Abstract
Cell therapy for patients who have intractable muscle disorders may require highly regenerative cells from young, healthy allogeneic donors. Mesenchymal stem cells are currently under clinical investigation because they are known to induce muscle regeneration and believed to be immune privileged, thus making them suitable for allogeneic applications. However, it is unclear whether allogeneic and myogenic-induced mesenchymal stem cells retain their immunomodulatory characteristics. Therefore, our aim was to evaluate the effects of mesenchymal stem cell differentiation on the immune characteristics of cells in vitro. We investigated the immunologic properties of mesenchymal stem cells after myogenic induction. Mesenchymal stem cells were obtained from C57BL/6 mice and the C3H/10T1/2 murine mesenchymal stem cell line. Two different 5-aza-2′-deoxycytidine doses (0.5 and 3 µM) were evaluated for their effects on mesenchymal stem cell skeletal myogenic differentiation potential, immune antigen expression, and mixed lymphocytic reactions. Using a mixed lymphocytic reaction, we determined the optimal splenocyte proliferation inhibition dose. The induction of regulatory T cells was markedly increased by the addition of 3 µM 5-aza-2′-deoxycytidine–treated mesenchymal stem cells. Myogenic-induced mesenchymal stem cells do not elicit alloreactive lymphocyte proliferative responses and are able to modulate immune responses. These findings support the hypothesis that myogenic-induced mesenchymal stem cells may be transplantable across allogeneic barriers.
Keywords: Mesenchymal stem cell, myogenic induction, immune modulation, regulatory T cells
Introduction
Bone marrow stem cells have been shown to participate in the regeneration of injured muscle.1,2 Bone marrow mesenchymal stem cells (MSCs) are conventionally defined as adherent nonhematopoietic cells expressing several common cell surface antigenic markers, such as CD44, CD73, CD90, and CD105, but not the hematopoietic markers CD34 and CD45.3 After treatment with inducing agents,4 MSCs can differentiate in vitro into adipocytes, chondrocytes, and osteocytes; and also into neurons, hepatocytes, pancreatic islets, and muscle.5 In addition, their ability to evade immunosurveillance after cell transplantation and to suppress the immune response has made MSCs a particularly attractive candidate for clinical use.6,7 In particular, it was observed that MSCs could suppress lymphocyte proliferation and activation in response to allogeneic activation or chemical stimulation in vitro and in vivo.8–10
MSCs are known to suppress alloantigen-induced T-cell functions in vitro and in vivo and seem to be a promising cell source for the treatment of graft versus host disease (GVHD), osteogenesis imperfect, acute myocardial infarction, and Duchenne muscular dystrophy (DMD).11–15 Little is known about the specificity of immunosuppression by MSCs, but these cells are not immunogenic and escape recognition by alloreactive T cells and other effector cells.16 There are several hypotheses on the immunosuppressive mechanism of MSCs.17–20 They are known to induce the increase in regulatory T (Treg) cell activity and also inhibit the maturation and motility of effector cells via production of cytokines such as fractalkine.21,22 Another theory states that MSCs induce overproduction of T helper (TH)-2-type cytokines such as interleukin (IL)-10, IL-12, and transforming growth factor (TGF)-β.23,24
Clinical interest in MSCs in cell therapy applications is based on their anti-inflammatory properties and ability to release cytokines into the surrounding environment, thereby modifying the developmental fate of neighboring cells. Thus, not only can MSCs themselves be induced to differentiate alone, for example, a myogenic pathway, in which they fuse with myotubes and promote the formation of new muscle fibers,15 but also in skeletal muscle. For example, they can induce the myogenic differentiation of neighboring satellite cells in the interstitial tissue.25 Nonetheless, the transplantation of cells already induced to differentiate along the pathway of interest would seem to offer a more direct and readily controllable therapeutic approach than conventional strategies that rely on the innate properties of MSCs to target undifferentiated cell populations.26 The compound 5-aza-2′-deoxycytidine (5-aza-CdR), an analog of cytidine, causes extensive demethylation of 5-methylcytosine, and reduced DNA methyltransferase (DNMT) activity in the cell27 has been used in several studies to induce differentiation of MSCs into striated muscle cells.28–30 In part, it appears that the mechanism by which 5-aza-CdR activates myogenic genes involves MyoD1.30 Here, we demonstrate that 5-aza-CdR-treated MSCs showed myogenic conversion. However, the effect of differentiation on the expression profile of immune proteins in allogeneic MSCs is largely unknown.
Allogeneic cell therapy that induces muscle regeneration and has immune-privileged status would provide numerous benefits to patients who have intractable muscle degenerative disorders. Toward this goal, this study aimed to evaluate allogeneic and myogenic-induced MSCs that modulate immune responses. We identified the effects of myogenic MSC induction on cellular antigen profile and lymphocyte proliferation and measured the induction of Treg cells.
Materials and methods
Animals and MSC isolation and culture
All animal work was approved by the Animal Care and Use Committee at Wake Forest University (protocol number: A10-082). Male C57BL/6 mice, 4 to 8 weeks old, were obtained from Charles River Laboratories (Wilmington, MA, USA), housed in a temperature- and light-controlled environment (12L:12D) and were provided food and water ad libitum. For bone marrow cell (BMC) isolation, C57BL/6 mice were killed by CO2 exposure, and their limbs were subsequently removed. Bone marrow was flushed from medullary cavities of both the femurs and tibiae with Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA) using a 25-gauge needle. For bone marrow MSC isolation, BMCs of C57BL/6 mice were plated at a density of 5 × 106/mL/cm2 and cultured in an MSC medium containing Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) medium with GlutaMax™-I (Invitrogen Life Technologies), 10% MSC-qualified fetal bovine serum (FBS; Invitrogen Life Technologies), and 5 µg/mL gentamicin (Invitrogen Life Technologies) at 37 °C in a 5% CO2 atmosphere. After 48 h, non-adherent cells were removed by washing with 1× phosphate buffered saline (PBS), and fresh medium was then added. The medium was changed weekly. When the culture was near confluence, the monolayer cells were washed twice with 1× PBS, and then they were lifted by incubation for 2–3 min at 37 °C with a 0.25% trypsin solution containing 0.01% ethylenediaminetetraacetic acid (EDTA; Invitrogen Life Technologies). Trypsin was neutralized by the addition of fresh complete medium. The resulting suspension was then expanded by plating it onto a new culture flask. Murine C3H10T1/2 cells (American Type Culture Collection (ATCC) no. CCL-26; Manassas, VA, USA) were cultured in DMEM supplemented with 10% FBS with 5% CO2 at 37°C.
Myogenic induction
Twice passaged C57BL/6 BM MSCs and murine C3H10T1/2 cells were seeded into 35-mm dishes at 5000 cells/dish. Beginning 24 h after seeding, these cultures were treated for 24 h with complete medium consisting of DMEM containing 10% FBS, 5% horse serum (HS; Hazleton Biologics, Inc., Lenexa, KS, USA), and 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen Life Technologies) containing two different concentrations (0.5 and 3 µM) of 5-aza-CdR (Sigma Chemical Co., St Louis, MO, USA) at 37 °C in a 5% CO2 atmosphere. After the cultures were washed twice with PBS, the medium was changed to complete medium without added 5-aza-CdR and subsequently changed twice a week for 2 weeks.
Antibodies
All antibodies used for fluorescence-activated cell sorting (FACS) or immunohistochemical analyses were obtained from BD Biosciences (San Jose, CA, USA), with the following exceptions: anti-desmin and anti-CD34 was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), anti-myosin heavy chain (MyHC; MF-20) was from the University of Iowa (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), anti-H-2Kk was from Biolegend (San Diego, CA, USA), anti-CD80 was from Abcam (Cambridge, MA, USA), and anti-CD86 was from LifeSpan BioSciences, Inc. (Seattle, WA, USA).
Immunohistochemical staining in myogenic-induced MSCs in vitro
Myogenic-induced MSCs were also analyzed for the expression of desmin and MyHC. Briefly, the cultured cells were fixed with cold methanol for 10 min. Nonspecific protein binding was blocked with Dako blocking solution (Dako, Carpinteria, CA, USA) for 30 min at room temperature. The samples were incubated overnight at 4 °C in goat polyclonal anti-desmin (1:50) or mouse monoclonal anti-MyHC (1:25) as a primary antibody. The cells were washed thoroughly with PBS and incubated with Alexa Fluor® 594–conjugated anti-goat (1:300) or anti-mouse (1:300) secondary antibodies (Invitrogen Life Technologies) for 30 min at room temperature. After washing with PBS, the cells were mounted in Vectashield® medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). Murine C2C12 myoblasts (ATCC) served as positive controls for characterizing myogenic induction. Undifferentiated cells and samples stained without primary antibodies served as negative controls. Samples were visualized using a Zeiss fluorescence microscope (Carl Zeiss, Inc., Jena, Germany).
Flow cytometry analysis in myogenic-induced MSCs in vitro
Myogenic-induced and undifferentiated MSCs and murine C3H10T1/2 cells were identified through expressions of desmin, MyHC, CD34, CD45, CD90, CD105, major histocompatibility complex (MHC) class I, MHC class II, CD80, and CD86 at day 14 of culture.
Aliquots of cultured MSCs and murine C3H10T1/2 cells were incubated with primary antibodies (anti-desmin, anti-MyHC, anti-CD34, anti-CD45, anti-CD90, anti-CD105, anti-H-2Kb/H-2Db, anti-H-2Kk, anti-I-A/I-E, anti-CD80, and anti-CD86) for 30 min and kept on ice. Fluorescence isothiocyanate (FITC)-conjugated secondary antibodies (anti-mouse IgG, anti-rabbit IgG anti, anti-goat IgG, and anti-rat IgG) were used to detect primary antibodies. Each fluorescence analysis included appropriate FITC-conjugated negative isotype controls.
The cells were fixed with 1% paraformaldehyde solution and then analyzed with a FACSCalibur™ flow cytometer (BD Biosciences).
Mixed leukocyte reaction
Mitomycin C (Mit C; 10 µg/mL; Sigma)–treated murine C3H10T1/2 cells (1 × 103 to 1 × 106 cells) were plated in round-bottom 96-well plates (Corning Inc., Corning, NY, USA) in a total volume of 0.2 mL of MSC medium and maintained 3 h before the T-cell proliferation assays. Splenocytes were isolated from C57BL/6 spleens. For preparation of spleen cell suspensions, spleens were removed and minced with a nylon mesh. After the cells were pelleted, the erythrocytes were lysed using hypotonic buffer containing 0.75% NH4Cl. Cells were washed two times in 1× PBS. Spleen cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA) according to the manufacturer’s protocol. Briefly, a spleen cell pellet was suspended in pre-warmed serum-free RPMI-1640 media containing 5 µM of CFSE for a final cell concentration of 10 × 107 cells/mL. The samples were incubated at 37 °C for 10 min, then centrifuged and washed with pre-warmed complete assay medium (RPMI-1640 supplemented with 10% fetal calf serum (FCS), 10 U/mL penicillin–streptomycin, 2 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 50 µM 2-mercaptoethanol, 1 mM nonessential amino acids (NEAA), and 1 mM sodium pyruvate), and then incubated at 37°C for 10 min to ensure complete probe modification. T-cell proliferation assays were performed in a total 0.2 mL of complete assay medium. To assess proliferation, the CFSE-labeled responding spleen cells (1 × 106) were cultured alone with phytohemagglutinin (PHA; 10 µg/mL; Sigma) as positive control or was cultured alone with Mit C as negative control or cocultured with Mit C–treated allogeneic MSCs either myogenic induced (5-aza-CdR-treated) or undifferentiated with PHA. Triplicate cultures were incubated at 37°C for 5 days. On day 5 of culture, the cells were harvested for staining with anti-CD3 allophycocyanin (APC) (BD Biosciences). Samples were acquired on a FACSCalibur flow cytometer (BD Biosciences) and data were gated on CD3+ events. The percent of T lymphocytes undergoing division has been identified as the precursor frequency (Pf) indicating how many cells divided at least once. CFSE analyses were determined using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Coculture of splenocyte with C3H10T1/2 cells
Splenocytes isolated from C57BL/6 mice were filtrated through nylon meshes and red blood cells were removed with NH4Cl lysis buffer. For coculturing of splenocyte and C3H10T1/2 cells, the C3H10T1/2 cells (1 × 104 to 1 × 106 cells) were plated onto the lower chambers of transwell culture plate (Corning Inc.) while the splenocytes (1 × 106) were introduced into the inserted upper chamber (0.4 µm pore size) with complete assay medium. After 5 days of coculture, the splenocytes in the inserted upper chambers were collected and analyzed for determining CD4+CD25+Foxp3+Treg cell.
Determination of phenotypes of Treg cells by FACS
Phenotypes of Treg cell population in splenocytes cocultured with C3H10T1/2 cells were determined by FACS analysis using Mouse Regulatory T Cell Staining Kit following the manufacturer’s protocol (eBioscience, San Diego, CA, USA). Briefly, cells were probed with anti-CD4-FITC and/or anti-CD25-APC for 30 min at 4°C. Next, following fixation, cells were probed for intracellular Foxp3 using phycoerythrin (PE)-labeled anti-Foxp3 antibody or isotype control antibody for 30 min at room temperature or 4°C overnight. Finally, cells were washed with permeabilization buffer and counted using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with FlowJo software (Tree Star).
Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM). The statistical significance was determined by one-way analysis of variance (ANOVA) followed by a Dunn’s multiple comparisons test, using GraphPad Prism 6.02 (GraphPad, San Diego, CA, USA). A p value <0.05 was considered significant.
Results
Evidence for myogenic induction
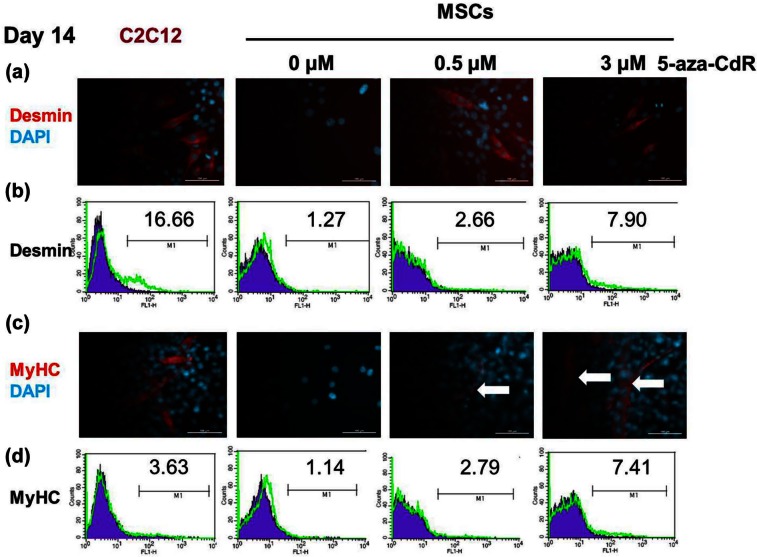
For characterization of BM MSCs and MSC cell line which were used in our experiment, the myogenic differential ability and immunologic markers of these cells were evaluated. Cells were isolated from mouse BM and murine C3H10T1/2 cell line, which contained heterogeneous populations of hematopoietic cells and then purified via adherence separation culturing. After 14 days, the cells from BM MSC and C3H10T1/2 cells were confirmed to have gone through myogenic induction (positive for desmin and MyHC). Immunophenotypic analysis also showed that the cultured cells manifested the typical MSC surface phenotypes. At day 14, the surface phenotype was defined as being positive for CD90 and CD105 and negative for CD34 and CD45. The murine MSC cell line C3H10T1/2 has already been shown to differentiate down the osteogenic, adipogenic, chondrogenic, and myogenic pathways30,31 as well as having the same immunosuppressive properties as bone marrow–derived primary human MSCs.32 MSCs cultured with 5-aza-CdR (to induce myogenic differentiation) formed myotube-like structures after 2 weeks (Figure 1(c) and Supplementary Figure S2A). Compared with untreated MSCs, 5-aza-CdR-treated MSCs exhibited upregulated expression of the myogenic-specific proteins desmin (Figure 1(a), (b) and Supplementary Figure S2C) and MyHC (Figure 1(c), (d) and Supplementary Figure S2B), suggesting that the 5-aza-CdR-treated MSCs acquired characteristics of myogenic cells. Flow cytometric analysis revealed that desmin was expressed by 7.9% of 3 µM 5-aza-CdR-treated MSCs, 2.66% of 0.5 µM 5-aza-CdR-treated MSCs, and 1.27% of untreated MSCs. Compared with C2C12 (16.66%), 3 µM 5-aza-CdR-treated MSCs exhibited almost half expression of desmin (Figure 1(b)). MyHC was expressed by 7.41% of 3 µM 5-aza-CdR-treated MSCs, 2.79% of 0.5 µM 5-aza-CdR-treated MSCs, and 1.14% of untreated MSCs. The expression of MyHC was more in 3 µM 5-aza-CdR-treated MSCs than in C2C12 (3.63%) (Figure 1(d)).
Figure 1.
Evidence for myogenic induction. MSCs were treated with 5-aza-CdR for 24 h and then cultured for 2 weeks to induce myogenic differentiation. (a–d) Immunostaining and flow cytometry on day 14. Compared with untreated MSCs (0 µM), 5-aza-CdR-treated MSCs exhibited upregulated expression of the myogenic-specific proteins desmin (a, b) and MyHC (c, d), suggesting that the 5-aza-CdR-treated MSCs acquired characteristics of myogenic cells. Myotube formation (arrow) was observed in 5-aza-CdR-treated MSCs, but not undifferentiated MSCs. DAPI is the nuclear staining. Flow cytometry: filled blue shows isotype control and empty green, corresponding antibody. Numbers represent percentages of cells staining positive for the given antibody. Data are representative of two independent experiments.
MSC: mesenchymal stem cell; 5-aza-CdR: 5-aza-2′-deoxycytidine; DAPI: 4,6-diamidino-2-phenylindole; MyHC: myosin heavy chain.
MSC markers and immune antigen expression
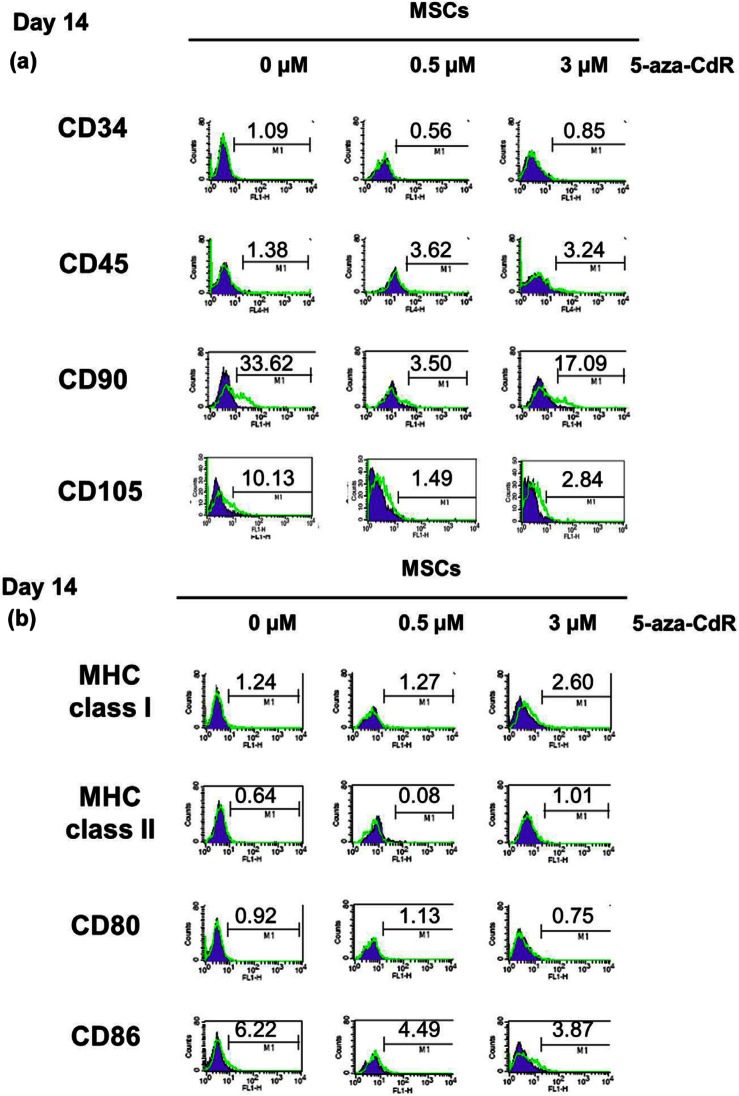
We carried out flow cytometry to directly compare the expression of MSC markers and immune-related surface markers between untreated MSCs and 5-aza-CdR-treated MSCs. Before experimental use, mouse BM MSCs and C3H10T1/2 were identified by the expression of CD 90 and CD105 antigens but not CD45 or CD34 antigens on day 0 (Supplementary Figure S1). We also confirmed that the cells we used in the experiment are able to differentiate into lipoblasts.33–36 Both 0.5 µM 5-aza-CdR-treated MSCs and 3 µM 5-aza-CdR-treated MSCs were negative for the hematopoietic progenitor/lineage markers CD34 and CD45 after 14 days myogenic induction. The expression of MSC antigens CD90 and CD105 decreased in 5-aza-CdR-treated MSCs. Compared with undifferentiated MSCs (33.62%), 3 µM 5-aza-CdR-treated MSCs exhibited half level of CD90 expression (17.09%). By contrast, 0.5 µM 5-aza-CdR-treated MSCs almost lost MSC phenotype (Figure 2(a)).
Figure 2.
MSC markers and immune antigen expression. We carried out flow cytometry to directly compare the expression of (a) MSC markers and (b) immune-related surface markers between untreated MSCs and 5-aza-CdR-treated MSCs on day 14. (a) Both 0.5 µM 5-aza-CdR-treated MSCs and 3 µM 5-aza-CdR-treated MSCs were negative for the hematopoietic progenitor/lineage markers CD34 and CD45 after 14 days myogenic induction. (b) Compared with undifferentiated MSCs, 5-aza-CdR-treated MSCs exhibited similar expression of MHC class I and negative expression of MHC class II. T-cell co-stimulatory marker CD80 has no difference between undifferentiated and 5-aza-CdR-treated MSCs. Undifferentiated MSCs were weakly positive for the co-stimulatory molecule CD86, but 5-aza-CdR-treated MSCs showed decreased expression of CD86. Flow cytometry: filled blue shows isotype control and empty green, corresponding antibody. Numbers represent percentages of cells staining positive for the given antibody. Data are representative of two independent experiments.
MSC: mesenchymal stem cell; 5-aza-CdR: 5-aza-2′-deoxycytidine; MHC: major histocompatibility complex.
Compared with undifferentiated MSCs, 5-aza-CdR-treated MSCs exhibited similar expression of MHC class I and negative expression of MHC class II. T-cell co-stimulatory marker CD80 has no difference between undifferentiated and 5-aza-CdR-treated MSCs. Undifferentiated MSCs were weakly positive for the co-stimulatory molecule CD86, but 5-aza-CdR-treated MSCs showed decreased expression of CD86 (Figure 2(b)).
Proliferative activity of CD3+ spleen cells cocultured with undifferentiated and 5-aza-CdR-treated myogenic-induced MSCs
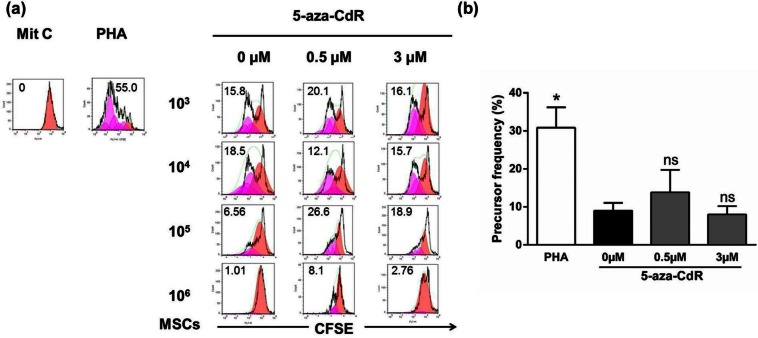
The ability of MSCs to inhibit activation of lymphocytes by allogeneic spleen cells from C57BL/6 mice and PHA was examined in a CFSE cell proliferation assay. Undifferentiated and 5-aza-CdR-treated MSCs were tested in this assay. Mit C–treated lymphocytes did not proliferate at all (negative control, Figure 3(a)). Stimulation with PHA induced obvious proliferation of lymphocytes (positive control, Figure 3(a)). Both undifferentiated and 3 µM 5-aza-CdR-treated MSCs induced exerted dose-dependent inhibition of lymphocyte activation (Figure 3(a)). MSCs, either undifferentiated or 3 µM 5-aza-CdR-treated MSCs, completely inhibited the proliferation of PHA-stimulated lymphocytes independently for a ratio MSCs:splenocytes equal to 1:1. By contrast, 0.5 µM 5-aza-CdR-treated MSCs did not inhibit lymphocyte activation until the 1:1 ratio.
Figure 3.
Proliferative activity of CD3+ spleen cells cocultured with undifferentiated and 5-aza-CdR-treated myogenic-induced MSCs. (a) To assess proliferation, the CFSE-labeled responding spleen cells (1 × 106) from C57BL/6 mice were cultured alone with PHA as positive control or was cultured alone with Mit C as negative control or cocultured with Mit C–treated allogeneic MSCs either myogenic induced (5-aza-CdR-treated) or undifferentiated with PHA. Triplicate cultures were incubated at 37 °C for 5 days. On day 5 of culture, the cells were harvested for staining with anti-CD3 APC. Samples were acquired on a fluorescence-activated cell sorting calibur flow cytometer and data were gated on CD3+ events. CFSE analyses were determined using FlowJo software. Numbers in histograms represent percent divided cells. (b) Precursor frequency of T lymphocytes in the presence of PHA was significantly greater than undifferentiated cells (0µM 5-aza-CdR, p < 0.05), whereas there was no significant difference between undifferentiated cells and those treated with 5-aza-CdR: 0 µM, 0.5 µM, and 3 µM (106 MSCs per treatment group). Data are representative of six independent experiments. Preliminary analysis of data for panels A and B was performed using the proliferation algorithm in the FlowJo software. Statistical analysis was determined by a one-way ANOVA followed by a Dunn’s multiple comparisons test.
5-Aza-CdR: 5-aza-2′-deoxycytidine; MSC: mesenchymal stem cell; CFSE: carboxyfluorescein diacetate succinimidyl ester; PHA: phytohemagglutinin; APC: allophycocyanin; Mit C: mitomycin C; ANOVA: analysis of variance; ns: not significant.
*p < 0.05.
Pf is a quantitative parameter representing the proliferative responsiveness of the T lymphocytes. Proliferative responsiveness of T lymphocytes in the presence of PHA were significantly greater than undifferentiated (cells in the presence of 0 µM 5-aza-CdR) MSCs (0 µM 5-aza-CdR: 8.97 ± 2.1%; PHA: 30.83 ± 5.3%; n = 6; p < 0.05; Figure 3(b)), whereas there was no significant difference between undifferentiated cells and those treated with 5-aza-CdR (0 µM 5-aza-CdR: 8.97 ± 2.1%; 0.5 µM 5-aza-CdR: 15.1 ± 6.8%; 3 µM 5-aza-CdR: 8.2 ± 2.4%; n = 6; not significant (ns); Figure 3(b)).
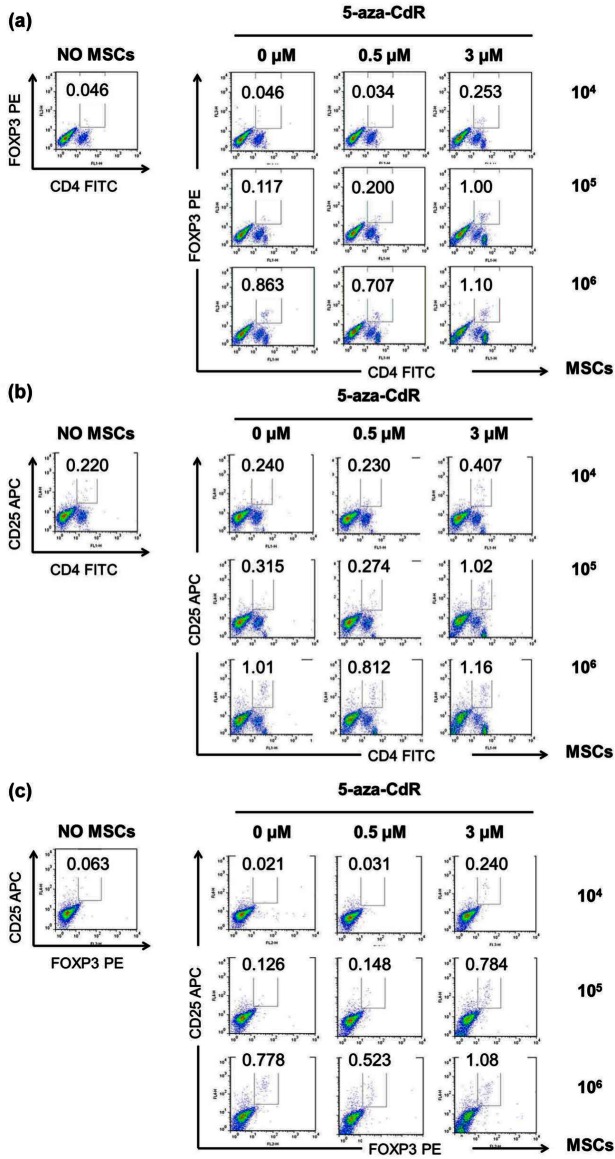
Induction of the Treg cells in response to various concentrations of undifferentiated and 5-aza-CdR-treated myogenic-induced MSCs
In order to assess the immunoregulatory function of MSCs related to Treg cells, we designed a transwell assay using the splenocytes from C57BL/6 mice in the presence of various concentrations of undifferentiated and 5-aza-CdR-treated myogenic-induced MSCs. Treg cells were increased with an increase in MSC dose from 104 to 106. Addition of undifferentiated MSCs and 5-aza-CdR-treated myogenic-induced MSCs dose dependently increased the proportion of CD4+Foxp3+ (Figure 4(a)), CD4+CD25+ (Figure 4(b)), and CD25+Foxp3+ (Figure 4(c)) expression in splenocyte culture by flow cytometry. In the presence of 3 µM 5-aza-CdR-treated MSCs, the proportion of CD4+Foxp3+, CD4+CD25+, and CD25+Foxp3+ expression in splenocytes showed highest.
Figure 4.
Induction of regulatory T cells in response to undifferentiated and 5-aza-CdR-treated MSCs. (a–c) After 5 days of coculture, splenocytes cocultured with MSCs were stained with antibodies against CD4, CD25, and Foxp3 and assessed by flow cytometry. Addition of undifferentiated MSCs and 5-aza-CdR-treated myogenic-induced MSCs dose dependently increased the proportion of (a) CD4+Foxp3+, (b) CD4+CD25+, and (c) CD25+Foxp3+ expression in splenocyte culture by flow cytometry. Numbers represent percentages of cells staining positive for the given antibody. Data are representative of two independent experiments with the same pattern of results.
5-Aza-CdR: 5-aza-2′-deoxycytidine; MSC: mesenchymal stem cell; PE: phycoerythrin; FITC: fluorescence isothiocyanate; APC: allophycocyanin.
Discussion
MSCs contribute to tissue repair and have been used for the regeneration of skeletal muscle and myocardium.37,38 MSCs are of interest because of their ability to differentiate to form myogenic cells in situ26 and may also be immune privileged (suitable for allogeneic applications). We assessed allogeneic and myogenic-induced MSCs for immunomodulatory properties, compared to undifferentiated MSCs.
Two different 5-aza-CdR doses (0.539 and 3 µM40) were evaluated for their effects on MSC myogenic differentiation potential, immune antigen expression, and mixed lymphocytic reactions. The 5-aza-CdR-treated MSCs acquired characteristics of myogenic cells and exhibited upregulated expression of late myogenic markers desmin and MyHC. MSCs cultured with 5-aza-CdR also formed myotube-like structures after 2 weeks. Compared with C2C12, 3 µM 5-aza-CdR-treated MSCs exhibited almost half expression of desmin. The expression of MyHC was more in 3 µM 5-aza-CdR-treated MSCs than in C2C12. In this study, 3 µM 5-aza-CdR-treated MSCs showed best result of myogenic induction.
We performed flow cytometry to directly compare the expression of MSC markers and immune-related surface markers between untreated MSCs and 5-aza-CdR-treated MSCs. Both 0.5 µM 5-aza-CdR-treated MSCs and 3 µM 5-aza-CdR-treated MSCs were negative for CD34 and CD45 after 14 days myogenic induction. The expression of MSC antigens CD90 and CD105 decreased in 5-aza-CdR-treated MSCs. Compared with undifferentiated MSCs, 3 µM 5-aza-CdR-treated MSCs exhibited half level of CD90 expression. By contrast, 0.5 µM 5-aza-CdR-treated MSCs almost lost MSC phenotype.
MSCs treated with 5-aza-CdR exhibited little expression of MHC class I and negative expression of MHC class II or T-cell co-stimulatory marker CD80, when compared with undifferentiated MSCs. While undifferentiated MSCs were weakly positive for the co-stimulatory molecule CD86, the 5-aza-CdR-treated MSCs showed decreased expression of CD86. This indicates that 5-aza-CdR-treated myogenic-induced MSCs, like undifferentiated MSCs, may not strongly activate rejection responses in allogeneic hosts. Once MSCs differentiate into skeletal muscle, they may lose their immunosuppressive properties. Nonetheless, the differentiated cells might be invisible to the host’s immune system because of their low-level expression of MHC class I. This would allow the long-term engraftment of MSCs without rejection following immunosuppressant-free transplantation.26 We further observed that 3 µM 5-aza-CdR-treated myogenic-induced MSCs, again like undifferentiated MSCs, inhibit PHA-induced activation of lymphocytes in a dose-dependent manner. Using a mixed lymphocytic reaction, we determined the optimal splenocyte proliferation inhibition dose. We titrated the proportion of MSCs relative to that of splenocytes. Under the 1:1 ratio of MSCs:splenocytes, splenocytes’ proliferation was inhibited with increase in MSC dose. By contrast, 0.5 µM 5-aza-CdR-treated MSCs did not inhibit lymphocyte activation until the 1:1 ratio and exhibited much stronger proliferation of lymphocytes compared to that of 3 µM 5-aza-CdR-treated MSCs. A major challenge in using MSCs for efficient tissue engineering or therapeutic cell transplantation is to direct their differentiation toward the desired lineage and to have the cells retain this phenotype.41 It should be noted that 5-aza-CdR is a demethylating agent, and as such, it could induce phenotypic changes that trigger the immune system even without cell differentiation.26 In this study, 0.5 µM 5-aza-CdR-treated MSCs showed lymphocyte activation and weak myogenic induction. This would be based on the loss of MSCs’ phenotype.
How MSCs modulate T-cell activation and hence the ultimate fate of the immune response is a critical unsolved question. But we hypothesized that MSCs induced activation and proliferation of Treg cells.42 Flow cytometry revealed that addition of undifferentiated MSCs and 5-aza-CdR-treated myogenic-induced MSCs dose dependently increased the proportion of CD4+CD25+Foxp3+ expression in splenocyte culture. The role of either soluble factors or cell contact-dependent mechanism in suppression of T-cell response and induction of Treg cells by MSCs is still an unsolved issue. We found that Treg cells increased in the presence of high concentration of MSCs in the present transwell study. Indeed, evidence is available that MSC-derived soluble factors played a role at high MSCs:responders ratio (1:1),32 whereas at lower ratios, cell contact was required to inhibit immune cell response.43 In addition, cell contact seems to play a non-redundant role in the induction of CD4+CD25+Foxp3+Treg cell.44 After culture of CD4+ T cells with MSCs, messenger RNA (mRNA) levels of CD25 and Foxp3 were only increased when cells were in close proximity.
In fact, myogenic induction, particularly 3 µM 5-aza-CdR-treated MSCs, enhances the expression of Treg cells. Numerous studies have demonstrated the ability of MSC to induce Treg cells.42,45–47 To our knowledge, this is the first report demonstrating the potential of myogenic-differentiated MSCs to induce Treg cells. High concentrations of local MSCs induce Treg cells which have been demonstrated to secrete TGF-β48 and IL-1042 and are engaged in Treg cell generation.49 Inhibition of innate immune activation is accomplished by MSCs through blocking dendritic cell (DC) maturation,50,51 suppression of macrophage activation,52 and augment IL-10 production,18 which directly inhibits inflammatory signaling. When DC activation is inhibited, MSCs hinder subsequent adaptive immunity by producing Treg cells and attenuating cytotoxic activities of CD8 cells.45
The compound 5-aza-CdR belongs to a class of cytosine analogs, which is developed as an inhibitor of DNA methylation and has been shown to have significant cytotoxic and antineoplastic activities in many experimental tumors.53,54 However, 5-Aza-CdR is reported to be noncarcinogenic and incorporates into DNA but not RNA or protein.55 Additionally, 5-aza-CdR is approved by the Food and Drug Administration (FDA) for the treatment of myelodysplastic syndrome (MDS) and is incorporated into the DNA of dividing cells where it specifically inhibits DNA methylation by forming covalent complexes with the DNMTs.56 Recently, several clinical trials of 5-aza-CdR have been reported, including a phase II study of 5-aza-CdR in patients with metastatic prostate cancer and a phase III study of 5-aza-CdR (decitabine) in patients with myelodysplasia.57 The treatment regimen used in our studies involved 24-h short-term application of 5-aza-CdR 2 weeks before the effects were analyzed. This treatment regime also has the advantage that any toxic effect of 5-aza-CdR would be confined to the 24-h in vitro treatment period since 5-aza-CdR would no longer be present at the end of culture. One could, therefore, envisage implantation of myogenic-induced MSCs into muscle defects or muscle degenerative disorders, without much toxicity concerns.
One limitation of this study is that although we maintained both untreated MSCs and 5-aza-CdR-treated MSCs in culture for 2 weeks, the prolonged cell culture conditions might have introduced factors that confounded the interpretation of MSCs’ antigen expression before and after myogenic differentiation. In addition, because we used unpurified spleen cells for the lymphocyte coculture experiments, we do not know whether 5-aza-CdR-treated myogenic-induced MSCs acted as antigen-presenting cells themselves or T cells activated via professional antigen-presenting cells.
In conclusion, the work described herein offers a method to induce allogeneic- and myogenic-differentiated MSCs that are not immunogenic. Myogenic-induced MSCs express little MHC class I, but not MHC class II. Furthermore, myogenic-induced MSCs have an immunomodulatory effect that do not elicit alloreactive lymphocyte proliferative responses and increased the induction of Treg cells. These findings support the view that myogenic-induced MSCs may be transplantable across allogeneic barriers as an immunomodulatory cell therapy source.
Supplementary Material
Acknowledgments
We would like to thank Dr Delrae M. Eckman for editorial assistance on this article. S.J., J.J.Y., and A.A. conceived and designed the experiments. S.J. and H.J.L. performed the experiments. S.J., J.D.J., and J.J.Y. analyzed the data. S.J., J.D.J., J.J.Y., and A.A. wrote and/or reviewed the article.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This study was supported by grant from the Joshua Frase Foundation.
References
- 1. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998; 279: 1528–1530. [DOI] [PubMed] [Google Scholar]
- 2. Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999; 401: 390–394. [DOI] [PubMed] [Google Scholar]
- 3. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 4. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71–74. [DOI] [PubMed] [Google Scholar]
- 5. Markert CD, Atala A, Cann JK, et al. Mesenchymal stem cells: emerging therapy for Duchenne muscular dystrophy. PM R 2009; 1: 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 2003; 5: 485–489. [DOI] [PubMed] [Google Scholar]
- 7. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 8. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003; 57: 11–20. [DOI] [PubMed] [Google Scholar]
- 10. Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 2004; 33: 597–604. [DOI] [PubMed] [Google Scholar]
- 11. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 12. Wolff D, Steiner B, Hildebrandt G, et al. Pharmaceutical and cellular strategies in prophylaxis and treatment of graft-versus-host disease. Curr Pharm Des 2009; 15: 1974–1997. [DOI] [PubMed] [Google Scholar]
- 13. Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 2002; 99: 8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mushtaq M, Oskouei BN, Hare JM. Cell therapy for heart disease: to genetically modify or not, that is the question. Circ Res 2011; 108: 398–401. [DOI] [PubMed] [Google Scholar]
- 15. Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005; 309: 314–317. [DOI] [PubMed] [Google Scholar]
- 16. Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med 2001; 52: 353–370. [DOI] [PubMed] [Google Scholar]
- 17. Zhao RC, Liao L, Han Q. Mechanisms of and perspectives on the mesenchymal stem cell in immunotherapy. J Lab Clin Med 2004; 143: 284–291. [DOI] [PubMed] [Google Scholar]
- 18. Nasef A, Chapel A, Mazurier C, et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr 2007; 13: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramasamy R, Tong CK, Seow HF, et al. The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol 2008; 251: 131–136. [DOI] [PubMed] [Google Scholar]
- 20. Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant 2001; 27: 777–783. [DOI] [PubMed] [Google Scholar]
- 21. Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 2005; 106: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 23. Reddy V, Winer AG, Eksioglu E, et al. Interleukin 12 is associated with reduced relapse without increased incidence of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 24. Skert C, Damiani D, Michelutti A, et al. Kinetics of Th1/Th2 cytokines and lymphocyte subsets to predict chronic GVHD after allo-SCT: results of a prospective study. Bone Marrow Transplant 2009; 44: 729–737. [DOI] [PubMed] [Google Scholar]
- 25. Ichim TE, Alexandrescu DT, Solano F, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol 2010; 260: 75–82. [DOI] [PubMed] [Google Scholar]
- 26. Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, et al. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol Ther 2012; 20: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haaf T, Schmid M. Experimental condensation inhibition in constitutive and facultative heterochromatin of mammalian chromosomes. Cytogenet Cell Genet 2000; 91: 113–123. [DOI] [PubMed] [Google Scholar]
- 28. Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999; 103: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukuda K. Application of mesenchymal stem cells for the regeneration of cardiomyocyte and its use for cell transplantation therapy. Hum Cell 2003; 16: 83–94. [DOI] [PubMed] [Google Scholar]
- 30. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995; 18: 1417–1426. [DOI] [PubMed] [Google Scholar]
- 31. Wang EA, Israel DI, Kelly S, et al. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 1993; 9: 57–71. [DOI] [PubMed] [Google Scholar]
- 32. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837–3844. [DOI] [PubMed] [Google Scholar]
- 33. Cho KA, Ju SY, Ryu KH, et al. Gene expression profile of mesenchymal stromal cells after co-culturing with injured liver tissue. Mol Med Rep 2009; 2: 51–61. [DOI] [PubMed] [Google Scholar]
- 34. Joo SY, Cho KA, Jung YJ, et al. Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy 2010; 12: 361–370. [DOI] [PubMed] [Google Scholar]
- 35. Jung YJ, Ju SY, Yoo ES, et al. MSC-DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy 2007; 9: 451–458. [DOI] [PubMed] [Google Scholar]
- 36. Ko E, Cho KA, Ju SY, et al. Mesenchymal stem cells inhibit the differentiation of CD4+ T cells into interleukin-17-secreting T cells. Acta Haematol 2008; 120: 165–167. [DOI] [PubMed] [Google Scholar]
- 37. De Bari C, Dell’Accio F, Vandenabeele F, et al. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol 2003; 160: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002; 105: 93–98. [DOI] [PubMed] [Google Scholar]
- 39. Nakatsuka R, Nozaki T, Uemura Y, et al. 5-Aza-2’-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol 2010; 55: 350–357. [DOI] [PubMed] [Google Scholar]
- 40. Kim BS, Chun SY, Lee JK, et al. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med 2012; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drost AC, Weng S, Feil G, et al. In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann N Y Acad Sci 2009; 1176: 135–143. [DOI] [PubMed] [Google Scholar]
- 42. Ye Z, Wang Y, Xie HY, et al. Immunosuppressive effects of rat mesenchymal stem cells: involvement of CD4+CD25+ regulatory T cells. Hepatobiliary Pancreat Dis Int 2008; 7: 608–614. [PubMed] [Google Scholar]
- 43. Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722–3729. [DOI] [PubMed] [Google Scholar]
- 44. English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 2009; 156: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008; 181: 3933–3946. [DOI] [PubMed] [Google Scholar]
- 46. Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008; 36: 309–318. [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010; 69: 241–248. [DOI] [PubMed] [Google Scholar]
- 48. Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 2007; 149: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev 2008; 7: 370–375. [DOI] [PubMed] [Google Scholar]
- 50. Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007; 25: 2025–2032. [DOI] [PubMed] [Google Scholar]
- 51. English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 2008; 115: 50–58. [DOI] [PubMed] [Google Scholar]
- 52. Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009; 15: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Natsume A, Wakabayashi T, Tsujimura K, et al. The DNA demethylating agent 5-aza-2’-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer 2008; 122: 2542–2553. [DOI] [PubMed] [Google Scholar]
- 54. Bender CM, Zingg JM, Jones PA. DNA methylation as a target for drug design. Pharm Res 1998; 15: 175–187. [DOI] [PubMed] [Google Scholar]
- 55. Glazer RI, Knode MC. 1-beta-D-arabinosyl-5-azacytosine. Cytocidal activity and effects on the synthesis and methylation of DNA in human colon carcinoma cells. Mol Pharmacol 1984; 26: 381–387. [PubMed] [Google Scholar]
- 56. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuendgen A, Lubbert M. Current status of epigenetic treatment in myelodysplastic syndromes. Ann Hematol 2008; 87: 601–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.