S. Food and Drug Administration is provided or should be inferred.
Christoph P. Hornik, Nidhi Tripathi, Reese H. Clark, William Rodriguez, and Debbie Avant have no relevant conflicts to disclose.
REFERENCES
- 1.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 2.Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. 1956;18(4):614–625. [PubMed] [Google Scholar]
- 3.Stewart DJ. The effects of tetracyclines upon the dentition. Br J Dermatol. 1964;76:374–378. doi: 10.1111/j.1365-2133.1964.tb14546.x. [DOI] [PubMed] [Google Scholar]
- 4.Burns LE, Hodgman JE, Cass AB. Fatal circulatory collapse in premature infants receiving chloramphenicol. N Engl J Med. 1959;261:1318–1321. doi: 10.1056/NEJM195912242612604. [DOI] [PubMed] [Google Scholar]
- 5.Yeh TF, Lin YJ, Huang CC, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics. 1998;101(5):E7. doi: 10.1542/peds.101.5.e7. [DOI] [PubMed] [Google Scholar]
- 6.Aranda JV, Clarkson S, Collinge JM. Changing pattern of drug utilization in a neonatal intensive care unit. Am J Perinatol. 1983;1(1):28–30. doi: 10.1055/s-2007-1000047. [DOI] [PubMed] [Google Scholar]
- 7.Du W, Warrier I, Tutag Lehr V, Salari V, Ostrea E, Aranda JV. Changing patterns of drug utilization in a neonatal intensive care population. Am J Perinatol. 2006;23(5):279–285. doi: 10.1055/s-2006-946719. [DOI] [PubMed] [Google Scholar]
- 8.Avenel S, Bomkratz A, Dassieu G, Janaud JC, Danan C. The incidence of prescriptions without marketing product license in a neonatal intensive care unit. Arch Pediatr. 2000;7(2):143–147. doi: 10.1016/s0929-693x(00)88083-5. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell CP, Stone RJ, Morley CJ. Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics. 2002;110(5):e52. doi: 10.1542/peds.110.5.e52. [DOI] [PubMed] [Google Scholar]
- 10.‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children's hospital. Pediatrics. 2001;108(5):1089–1093. doi: 10.1542/peds.108.5.1089. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration Modernization Act, Pub L No 105–115, 111 Stat 2296 1997.
- 12.Pediatric Rule, 21 CFR 31455, 21 CFR 60127, 21 CFR, 201, 21 CFR 312, 21 CFR 314, 21 CFR 601. Code of Federal Regulations.
- 13.Best Pharmaceuticals for Children Act, Pub L No 107–109, 115 Stat 1408 2002.
- 14.Pediatric Research Equity Act, Pub L No 108–155, 117 Stat 1936–1943 2003.
- 15.U.S. Food and Drug Administration Safety and Innovation Act of 2012 (Public Law 112–114).
- 16.FDA Pediatric Labeling Changes Table. U.S. Department of Health and Human Services. Food and Drug Administration Website. www.fda.gov.
- 17.Sachs AN, Avant D, Lee CS, Rodriguez W, Murphy MD. Pediatric information in drug product labeling. JAMA. 2012;307(18):1914–1915. doi: 10.1001/jama.2012.3435. [DOI] [PubMed] [Google Scholar]
- 18.Medical, Statistical, and Clinical Pharmacology Reviews of Pediatric Studies Conducted under Section 505A and 505B of the Federal Food, Drug, and Cosmetic Act (the Act), as amended by the FDA Amendments Act of 2007, U.S. Department of Health and Human Services, Food and Drug Administration Website. www.fda.gov.
- 19.Summaries of Medical and Clinical Pharmacology Reviews of Pediatric Studies Conducted under Section 505A and 505B of the Federal Food, Drug, and Cosmetic Act (the Act), as amended by the Best Pharmaceuticals for Children Act of 2002, US Department of Health and Human Services, Food and Drug Administration Website. www.fda.gov.
- 20.Drugs@FDA. U.S. Department of Health and Human Services. [Accessed June 11, 2013];Food and Drug Administration website. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/.
- 21.Standl T, Lochbuehler H, Galli C, Reich A, Dietrich G, Hagemann H. HES 130/0.4 (Voluven) or human albumin in children younger than 2 yr undergoing non-cardiac surgery. A prospective, randomized, open label, multicentre trial. Eur J Anaesthesiol. 2008;25(6):437–445. doi: 10.1017/S0265021508003888. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs A, Cowles MK, Britto P, et al. Pharmacokinetics of didanosine and drug resistance mutations in infants exposed to zidovudine during gestation or postnatally and treated with didanosine or zidovudine in the first three months of life. Pediatr Infect Dis J. 2005;24(6):503–509. doi: 10.1097/01.inf.0000164787.63237.0b. [DOI] [PubMed] [Google Scholar]
- 23.Omari T, Lundborg P, Sandstrom M, et al. Pharmacodynamics and systemic exposure of esomeprazole in preterm infants and term neonates with gastroesophageal reflux disease. J Pediatr. 2009;155(2):222–228. doi: 10.1016/j.jpeds.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 24.James LP, Marotti T, Stowe CD, Farrar HC, Taylor BJ, Kearns GL. Pharmacokinetics and pharmacodynamics of famotidine in infants. J Clin Pharmacol. 1998;38(12):1089–1095. [PubMed] [Google Scholar]
- 25.Orenstein SR, Shalaby TM, Devandry SN, et al. Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial. Aliment Pharmacol Ther. 2003;17(9):1097–1107. doi: 10.1046/j.1365-2036.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 26.Moodley D, Pillay K, Naidoo K, et al. Pharmacokinetics of zidovudine and lamivudine in neonates following coadministration of oral doses every 12 hours. J Clin Pharmacol. 2001;41(7):732–741. doi: 10.1177/00912700122010636. [DOI] [PubMed] [Google Scholar]
- 27.Moodley J, Moodley D, Pillay K, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178(5):1327–1333. doi: 10.1086/314431. [DOI] [PubMed] [Google Scholar]
- 28.Springer M, Atkinson S, North J, Raanan M. Safety and pharmacodynamics of lansoprazole in patients with gastroesophageal reflux disease aged <1 year. Paediatr Drugs. 2008;10(4):255–263. doi: 10.2165/00148581-200810040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Kukulka M, Witt G, Sutkowski-Markmann D, North J, Atkinson S. Age-dependent pharmacokinetics of lansoprazole in neonates and infants. Paediatr Drugs. 2008;10(4):265–274. doi: 10.2165/00148581-200810040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan SL, Deville JG, Yogev R, et al. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr Infect Dis J. 2003;22(8):677–686. doi: 10.1097/01.inf.0000078160.29072.42. [DOI] [PubMed] [Google Scholar]
- 31.Kearns GL, Jungbluth GL, Abdel-Rahman SM, et al. Impact of ontogeny on linezolid disposition in neonates and infants. Clin Pharmacol Ther. 2003;74(5):413–422. doi: 10.1016/S0009-9236(03)00226-1. [DOI] [PubMed] [Google Scholar]
- 32.Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J. 2009;28(3):215–219. doi: 10.1097/INF.0b013e31818cc053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver LH, Woodside AM, Montgomery DB. Clinical safety of moxifloxacin ophthalmic solution 0.5% (VIGAMOX) in pediatric and nonpediatric patients with bacterial conjunctivitis. Surv Ophthalmol. 2005;50(Suppl 1):S55–S63. doi: 10.1016/j.survophthal.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Bryson YJ, Mirochnick M, Stek A, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9(2):115–125. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luzuriaga K, McManus M, Mofenson L, et al. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350(24):2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 36.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355(4):343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 37.Kinsella JP, Cutter GR, Walsh WF, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355(4):354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 38.Mercier JC, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376(9738):346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 39.Ward RM, Tammara B, Sullivan SE, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD) Eur J Clin Pharmacol. 2010;66(6):555–561. doi: 10.1007/s00228-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 40.Wells TG, Heulitt MJ, Taylor BJ, Fasules JW, Kearns GL. Pharmacokinetics and pharmacodynamics of ranitidine in neonates treated with extracorporeal membrane oxygenation. J Clin Pharmacol. 1998;38(5):402–407. doi: 10.1002/j.1552-4604.1998.tb04443.x. [DOI] [PubMed] [Google Scholar]
- 41.Davis PJ, Galinkin J, McGowan FX, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. Anesth Analg. 2001;93(6):1380–1386. doi: 10.1097/00000539-200112000-00006. table of contents. [DOI] [PubMed] [Google Scholar]
- 42.Galinkin JL, Davis PJ, McGowan FX, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. II. Perioperative breathing patterns in neonates and infants with pyloric stenosis. Anesth Analg. 2001;93(6):1387–1392. doi: 10.1097/00000539-200112000-00007. table of contents. [DOI] [PubMed] [Google Scholar]
- 43.Russell IA, Miller Hance WC, Gregory G, et al. The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg. 2001;92(5):1152–1158. doi: 10.1097/00000539-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Saul JP, Ross B, Schaffer MS, et al. Pharmacokinetics and pharmacodynamics of sotalol in a pediatric population with supraventricular and ventricular tachyarrhythmia. Clin Pharmacol Ther. 2001;69(3):145–157. doi: 10.1067/mcp.2001.113795. [DOI] [PubMed] [Google Scholar]
- 45.Saul JP, Schaffer MS, Karpawich PP, et al. Single-dose pharmacokinetics of sotalol in a pediatric population with supraventricular and/or ventricular tachyarrhythmia. J Clin Pharmacol. 2001;41(1):35–43. doi: 10.1177/00912700122009818. [DOI] [PubMed] [Google Scholar]
- 46.Shi J, Ludden TM, Melikian AP, Gastonguay MR, Hinderling PH. Population pharmacokinetics and pharmacodynamics of sotalol in pediatric patients with supraventricular or ventricular tachyarrhythmia. J Pharmacokinet Pharmacodyn. 2001;28(6):555–575. doi: 10.1023/a:1014412521191. [DOI] [PubMed] [Google Scholar]
- 47.Rongkavilit C, Thaithumyanon P, Chuenyam T, et al. Pharmacokinetics of stavudine and didanosine coadministered with nelfinavir in human immunodeficiency virus-exposed neonates. Antimicrob Agents Chemother. 2001;45(12):3585–3590. doi: 10.1128/AAC.45.12.3585-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimberlin DW, Acosta EP, Sanchez PJ, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis. 2008;197(6):836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- 49.Laughon MM, Benjamin DK, Jr, Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malcolm WF, Cotten CM. Metoclopramide, H2 blockers, and proton pump inhibitors: pharmacotherapy for gastroesophageal reflux in neonates. Clin Perinatol. 2012;39(1):99–109. doi: 10.1016/j.clp.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Cole FS, Alleyne C, Barks JD, et al. NIH consensus development conference: inhaled nitric oxide therapy for premature infants. NIH Consens State Sci Statements. 2010;27(5):1–34. Review. [PubMed] [Google Scholar]