Abstract
Purpose
Rhabdomyosarcoma (RMS) is a pediatric sarcoma rarely occurring in adults. For unknown reasons, adults with RMS have worse outcomes.
Methods
We analyzed data from all patients who presented to XXXXXXXX between 1990 and 2011 with RMS diagnosed at age 16 or older. 148 patients met study criteria. Ten were excluded for lack of adequate data.
Results
Median age was 28 yrs. Tumor histology was: embryonal 54%, alveolar 33%, pleomorphic 12%, and NOS 2%. The tumor site was unfavorable in 67% of patients. 33 patients (24%) were low risk, 61 (44%) intermediate risk, and 44 (32%) high risk. 46% were treated on or according to a prospective RMS protocol. Five-year overall survival (OS) was 45% for non-metastatic patients. Failure rates at 5 years for non-metastatic patients were 34% locally and 42% distantly. Among patients with non-metastatic disease (n=94), significant factors associated with OS were histology, site, risk group, age, and protocol treatment. On multivariate analysis, risk group and protocol treatment were significant after adjusting for age. Five-yr OS was 54% for protocol patients vs 36% for non-protocol patients.
Conclusions
Survival in non-metastatic adult patients was significantly improved for those treated on RMS protocols, most of which are now open to adults.
Keywords: rhabdomyosarcoma, adolescents, adults, soft tissue sarcoma, age
Introduction
Rhabdomyosarcoma (RMS) is a rare malignancy, with an incidence of 4.5 per million people younger than 20 years in the United States. It is largely a disease of childhood with greater than 50% of cases diagnosed before the age of 10 [14]. Whereas the five-year overall survival rates of RMS in the pediatric population have improved in recent years to around 70%, the survival of adult populations with RMS is consistently lower with 5-year overall survival rates ranging from 40%–54% [16] In many pediatric studies, age itself is identified as a poor prognostic feature, with children greater than 10 demonstrating lower rates of survival than children 1–9 years of age [5]. Some of this difference in outcome between children and adults has been attributed to increased incidence of poor prognostic features in adults such as unfavorable primary site, unfavorable histology, and higher rates of regional and distant spread [17]. The extent to which discrepancies in the treatment of the pediatric and adult population with RMS plays a role in the lower survival rates among adults is unknown [19].
To better understand the role of demographic, clinical, and treatment variables in adult patients with RMS, we analyzed a large cohort of patients with RMS with a focus on both prognostic variables and treatment parameters.
Materials and Methods
Patients
We analyzed data from all patients who presented to XXXXXXXX (XXXXXX) between 1990 and 2011 who were diagnosed with RMS at age 16 or older. Details regarding demographic features, tumor characteristics, surgery, radiation, and chemotherapy were collected from the electronic medical record and the patients’ charts. The variables that were analyzed include age at diagnosis, histology, primary site distribution, Children’s Oncology Group (COG) risk group, performance status, chemotherapy regimen, whether patients received definitive surgery or radiation, and participation on a prospective RMS protocol.
For the purposes of statistical analyses, histology was classified as favorable and unfavorable with embryonal classified as favorable and alveolar, pleomorphic, and not otherwise specified (NOS) classified as unfavorable. Similarly, for primary site, favorable primary site included genitourinary (non-bladder or prostate), head and neck (non-parameningeal), and orbit. COG risk group was defined according to COG guidelines. All patients with metastatic disease were classified as high risk. Low risk patients included all patients with embryonal RMS and localized disease with the exception of patients with unresected, unfavorable site tumors. These patients as well as all patients with alveolar, pleomorphic, or NOS histology and localized disease, were categorized as intermediate risk. The protocols on which some patients were enrolled include both national protocols such as the COG protocol ARST 0331 and XXXXXXXX protocols designed for patients with RMS.
Statistical analyses
Overall survival (OS), local failure (LF), and distant metastasis (DM) were the endpoints used in this study. These endpoints were calculated from date of diagnosis. Kaplan-Meier method was used to calculate the OS rates and a competing risks analysis was used to calculate the cumulative incidence rates of LF and DM. OS was compared using the log-rank test for categorical variables and Cox proportional hazards regression model for continuous variables. Cumulative incidence curves of LF and DM were compared using Gray’s method. Multivariate analysis (MVA) using the Cox proportional hazards regression method using forward stepwise selection was used to identify prognostic factors for the endpoints. Variables with p-values <0.10 on univariate analysis were considered in the MVA. A p-value <0.05 was considered statistically significant. Age at presentation was treated as a continuous variable. Statistical analysis was performed with the software SAS version 9.2 (SAS Institute Cary, NC) and R version 2.92 package.
Results
Patients
Of the 148 patients who met study criteria, ten were excluded for lack of adequate data. The median follow up was 43 (range 1–254) months for surviving patients. Demographic and clinical characteristics are shown in Table 1. Median age was 28 (range 16–86) years. Embryonal tumors were present in 74 patients (54%), alveolar in 45 patients (33%), pleomorphic in 16 patients (12%), and NOS in 3 patients (2%). The majority of patients had an unfavorable primary site with 24 patients with parameningeal tumors (17%) and 30 patients with tumors of the extremity (22%). When stratified by COG risk group, there were 33 patients with low-risk disease (24%), 61 patients with intermediate-risk disease (44%), and 44 patients with high-risk disease (32%). KPS data was available in 117 patients with a median KPS of 80%.
TABLE 1.
Demographic and Clinical Characteristics of 138 Patients with Adult Rhabdomyosarcoma
| Descriptor | No. | % |
|---|---|---|
| Gender | ||
| Female | 60 | 43 |
| Male | 78 | 57 |
| Age | ||
| <21 | 41 | 30 |
| 21–40 | 61 | 44 |
| >40 | 36 | 26 |
| KPS | ||
| >70% | 90 | 77 |
| 27 | 23 | |
| ≤70 | 15 | 11 |
| Primary site | 29 | 21 |
| Orbit | 24 | 17 |
| Head and neck | 6 | 4 |
| GU non-BP | 30 | 22 |
| Parameningeal | 34 | 25 |
| GU BP | ||
| Extremity | ||
| Other | ||
| Histology | ||
| Embryonal | 74 | 54 |
| Alveolar | 45 | 33 |
| Pleomorphic | 16 | 12 |
| NOS | 3 | 2 |
| IRS Group | ||
| I | 20 | 14 |
| II | 25 | 18 |
| III | 49 | 36 |
| IV | 44 | 32 |
| COG risk group | ||
| Low-risk | 33 | 24 |
| Intermediate-risk | 61 | 44 |
| High-risk | 44 | 32 |
| Treatment | ||
| Chemotherapy | 125 | 91 |
| Surgery | 90 | 65 |
| Radiation (any) | 101 | 73 |
| Radiation to primary site | 90 | 65 |
| Chemotherapy | ||
| None | 13 | 9 |
| VAC | 33 | 24 |
| VADRC | 72 | 52 |
| Ifos/ADR | 5 | 4 |
| MAID | 4 | 3 |
| Unknown | 2 | 1 |
| Other | 9 | 7 |
| On or according to protocol | 64 | 46 |
Abbreviations: GU BP, genitourinary bladder/prostate; GU non-BP, genitourinary non-bladder/prostate; NOS, not otherwise specified; IRS, Intergroup Rhabdomyosarcoma Study; COG, Children’s Oncology Group; VAC, vincristine, dactinomycin, cyclophosphamide; VADRC, vincristine, doxorubicin, cyclophosphamide; Ifos, ifosfamide; ADR, doxorubicin; MAID, mesna, doxorubicin, ifosfamide, and dacarbazine.
Treatment
The majority of patients were treated with multimodality treatment consisting of chemotherapy, surgery, and radiation. Thirteen patients (9%) did not receive chemotherapy. Of these, 2 refused adjuvant treatment and 2 were dead of disease before additional treatment was administered. The remainder had small tumors with gross total resections with the majority receiving adjuvant radiation. The chemotherapy regimens utilized were diverse. Of the patients who received chemotherapy (n=125), 72 patients (58%) received cyclophosphamide, doxorubicin and vincristine and 33 patients (26%) received cyclophosphamide, dactinomycin and vincristine. The remainder of patients was treated with other regimens including ifosfamide and doxorubicin and mesna, doxorubicin, ifosfamide, and dacarbazine (MAID).
Radiation was administered in 101 patients (73%) with ninety of these patients (89%) receiving radiation to their primary tumor site. Of the patients who received radiation to their primary tumors, the median radiation dose was 50.4Gy (range: 10Gy–70.3Gy). Ten patients (7%) received intraoperative radiation, 10 patients received whole brain irradiation or craniospinal irradiation (7%), and 4 patients (3%) were treated with whole lung radiation.
Of patients without metastatic disease at diagnosis (n=94), fifty-eight patients (62%) had a complete resection of their primary tumor. Fifteen of these patients received neoadjuvant chemotherapy prior to surgery.
Sixty-four (46%) patients were treated on or according to a prospective RMS protocol. Patients treated according to a protocol were designated as such at time of treatment.
Outcomes
Five-year overall survival for the entire cohort was 34%. On univariate analysis, low and intermediate risk group (p<.0001), embryonal histology (p=.005), favorable primary site (p=.0009), performance status (p=.02), and no chemotherapy (p=.01) were significantly associated with improved overall survival. Protocol participation was not significant (p=.33). On MVA, risk group (low vs. high, HR 0.29, 95% CI 0.13–0.63, p=.002) and favorable primary site (vs. unfavorable HR .35, 95% CI .20-.63, p=.0004) remained significant for overall survival.
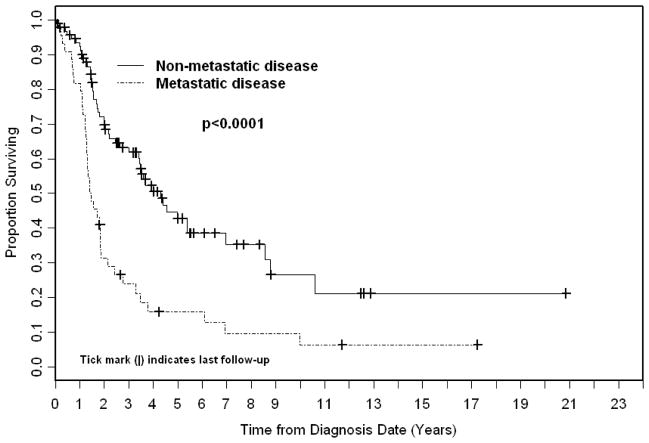
Among patients with non-metastatic disease at presentation (n=94), five year overall survival was 45% (Figure 1). On univariate analysis, younger age (p=.02), lower risk group (p=.01), embryonal histology (p=.04), favorable primary site (p=.02), and protocol participation (p=.03) were associated with improved overall survival (Table 2). On MVA, risk group (intermediate vs. low, HR 2.78, 95% CI 1.40–5.53, p=.004) and protocol participation as stratified by age remained significant. Since protocol patients tended to be younger than non-protocol patients, (mean age of protocol patients 22 vs. 43 for non-protocol patients), patients were stratified as age ≤ 44 on protocol and age ≤ 44 not on protocol thereby controlling the difference in age between the two groups. On MVA for overall survival, patients ≤ 44 not on protocol experienced significant shorter OS (HR 2.16, 95% CI 1.04–4.49, p=.04) than patients of the same age group treated on protocol. Patients at the older age group (≥45 years), none of whom participated on protocols, had an even shorter OS (HR 3.03, 95% CI 1.43–6.46, p=.004) compared to protocol patients.
FIGURE 1.
Kaplan Meier overall survival stratified by disease status.
TABLE 2.
Univariate and Multivariate Analysis of OS Using Cox Proportional Hazards Regression Model in Patients with Non-metastatic Disease (n=94)
| Factor | HR (95% CI) | P-value |
|---|---|---|
| Univariate Analysis | ||
| Age (years) | 0.02 | |
| Risk Group (low vs. intermediate) | 0.01 | |
| Histology (favorable vs. unfavorable) | 0.04 | |
| Site (favorable vs. unfavorable) | 0.02 | |
| Chemotherapy (no vs. VADRC vs. VAC vs. other) | 0.14 | |
| Protocol (yes vs. no) | 0.03 | |
| KPS (>70% vs. ≤ 70% | 0.16 | |
| Multivariate Analysis | ||
| COG Risk Group | ||
| Low (reference) | 1.00 | |
| Intermediate | 2.78 (1.40–5.53) | 0.004 |
| Protocol | ||
| Age ≤44, on protocol (reference) | 1.00 | |
| Age ≤44, not on protocol | 2.16 (1.04–4.49) | 0.04 |
| Age >45, not on protocol | 3.03 (1.43–6.46) | 0.004 |
Abbreviations: COG, Children’s Oncology Group; VAC, vincristine, dactinomycin, cyclophosphamide; VADRC, vincristine, doxorubicin, cyclophosphamide.
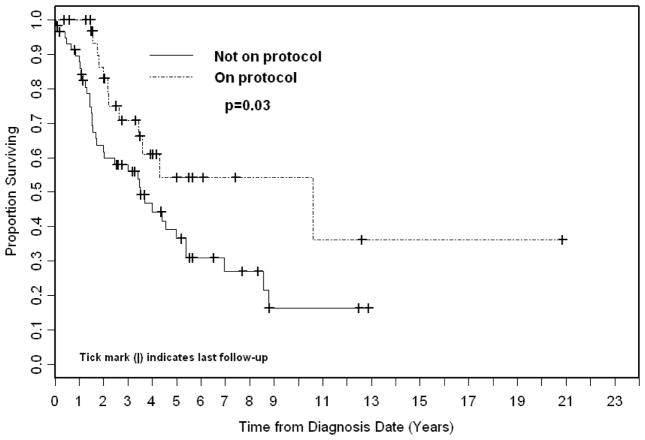
OS at 3 years, 4 years, and 5 years for patients with non-metastatic disease who participated on or according to prospective RMS protocols (n=35) was 71%, 61%, and 54%, respectively, compared with 56%, 44%, and 36% for all patients with non-metastatic disease treated off-protocol (Figure 2). There were no differences between non-metastatic protocol patients and non-protocol patients with regard to risk group (low vs. intermediate), KPS, histology (favorable vs. unfavorable), and primary site (favorable vs. unfavorable) (table 3). There was a significant difference with regard to age which was accounted for in MVA as discussed above. There was also a significant difference in chemotherapy regimens between the two groups with more protocol patients receiving cyclophosphamide, doxorubicin and vincristine (71% vs. 20% for non-protocol patients). However, chemotherapy regimen for non-metastatic patients was not significant on univariate analysis for overall survival (p=.14).
FIGURE 2.
Kaplan Meier Overall Survival stratified by protocol for non-metastatic patients.
TABLE 3.
Comparison of Patient Characteristics of Patients with Non-metastatic Disease Treated On or According to vs. Off Protocol
| Factor | Protocol, n (%) | P-value | |
|---|---|---|---|
| No (n=59) | Yes (n=35) | ||
| Age (years), mean ± SD | 43.4 ± 18.0 | 21.7 ± 5.3 | <0.0001 |
| COG risk groups | |||
| Low | 21 (36) | 12 (34) | 0.90 |
| Intermediate | 38 (64) | 23 (66) | |
| Performance Status, median | 90 | 90 | 0.41 |
| Histology | |||
| Favorable | 35 (59) | 21 (60) | 0.95 |
| Unfavorable | 24 (41) | 14 (40) | |
| Site | |||
| Favorable | 20 (34) | 15 (43) | 0.39 |
| Unfavorable | 39 (66) | 20 (57) | |
| Chemo | |||
| None | 13 (22) | 0 (0) | <0.0001 |
| VAC | 12 (20) | 25 (71) | |
| VADRC | 20 (34) | 10 (29) | |
| All others | 14 (24) | 0 (0) | |
Abbreviations: COG, Children’s Oncology Group; VAC, vincristine, dactinomycin, cyclophosphamide; VADRC, vincristine, doxorubicin, cyclophosphamide.
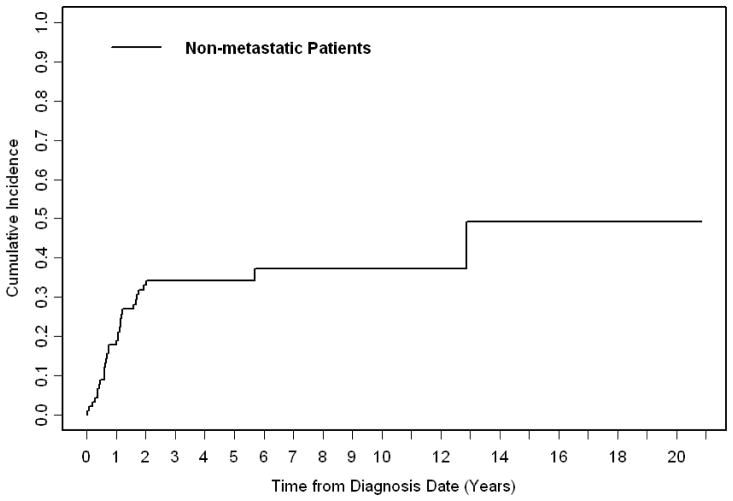
Local failure rate at five years for non-metastatic patients was 34% (Figure 3) with unfavorable histology being the only significant predictor of local failure. Local failure did not differ between patients treated with surgery vs. definitive radiation treatment (p=.37). Distant metastasis rates at five years was 42% with low risk group (p=.003), embryonal histology (p=.0003), and favorable primary site (p=.002) all significantly associated with improved distant metastasis free survival on univariate analysis. On MVA, embryonal histology (vs. alveolar/pleomorphic, HR .39, 95% CI .18 – .84, p=.02, favorable primary site (vs. unfavorable, HR .27, 95%CI .12 – .59, p=.001), no chemotherapy (as opposed to any chemotherapy, HR .07, 95%CI .01 – .45, p=.005), and protocol participation (vs. no protocol participation, HR .32, 95% CI .15 – .69, p=.004) were significant predictors of distant metastasis free survival.
FIGURE 3.
Cumulative incidence of local failure for non-metastatic patients.
Of patients with parameningeal RMS (n=24), leptomeningeal failure occurred in 4 patients (17%). Three of these patients had embryonal histology and one had alveolar histology. Four patients (13%) with extremity RMS (n=30), all with alveolar histology, also developed leptomeningeal disease. Two patients (1%) developed secondary AML.
Discussion
RMS is the most common pediatric sarcoma and large, multi-institutional trials have detailed its biologic and clinical characteristics. In particular, the Intergroup Rhabdomyosarcoma Studies, provide data on thousands of children (age <21 years of age) with long-term survival data [4,5,12,13]. With regard to adult RMS, much of the published literature is from single-institution series which report on clinical parameters and survival data for adults with RMS.
The data from these large retrospective studies consistently demonstrated poor outcomes in adults with RMS. Five-year overall survival from single institution studies were in the range of 31% to 44% [1,6,7,9,11]. These studies all defined adults as older than age 15–18 and thus represented similar aged patients. Our five-year OS rate of 34% for the entire cohort is in line with previously published data. A large-comparison of adult and pediatric RMS using the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2005 compared 1,071 patients age > 19 with 1,529 children [17]. Five-year OS was 27% in the adult population and 61% in the pediatric population. Although the adult OS rate of 27% was lower than the 31%–44% in the retrospective studies cited above and the pediatric OS rate of 61% was lower than historical controls of 70%–80% [5,16], this study nonetheless provided comparative survival data from large numbers of adult and pediatric patients that demonstrated that adults had considerably lower survival rates than children.
Age itself is prognostic in both adult and pediatric series. In the IRS-IV study that included 883 patients <21 years of age, children age 1–9 with non-metastatic disease had a significantly improved 3-year failure-free survival (83%) as compared to children age 10 or older (68%) [5]. Within series of adult patients, there are those studies that found that advanced age itself is a predictor for lower survival [9,10] and others that found that age was not a predictor of survival among the cohort of adult patients [6,11]. In our study, age was not a significant predictor of OS for the entire cohort but was significant for OS in non-metastatic patients.
In terms of other prognostic factors that predict for better outcomes in adults, we found that the predictors of better outcome in children were similar to those found in adults, such as low-risk group, favorable histology, and favorable primary site, all of which were significant on univariate analysis for OS, though histology lost significance on MVA for the entire cohort and primary site and histology lost significance on MVA in non-metastatic patients. The fact that histology was not significant on MVA is in keeping with previous analyses showing that these prognostic factors in children may be less important in adults [9,17].
One explanation for the poorer survival seen in adults is that adults have an increased incidence of poor prognostic features as compared to children. A previous series from our institution of 84 patients noted that pleomorphic subtype was more common among adult patients, and was present in 42% of patients greater than age 40 [9]. Series from both MD Anderson and the University of Iowa found pleomorphic histology to be the most common histology with rates of 43% and 36%, respectively [11,15]. One recent study compared children <15 with children ages 15–19 treated on the Italian Soft Tissue Sarcoma Committee (STSC) protocols and found that adolescents have higher rates of alveolar RMS (47.4% vs. 32.6%) and higher rates of lymph node infiltration and metastatic disease at time of diagnosis [2]. Similarly, the SEER analysis showed that adults were more likely to have tumors at unfavorable sites (65% in adults vs. 55% in children), and pleomorphic or NOS histology (19% and 43% respectively in adults). This study did not find that adults were more likely to have regional and distant spread at presentation [17]. Like the SEER data, we found a large percentage of patients (67%) with an unfavorable primary site. However, our study also included a lower percentage of adults with unfavorable histologies (47% unfavorable) which is in contrast to the SEER data where 79% of adults had unfavorable histologies as compared to 42% of children. Of note, the SEER analysis classified 43% of adults with NOS histology, which may falsely overrepresent the proportion with unfavorable histology. Rates of favorable and unfavorable histologies in the third IRS study (n=1,062, age <21) were 59% and 29%, respectively, with 13% of specimens classified as other due to inadequate tissue [4].
Another possibility for the lower survival rates observed in adolescents with RMS is that adolescents perhaps receive suboptimal chemotherapy due to differences in pharmacology between adolescents, pediatric patients, and adults [18]. A recent study from the Children’s Oncology Group (COG) investigated this hypothesis by exploring the rates of adverse events in the IRS-IV study by age and treatment regimen. They found that adolescents >15 years of age experienced less hematologic toxicity and more peripheral nervous system toxicity compared to younger children. This study noted that the total dose of each chemotherapy agent was similar in all age groups and thus could not link the lower survival seen in adolescents to the differences in toxicity which were observed [8].
In a similar vein, it has been proposed that suboptimal treatment in adults as compared to children accounts for the poor OS seen in adult RMS. One retrospective analysis from Italy attempted to retrospectively score the adequacy of the patient’s treatment based on standards of care established by the IRS and Italian Cooperative Group (ICG) for children. This group found that among patients with high scores for appropriate treatment, 5-year OS was 61% [7]. This study is limited by its retrospective scoring of treatment adequacy which is inherently prone to bias. A more recent study from the Italian Soft Tissue Sarcoma Committee (STSC) investigated patients treated on four STSC protocols stratified by age <15 and age 15–19. All patients included in this study received treatment as determined by a prospective protocol and no retrospective scoring was necessary. Five-year OS was 69% in children versus 57% in adolescents. In patients with localized disease, OS rates were similar (77% versus 79%). The authors noted that survival was satisfactory for adolescents enrolled in STSC protocols [2].
We also noted an improved survival among patients treated on or according to a prospective RMS protocol. Unlike the study by Ferrari et al., we do not retrospectively score adequacy of treatment but use treatment on or according to protocol as an objective criterion. Among patients with non-metastatic disease treated on an RMS protocol, 3-year, 4-year and 5-year OS was 71%, 61%, and 54%, respectively as compared to 56%, 44% and 36% for all patients with non-metastatic disease treated off-protocol as demonstrated in Figure 2. In the IRS-IV series, patients age 10 or greater with non-metastatic disease had a 3-year FFS rate of 68% which is comparable to our 3-year OS rate of 71% [5]. The mean age of patients treated on protocol in our study was 22 with a range of 16–43. We therefore captured an older age group than the adolescent population studied in IRS IV in which all patients were < 21 or in the Italian STSC study which included patients ≤18.
The study from the Italian STSC computes the expected vs. actual enrollment on protocols and found that this number was only 27% for adolescents as compared to 90% of children [2]. A study from the United States that used the SEER database to investigate national survival trends of young adults with sarcoma, found that there was a direct correlation between age-dependent survival improvement and clinical-trial participation. For patients with soft tissue sarcoma, the least survival improvement occurred between ages 15 and 45. Furthermore, the proportion of young adults with soft tissue sarcomas participating on clinical trials was lowest among patients age 25–45 in which it was only 6–14% of the estimated accrual proportion for all cancers [3].
Taken together, our data along with the Italian STSC data, especially when viewed in light of national and international trends in survival improvement and protocol participation, provide evidence for the enrollment of older adolescent and adult patients with non-metastatic RMS on or according to prospective protocols. The inclusion of patients up to age 50 on current COG sarcoma protocols enables the inclusion of the majority of these patients on study. Irrespective of histology, primary site, and age, our study points to a survival advantage with protocol participation.
This study also highlights other areas for improvement in the treatment of adult RMS. Though the absolute numbers are small, we found that leptomeningeal failure occurred in 17% of patients with parameningeal tumors which emphasizes the need for novel central nervous system (CNS) therapies. Prospective studies looking at CNS directed prophylactic therapies are warranted given the high rates of CNS failure seen in adults with parameningeal RMS. We also observed a high rate of leptomeningeal disease among patients with alveolar RMS of the extremities, indicating another population at risk for CNS failure.
Conclusions
The results of the current study indicate that adults with RMS have increased rates of poor prognostic features such as unfavorable primary site. For the entire cohort, overall survival is poor as compared to pediatric survival rates as demonstrated in multiple large-scale studies. However, for a subset of non-metastatic patients treated on prospective RMS protocols, survival is significantly improved and approaches survival rates seen in the pediatric population.
This study is a large retrospective series of adult patients with rhabdomyosarcoma (RMS). For unknown reasons, adults with RMS have worse outcomes. This series correlates both prognostic features and treatment variables with outcome. There is no difference between patients who received definitive surgery or radiation for local control. We find that survival in non-metastatic adult patients with rhabdomyosarcoma was significantly improved for those treated on RMS protocols, most of which are now open to adults.
Acknowledgments
Funding: None
Thank you to Lawrence A. Herman for his editorial assistance with this manuscript.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn HK, et al. Analysis of prognostic factors of pediatric-type sarcomas in adult patients. Oncology. 2011;80:21–28. doi: 10.1159/000327222. [DOI] [PubMed] [Google Scholar]
- 2.Bisogno G, et al. Rhabdomyosarcoma in adolescents: A report from the aieop soft tissue sarcoma committee. Cancer. 2012;118:821–827. doi: 10.1002/cncr.26355. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, et al. National survival trends of young adults with sarcoma: Lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103:1891–1897. doi: 10.1002/cncr.20995. [DOI] [PubMed] [Google Scholar]
- 4.Crist W, et al. The third intergroup rhabdomyosarcoma study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 5.Crist WM, et al. Intergroup rhabdomyosarcoma study-iv: Results for patients with nonmetastatic disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 6.Esnaola NF, et al. Response to chemotherapy and predictors of survival in adult rhabdomyosarcoma. Annals of surgery. 2001;234:215–223. doi: 10.1097/00000658-200108000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari A, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98:571–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AA, et al. Patterns of chemotherapy-induced toxicities in younger children and adolescents with rhabdomyosarcoma: A report from the children’s oncology group soft tissue sarcoma committee. Cancer. 2012;118:1130–1137. doi: 10.1002/cncr.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins WG, et al. Clinicopathologic analysis of patients with adult rhabdomyosarcoma. Cancer. 2001;91:794–803. [PubMed] [Google Scholar]
- 10.La Quaglia MP, et al. The effect of age at diagnosis on outcome in rhabdomyosarcoma. Cancer. 1994;73:109–117. doi: 10.1002/1097-0142(19940101)73:1<109::aid-cncr2820730120>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Little DJ, et al. Adult rhabdomyosarcoma: Outcome following multimodality treatment. Cancer. 2002;95:377–388. doi: 10.1002/cncr.10669. [DOI] [PubMed] [Google Scholar]
- 12.Maurer HM, et al. The intergroup rhabdomyosarcoma study-i. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Maurer HM, et al. The intergroup rhabdomyosarcoma study-ii. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Ognjanovic S, et al. Trends in childhood rhabdomyosarcoma incidence and survival in the united states, 1975–2005. Cancer. 2009;115:4218–4226. doi: 10.1002/cncr.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon JH, et al. Presentation, prognostic factors and patterns of failure in adult rhabdomyosarcoma. Sarcoma. 2003;7:1–7. doi: 10.1080/1357714031000114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MA, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sultan I, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2,600 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 18.Veal GJ, Hartford CM, Stewart CF. Clinical pharmacology in the adolescent oncology patient. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4790–4799. doi: 10.1200/JCO.2010.28.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolden SL, Alektiar KM. Sarcomas across the age spectrum. Seminars in radiation oncology. 2010;20:45–51. doi: 10.1016/j.semradonc.2009.09.003. [DOI] [PubMed] [Google Scholar]