Abstract
We examined racial differences in gout incidence among black and white participants in a longitudinal, population-based cohort and tested whether racial differences were explained by higher levels of serum urate. The Atherosclerosis Risk in Communities Study is a prospective, US population–based cohort study of middle-aged adults enrolled between 1987 and 1989 with ongoing annual follow-up through 2012. We estimated the adjusted hazard ratios and 95% confidence intervals of incident gout by race among 11,963 men and women using adjusted Cox proportional hazards models. The cohort was 23.6% black. The incidence rate of gout was 8.4 per 10,000 person-years (15.5/10,000 person-years for black men, 12.0/10,000 person-years for black women, 9.4/10,000 person-years for white men, and 5.0/10,000 person-years for white women; P < 0.001). Black participants had an increased risk of incident gout (for women, adjusted hazard ratio (HR) = 1.69, 95% confidence interval (CI): 1.29, 2.22; for men, adjusted HR = 1.92, 95% CI: 1.44, 2.56). Upon further adjustment for uric acid levels, there was modest attenuation of the association of race with incident gout (for women, adjusted HR = 1.62, 95% CI: 1.24, 2.22; for men, adjusted HR = 1.49, 95% CI: 1.11, 2.00) compared with white participants. In this US population–based cohort, black women and black men were at increased risk of developing gout during middle and older ages compared with whites, which appears, particularly in men, to be partly related to higher urate levels in middle-aged blacks.
Keywords: gout, inflammatory arthritis, race, uric acid
Gout is a leading form of inflammatory arthritis, affecting an estimated 8 million US adults and accounting for almost 4 million outpatient visits every year (1, 2). Gout is characterized by severe joint pain and can lead to irreversible joint damage if untreated (3). This inflammatory arthropathy causes a substantial economic burden (4, 5) and is associated with a poor overall quality of life (6, 7). Gout has been considered a disease of men because the prevalence of gout in men is more than twice that of women (8). Most epidemiologic studies of gout incidence have focused on white men and health care professionals (9–13) and have been limited in geographical and racial diversity (14).
However, women and men, regardless of race, experience gout (14–16). Cross-sectional, nationally representative data suggest that the prevalence of gout is 5.0% in blacks compared with 4.0% in whites (1). One previous prospective study that was not population based demonstrated a 2-fold excess risk of gout in black male physicians compared with their white counterparts; however, the difference in risk was attributed to differences in hypertension status (13). Racial differences in gout incidence have not been studied in a population-based cohort. As the prevalence and incidence of gout continue to increase in both women and men (17, 18), there is a greater need to quantify the risk of gout in men and women of both white and black races. However, it is unclear whether there are differences in the incidence of gout associated with race and whether such differences are attributable to variation in gout risk factors.
The goal of this study was to establish whether there are racial differences in the incidence of gout independent of known risk factors in a US population–based setting by using data from the Atherosclerosis Risk in Communities (ARIC) Study. Additionally, we explored whether the observed association of race and gout was explained by racial differences in serum urate levels.
METHODS
Study population and study design
The ARIC Study is an ongoing, prospective US population–based cohort of 15,792 persons aged 45–64 years. Participants were selected from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). Although the Jackson, Mississippi, site recruited only black participants, black participants were recruited from all sites.
Participants took part in examinations starting with a baseline visit (in 1987–1989) and 3 follow-up visits (visit 2 in 1990–1992, visit 3 in 1993–1995, and visit 4 in 1996–1998). At cohort entry, participants' sociodemographic characteristics, smoking status, alcohol-drinking habits, medication use, and medical history were assessed through a home interview. Details of the ARIC Study cohort have been published elsewhere (19). Participants were contacted annually as part of the annual follow-up through 2012. Institutional review boards of the participating institutions approved the study protocols. All study participants provided written informed consent.
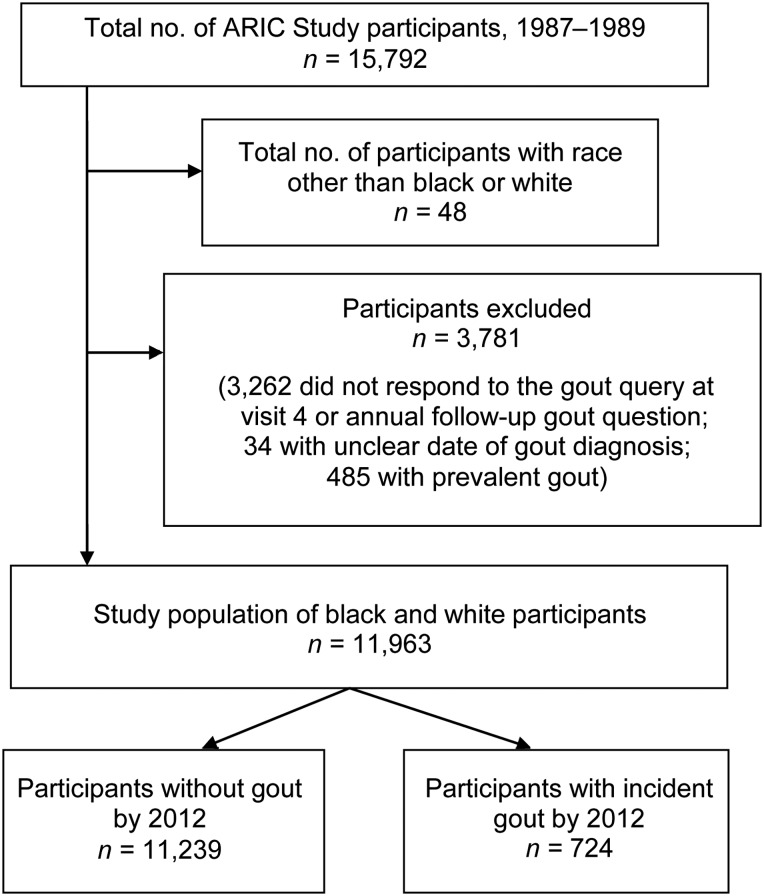
The present study used data from ARIC Study participants who self-reported white or black race, responded to the gout query at visit 4 or during annual follow-up, reported a date of gout onset, and were free of gout at cohort entry, as detailed in Figure 1. There were 11,963 participants included in this analysis.
Figure 1.
Flowchart of Atherosclerosis Risk in Communities (ARIC) Study (United States, 1989–2012) participants showing the number of participants excluded for the analysis of race and gout.
Assessment of gout
Incident gout was defined on the basis of self-reports of physician-diagnosed gout at visit 4 or the annual follow-up contact in 2011–2012; this was the only annual follow-up contact that included the gout query. Only participants who reported onset after cohort entry were considered incident cases of gout. Self-reports of physician diagnoses of gout have been shown to be a reliable and a sensitive measure of gout (20).
Assessment of race and covariates
All covariates were assessed at cohort entry by using standard protocols (19, 21). Baseline covariates were included in this analysis to represent gout risk factors in middle age. All participants self-reported race from the following options: Asian, black, American Indian, or white. Body mass index (weight (kg)/height (m)2) (22) and blood pressure (23) were measured according to published methods. A second anthropometric measure, waist-to-hip ratio, was similarly measured. Hypertension was defined as measured systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of a medication to treat hypertension. Trained interviewers collected information on the medications that participants used in the 2 weeks prior to the visit, including diuretic use. Participants reported their educational level, alcohol use, weight at age 25 years, and smoking status.
Diet was assessed with an interviewer-administered 66-item semiquantitative food frequency questionnaire based on the validated Willett 61-item questionnaire (24). The reproducibility of this dietary questionnaire in this cohort has been previously reported (25). We focused on intakes of animal protein, organ meat, shellfish, and alcohol as dietary covariates of interest. Alcohol intake was quantified as the amount of regular intake per week.
Central laboratories performed analyses on baseline fasting specimens by using conventional assays to obtain serum uric acid, glucose, and creatinine values (26). Uric acid was measured by the uricase method (27) and standardized across laboratories and visits. The reliability coefficient of uric acid was 0.91, and the within-person variability was 7.2 (28). Diabetes mellitus was defined as a fasting glucose level of 126 mg/dL or greater, a nonfasting glucose level of 200 mg/dL or greater, a reported physician diagnosis of diabetes, or a history of medication use for diabetes. Serum creatinine was measured by using a modified kinetic Jaffé reaction. Estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation (29, 30). Serum creatinine concentration was corrected for interlaboratory differences and calibrated to the Cleveland Clinic measurement (31, 32). We classified participants into 3 groups on the basis of the following thresholds of eGFR (mL/minute 1.73 m2): ≥90 (stage 1, normal kidney function), 60–89 (stage 2, mild reduced kidney function), or <60 (stage 3 or higher, reduced kidney function).
Coronary heart disease was defined by the presence of any of the following observations or procedures: adjudicated myocardial infarction by electrocardiography or self-report of myocardial infarction, heart or arterial surgery, coronary bypass, balloon angioplasty, or angioplasty of coronary arteries. Heart failure was defined by the use of a medication for heart failure or fulfillment of the Gothenburg criteria (33).
Statistical analysis
We estimated hazard ratios and 95% confidence intervals by using Cox proportional hazards modeling with age as the time scale and Efron approximation for ties (34). Sex-specific models were sequentially adjusted for confounders. The 4 core models, each of which included age in years as the time scale, were as follows: model 1, unadjusted (with age in years as the time scale); model 2, adjusted for body mass index, protein intake, organ meat intake, shellfish intake, alcohol intake, income, educational level, cigarette use, diabetes, renal function, and menopausal status (for women); model 3, adjusted for the parameters in model 2 and hypertension, diuretic use, waist-to-hip ratio, and change in weight from age 25 years to cohort entry; and model 4, adjusted for the parameters in model 3 and uric acid level to test for mediation. We tested whether an interaction between race and sex was statistically significant using the Wald test of the interaction term in a model that included both men and women. Additionally, more than 10% of income and alcohol intake data were missing at cohort entry, and the missing values were imputed by using multiple imputation. Assumptions of the Cox proportional hazards model were confirmed by visual inspection of the complementary log-log plots in the sex-stratified models; although the cumulative incidence curves crossed for white men and black women, this did not violate the proportional hazards assumption because all models were stratified by sex.
Statistical analysis: race and uric acid levels
Additionally, we tested whether mean serum urate (measured at cohort entry) differed by race for men and women by using linear regression with adjustment for confounders (model 3). All analyses were conducted using Stata SE, version 12.1, software (StataCorp LP, College Station, Texas). All reported P values are 2-sided.
Sensitivity analyses
We tested the sensitivity of our results by using the following procedures: 1) adjustment for time-varying covariates (body mass index, hypertension, diuretic use, alcohol intake, smoking status, and eGFR); 2) modification of incident case definition to participants who self-reported gout and additionally reported taking a medication used to treat gout (allopurinol, probenecid, or colchicine); 3) exclusion of those with baseline hyperuricemia; 4) exclusion of those with gout diagnosed within 2 years of enrollment; and 5) adjustment for urate levels measured at visit 2. Additionally, we tested the association of race and incident gout in participants in the Forsyth County, North Carolina, site, which had the highest number of black participants outside Jackson, Mississippi, to ensure that our results were not driven by geographical differences.
RESULTS
Participant characteristics
The study population included 11,963 black or white participants (Figure 1); 23.6% were black. For both women and men, black participants were, on average, younger (for women, mean age = 52.7 vs. 53.6 years, P < 0.001; for men, mean age = 52.8 vs. 54.3 years, P < 0.001), more likely to be obese (for women, 46.5% vs. 22.0%, P < 0.001; for men, 27.2% vs. 20.8%, P < 0.001), more likely to have had greater weight gain between age 25 years and cohort entry (for women, mean weight gain = 47.4 vs. 26.8 lbs, P < 0.001; for men, mean weight gain = 25.1 vs. 21.4 lbs, P < 0.001) (1 lb = 0.45 kg), more likely to have family incomes of less than $16,000/year (for women, 53.8% vs. 13.2%, P < 0.001; for men, 35.8% vs. 6.6%, P < 0.001), more likely to have less than high school education (for women, 35.8% vs. 13.9%, P < 0.001; for men, 36.5% vs. 15.5%, P < 0.001), and more likely to have chronic medical conditions (Table 1) than white participants. However, white participants were less likely to have normal kidney function (for women, 57.1% vs. 74.8%, P < 0.001; for men, 49.9% vs. 63.6%, P < 0.001).
Table 1.
Characteristics by Race and Sex of 11,963 Participants in the ARIC Study at Cohort Entry, 1987–1989
| Risk Factor | Women |
Men |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White (n = 5,009) |
Black (n = 1,852) |
P Value | White (n = 4,134) |
Black (n = 968) |
P Value | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |||
| Age, years | 53.6 (5.6) | 52.7 (5.7) | <0.001 | 54.3 (5.7) | 52.8 (5.8) | <0.001 | ||||
| Adiposity | ||||||||||
| Obese | 22.0 | 46.5 | <0.001 | 20.8 | 27.2 | <0.001 | ||||
| Body mass indexa | 26.5 (5.3) | 30.5 (6.3) | <0.001 | 27.3 (3.9) | 27.6 (4.5) | 0.04 | ||||
| Waist-to-hip ratio | 0.89 (0.08) | 0.90 (0.08) | <0.001 | 0.97 (0.05) | 0.93 (0.05) | <0.001 | ||||
| Weight change, lbsb | 26.8 (25) | 47.4 (32) | <0.001 | 21.4 (23) | 25.1 (29) | <0.001 | ||||
| Dietary intakes | ||||||||||
| Protein, g/day | 52.9 (24) | 51.2 (22) | 0.01 | 56.0 (24) | 55.2 (24) | 0.40 | ||||
| Organ meat, >2 servings/month | 7.6 | 24.6 | <0.001 | 9.3 | 28.3 | <0.001 | ||||
| Shellfish, >1 serving/week | 7.1 | 12.3 | <0.001 | 9.0 | 15.2 | <0.001 | ||||
| Alcohol, drinks/day | 0.4 (0.7) | 0.5 (0.9) | 0.01 | 1.0 (1.3) | 1.3 (1.8) | <0.001 | ||||
| Smoking status | <0.001 | <0.001 | ||||||||
| Current | 21.6 | 22.4 | 21.5 | 32.1 | ||||||
| Former | 25.1 | 17.9 | 48.3 | 34.4 | ||||||
| Never | 53.3 | 59.7 | 30.2 | 33.5 | ||||||
| Family income | <0.001 | <0.001 | ||||||||
| <$16,000 | 13.2 | 53.8 | 6.6 | 35.8 | ||||||
| $16,000–$24,999 | 15.3 | 19.0 | 11.6 | 19.8 | ||||||
| $25,000–$34,999 | 20.2 | 12.5 | 19.9 | 15.7 | ||||||
| ≥$35,000 | 51.3 | 14.7 | 61.9 | 28.7 | ||||||
| Education | <0.001 | <0.001 | ||||||||
| <12 years | 13.9 | 35.8 | 15.5 | 36.5 | ||||||
| 12–16 years | 51.5 | 31.2 | 39.6 | 27.0 | ||||||
| 17–21 years | 34.6 | 33.0 | 44.9 | 36.5 | ||||||
| Pre- or perimenopausal | 33.0 | 24.0 | <0.001 | |||||||
| Chronic conditions | ||||||||||
| Coronary heart disease | 1.1 | 1.9 | 0.01 | 6.4 | 3.3 | <0.001 | ||||
| Congestive heart failure | 3.7 | 6.6 | <0.001 | 1.9 | 2.3 | 0.36 | ||||
| Hypertension | 23.6 | 53.0 | <0.001 | 25.0 | 46.9 | <0.001 | ||||
| Diabetes mellitus | 6.3 | 15.2 | <0.001 | 8.0 | 12.9 | <0.001 | ||||
| Categorical eGFRc | <0.001 | <0.001 | ||||||||
| ≥90 | 57.0 | 74.8 | 49.9 | 63.6 | ||||||
| 60–89 | 40.8 | 23.2 | 48.4 | 34.1 | ||||||
| <60 | 2.2 | 2.0 | 1.7 | 2.3 | ||||||
| Diuretic use | 15.9 | 28.3 | <0.001 | 9.0 | 16.3 | <0.001 | ||||
| Uric acid, mg/dL | 4.4 (1.3) | 4.9 (1.4) | <0.001 | 5.8 (1.3) | 6.0 (1.5) | <0.001 | ||||
Abbreviations: ARIC, Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; SD, standard deviation.
a Weight (kg)/height (m)2.
b Change in weight from age 25 years to cohort entry; 1 lb = 0.45 kg.
c eGFR is measured as mL/minute / 1.73 m2.
Race and gout
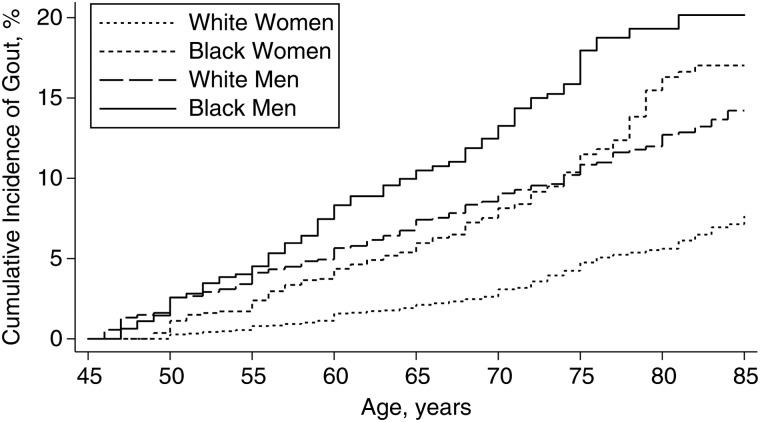
The overall incidence rates of gout were 8.4 per 10,000 person-years in the cohort; 12.0 per 10,000 person-years for black women, 5.0 per 10,000 person-years for white women, 15.5 per 10,000 person-years for black men, and 9.4 per 10,000 person-years for white men (P < 0.001). However, the cumulative incidence for black women was higher than that for white men in older age (Figure 2).
Figure 2.
Cumulative incidence of gout between 1987 and 2012 in the US-based Atherosclerosis Risk in Communities Study by race and sex. The cumulative incidence of gout by age was plotted using the Kaplan Meier method according to race and sex strata. The log-rank P value was less than 0.001.
Black participants were at increased risk of developing gout after adjustment for demographic, dietary, clinical, and socioeconomic confounders (Table 2) (for women, hazard ratio (HR) = 1.69, 95% confidence interval (CI): 1.29, 2.22; for men, HR = 1.92, 95% CI: 1.44, 2.56). After further adjustment for uric acid levels, there was some attenuation, particularly among men, of the measures of association with gout incidence, although the results remained statistically significant (in model 4, for women, HR = 1.62, 95% CI: 1.24, 2.12; for men, HR = 1.49, 95% CI: 1.11, 2.00), suggesting partial mediation of the association of race with incident gout due to serum urate level. Notably, sex did not modify the relationship between race and incident gout after adjustment for confounders (unadjusted P for interaction = 0.04; adjusted P for interaction > 0.05).
Table 2.
Association of Race With Incident Gout by Race and Sex in the US-Based Atherosclerosis Risk in Communities Study, 1987–2012
| Model | Women |
Men |
P Value for Interaction | ||||
|---|---|---|---|---|---|---|---|
| White (n = 5,009) | Black (n = 1,852) |
White (n = 4,134) | Black (n = 968) |
||||
| HR | 95% CI | HR | 95% CI | ||||
| 1a | Referent | 2.51 | 2.03, 3.12 | Referent | 1.73 | 1.37, 2.18 | 0.04 |
| 2b | Referent | 1.80 | 1.38, 2.36 | Referent | 1.84 | 1.41, 2.39 | 0.64 |
| 3c | Referent | 1.69 | 1.29, 2.22 | Referent | 1.92 | 1.44, 2.56 | 0.81 |
| 4d | Referent | 1.62 | 1.24, 2.12 | Referent | 1.49 | 1.11, 2.00 | 0.34 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Unadjusted model (age was used as the time scale).
b Adjusted for body mass index (weight (kg)/height (m)2); intakes of protein, organ meat, and shellfish; daily intake of alcoholic drinks; income; educational level; cigarette use; diabetes; renal function (estimated glomerular filtration rate categories of ≥90, 60–89, or <60 mL/minute/1.73 m2); coronary heart disease; waist-to-hip ratio; weight change between age 25 years and cohort entry; and menopause (women only).
c Adjusted for model 2 covariates plus hypertension and diuretic use.
d Adjusted for model 3 covariates plus serum urate level at cohort entry.
Sensitivity analyses
The results from the adjusted model (model 3) did not differ when we adjusted for time-varying covariates (for women, HR = 1.73, 95% CI: 1.29, 2.32; for men, HR = 2.22, 95% CI: 1.66, 2.98), varied the case definition of gout (for women, HR = 1.70, 95% CI: 1.29, 2.24; for men, HR = 1.90, 95% CI: 1.43, 2.52), excluded those with baseline hyperuricemia (for women, HR = 1.76, 95% CI: 1.33, 2.34; for men, HR = 2.08, 95% CI: 1.54, 2.80), excluded gout diagnosed within 2 years of enrollment (for women, HR = 1.82, 95% CI: 1.18, 2.80; for men, HR = 2.73, 95% CI: 1.60, 4.60), and adjusted for urate levels measured at visit 2, as well as at baseline (for women, HR = 1.49, 95% CI: 1.15, 1.94; for men, HR = 1.35, 95% CI: 1.02, 1.77). Additionally, we tested whether the results were driven by the Jackson, Mississippi, center, which enrolled only black participants; among participants in Forsyth County, North Carolina, the association of race and gout was similar to that in the whole cohort.
Race and uric acid values
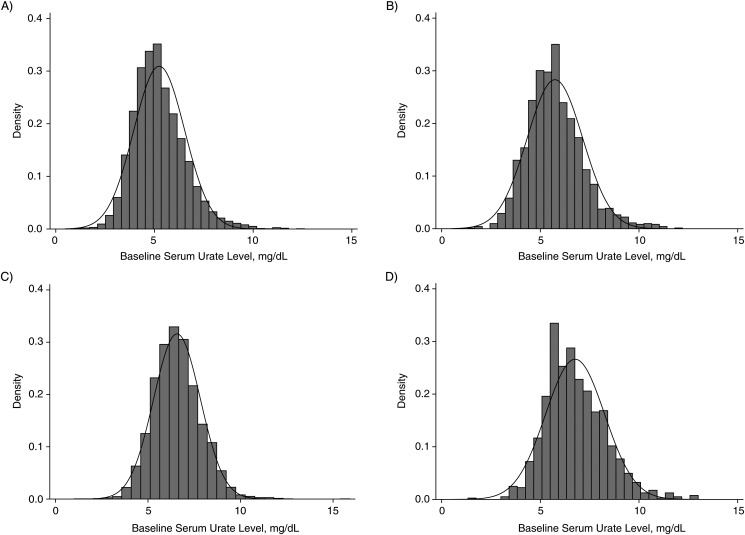
After stratification by sex, mean serum urate levels were higher in black participants (for women, 4.9 vs. 4.4 mg/dL, P < 0.001; for men, 6.0 vs. 5.8 mg/dL, P < 0.001) than in white participants, although these differences may not be clinically significant (Table 1 and Figure 3). The adjusted mean differences in uric acid levels for black versus white participants were 0.26 mg/dL (95% CI: 0.12, 0.39) in women and 0.38 mg/dL (95% CI: 0.24, 0.52) in men.
Figure 3.
Distribution of serum urate (in mg/dL) at cohort entry (1987–1989) in the US-based Atherosclerosis Risk in Communities Study by race and sex. The density of serum urate levels at cohort entry (baseline) and normal distribution were plotted by race and sex for A) white women; B) white men; C) black women; and D) black men.
DISCUSSION
In this US population–based cohort of middle-aged adults, there was strong evidence of a racial difference in the incidence of gout. Black men and black women were more likely to develop gout during middle age and older age compared with their white counterparts, even after accounting for known differences in gout risk factors between groups. Strikingly, the incidence of gout was higher in black women than in white men in older age. Furthermore, although uric acid levels were higher in black participants, the greater incidence of gout among black participants compared with white participants in the ARIC Study persisted and remained approximately 1.5 times greater, even after adjustment for serum urate levels.
To our knowledge, this is the first population-based study in the United States to evaluate the association of race with incidence of gout. Previously, Hochberg et al. (13) examined racial differences in gout risk in an observational cohort and reported a 2-fold excess risk of incident gout in black male physicians compared with their white counterparts. In contrast to our study, the risk appeared to be largely mediated by hypertension or antihypertensive medications. Additionally, this prior report was restricted to physicians, did not control for known dietary risk factors or renal insufficiency, and did not report or adjust for serum urate levels. Notably, the present study is a US biracial population–based study of middle-aged adults from varying socioeconomic backgrounds, and it controlled for numerous potential confounders of the relationship between race and incident gout, including dietary differences, renal function, socioeconomic measures, and measured levels of urate.
Few previous investigations have focused on racial differences in uric acid levels in a US population–based setting, and others have been cross-sectional. Previous data from the ARIC Study have shown that blacks are more likely than whites to have uric acid values in the higher quartiles (35, 36). Additionally, 1 study of younger adults found that black men and black women had lower serum urate levels; however, during follow-up, black women were at increased risk of developing hyperuricemia (37). The racial differences in serum urate levels observed in the ARIC Study cohort pertain to middle-aged and older adults, which may explain why they differ from the aforementioned prior reports of younger adults.
Hyperuricemia is a clear and well-known risk factor for gout (16, 38). Thus, we hypothesized and then demonstrated that the elevated incidence of gout among blacks compared with whites was attenuated after controlling for uric acid levels. However, black race was associated with an increased risk of gout in the fully adjusted models. Our novel finding was that there appear to be racial differences in uric acid levels, above and beyond potential comorbidities, and that these differences in uric acid values may partially mediate the observed higher risk for gout in blacks, particularly in men. Previous analyses have demonstrated that there are genetic differences in renal urate handling that increase the risk of hyperuricemia and gout (39, 40). The frequencies of these polymorphisms differ between populations (39). In the ARIC Study (39), 2 loci (solute carrier family 2 (facilitated glucose transporter), member 9 and ATP-binding cassette, subfamily G (WHITE), member 2) were associated with uric acid concentration in white and black participants. In contrast, solute carrier family 18 (organic anion transporter), member 3 was associated with uric acid levels only in whites. This suggests that there are differences in known and unknown genetic loci for uric acid values between whites and blacks. Although it is possible that these genetic differences may play a role in differences in gout risk between populations, it is equally possible that the relationship between race and risk for elevated uric acid and gout was caused in part by our inability to fully adjust for environmental risk factors for gout that differ between the 2 races. For example, differences in socioeconomic status may lead to health disparities that are not fully captured through measures of education and income; residual confounding has been previously identified as a threat to causal inference in studies of racial differences and racial disparities (41, 42).
Several strengths and potential limitations of the present study deserve comment. This study is the first epidemiologic investigation of the association of black race with gout incidence in a US population–based setting, which allowed us to estimate the association of race and gout in both men and women. Our study had more black participants with gout than any other prior report and contained detailed information on potential risk factors, including dietary factors, serum creatinine levels, serum urate levels, and socioeconomic data. Although there have been both population-based and longitudinal studies of race and gout, this is the first study that is both population-based and longitudinal, which allowed us to ascertain whether race was associated with the incidence of gout. Additionally, this is the only study to examine whether differences in gout incidence by race are attributable to racial differences in levels of serum urate.
There are a number of limitations to recognize. Our definition of gout did not require observation of monosodium urate crystals in joint fluid or fulfillment of the American College of Rheumatology criteria (43) for gout. However, in population-based studies, synovial fluid analysis is logistically challenging and unethical in asymptomatic participants. Our previous study suggested that self-reported gout is a reliable and valid measurement (20). However, we noted that there were differences in the validity of self-reports of physician-diagnosed gout by race; thus, we conducted a sensitivity analysis in which incident gout was defined by both treatment using gout-specific medications and by self-report. No differences in the association of gout and race were observed. Additionally, the outcome of interest was reported at visit 4 and during the most recent annual follow-up call; therefore, the exclusion criterion with the greatest impact on sample size was the report of gout status, which may introduce selection bias. The majority of unanswered gout queries were due to nonattendance at visit 4 and no annual follow-up contact in 2011–2012. Black participants were less likely to respond to the gout query; therefore, it is likely that our results underestimate the association of race and gout. The mediation analysis adjusted for urate levels at baseline and visit 2 (as a sensitivity analyses), which may not correctly classify urate levels of participants who developed gout in older age. Measurement error of lifetime uric acid levels and potential residual confounding by other confounders, such as dietary factors, may affect the findings of the mediation analysis.
Our results may be representative of only US white and black populations because there were too few ARIC Study participants who reported other races, such as Hispanic or Asian American. Furthermore, our results were not driven by geographical variations in gout incidence, because the majority of black participants were from Jackson, Mississippi; we noted a similar association when the analysis was limited to participants from Forsyth County, North Carolina.
This study suggests that black race is associated with an increased risk of incident gout, and that these racial differences are demonstrable, above and beyond the observed racial differences in serum urate level. Future studies should investigate the social and biological mechanisms that contribute to racial differences in gout burden. Importantly, physicians and health care professionals should be aware that not only black men but also black women are more likely to develop gout than their white counterparts, especially during older age.
ACKNOWLEDGMENTS
Author affiliations: United States Food and Drug Administration, Silver Spring, Maryland (Janet W. Maynard); Division of Rheumatology, Johns Hopkins School of Medicine, Baltimore, Maryland (Janet W. Maynard, Allan C. Gelber, Alan N. Baer); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Mara A. McAdams-DeMarco, Linda Kao, Allan C. Gelber, Josef Coresh); and Department of Surgery, Johns Hopkins School of Medicine, Baltimore, Maryland (Mara A. McAdams-DeMarco, Andrew Law).
Janet W. Maynard and Mara A. McAdams-DeMarco contributed equally to the manuscript and should both be considered first authors.
This work was supported by Takeda Pharmaceuticals North America, Inc. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). M.A.M.-D. was jointly funded by the Arthritis National Research Foundation and the American Federation for Aging Research.
We thank the ARIC Study staff for their dedication to this study.
An earlier version of this work was presented at the American College of Rheumatology Annual Meeting in Atlanta, Georgia in November 6–11, 2010.
The sponsor of this research project (Takeda Pharmaceuticals North America, Inc.) was not involved in the design and conduct of the study or the collection, management, analysis, or interpretation of the data; nor were they involved in the preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol. 2008;35(3):498–501. [PubMed] [Google Scholar]
- 3.Sundy JS, Hershfield MS. Uricase and other novel agents for the management of patients with treatment-failure gout. Curr Rheumatol Rep. 2007;9(3):258–264. doi: 10.1007/s11926-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 4.Halpern R, Mody RR, Fuldeore MJ, et al. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin. 2009;25(7):1711–1719. doi: 10.1185/03007990903017966. [DOI] [PubMed] [Google Scholar]
- 5.Halpern R, Fuldeore MJ, Mody RR, et al. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol. 2009;15(1):3–7. doi: 10.1097/RHU.0b013e3181945d2c. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA. Quality of life and quality of care for patients with gout. Curr Rheumatol Rep. 2009;11(2):154–160. doi: 10.1007/s11926-009-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology (Oxford) 2007;46(9):1441–1444. doi: 10.1093/rheumatology/kem150. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742–748. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277–1281. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 12.Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum. 2007;56(6):2049–2055. doi: 10.1002/art.22712. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC, Thomas J, Thomas DJ, et al. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38(5):628–632. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 14.Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two-year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069–1076. doi: 10.1002/art.27338. [DOI] [PubMed] [Google Scholar]
- 15.Juraschek SP, Miller ER, 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res. 2013;65(1):127–132. doi: 10.1002/acr.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard JW, McAdams DeMarco MA, Baer AN, et al. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Med. 2012;125(7):717 e9–717 e17. doi: 10.1016/j.amjmed.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: Is the incidence rising? J Rheumatol. 2002;29(11):2403–2406. [PubMed] [Google Scholar]
- 18.Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3(8):443–449. doi: 10.1038/ncprheum0556. [DOI] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 20.McAdams MA, Maynard JW, Baer AN, et al. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the Atherosclerosis Risk in the Community cohorts. J Rheumatol. 2011;38(1):135–141. doi: 10.3899/jrheum.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) study investigators. J Clin Epidemiol. 1996;49(12):1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 22.National Heart Lung and Blood Institute. Atherosclerosis Risk in Communitiesy Study. Operations Manual No. 2: Cohort Component Procedures, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 23.National Heart Lung and Blood Institute. Atherosclerosis Risk in Communities Study. Operations Manual No. 11: Sitting Blood Pressure, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Stevens J, Metcalf PA, Dennis BH, et al. Reliability of a food frequency questionnaire by ethnicity, gender, age, and education. Nutr Res. 1996;16(5):735–745. [Google Scholar]
- 26.National Heart Lung and Blood Institute. Atherosclerosis Risk in Communities Study. Operations Manual No. 10: Clinical Chemistry Determinations, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 27.Iribarren C, Folsom AR, Eckfeldt JH, et al. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol. 1996;6(4):331–340. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 28.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astor BC, Arnett DK, Brown A, et al. Association of kidney function and hemoglobin with left ventricular morphology among African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2004;43(5):836–845. doi: 10.1053/j.ajkd.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 31.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 34.Hertz-Picciotto I, Rockhill B. Validity and efficiency of approximation methods for tied survival times in Cox regression. Biometrics. 1977;53(3):1151–1156. [PubMed] [Google Scholar]
- 35.Hozawa A, Folsom AR, Ibrahim H, et al. Serum uric acid and risk of ischemic stroke: the ARIC Study. Atherosclerosis. 2006;187(2):401–407. doi: 10.1016/j.atherosclerosis.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Moriarity JT, Folsom AR, Iribarren C, et al. Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2000;10(3):136–143. doi: 10.1016/s1047-2797(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 37.Gaffo AL, Jacobs DR, Jr, Lewis CE, et al. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res Ther. 2012;14(1):R4. doi: 10.1186/ar3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421–426. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 39.Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Kottgen A, Dehghan A, et al. Multiple genetic loci influence serum urate and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628. [PubMed] [Google Scholar]
- 42.Kaufman JS. Epidemiologic analysis of racial/ethnic disparities: some fundamental issues and a cautionary example. Soc Sci Med. 2008;66(8):1659–1669. doi: 10.1016/j.socscimed.2007.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]