Abstract
Rationale
Serotonin (5-HT) neurotransmission is intimately linked to anxiety and depression and a diverse body of evidence supports the involvement of the main inhibitory serotonergic receptor, the serotonin-1A (5-HT1A) subtype, in both disorders.
Objectives
In this review, we examine the function of 5-HT1A receptor sub-populations and re-interpret our understanding of their role in mental illness in light of new data, separating both spatial (autoreceptor vs heteroreceptor) and the temporal (developmental vs adult) roles of the endogenous 5-HT1A receptors, emphasizing their distinct actions in mediating anxiety and depression-like behaviors.
Results
It is difficult to unambiguously distinguish the effects of different populations of the 5-HT1A receptors with traditional genetic animal models and pharmacological approaches. However, with the advent of novel genetic systems and subpopulation-selective pharmacological agents, direct evidence for distinct roles of these populations in governing emotion related behavior are emerging.
Conclusions
There is strong and growing evidence for a functional dissociation between auto and heteroreceptor populations in mediating anxiety and depressive-like behaviors respectively. Furthermore, while it is well established that 5-HT1A receptors act developmentally to establish normal anxiety-like behaviors, the developmental role of 5-HT1A heteroreceptors is less clear, and the specific mechanisms underlying the developmental role of each subpopulation are likely to be key elements determining mood control in adult subjects.
Keywords: 5-HT1A, autoreceptor, heteroreceptor, anxiety, depression, 5-HT, polymorphism, development
1. Introduction
Depression and anxiety are amongst the worlds leading public health problems today. Indeed, approximately 35 million adults in the US population (16%) are likely to suffer from depression at some time in their lives (Samuels et al. 2011) and the World Health Organization considers that unipolar depression will be the 2nd-highest cause of illness-induced disability by the year 2020 (Organization WH 2011). Although antidepressants are one of the most commonly used groups of therapeutic agents worldwide, less than half of depressed patients show full symptom remission and at least one quarter show treatment-resistance to current antidepressants (Corey-Lisle et al. 2004; Samuels et al. 2011). As concerns anxiety, an authoritative survey in the European Union found that it was the most prevalent of mental health disorders (12 month prevalence 14%) affecting over 60 million people each year (Wittchen et al., 2011) and entailing a massive economic burden of over 65 billion euros annually (Gustavsson et al. 2011). Despite extensive efforts searching for novel anxiolytic agents, advances have been incremental, notably due to the limitations of classical animal models (Griebel and Holmes 2013).
Such considerations emphasize the need for improved understanding of the mechanisms of action underlying depressive and anxiety states. Thus, whilst it is known that the serotonergic system plays an important role in the etiology and treatment of mood and anxiety disorders (Charney et al. 1998; Nemeroff 2002; Rush et al. 2000), the precise means by which this occurs are the subject of ongoing study.
Direct experimental evidence in humans implicating 5-HT in mood disorders came from studies of tryptophan depletion (Young et al. 1985), reviewed by (Ruhe et al. 2007). More recently, multiple lines of evidence implicate dysregulated 5-HT neurotransmission as a primary defect in mood and anxiety disorders (aan het Rot et al. 2009; Durant et al. 2010; Ganasen and Stein 2010; Jans et al. 2007; Ravindran and Stein 2010). Furthermore, complementary data suggesting a role for 5-HT in mood disorders, or at least in the recovery from mood disorders, comes from the use of selective serotonin reuptake inhibitors (SSRIs) and other serotonergic agents as first-line treatments. These drugs are believed to exert their therapeutic action by increasing 5-HT levels and facilitating 5-HT neurotransmission (Gartside et al. 1995). However, evidence from animal models suggests that SSRI treatment during early development increases anxiety or depression later in adulthood, in contrast to the well-known beneficial effects of SSRIs in adults (Ansorge et al. 2008; Caspi et al. 2003; Lira et al. 2003; Oberlander et al. 2009; Oliver et al. 2008). These results suggest that 5-HT may impact immature and mature mood-related circuitry differently. Furthermore, it is important to underscore that 5-HT’s effect on mood occurs in the context of multiple other neuromodulatory systems such as noradrenaline, dopamine and neurotrophins that may have distinct yet related effects on mood regulation, and which have their own developmental trajectories (Benes et al. 2000; Blier 2003; Murrin et al. 2007; Covington et al. 2010; Duman 2004; Durant et al. 2010; Nestler and Carlezon 2006; Nikolaus et al. 2010; Skolnick et al. 2009).
Serotonergic neurons are mainly located in the dorsal and median raphe of brainstem (DRN and MRN, respectively) (Barnes and Sharp 1999). Projections from these neurons release serotonin throughout the entire forebrain and brainstem and modulate a variety of neuronal activities, but there are other raphe nuclei that also provide innervations to the midbrain (Bockaert et al. 2006). The largely neuromodulatory effects of 5-HT are mediated through 14 receptor subtypes that are grouped into sub-families based on their primary signaling mechanism (Hoyer and Martin 1997). Here we focus on the 5-HT1A receptor, as evidence from both human and rodent studies suggest that it may play a particularly important role in both the etiology of these disorders and their treatment (Akimova et al. 2009; Gordon and Hen 2004; Hirvonen et al. 2008; Le Francois et al. 2008; Lesch and Gutknecht 2004; Strobel et al. 2003).
The 5-HT1A receptor is a major inhibitory G-protein coupled receptor subtype that exists in two major populations in the nervous system (autoreceptor and heteroreceptor), and functions by coupling to Gi/Go proteins that control numerous intracellular signaling cascades, including inhibition of cAMP formation, inactivation of calcium channels, and activation of potassium channels (Barnes and Sharp 1999). The 5-HT1A autoreceptor resides on the soma and dendrites of serotonin neurons in the raphe nuclei, where its activation hyperpolarizes and reduces the firing rate of these cells, and thereby serotonin extracellular levels in its projection areas (Hjorth and Sharp 1991; Meller et al. 1990; Sprouse and Aghajanian, 1986; Verge et al. 1985; Wang and Aghajanian 1977). Studies from several laboratories suggest that the molecular signaling mechanisms of 5-HT1A receptors in the raphe nuclei are distinct from those in other brain regions and may be preferentially mediated by coupling to Gαi3 G-protein subunits leading to partial inhibition of adenylyl cyclase (Liu et al. 1999; Marazziti et al. 2002; Palego et al. 1999; Valdizan et al. 2010). Local release of 5-HT in the raphe nuclei from axonal collaterals or crosstalk between different 5-HT neurons will thus diminish neuronal firing and produce a negative feedback regulation of transmitter release, and may add an extra level of topographical specification (Adell et al. 1991; Artigas et al. 1996; Bang et al. 2012). Consistent with their role in regulating serotonergic tone, autoreceptors limit the initial increase of 5-HT extracellular levels induced by SSRIs (Hervás et al. 2000; Hjorth and Auebarch, 1994; Hjorth et al., 1996; Rollema et al. 1996), delaying the therapeutic response (Artigas et al. 1996; Blier and De Montigny 1983; Gardier et al. 1996). This effect is gradually overcome by desensitization of 5-HT1A autoreceptors in the raphe nuclei (Dawson et al. 2000), allowing the firing rate of serotonergic neurons to recover (Blier and De Montigny 1983; El Mansari et al. 2005).
Postsynaptic 5-HT1A heteroreceptors are expressed in target areas receiving serotonergic innervation. These heteroreceptors are mainly located on pyramidal neurons and on GABAergic interneurons (Artigas et al. 2006; Azmitia et al. 1996; Palchaudhuri and Flugge 2005; Santana et al. 2004). They are highly expressed in brain regions implicated in the regulation of mood and anxiety, such as the prefrontal cortex, hippocampus (HP), and amygdala (Beck et al. 1992; Hamon et al. 1990; Pompeiano et al., 1992; Riad et al. 2000). Activation of 5-HT1A heteroreceptors in these areas mediates a hyperpolarizing response to released serotonin on pyramidal neurons (Andrade et al., 1986; Hamon et al. 1990; Riad et al. 2000), an effect that may be mediated by coupling of the receptors mainly to Gαo subunits in the hippocampus, and equally to Gαo and Gαi3 in cerebral cortex (Mannoury la Cour et al. 2001), unlike 5-HT1A autoreceptors that may couple preferentially to Gαi3. Moreover, there is a second indirect mechanism regulating serotonergic neurotransmission that involves 5-HT1A heteroreceptors in the mPFC-raphe pathway (Celada et al. 2001; Hajos et al. 1999). Hence, 5-HT1A receptors are powerful modulators of 5-HT function through their distinct populations, likely exerting differential effects both by their distinct anatomical localizations as well as by distinct Gα subunit coupling that may account for regional differences in activation versus inhibition of downstream signaling targets.
In the present review, we focus on the evidence that demonstrates a distinct role of 5-HT1A autoreceptors versus heteroreceptors in the initial establishment of mood and anxiety homeostasis as well as their developmental requirement in affective illness.
2. Role of 5-HT1A receptors in depression and anxiety
2.1 Human studies
Dysregulation of 5-HT1A receptors occurs in patients suffering from depression and related mood disorders. While there has been considerable discrepancy amongst postmortem and human imaging studies of 5-HT1A receptor levels in depression there is growing evidence supporting an increase of 5-HT1A autoreceptors and a decrease of heteroreceptors in major depression (Arranz et al. 1994; Boldrini et al. 2008; Cheetham et al. 1990; Dillon et al. 1991; Joyce et al. 1993; Lowther et al. 1997; Matsubara et al. 1991; Parsey et al. 2010; Stockmeier et al. 1996).
Increases in 5-HT1A autoreceptor density in the midbrain have been demonstrated in depressed suicide patients (Stockmeier et al. 1998). Boldrini et al., (2008) confirmed and extended this observation by using (H3) 8-OH-DPAT autoradiography and reported an increase in rostral divisions of the dorsal raphe nuclei, which project to the prefrontal cortex, but decreased levels of 5-HT1A autoreceptors in the caudal dorsal raphe nuclei. An increase in autoreceptor levels in rostral divisions might result in a decrease serotonin activity in projection areas by lowering firing rate. However, disagreement exists within the postmortem literature regarding this subpopulation, suggesting that a more complex pattern of 5-HT1A autoreceptor binding abnormalities exists in depression. Indeed, reductions in 5-HT1A autoreceptor binding have also been reported in different PET studies using primary depressives as subjects (Drevets et al. 1999; Drevets et al. 2007; Meltzer et al. 2004).
5-HT1A receptors have been also examined in a number of cerebral cortical and subcortical areas in subjects with a history of mood disorders. PET studies revealed decreased 5-HT1A heteroreceptor levels in the orbitofrontal, anterior cingulate, occipital and parietal cortex in untreated or treated depressed patients and it was also decreased in patients with remitted depressive episodes and unmedicated subjects (Bhagwagar et al. 2004; Drevets et al. 2000; Drevets et al. 2007; Sargent et al. 2000). Furthermore, reduced 5-HT1A heteroreceptor levels have also been reported in patients with social anxiety disorders (Lanzenberger et al. 2007), as well as in cortical regions from patients suffering from panic disorder (Nash et al. 1998; Neumeister et al. 2004), although not all studies are in agreement (see Meltzer et al. 2004; Parsey et al. 2006, Parsey 2010).
Neevertheless, despite some discrepant findings, the overall evidence suggests that 5-HT1A receptor function is altered in clinical populations when compared to controls. Furthermore, it should be underscored that the observed abnormalities in 5-HT1A receptor levels are found in a number of affective and anxiety-related disorders (Neumeister et al. 2004), suggesting that these findings may reflect a general vulnerability factor for psychopathology.
2.2 5-HT1A Polymorphism
Stress diathesis theories of depression predict that an individual’s sensitivity to stressful events depends on their genetic makeup (Costello et al. 2002; Monroe and Simons 1991) and such predictions are now increasingly supported by experimental evidence. Indeed, in the case of 5-HT1A receptors, a functional C(-1019)G single nucleotide polymorphism (SNP) in the transcriptional control region of the HTR1A gene (HTR1A-1019) has been associated with a number of mood-related variables, including depression, risk of suicide, response to antidepressant treatment, and amygdala reactivity (Fakra et al. 2009; Le Francois et al. 2008; Lesch and Gutknecht 2004; Strobel et al. 2003). Lemonde and co-workers (2003) were the first to report that the G/G genotype is associated with major depression and suicide in two different cohorts. This association has been replicated and extended in most subsequent studies (Anttila et al. 2007; Kraus et al. 2007; Neff et al. 2009; Parsey et al. 2006). The 5-HT1A G(-1019) allele has also been associated with anxiety (Choi et al. 2010; Domschke et al. 2006; Fakra et al. 2009). More recently, it has been suggested that the HTR1A G allele of the polymorphism is associated to the frequent clinical presentation of comorbid major depression and anxiety, suggesting a common genetic background for mixed depression and anxiety state (Molina et al. 2011). Furthermore, the G allele of the polymorphism has also been associated with several mood disorders, such as panic disorder (Rothe et al. 2004; Strobel et al. 2003) and panic attack (Huang et al. 2004). Perhaps not surprisingly for a complex pyschiatric disorder, not all studies have found a clear association of the G allele of the polymorphism with depression (Arias et al. 2002; Hettema et al. 2008; Huang et al. 2004). These discrepancies could be related to different variables such as the frequency of the risk allele, ethnicity or disease in the population studied. However, the overall message that emerges from literature suggests that the 5-HT1A receptor G(–1019) allele is a risk allele for depression and related mood disorders.
Not only is there evidence for increased risk for mood disorders, but patients homozygous for the G allele consistently have a reduced response to SSRIs treatment (Arias et al. 2005; Lemonde et al. 2004; Parsey et al. 2006) compared to patients with the C/C genotype (Hong et al. 2006; Serreti et al. 2004; Yu et al. 2006) but see (Levin et al. 2007). Overall, these finding suggest that genetic variations in the HTR1A gene may contribute not only to susceptibility to depression but also to individual differences in responsiveness to antidepressant treatment.
At the molecular level, the C(-1019)G polymorphism is located in a 26-bp palindrome region within the repressor/enhancer region of the HTR1A promoter (Albert et al. 1996; Albert and Lemonde 2004). This region is recognized by a number of transcription factors including Deaf-1 and Hes5, that act as repressors of the C- but not the G allele of the 5-HT1A polymorphism in the raphe. While Hes5 also appears to function as a repressor of heteroreceptor populations, Deaf-1 may enhance expression of 5-HT1A heteroreceptors in C- allele carriers (Lemonde et al. 2003, Czesak et al. 2006). In addition to the effects of Deaf-1 and Hes-5, Hes1 is also a repressor of 5-HT1A autoreceptors, both in vitro and in vivo (Jacobsen et al., 2008). Thus, although initial in vitro reports suggested that this polymorphism could impact autoreceptor levels (Lemonde et al. 2003), a subsequent in vivo study reported that the G allele resulted in increased 5-HT1A expression in both the raphe and other brain regions of antidepressant naive depressed patients (Parsey et al. 2006). Furthermore, a subsequent study showed the opposite regulation of heteroreceptor compared to autoreceptor by the G-allele (Czesak et al. 2006) and this was further confirmed in vivo using Deaf1-null mouse model lacking the key transcription factor thought to act at this polymorphism in adults (Czesak et al. 2012). In this model, raphe 5-HT1A receptor RNA and protein were increased by 50%, while in prefrontal cortex but not hippocampus a smaller 30% reduction in RNA was observed. However, whether the effects on forebrain levels observed in vivo are primary or secondary to changes in autoreceptor levels remains to be elucidated.
In summary, this model predicts that having relatively higher levels of 5-HT1A autoreceptors results in increased susceptibility to depression and decreased response to treatment. A recent study looking at mice that differed only in their level of autoreceptors supports such a predicition (Richardson-Jones et al. 2010).
2.3 Preclinical pharmacological studies
Data from the depression and anxiety literature provide evidence that 5-HT1A receptors are involved in both disorders. Indeed, several clinically approved drugs, including buspirone and tandospirone, likely mediate their anxiolytic properties via prominent 5-HT1A partial agonist activity (Lacivita et al. 2008). Other drugs, such as flesinoxan or flibanserin, exhibit high agonist efficacy at 5-HT1A receptors and have proved active in clinical trials as antidepressants (Pitchot et al., 2005). Further evidence for the involvement of 5-HT1A receptors in regulation of depressive states comes from co-treatment of depressed subjects with SSRIs and the 5-HT1A weak partial agonist, pindolol. The latter drug (which is also an adrenergic beta-blocker) preferentially occupies 5-HT1A autoreceptors, thus preventing feedback inhibition of serotonin release and accelerating antidepressant response in most though not all studies (Celada et al. 2013; Portella et al. 2011). Consistent with this mechanistic interpretation, co-treatment of depressed patients with buspirone and pindolol elicited an antidepressant effect (McAllister and Massey 2003), whereas buspirone lacks antidepressant efficacy by itself, likely because its insufficient partial agonist efficacy at post-synaptic receptors (and full agonist activity at 5-HT1A autoreceptors) (Celada et al. 2013). Furthermore, vilazodone, a combined SSRI and 5-HT1A receptor partial agonist (Sorbera et al. 2001) exhibits anxiolytic and antidepressant-like effects (Bartoszyk et al. 1997; Page et al. 2002).
Taken together, these observations support the importance of 5-HT1A receptors in control of mood disorders in a clinical context and have spurred investigation of 5-HT1A receptor function using animal models. In particular efforts have been made to probe 5-HT1A receptor function using pharmacological and genetic approaches.
The existence of specific 5-HT1A ligands has made it possible to study the function of this receptor (Fletcher et al. 1996; Hamon et al. 1990). For example, pharmacological studies demonstrate that 5-HT1A receptor partial agonists such as buspirone exert modest antidepressant and anxiolytic effects in animal studies (Detke et al. 1995; Lucki 1991) and the behavioral effects of imperfectly selective agonists, such as 8-OH-DPAT are absent in the 5-HT1A KO mice during the novelty supressed feeding test (Santarelli et al. 2003). However, these ligands bind to both 5-HT1A autoreceptors and heteroreceptors (Yocca 1990), making it difficult to determine which subpopulation mediates specific behavioral effects. Despite this, behavioral models of stress have consistently shown that activation of 5-HT1A heteroreceptors produce similar changes to conventional antidepressants (Lucki 1991) and several preclinical studies have suggested that 5-HT1A heteroreceptors are particularly important to the antidepressant response (Blier and de Montigny 1994; De Vry 1995).
In order to circumvent the limitation of systemic injections, investigators have used localized infusions of 5-HT1A ligands into restricted brain regions in an attempt to selectively activate 5-HT1A autoreceptors or heteroreceptors. For example, it has been reported that infusion of 5-HT1A agonists into the DRN increased social interaction (SI), suggesting that the drug-induced increases in SI reflected decreases in anxiety (Higgins et al. 1992). Furthermore, the 5-HT1A agonist, 8-OH-DPAT, has been acutely injected into restricted brain areas such as MRN and DRN resulting in an anxiolytic action (Andrews et al. 1994; De Almeida et al. 1998; File et al. 1996; Hogg et al. 1994). On the other hand, acute stimulation of the post-synaptic 5-HT1A receptors in the dorsal HP results in an anxiogenic effect in the same tasks in which the knock-outs behave abnormally (Andrews et al. 1994; File and Gonzalez 1996; File et al. 1996; Stefanski et al. 1993). Thus, results from localized infusions suggest that stimulating auto and heteroreceptors may result in opposing phenotypes, a conclusion that would be difficult to reach from systemic administration experiments.
More recently, pharmacological investigation of 5-HT1A receptors has advanced with the availability of a new generation of agonists that preferentially target 5-HT1A subpopulations. Indeed, the novel drug, F15599, exhibits a pronounced preference for activation of 5-HT1A heteroreceptors in the frontal cortex, whereas its chemical congener, F13714 has the opposite profile, potently activating 5-HT1A autoreceptors in the raphe. Data supporting this assertion have been generated in models of c-Fos expression in different brain regions, in electrophysiology tests measuring electrical activity of DRN and pyramidal neurons, and in microdialysis experiments, measuring dopamine release as an index of cortical heteroreceptors activation and 5-HT release as an index of autoreceptor activation (Newman-Tancredi 2011). Both F15599 and F13714 drugs are highly selective for 5-HT1A receptors and their activity in a range of pharmacological models is antagonized by selective 5-HT1A antagonists. The possibility of interactions by F15599 and F13714 at cross-reacting sites can therefore be discounted and the capacity of the drugs to target receptor subpopulations may be attributed to the “biased agonist” profile of these drugs. Indeed, F15599 has a distinctive profile of in vitro signaling in cellular tests of G-protein activation, adenylyl cyclase inhibition, ERK1/2 phosphorylation and receptor internalization (Newman-Tancredi et al. 2009). F15599 showed a marked potency for ERK1/2 phosphorylation whereas other 5-HT1A agonists, such as F13714 and 5-HT, did not discriminate (Newman-Tancredi et al. 2009). Interestingly, preferential stimulation of ERK phosphorylation may lead to improved antidepressant efficacy, because ERK phosphorylation deficits are associated with depressed mood. Indeed, deficits in ERK expression and phosphorylation are seen in post-mortem brain of depressed suicide victims (Dwivedi et al. 2001; Dwivedi et al. 2009). In rat, chronic stress-induced depression elicits deficits in ERK phosphorylation which are fluoxetine-reversible (Qi et al. 2006; Qi et al. 2008). Conversely, chronic administration of a ERK inhibitor elicits anhedonia and anxiety-like behavior (Qi et al. 2009). Consistent with these observations, the potent phosphoryation of ERK1/2 elicited by F15599 in vitro and also demonstrated in ex vivo studies of frontal cortex tissue (Newman-Tancredi et al. 2009) may underlie its antidepressant-like effects of F15599. Indeed, F15599 exhibits antidepressant-like properties in rodent models of mood deficit (FST and ultrasonic vocalization) (Assié et al. 2010), and demonstrates beneficial activity on cognitive function in rats treated with the psychotomimetic drug, phencyclidine (Depoortère et al. 2010).
Taken together, the above observations suggest that availability of highly selective biased agonists should facilitate the pharmacological characterization of the role of 5-HT1A receptor subpopulations.
2.4 Preclinical genetic approaches
In addition to pharmacologic approaches, genetic strategies have also been used to assess 5-HT1A function, initially with transgenic and KO mice and later with techniques capable of regulating the expression of receptors in a tissue-specific and temporally specific manner. In 1998, three different lines of mice lacking the 5-HT1A receptors were generated (Heisler et al. 1998; Parks et al. 1998; Ramboz et al. 1998). In each of the three studies, 5-HT1A knockout mice exhibited an anxiety-like phenotype in behavioral conflict tests such as open field, elevated plus maze, zero maze and novelty-suppressed feeding test, a phenotype that is also present in the heterozygote 5-HT1A receptor knockout mice, that expressed approximately one-half of wild-type receptor density, indicating that a partial receptor deficit is sufficient to elicit the anxious behavior (Ramboz et al. 1998). The impaired performance of 5-HT1A knockout mice is likely due to an enhanced fear response to threatening context, but not due to a deficit in exploratory drive (Klemenhagen et al. 2006). Interestingly, despite the association of 5-HT1A function with depression in humans, 5-HT1A knockout mice did not display a prominent depression-like phenotype. Moreover, 5-HT1A KO mice display increased physiological responses to acute stress (Van Bogaert et al. 2006). However, these behavioural alterations are not correlated with 5-HT or 5-HIAA (5-hydroxyindoleacetic acid, the major 5-HT metabolite) brain tissue levels. Furthermore, microdialysis studies have not shown alterations in basal 5-HT extracelullar levels in 5-HT1A KO mice in different brain areas such as hippocampus, striatum, raphe nuclei and frontal cortex (Bortolozzi et al. 2004; Guilloux et al. 2006, Knobelman et al. 2001). These results demostrate that genetic deletion of 5-HT1A receptors leads to an enhanced anxiety phenotype without affecting 5-HT levels (Ramboz et al. 1998), suggesting either a lack of tonic control of 5-HT1A autoreceptors on nerve terminal 5-HT release, or developmental compensation (see below). Indeed, despite findings that serotonin levels are unchanged, there is evidence to suggest increased 5-HT turnover, indicating increased activity of serotonergic neurons or compensatory changes due to the lack of 5-HT1A receptors (Ase et al. 2000).
Pharmacological studies have also provided insight into the role of 5-HT1A receptors in the regulation of 5-HT levels in KO mice. Indeed, SSRIs increase dialysate 5-HT levels in both the frontal cortex and raphe nuclei areas, but this effect is greater in 5-HT1A KO mice (Bortolozzi et al. 2004; Guilloux et al. 2006; Knobelman et al. 2001), suggesting the absence of an inhibitory feedback control over 5-HT release. Interestingly, 5-HT1A KO mice respond to tricyclic antidepressants (TCAs), but not to the SSRI fluoxetine, in the tail suspension test and the novelty supressed feeding test, suggesting that the 5-HT1A receptors are a critical component in the mechanism of action of SSRIs but not TCAs (Mayorga et al 2001; Santarelli et al. 2003). In contrast to the behavioral changes observed in mice lacking the 5-HT1A receptor, a transgenic line overexpressing the murine 5-HT1A receptor in the central nervous system under control of its endogenous promoter (Kusserow et al. 2004) had reduced anxiety-like behavior, reduced 5-HIAA/5-HT ratio in several brain areas and elevated serotonin levels in the hippocampus and striatum. The behavioural data from this study suggest the opposite phenotype of 5-HT1A knockout mice and an inverse correlation between 5-HT1A receptor levels and anxiety.
Since 5-HT1A receptors can influence anxiety and depression by impacting either 5-HT levels (as an autoreceptor) or the limbic response to released 5-HT (as a heteroreceptor), it is important to understand the role of these receptor populations in maintaining normal levels of anxiety and depression. Gross et al. (2002), using a gain-of-function approach, ectopically expressed 5-HT1A receptors using a vector driven by CamKII promoter. This results in 5-HT1A overexpression in pyramidal excitatory neurons, but not GABAergic interneurons, in forebrain areas such as the cortex, hippocampus, striatum and lateral amygdala, in the absence of autoreceptors. They found that this 5-HT1A receptor expression pattern reversed the increased anxiety behavior in 5-HT1A KO mice, leading to the hypothesis that endogenous 5-HT1A heteroreceptors in the forebrain may control the normal establishment of anxiety-like behavior (Akimova et al. 2009; Goodfellow et al. 2009; Gross and Hen 2004; Zhang et al. 2010). However, it is worth noting that this phenotypic reversal occurs in the context of missing autoreceptors.
More recently we developed another transgenic system to independently assess the function of 5-HT1A autoreceptors and heteroreceptors (Richardson-Jones et al. 2011). This system provides a number of advances over classic KO and previous transgenic technology. This study demonstrated that supression of endogenous heteroreceptors is not sufficient to impact anxiety-like behavior. However loss of autoreceptors impacts anxiety in the adult, suggesting that the anxious-like phenotype of the 5-HT1A KO mouse likely results from increased serotonergic neuron excitability during development (Reviewed below). Furthermore mice lacking 5-HT1A heteroreceptors throughout life displayed decreased mobility in the FST, or increased behavioral despair, in adulthood. Surprisingly, whole brain knockout mice display higher mobility time in the FST and TST, suggesting that the absence of 5-HT1A receptors could result in an “antidepressant-like” effect. In contrast loss of 5-HT1A autoreceptors throughout life did not impact behavior in the FST in adulthood.
These results provided the first direct genetic evidence for distinct roles of the two endogenous receptor populations in mediating anxious or depression like phenotypes, providing evidence that autoreceptors could impact establishment of anxiety-like behaviour, with heteroreceptors affecting behavior in the forced swim test, a depression related test.
3. Developmental requirement of 5-HT1A receptor
The serotonergic system is perfectly poised to play an important role in sculpting the circuitry that subserves anxiety and depression. First, serotonin has clearly been implicated in development at key periods in a number of systems at different developmental timepoints (Benes et al. 2000; Trowbridge et al. 2011; van Kleef et al. 2012). For example, serotonin is known to play a critical role in the development of whisker barrel fields in the somatosensory cortex of the mouse. The critical period for this process appears to be postnatal days 1–6 (Fox 1992). More recently, it has been suggested that the post-synaptic 5-HT1A receptor appears to be critical in the development of the barrel cortex organization (Maya-Vetencourt et al. 2008; Maya-Vetencourt et al. 2011). At a later stage, Nakamura and colleagues have identified TPH1, an enzyme involved in the rate-limiting step for serotonin synthesis as playing a role in the maturation of sensorimotor gating (Nakamura et al. 2006). TPH1 is specifically required during postnatal days 21–24 for proper maturation of the circuit. Thus it is clear that serotonin has distinct, clearly defined roles in circuit maturation at different times during development.
In the case of the 5-HT1A receptor, numerous studies have implicated this receptor in the development of normal anxiety-like behavior (Gross et al. 2002; Heisler et al. 1998; Parks et al. 1998; Ramboz et al. 1998). In particular, there is evidence to suggest that normal expression of the 5-HT1A receptor is required in the 2nd and 3rd week of life for the emergence of normal anxiety, with mice lacking functional 5-HT1A receptors during this time developing pathological levels of anxiety (Gross et al., 2002; Leonardo and Hen, 2008). Furthermore, the same behavioral phenotype is also seen with pharmacological blockade of 5-HT1A receptors during postnatal development (Lo Iacono and Gross 2008). These studies have also demonstrated that disruption of the 5-HT1A receptor in adulthood does not result in an anxiety phenotype, suggesting that the phenotype of the 5-HT1A knockout mouse is due to its absence during a critical developmental window. Therefore, these data firmly establish the opening of a critical window in the second and third postnatal week.
The requirement for 5-HT1A receptors in the third week of life coincides with the emergence of behaviors that are consistent with conflict-based anxiety. Thus, exploration of and habituation to novel environments emerge at this time (Murrin et al. 2007). In addition, postnatal day 21 is the earliest time-point in which behavioral differences in anxiety measures can be detected in the 5-HT1A knockout mice (Kristin Klemenhagen, personal communication). As a result, it is reasonable to assume that 5-HT1A receptors during this time period play a role in establishing the circuits that mediate these behaviors. These results also suggest that by the end of the third week of life in the mouse, circuitry capable of mediating anxiety-like behavior is in place. Indeed, genetic attempts to “rescue” normal levels of anxiety after this p21 period have not been successful (Gross et al. 2002). This data leads to the conclusion that if the circuits do not form properly in the first place, they cannot be rescued later. Similarly, the lack of an anxiety phenotype in mice that do not have 5-HT1A receptors in adulthood suggests that once formed, the circuits are either sufficiently stable to withstand the loss of 5-HT1A receptors, or that 5-HT1A receptors play a different role in adulthood than they do in development.
Given the evidence that the 5-HT1A receptor is important for normal development of mood control, it is reasonable to hypothesize that at least some of these effects could be linked to different 5-HT1A receptor subpopulations. Therefore, defining the stage-specific effects of 5-HT1A autoreceptors and heteroreceptors in the establishment and maturation of circuits that sub-serve anxiety and depression-related behavior in the mouse, would inform hypotheses concerning mechanisms through which variation in 5-HT1A receptor function leads to related phenotypes in humans.
A few studies have attempted to dissect the developmental requirements of 5-HT1A autoreceptors and heteroreceptors. For example, as mentioned above, overexpression of 5-HT1A heteroreceptors after p21 (in the absence of autoreceptors) using a gain of function approach in a knockout background resulted in anxiety levels that are indistiguishable from whole brain knockout animals. Conversely, earlier expression (p15) of the heteroreceptor in the absence of autoreceptors leads to normal anxiety levels in adulthood. These results suggest that normal anxiety-like behaviour in the adult requires the proper establishment of circuitry in the early postnatal period and cannot be rescued later (Gross et al. 2002). Likewise, another report showed, using a loss-of-function approach, that supression of endogenous 5-HT1A autoreceptors throughout life is sufficient to increase anxiety-like behavior in the adult (Richardson-Jones et al. 2011). However, modulating 5-HT1A autoreceptors in adulthood does not impact anxiety-like behavior (Richardson-Jones et al. 2010). While seemingly in conflict regarding the population of receptors involved, these data taken together are consistent with a developmental role for 5-HT1A autoreceptors in the establishment of anxiety-related circuitry (Gross et al. 2002; Lo Iacono and Gross 2008). These findings further suggest that serotonin plays a critical role in the maturation and/or development of circuits that influence the processing of anxiety-related cues in adulthood. Furthermore, these results suggest the opening of a critical window in the second and third postnatal week and that this plasticity no longer remains in adult animals. The question of how long these plastic periods last remains to be answered.
The apparent conflict in the population responsible for the anxious phenotype can be resolved by closely examining the different experimental approaches taken in each experiment and the information that each provides. The 5-HT1A autoreceptor knockout mouse was a loss of function model that looked at the effect of disrupting endogenous receptors: absence of 5-HT1A autoreceptors appears to be the dominant element that determines the phenotype of the constitutive knockout mice. In the case of the heteroreceptor overexpression study, a gain of function approach was used to demonstrate that ectopic/overexpression of 5-HT1A heteroreceptors in the forebrain can normalize anxious behavior in a knockout mouse. In a loss of function approach, another study (Richardson-Jones et al. 2011) demonstrated that suppression of heteroreceptor expression is not sufficient to recapitulate the anxious phenotype of the constitutive 5-HT1A knockout mouse. Thus, the data are most consistent with a loss of autoreceptors resulting in increased anxiety through increased serotonergic signalling from a disinhibited raphe. The gain of function experiment suggests that this anxiety can be rescued in these ‘raphe disinhibited’ mice by increasing signalling through 5-HT1A heteroreceptors which are the major inhibitory serotonin receptor in the forebrain. Thus there is a fine balance between serotonin levels and inhibitory receptors that seems to be established during this early period.
Regarding 5-HT1A heteroreceptors, there is only one study examining the developmental requirement of this receptor (Richardson-Jones et al. 2011). This study showed that supression of this receptor during development leads to an increased behavioral despair in adulthood. In contrast, this phenotype was not observed when heteroreceptor suppression was initiated in adulthood, suggesting that 5-HT1A heteroreceptors act developmentally to establish the circuitry underlying the behavioral response to forced swim stress without affecting conflict based anxiety paradigms (Figure 2). Furthermore, it should be noted that supression of 5-HT1A heteroreceptors results in behavioral despair but not anxiety, while ectopic overexpression in the forebrain of this receptor during development rescues the anxious phenotype of whole-brain 5-HT1A KO mice. Future studies should be directed to the suppression of interneuronal vs. pyramidal 5-HT1A receptors, potentially elucidating their distinct in the etiology of anxiety and depression.
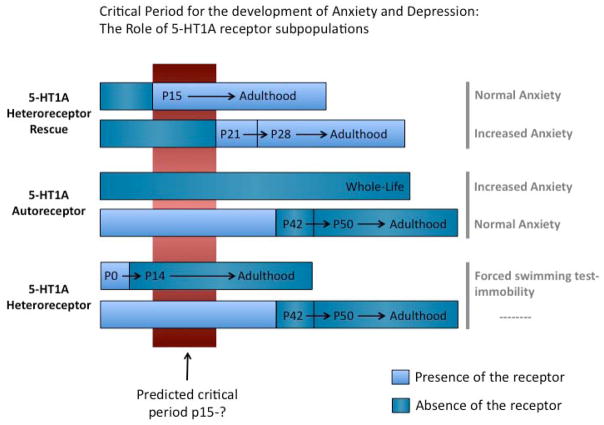
Figure 2.
Figure summarizes data supporting a critical role for 5-HT1A autoreceptors and heteroreceptors in establishing normal anxiety and depressive-like behavior circuits. (top) Transgenic forebrain expression of 5-HT1A heteroreceptors in a knockout background beginning at day 15 is sufficient to rescue normal behavior, while graded re-expression of the heteroreceptor beginning a P21 results in an anxious phenotype (Gross et al. 2002). (Middle) Supression of 5-HT1A autoreceptors throughout life resulted in an increase anxiety-like behavior in the adult. Conversely, loss of endogenous autoreceptors in adulthood is not sufficient to impact anxiety-like behavior (Richardson-Jones et al. 2011). (Bottom) Supression of 5-HT1A heteroreceptors throughout life resulted in an increase immobilty in the forced swim test in the adult. Conversely, loss of endogenous heteroreceptors in adulthood is not sufficient to impact behavior (Richardson-Jones et al. 2011). These results suggest that a critical period exists beginning on the P15. The end of this critical period remains unclear.
In humans it is increasingly accepted that developing circuits are sensitive to environmental insults, with different circuits being sensitive at distinct points in development. In addition, it is increasingly clear that some individuals are more sensitive to these environmental insults than are others. As described above, there is a growing body of literature describing the effiects of a functional polymorphism in the promoter of the human 5-HT1A receptor (Lemonde et al. 2003). The polymorphism C( 1019)G, is thought to result in altered levels of 5-HT1A receptor expression and is thought to moderate susceptibility to stress (Albert and Lemonde 2004). The time course of susceptibility has not been mapped out for this polymorphism. Given the evidence that 5-HT1A receptors are important during normal development in the mouse, at least some of the effects seen from this polymorphism in human populations may be due to alterations in circuit formation that occurred during critical developmental periods.
4. Conclusions
The elucidation of the role of 5-HT1A receptors in the development and/or stabilization of circuitry that mediates emotional behaviors has been complicated by the fact that the receptor exists as two distinct populations, having the dual ability to modulate both global serotonin levels, and local responses to released serotonin. However, various approaches are being used to cast light on the role of 5-HT1A auto-and heteroreceptors in mood disorders. Experimental strategies include pharmacological approaches, using local administration by microinjection or using novel biased agonists targeting receptor subpopulations, and new transgenic approaches that allow independent manipulation of endogenous autoreceptors and heteroreceptors. Results from these new experimental strategies, in conjunction with results from more classic approaches, have provided new perspectives on how 5-HT1A receptors subpopulations differentially influence anxiety and depression.
However, although it is well established that 5-HT1A receptors act developmentally to establish normal anxiety and depressive-like behaviors, the specific mechanisms underlying the developmental role of each subpopulation requires further investigation. In addition, although disruption of 5-HT1A receptor functioning during adulthood does not result in a prolonged anxious or depressive phenotype, the 5-HT1A receptor may still play a role in regulation the plasticity of behavior once circuits mediating anxiety are functional. It is possible that there is a time after the circuits are developed when the system remains plastic and enduring, even permanent changes to be effected by alterations in 5-HT1A receptor function. This may be true for the development of pathological states but also for therapeutic interventions, potentially opening new avenues for improved treatment of debilitating mood deficit disorders
Figure 1.
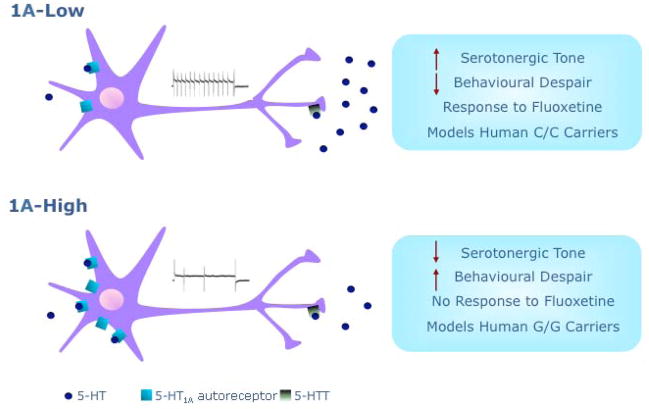
Model of 5-HT1A autoreceptor effects on the serotonergic raphe nuclei. Schematic depicting representative raphe neurons in 1A-High and 1A-Low animals, emphasizing the differences between the two groups. (Top) In 1A-Low mice, low levels of somatodendritic 5-HT1A autoreceptors result in weak negative feedback, resulting in higher firing rates of raphe neurons and concomitant increased release of serotonin. (Bottom) Conversely, 1A-High mice have lower basal firing rate and high levels of somatodendritic 5-HT1A autoreceptor, which exert robust inhibitory effects on raphe firing. This results in a greater behavioral despair in response to stress, compared to 1A-Low mice. While 1A-High mice do not respond behaviorally to treatment with the antidepressant fluoxetine, 1A-Low mice display a robust behavioral response. 1A-High and 1A-Low mice provide a mechanistic model for humans carrying, respectively, the G/G and C/C alleles of the Htr1aI C(-1019)G polymorphism. Adapted from Richardson-Jones et al. 2010.
Acknowledgments
AGG is a recipent of a Spanish Ministry of Science postdoctoral fellowship. EDL is supported through NIH RO1 MH91427. We are grateful to Alex Dranovsky for helpful discussions and critical reading of the manuscript.
References
- aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180(3):305–13. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A, Carceller A, Artigas F. Regional distribution of extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the brain of freely moving rats. J Neurochem. 1991;56:709–12. doi: 10.1111/j.1471-4159.1991.tb08208.x. [DOI] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–35. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;1:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–93. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Albert PR, Le Francois B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234(4781):1261–5. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharmacol. 1994;264(3):259–64. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28(1):199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J Neural Transm. 2007;114:1065–8. doi: 10.1007/s00702-007-0705-9. [DOI] [PubMed] [Google Scholar]
- Arias B, Arranz MJ, Gasto C, Catalan R, Pintor L, Gutierrez B, Kerwin RW, Fananas L. Analysis of structural polymorphisms and C-1018G promoter variant of the 5-HT(1A) receptor gene as putative risk factors in major depression. Mol Psychiatry. 2002;7:930–2. doi: 10.1038/sj.mp.4001146. [DOI] [PubMed] [Google Scholar]
- Arias B, Catalan R, Gasto C, Gutierrez B, Fananas L. Evidence for a combined genetic effect of the 5-HT1A receptor and serotonin transporter genes in the clinical outcome of major depressive patients treated with citalopram. J Psychopharmacol. 2005;19:166–72. doi: 10.1177/0269881105049037. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35:457–63. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Artigas F, Bel N, Casanovas JM, Romero L. Adaptative changes of the serotonergic system after antidepressant treatments. Adv Exp Med Biol. 1996;398:51–9. doi: 10.1007/978-1-4613-0381-7_6. [DOI] [PubMed] [Google Scholar]
- Artigas F, Adell A, Celada P. Pindolol augmentation of antidepressant response. Curr Drug Targets. 2006;7:139–47. doi: 10.2174/138945006775515446. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem. 2000;75(6):2415–26. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. F15599, a highly selective post-synaptic 5-HT(1A) receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol. 2010 Nov;13(10):1285–98. doi: 10.1017/S1461145709991222. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–3. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012 Jan;35(1):85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bartoszyk GD, Hegenbart R, Ziegler H. EMD 68843, a serotonin reuptake inhibitor with selective presynaptic 5-HT1A receptor agonistic properties. Eur J Pharmacol. 1997;322(2–3):147–53. doi: 10.1016/s0014-2999(96)00999-5. [DOI] [PubMed] [Google Scholar]
- Beck SG, Choi KC, List TJ. Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther. 1992;263:350–9. [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000 Oct;10(10):1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–96. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci. 1983;3:1270–8. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Tardif D. Short-term lithium treatment enhances responsiveness of postsynaptic 5-HT1A receptors without altering 5-HT autoreceptor sensitivity: an electrophysiological study in the rat brain. Synapse. 1987;1:225–32. doi: 10.1002/syn.890010302. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–6. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003 Mar;13(2):57–66. doi: 10.1016/s0924-977x(02)00173-6. 2003. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–42. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Amargos-Bosch M, Toth M, Artigas F, Adell A. In vivo efflux of serotonin in the dorsal raphe nucleus of 5-HT1A receptor knockout mice. J Neurochem. 2004;88:1373–9. doi: 10.1046/j.1471-4159.2003.02267.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Castañé A, Semakova J, Santana N, Alvarado G, Cortés R, Ferrés-Coy A, Fernández G, Carmona MC, Toth M, Perales JC, Montefeltro A, Artigas F. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol Psychiatry. 2012 Jun;17(6):612–23. doi: 10.1038/mp.2011.92. 2012. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–53. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Vilaro MT, Mengod G, Artigas F. Differential regulation of somatodendritic serotonin 5-HT1A receptors by 2-week treatments with the selective agonists alnespirone (S-20499) and 8-hydroxy-2-(Di-n-propylamino)tetralin: microdialysis and autoradiographic studies in rat brain. J Neurochem. 1999;72:262–72. doi: 10.1046/j.1471-4159.1999.0720262.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–13. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A Receptors as Targets for Agents to Treat Psychiatric Disorders: Rationale and Current Status of Research. CNS Drugs. 2013 Jun 12; doi: 10.1007/s40263-013-0071-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl 14):11–4. [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT1 binding sites in depressed suicides. Psychopharmacology (Berl) 1990;102:544–8. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- Choi WS, Lee BH, Yang JC, Kim YK. Association Study between 5-HT1A Receptor Gene C(-1019)G Polymorphism and Panic Disorder in a Korean Population. Psychiatry Investig. 2010;7:141–6. doi: 10.4306/pi.2010.7.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Archives of internal medicine. 2004;164:1197–204. doi: 10.1001/archinte.164.11.1197. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529–42. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Vialou V, Nestler EJ. From synapse to nucleus: novel targets for treating depression. Neuropharmacology. 2010;58(4–5):683–93. doi: 10.1016/j.neuropharm.2009.12.004. Epub 2009 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006;26:1864–71. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesak M, Le François B, Millar AM, Deria M, Daigle M, Visvader JE, Anisman H, Albert PR. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J Biol Chem. 2012 Feb 24;287(9):6615–27. doi: 10.1074/jbc.M111.293027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ. The role of 5-HT(1A) and 5-HT(1B/1D) receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: the mechanism of action of (+/-)pindolol. Neuropharmacology. 2000;39:1044–52. doi: 10.1016/s0028-3908(99)00192-6. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, Charchat H, Lucion AB. 8-OH-DPAT in the median raphe nucleus decreases while in the medial septal area it may increase anxiety in female rats. Neurosci Biobehav Rev. 1998;23(2):259–64. doi: 10.1016/s0149-7634(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol. 2010 Sep;20(9):641–54. doi: 10.1016/j.euroneuro.2010.04.005. Epub 2010 May 21. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl) 1995 Sep;121(1):1–26. doi: 10.1007/BF02245588. Review. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Wieland S, Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berl) 1995;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, Hohoff C, Kersting A, Engelien A, Arolt V, Heindel W, Deckert J. Association of the functional -1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol. 2006;9:349–55. doi: 10.1017/S1461145705005869. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–77. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56(3):140–5. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Durant C, Christmas D, Nutt D. The pharmacology of anxiety. Curr Top Behav Neurosci. 2010;2:303–30. doi: 10.1007/7854_2009_8. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the postmortem brain of depressed suicide subjects. J Neurochem. 2001 May;77(3):916–28. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1) Int J Neuropsychopharmacol. 2009 Nov;12(10):1337–54. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Blier P. Responsiveness of 5-HT(1A) and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry Neurosci. 2005;30:268–74. [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16(15):4810–5. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphé and ventral hippocampus. Pharmacol Biochem Behav. 1996 May;54(1):123–8. doi: 10.1016/0091-3057(95)02108-6. 1996. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–53. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–38. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganasen KA, Stein DJ. Pharmacotherapy of social anxiety disorder. Curr Top Behav Neurosci. 2010;2:487–503. doi: 10.1007/7854_2009_1. [DOI] [PubMed] [Google Scholar]
- Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajós M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115(6):1064–70. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci. 2009;29:10094–103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12(9):667–87. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–63. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–52. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, David DJ, Guiard BP, Chenu F, Reperant C, Toth M, Bourin M, Gardier AM. Blockade of 5-HT1A receptors by (+/−)-pindolol potentiates cortical 5-HT outflow, but not antidepressant-like activity of paroxetine: microdialysis and behavioral approaches in 5-HT1A receptor knockout mice. Neuropsychopharmacology. 2006;31:2162–72. doi: 10.1038/sj.npp.1301019. [DOI] [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jönsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, Van den Bergh P, van Os J, Vos P, Xu W, Wittchen HU, Jönsson B, Olesen J CDBE2010 Study Group. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718–79. doi: 10.1016/j.euroneuro.2011.08.008. Epub 2011 Sep 15. Erratum in: Eur Neuropsychopharmacol. 2012 Mar;22(3):237–8. den Bergh, Peter Van [corrected to Van den Bergh, Peter] [DOI] [PubMed] [Google Scholar]
- Hajos M, Hajos-Korcsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–50. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Gozlan H, el Mestikawy S, Emerit MB, Bolanos F, Schechter L. The central 5-HT1A receptors: pharmacological, biochemical, functional, and regulatory properties. Ann N Y Acad Sci. 1990;600:114–29. doi: 10.1111/j.1749-6632.1990.tb16877.x. discussion 129–31. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–54. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas I, Queiroz CM, Adell A, Artigas F. Role of uptake inhibition and autoreceptor activation in the control of 5-HT release in the frontal cortex and dorsal hippocampus of the rat. Br J Pharmacol. 2000;130:160–6. doi: 10.1038/sj.bjp.0703297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, An SS, van den Oord EJ, Neale MC, Kendler KS, Chen X. Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:661–6. doi: 10.1002/ajmg.b.30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR. Effect of 5-HT1A receptor agonists in two models of anxiety after dorsal raphe injection. Psychopharmacology (Berl) 1992;106(2):261–7. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–76. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–86. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Auerbach SB. Further evidence for the importance of 5-HT1A autoreceptors in the action of selective serotonin reuptake inhibitors. Eur J Pharmacol. 1994;260(2–3):251–5. doi: 10.1016/0014-2999(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Milano S. Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or beta-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. Eur J Pharmacol. 1996;316(1):43–7. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- Hogg S, Andrews N, File SE. Contrasting behavioural effects of 8-OH DPAT in the dorsal raphé nucleus and ventral hippocampus. Neuropharmacology. 1994;33(3–4):343–8. doi: 10.1016/0028-3908(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Martin G. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–28. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, Brodsky B, Arango V, Brent DA, Mann JJ. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–51. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Vanderluit JL, Slack RS, Albert PR. HES1 regulates 5-HT1A receptor gene transcription at a functional polymorphism: essential role in developmental expression. Mol Cell Neurosci. 2008 Jul;38(3):349–58. doi: 10.1016/j.mcn.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12(6):522–43. doi: 10.1038/sj.mp.4001920. Epub 2006 Dec 12. [DOI] [PubMed] [Google Scholar]
- Jonnakuty C, Gragnoli C. What do we know about serotonin? J Cell Physiol. 2008;217:301–306. doi: 10.1002/jcp.21533. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8:315–36. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–11. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Blendy JA, Lucki I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine(1A) or 5-hydroxytryptamine(1B) receptor in the mouse. J Pharmacol Exp Ther. 2001;298(3):1092–100. [PubMed] [Google Scholar]
- Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–86. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- Kusserow H, Davies B, Hortnagl H, Voigt I, Stroh T, Bert B, Deng DR, Fink H, Veh RW, Theuring F. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res. 2004;129:104–16. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Lacivita E, Leopoldo M, Berardi F, Perrone R. 5-HT1A receptor, an old target for new therapeutic agents. Curr Top Med Chem. 2008;8(12):1024–34. doi: 10.2174/156802608785161385. [DOI] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight Pharmacol., Biochem. Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61(9):1081–9. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–85. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–22. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7:501–6. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33:134–40. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol. 2004;7:381–5. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- Levin GM, Bowles TM, Ehret MJ, Langaee T, Tan JY, Johnson JA, Millard WJ. Assessment of human serotonin 1A receptor polymorphisms and SSRI responsiveness. Mol Diagn Ther. 2007;11:155–60. doi: 10.1007/BF03256237. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu YF, Ghahremani MH, Rasenick MM, Jakobs KH, Albert PR. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Galphai protein expression. Gi subtype specificity of the 5-HT1A receptor. J Biol Chem. 1999;274:16444–16450. doi: 10.1074/jbc.274.23.16444. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–7. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Ann N Y Acad Sci. 1997;836:106–34. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- Lucki I. Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry. 1991;52(Suppl):24–31. [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci. 2001;21:2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Palego L, Giromella A, Mazzoni MR, Borsini F, Mayer N, Naccarato AG, Lucacchini A, Cassano GB. Region-dependent effects of flibanserin and buspirone on adenylyl cyclase activity in the human brain. Int J Neuro- psychopharmacol. 2002;5:131–140. doi: 10.1017/S1461145702002869. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–20. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect. 1991;85:181–94. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001 Sep;298(3):1101–7. [PubMed] [Google Scholar]
- McAllister-Williams RH, Massey AE. EEG effects of buspirone and pindolol: a method of examining 5-HT1A receptor function in humans. Psychopharmacology (Berl) 2003 Mar;166(3):284–93. doi: 10.1007/s00213-002-1339-0. Epub 2003 Feb 13. [DOI] [PubMed] [Google Scholar]
- McEwen B, Chao H, Spencer R, Brinton R, Macisaac L, Harrelson A. Corticosteroid receptors in brain: relationship of receptors to effects in stress and aging. Ann N Y Acad Sci. 1987;512:394–401. doi: 10.1111/j.1749-6632.1987.tb24975.x. [DOI] [PubMed] [Google Scholar]
- Meller E, Goldstein M, Bohmaker K. Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol. 1990;37:231–7. [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Molina E, Cervilla J, Rivera M, Torres F, Bellon JA, Moreno B, King M, Nazareth I, Gutierrez B. Polymorphic variation at the serotonin 1-A receptor gene is associated with comorbid depression and generalized anxiety. Psychiatr Genet. 2011;21:195–201. doi: 10.1097/YPG.0b013e3283457a48. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110(3):406–25. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–36. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H, Hirose S. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 2006;26:530–4. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193(3):229–34. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Neff CD, Abkevich V, Packer JC, Chen Y, Potter J, Riley R, Davenport C, DeGrado Warren J, Jammulapati S, Bhathena A, Choi WS, Kroeger PE, Metzger RE, Gutin A, Skolnick MH, Shattuck D, Katz DA. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry. 2009;14:621–30. doi: 10.1038/mp.2008.8. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Comorbidity of mood and anxiety disorders: the rule, not the exception? Am J Psychiatry. 2002;159:3–4. doi: 10.1176/appi.ajp.159.1.3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. Epub 2006 Mar 29. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–91. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009 Jan;156(2):338–53. doi: 10.1111/j.1476-5381.2008.00001.x. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A. Biased agonism at serotonin 5-HT1A receptors: preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry. 2011 Apr;1(2):149–164. [Google Scholar]
- Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders-results from in vivo imaging studies. Rev Neurosci. 2010;21(2):119–39. doi: 10.1515/revneuro.2010.21.2.119. Review. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Organization WH. Mental Health Disorders Management: Depression. 2011 http://wwwwhoint/mental_health/management/depression/definition/en/
- Palchaudhuri M, Flugge G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005;321:159–72. doi: 10.1007/s00441-005-1112-x. [DOI] [PubMed] [Google Scholar]
- Page ME, Cryan JF, Sullivan A, Dalvi A, Saucy B, Manning DR, Lucki I. Behavioral and neurochemical effects of 5-(4-[4-(5-Cyano-3-indolyl)-butyl)-butyl]-1-piperazinyl)-benzofuran-2-carboxamide (EMD 68843): a combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine(1A) receptor partial agonist. J Pharmacol Exp Ther. 2002;302(3):1220–7. doi: 10.1124/jpet.102.034280. [DOI] [PubMed] [Google Scholar]
- Palego L, Giromella A, Marazziti D, Borsini F, Naccarato AG, Giannaccini G, Lucacchini A, Cassano GB, Mazzoni MR. Effects of postmortem delay on serotonin and (+)8-OH- DPAT-mediated inhibition of adenylyl cyclase activity in rat and human brain tissues. Brain Res. 1999;816:165–174. doi: 10.1016/s0006-8993(98)01156-1. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–9. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–9. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Parsey RV. Serotonin receptor imaging: clinically useful? J Nucl Med. 2010:1495–8. doi: 10.2967/jnumed.109.068908. Epub 2010 Sep 16. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–8. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, Pinto E, Reggers J, Fuchs S, Ansseau M. 5-Hydroxytryptamine 1A receptors, major depression, and suicidal behavior. Biol Psychiatry. 2005 Dec 1;58(11):854–8. doi: 10.1016/j.biopsych.2005.05.042. Epub 2005 Sep 1. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12(2):440–53. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portella MJ, de Diego-Adeliño J, Ballesteros J, Puigdemont D, Oller S, Santos B, Álvarez E, Artigas F, Pérez V. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect?. A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry. 2011;72(7):962–9. doi: 10.4088/JCP.09m05827blu. Epub 2010 Oct 19. [DOI] [PubMed] [Google Scholar]
- Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res. 2006 Dec 15;175(2):233–40. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008 Aug;31(2):278–85. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Qi X, Lin W, Wang D, Pan Y, Wang W, Sun M. A role for the extracellular signal-regulated kinase signal pathway in depressive-like behavior. Behav Brain Res. 2009 May 16;199(2):203–9. doi: 10.1016/j.bbr.2008.11.051. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–81. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;71(7):839–54. doi: 10.4088/JCP.10r06218blu. Review. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–94. [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–18. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Clarke T, Sprouse JS, Schulz DW. Combined administration of a 5-hydroxytryptamine 5-HT1D antagonist and a 5-HT reuptake inhibitor synergistically increases 5-HT release in guinea pig hypothalamus in vivo. J Neurochem. 1996;67:2204–7. doi: 10.1046/j.1471-4159.1996.67052204.x. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Jacob C, Rietschel M, Nothen MM, Garritsen H, Fimmers R, Deckert J, Lesch KP. Association of a functional 1019C>G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia. Int J Neuropsychopharmacol. 2004;7:189–92. doi: 10.1017/S1461145703004061. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Rush AJ. The use of placebos in unipolar major depression: the current status. Biol Psychiatry. 2000;47:745–7. doi: 10.1016/s0006-3223(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Leonardo ED, Gadient R, Williams A, Zhou J, David DJ, Gardier AM, Wong EH, Hen R. Modeling treatment-resistant depression. Neuropharmacology. 2011 Sep;61(3):408–13. doi: 10.1016/j.neuropharm.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–9. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Serretti A, Artioli P, Lorenzi C, Pirovano A, Tubazio V, Zanardi R. The C(-1019)G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol. 2004;7:453–60. doi: 10.1017/S1461145704004687. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–36. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30(11):563–9. doi: 10.1016/j.tips.2009.09.002. Review. [DOI] [PubMed] [Google Scholar]
- Sorbera LA, Rabasseda X, Silvestre J, Castaner J. Vilazodone hydrochloride–Antidepressant–5-HT1A partial agonist–5-HT reuptake inhibitor. Drugs Future. 2001;26:247–252. [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, Moser U, Holik A, Pezawas L, Kletter K, Kasper S. Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2009;14:1040–50. doi: 10.1038/mp.2008.35. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (-)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–8. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Stefański R, Pałejko W, Bidziński A, Kostowski W, PłaŸnik A. Serotonergic innervation of the hippocampus and nucleus accumbens septi and the anxiolytic-like action of midazolam and 5-HT1A receptor agonists. Neuropharmacology. 1993;32(10):977–85. doi: 10.1016/0028-3908(93)90062-8. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Haycock JW, Thompson PA, Lowy MT. Quantitative subregional distribution of serotonin1A receptors and serotonin transporters in the human dorsal raphe. Brain Res. 1996;727:1–12. doi: 10.1016/0006-8993(96)00239-9. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;15;18(18):7394–401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, Brocke B, Lesch KP. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Trowbridge S, Narboux-Neme N, Gaspar P. Genetic models of serotonin (5-HT) depletion: What do they tell us about the developmental role of 5-HT? Anat Rec. 2011;294:1615–1623. doi: 10.1002/ar.21248. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdizan EM, Castro E, Pazos A. Agonist- dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuro- psychopharmacol. 2010;13:835–843. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Van Bogaert M, Oosting R, Toth M, Groenink L, van Oorschot R, Olivier B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: modulation by serotonergic and GABAA-ergic drugs. Eur J Pharmacol. 2006;550:84–90. doi: 10.1016/j.ejphar.2006.08.058. [DOI] [PubMed] [Google Scholar]
- van Kleef ES, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development Eur. J Neurosci. 2012;35:1563–1572. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–59. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–4. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Inhibiton of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway. Brain Res. 1977;120:85–102. doi: 10.1016/0006-8993(77)90499-1. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation Pharmacol. Rev. 2012;64:359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocca FD. Neurochemistry and neurophysiology of buspirone and gepirone: interactions at presynaptic and postsynaptic 5-HT1A receptors. J Clin Psychopharmacol. 1990;10:6S–12S. doi: 10.1097/00004714-199006001-00003. [DOI] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173–7. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Liou YJ, Hong CJ, Chen TJ. Association study of two serotonin 1A receptor gene polymorphisms and fluoxetine treatment response in Chinese major depressive disorders. Eur Neuropsychopharmacol. 2006;16:498–503. doi: 10.1016/j.euroneuro.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, Wu DL, Zhu LJ, Zhu DY. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J Neurosci. 2010;30:2433–41. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]