Abstract
Gastric cancer (GC) imposes a significant health burden around the globe despite its declining incidence. GC is often diagnosed in advanced stages and carries a poor prognosis. In depth understanding of molecular underpinnings of GC has lagged behind many other cancers of its magnitude, as a result our knowledge base for identifying germline susceptibility traits for risk and somatic drivers of progression (to identify novel therapeutic targets) is limited. A few germline (PLCE1) and somatic (ERBB2, ERBB3, PTEN, PI3K/AKT/mTOR, FGF, TP53, CDH1, and c-MET) alterations are emerging and some are being pursued in the clinic. Novel somatic gene targets, Arid1a, FAT4, and MLL/MLL3 are of interest. Clinically, variations in the therapeutic approaches for localized GC are evident by geographic regions. These are driven by preferences for the adjunctive strategies and the extent of surgery coupled with philosophical divides. However, there is a greater uniformity in approaches to metastatic cancer, an incurable condition. Having realized only modest successes, the momentum is building for carrying out more phase 3 comparative trials and some are using biomarker-based patient selection. Overall, rapid progress in biotechnology is improving our molecular understanding and can help with new drug discovery. The future prospects are excellent for defining biomarker-based subsets of patients and application of specific therapeutics. However, many challenges remain to be tackled. Here we review representative molecular and clinical dimensions of GC.
Review
The objective of this review is to adequately highlight advances in molecular and clinical arenas that reflect the current understanding and it is intentionally not encyclopedic. Details of preventive strategies, impact of new classifications, and nuances of surgery and radiation therapeutics are beyond the scope of this review.
1. Epidemiology
Globally, the incidence of GC ranks 4th in men and 5th in women, but its death rate is next to lung cancer.1 In 2008, there were ~989,600 (8% of all cancers) new GC cases worldwide and 738,000 (10% of all cancer deaths) deaths. 70% of deaths occurred in the developing regions with China having ~40% of them.1 The endemic regions are in Asia, Eastern Europe and South America. The incidence of GC has declined over time,2 due to improving living standards.2–5 The exemplary early detection strategy has reduced the GC death rate in Japan.6 Helicobacter pylori (HP) infection as a risk factor is of importance for preventive strategies.7
2. Risk factors
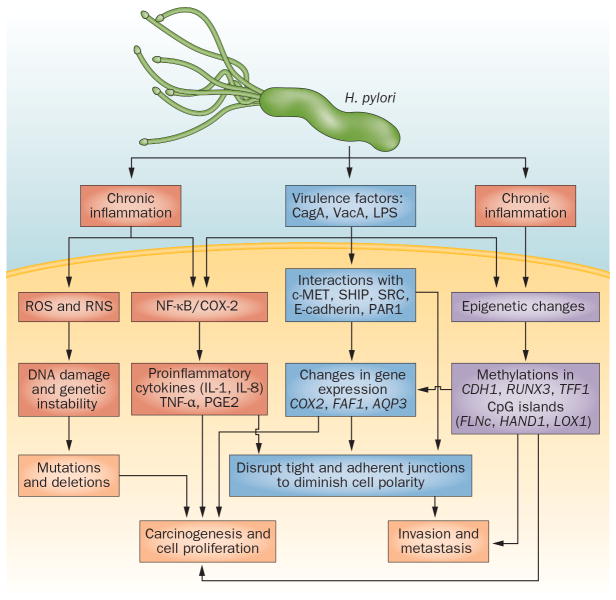
Risk factors include old age, smoking, alcohol, above normal body weight, high salt and or fat consumption, low vegetables and fruits consumption, low economic status, pernicious anemia, other chronic gastric diseases, and HP infection.5,8 Of these risk factors, the biology of HP is fascinating (Figure 1).
Figure 1. Molecular carcinogenesis of Helicobacter pylori in gastric cancer.
H. pylori and its several virulence factors, such as CagA, interact with gastric epithelial cells to induce chronic inflammation, mucosal damage and multiple alterations in gene expression and genetic and epigenetic changes, eventually leading to gastric carcinogenesis. Abbreviations: COX-2, cyclooxygenase-2; CpG island, areas of cytosine and guanine repeats; LPS, lipopolysaccharide; RNS, reactive nitrogen species; ROS, reactive oxygen species; VacA, vacuolating cytoxin A.
HP infection increases the risk 3–6 fold9 and is more associated with distal GC and intestinal histologic phenotype.10 Chronic active gastritis is an integral part of HP-related GC.10,11 HP’s attachment to gastric epithelial cells leads to inflammation and an increase in the reactive oxygen or nitrogen species causing tissue damage.12,13 CagA, an oncoprotein, producing HP species are carcinogenic.11,14,15 CagA is encoded by cag PAI and is translocated by HP into the host epithelial cytosol.14,16 Phosphorylated CagA (by Src and c-Abl kinases),17,18 forms a complex with the SRC homology 2-domain (SH2)-containing tyrosine phosphatase SHP-2 in a phosphorylation-dependent manner, resulting in cytoskeletal reorganization that can induce cell transformation to GC.19 CagA activates the ERK/MAP kinase cascade, resulting in Elk-1 phosphorylation and increased c-fos transcription.20 In addition, CagA promotes invasion through activation of hepatocyte growth factor/scatter factor receptor c-MET.21 Its induction of Toll-like receptors (TLRs) leads to proliferation.22 CagA induces E-cadherin-mediated impairment of cell adhesion junctions leading to cytoplasmic and nuclear accumulations of β-catenin.23 CagA binds Crk adaptor proteins (Crk-II, Crk-I, and Crk-L)24 and kinase PAR1,25 eliciting loss of cell polarity. CagA stimulates cytokines IL-8, IL-1 and TNF-alpha26–28 through NF-kB in epithelium.29 Pro-inflammatory IL-1 gene cluster polymorphisms (IL-1B, encoding IL-1B and IL-IRN, and its receptor antagonist) increase the risk of non-cardia GC.30,31 CagA also upregulates cyclo-oxygenase-2 (COX-2)26,32 that is overexpressed in GC. COX-2 induced prostaglandins are oncogenic.33,34 HP alters the Fas-associated factor 1 (FAF1) that promotes apoptosis but it is reduced in GC.35 HP also mediates increases in another oncoprotein, aquaporin 3 (AQP3).36
HP alters DNA methylation of E-cadherin (CDH1), an oncogenic event.37,38 HP promotes methylation of tumor suppressor TFF239 and RUNX340 and likely 6 other tumor suppressors (FLNc, HAND1, THBD, p41ARC, HRASLs and LOX).41 It would appear that HP is clearly carcinogenic but in susceptible individuals.
3. Single nucleotide polymorphisms (SNP) and Genome-wide association studies (GWAS)
Genetic susceptibility can be critical, for example, all endemic areas have high prevalence of HP but have few GC cases.42 Rare germline mutations in CDH-1 lead to familial GC.43,44 SNPs can facilitate GC but one adverse allele may be a weak contributor, however, multiple adverse alleles can increase the risk.45 Prior SNP investigation have focused on genes involved in mucosal protection against HP (e.g., IL1B, IL1RN, and TNF-α), carcinogen metabolism (e.g., CYP2E1 and GSTM1), deoxynucleotide synthesis, DNA repair (e.g., MTHFR and XRCC1), and tumor suppressors (e.g., TP53 and CDH1). However, these have had limited yield and none can be used clinically.
GWAS can scan the whole genome for implicating SNPs. A Japanese group documented that PSCA was associated with diffuse GC.46 They genotyped 188 cases and 752 controls for 85,576 SNPs and then replicated in 749 cases and 750 controls for 2,753 SNPs. The intronic rs2976392 SNP in PSCA was identified as the risk allele and the SNP was in diseuilibrium with rs2294008 located in exon 1. The second study included 1,077 esophageal cancer cases and 1,733 controls leading to 18 hits that were validated in 2,766 cases of cardia GC and PLCE1rs2274223 and C20orf54 rs1304295 SNPs were associated with GC risk.47 The third study included 2,240 GC cases and 3,302 controls and identified the PLCE1rs2274223 SNP as a risk allele for cardia GC.48 PLCE1 SNPs were associated with GC risk,49,50 and prognosis of Chinese patients51 but not Caucasian patients.52 The fourth GWAS in China included 1,006 cases and 2,273 controls and replicated in 3,288 cases and 3,069 controls; SNP rs13361707 located between PTGER4 and PRKAA1 and the ZBTB20 rs9841504SNP were associated with risk.53 Table 1 sumerizes the current GWAS results. Clearly, we have a long way to go.
Table 1.
Representative GWAS studies in gastric cancer
| Type of gastric cancer | Genes | Chr no. | Significant SNP identified by GWAS | Ref no. | Confirmed by other studies | Ref no. |
|---|---|---|---|---|---|---|
| Non-cardia Diffuse | PSCA | 8q24.3 | rs2976392A>G rs2294008 |
46 | Yes | 53,185–189 |
| Diffuse | MUC1 | 1q22 | rs2070803G>A | 46l | Yes | 52,190 |
| Cardia, non-cardia | PLCE1 | 10q23 | rs2274223A>G | 47,48 | Yes | 49,186,191 |
| Cardia, non-cardia | C20orf54 | 20p13 | rs1304295 | 47 | No | 52 |
| Non-cardia | PTGER4 or PRKAA1 | 5p13.1 | rs13361707T>C | 53 | Yes | 189 |
| Non-cardia | ZBTB20 | 3q13.3 | rs9841504C>G | 53 | No |
GWASs have identified previously unknown genes, for example, the PLCE1 is not known to be involved in GC, but its oncogenic role in skin and intestine is reported.47
4. Gastric cancer stem cells (GCSCs) and Aberrant signaling pathways (Figure 2)
Gastric carcinogenesis is complex and not fully characterized.54 Although, intestinal GC (IGC) develops after systematic progression from the pre-neoplastic stages, diffuse GC (DGC) is thought to arise de novo as the result of downregulation (mutation or promoter methylation) of CDH1,55,56 thereby permitting tumorigenesis and progression. Nevertheless, accumulated genetic alterations (mutations, amplifications, insertions, deletions, and or recombinations) lead to GC.57,58 More alterations accumulate as GC progresses. Cancer is hierarchically organized with ample plasticity. There is increasing evidence for the existence of GCSC to initiate tumor by self-renewal and differentiation. The origin of human GCSCs is still unclear, but may be the mesenchymal stem cells in bone marrow.59,60
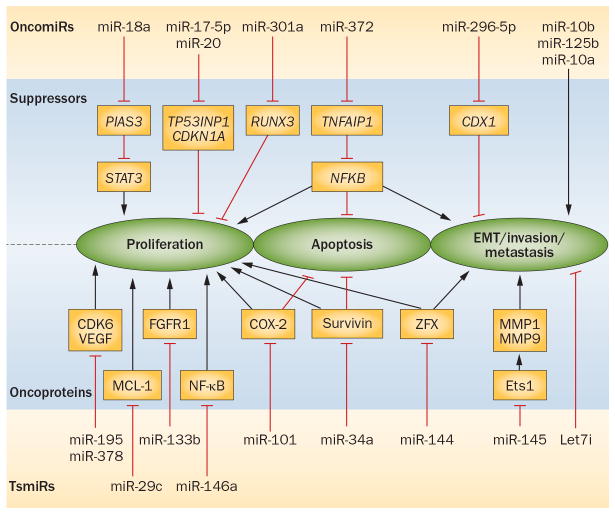
Figure 2. microRNA targets and functions in gastric cancer.
Some oncomiRs are overexpressed in tumours and inhibit tumour suppressors, leading to cell proliferation, invasion and reduced apoptosis. By contrast, tsmiRs normally target oncogenes that are downregulated in tumours and facilitate the activity of their target oncogenes. Abbreviations: EMT, epithelial–mesenchymal transition; miR, microRNA; oncomiRs, oncogenic microRNAs; tsmiRs, tumour-suppressor microRNAs.
CSCs can undergo epithelial mesenchymal transition (EMT), activate oncogenic pathways61 and embryogenesis signaling pathways62 essential for self renewal and maintenance. The combination of EMT, CSC, and drug resistance forms the axis of evil.63 EMT leads to the CSC-like phenotype.64 CSCs depend on the Wnt, Notch, and Hedgehog (Hh) pathways.65 Four pleiotropic transcriptional factors (Snail, Slug, Twist, and Zeb1/2) orchestrate the EMT and related processes.66 c-MET and TGF-β signaling can be critical for EMT. c-MET activation can induce the reprogramming transcription factors known to support embryonic stem cells and induce differentiated cells to form the pluripotent stem (iPS) cells.67
TGFβ can be pro-oncogenic by inducing matrix deposition, immunosuppression, and EMT.68–70 TGF-β signaling drives EMT and CSC self-renewal mediated by targeting microRNAs,66 upregulating Snail family members, and repression of E-cadherin.63 Downstream of c-MET and TGF-β receptor, PI3K/Akt/mTOR signaling conveys pro-survival messages for CSC expansion and maintenance.71 There has been interest in targeting mTOR with metformin to inhibit CSCs.72 Ras and Hh help maintain CSCs.73,74 GCSCs express CD133, CD44, ALDH1 (aldehyde dehydrogenase 1) and ABCG2 (ATP-binding cassette sub-family G member 2); CD44 and ALDH are associated with therapy resistance and can be exploited therapeutically upon understanding the underlying mechanisms.75
Members of the human epidermal receptor (HER) family have been of interest.76 Oncogenic properties are conferred through the RAS/MEK/MAPK and PI3K/Akt/mTOR pathways.77,78. Overexpression of HER2 is due to HER2 amplification and this is more prevalent in IGC than DGC. 79,80
HER2 interacts with EGFR, HER381, and IGF1R.82 These genes are amplified and/or overexpressed83,84 or acquire activating mutations.85–87
Constitutive activation of c-MET triggers proliferation and anti-apoptotic signals.88 Amplification/overexpression of c-MET rather than mutated gene can activate receptor tyrosine kinase.89,90 c-MET overexpression/amplification is more common in IGC91,92 but its amplification has been reported in DGC cells.93. Amplified c-MET cross talk can activate EGFR, HER2, and HER3 to establish a signaling network leading to constitutive PI3K/AKT signaling.94–96 GCs with c-MET overexpression coexpress EGFR, HER-3, or both;97 clinically relevant for the dual inhibition strategies.93,96,97
PI3K/AKT/mTOR pathway is frequently altered as a results of amplification or overexpression (PIK3CA, Akt1), activating mutations (PIK3CA)55,98 of components, or loss of PTEN.99 Overexpression of phospho-mTOR can occur in DGC.55 HER3 and FGFR amplification in DGC is another mechanism for PI3K/Akt activation.100,101
TGFβ is overexpressed in DGC102 and it stimulates collagen synthesis and subsequent fibrosis. TGFβ can be anti-apoptotic through transactivation of EGFR.103 The bone morphogenetic proteins (BMPs), members of TGF-β superfamily activate PI3K/Akt.104 There has been considerable interest in the inhibition of angiogenesis. In that regard, VEGF and VEGFR overexpression is common in IGC through activation of NFκB by HP. 105,106
While HERs, c-MET, PI3K/AKT/mTOR, VEGFRs, VEGF have been targeted, TGFβ and CDH1 are not targetable. There are also several novel targets worth mentioning. Chromatin modifiers such as ARID1A, MLL3, MLL, and FAT4 (cell adhesion) are of increasing interest and importance.107,108
While some efforts have been made to genotype IGC and DGC (and identify novel subtypes)109–120, clinically relevant and robust molecular subtypes have yet to emerge. IGC and DGC genotypes in one study121 resulted in homogeneity in response to therapy than did the phenotypes.
5. MicroRNAs
miRNAs play a role in tumorigenesis, tumor progression, and metastasis. Here we update (Figure 3) our recent review.122 Oncogenic miRNAs (oncoMIRs): OncoMIRs are overexpressed and inhibit tumor suppressors leading to cell proliferation, invasion, and reduced apoptosis. For example, overexpression of miR-296-5p in GC cells increased cell proliferation and inhibition of apoptosis by repression of tumor suppressor CDX1.123 Overexpressed miR-301a directly targets tumor suppressor RUNX3.124 miR-17-5p/20a targets p21 and p53-induced nuclear protein 1 (TP53INP1).125 miR-18a levels were correlated with those of survivin, Bcl-xL, and c-Myc (downstream targets of STAT3 and negatively regulated by PIAS3; thus, miR-18a acts as an oncoMIR by negatively regulating PIAS3.126 microRNA-372 is oncogenic as it targets TNFAIP1 and modulates NFkB signaling in GC cells.127 IRX1, a newly identified tumor suppressor gene, is inactivated by miR-544.128 miR-10b is highly expressed in IGC is associated with the depth of invasion, lymph node, and metastatic progression.129 Tumor suppressor miRNAs (TsMIRs): TsMIRs are downregulated miRNAs, thus facilitating activity of target oncogenes. miR-195 and miR-378 are downregulated in GC and GC cells and their target oncogenes are CDK6 and VEGF.130 miR-133b’s oncogene target is FGFR1 often amplified in DGC.131 miR-29c‘s oncogene target is Mcl-1 and activation of miR-29c by celecoxib represses Mcl-1 and promotes apoptosis of GC cells.132 miR-34a is downregulated in GC cells and its oncogene target is survivin.133 miR-145 suppresses v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1) by binding to 3′-UTR and reducing the oncogenic processes.134 Let-7i is frequently downregulated in most tumors and is prognostic of lymphatic invasion, nodal metastasis, and poor pathologic in GC.135 ZFX has a role the maintenance of CSCs and it is the target of miR-144 in GC.136 miR-101 targets the 3′-UTR of COX-2 mRNA and its downregulation in GC correlates with COX-2 overexpression and proliferation.137 microRNA-146a inhibits NF-kappaB by targeting CARD10 and COPS8 in GC.138
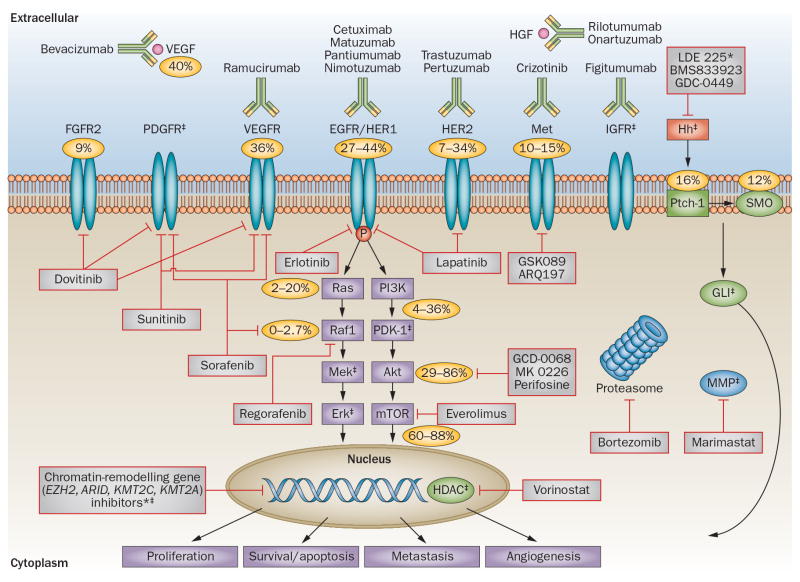
Figure 3. Targeted therapy in gastric cancer.
Percentages signify the overall molecular characteristics in the disease: FGFR2 amplification (9%), VEGF/VEGFR overexpression (36–40%), EGFR amplification and overexpression (27–44%), HER-2 amplification and overexpression (7–34%), c-MET amplification (10–15%), kRAS mutation (2–20%), Raf mutation (0–3%), PI3K mutation (4–36%), phospho-Akt expression (29–86%), phospho-mTOR expression (60–88%), PTCH1 overexpression (16%), SMO overexpression (12%) and HER3 mutations (10%, not shown). *No clinical trials of these agents have yet been reported in gastric cancer. ‡No known numbers or percentages for these genes and pathways. Abbreviations: EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GLI, glioma-associated oncogene family zinc finger 1; HDAC, histone deacetylase; HER, human epidermal growth factor receptor; HGF, hepatocyte growth factor; Hh, Hedgehog; IGFR, insulin-like growth factor receptor; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; Ptch-1, protein patched homolog 1; Smo, smoothened; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
miRNAs as biomarkers: miRNAs are stable in serum, plasma, gastric juice, and other body fluids.139 miR-21 and miR-106a were overexpressed in GC and gastric juice compared to normal controls.140 Additionally, miR-421 in gastric juice of GC patients was higher than in controls (P < 0.001) and it resulted in early diagnosis of GC compared by serum carcinoembryonic antigen.141 Plasma miR-106b, miR-20a, and miR-221 levels were elevated in GC patients than in healthy controls (P < 0.05).142 Plasma levels of miRNA-199a-3p was associated with tumor invasion, malignant node, and metastases.143 Circulating miR-17-5p and miR-20a (miR-17-5p/20a) have been detected in GC patients and both miRs correlated with clinical variables.144 The miR-200c expression level in blood in GC patients were significantly higher than in normal controls (p = 0.018). Clearly, research on several miRs needs to be fully developed and holds promise.
6. Mechanisms of resistance using HER2 as an example
The major reason for treatment failure in patients is the occurrence of primary and or secondary resistance. We focus on secondary resistance and use her2 inhibition as an example. The predominant mechanism is the compensatory signaling by other cell surface receptors.145 HER2 overexpressing cells when inhibited reprogram other oncogenes, including IGF-IR and c-MET, growth differentiation factor 15 (GDF15), and other members of the ERBB family.146 The IGF-IR-mediated resistance involves the PI3K pathway, leading to enhanced degradation of p27Kip1.82,147,148 While activated MET mediates resistance in GC cells149,150 by restoring shared downstream signaling in the MAPK and AKT pathways.149 Increased levels of EGFR and HER3 ligands can overcome HER2 inhibition.63,100,146 Constitutively activated p95HER2, truncated HER2 receptor, is the most intriguing mechanism of resistance in response to the blockade of the extracellular domain of HER2.151 Membrane mucins such as Muc4 interact with HER2 in HER2-overexpressing breast cancer cells resulting in epitope masking that blocks trastuzumab binding.152 Other proteins that confer resistance include focal adhesion kinase (FAK), and Src, as well as alterations in cell cycle regulators.153 Constitutive activation of PI3K due to activating PIK3CA mutations,57 reduced PTEN expression,154 or deregulated signaling can induce resistance to HER2 inhibition.57,155,156 Finally, STAT3 activation can mediate resistance as a result of production of IL-6.157 All this suggests that cancer cells have many redundant mechanisms to overcome therapy resistance and we have considerable work ahead of us. Including exploring drug conjugates and immune modulation.
7. Clinical Dimensions
7.1 Regional differences
The epidemiology and location of the primary GC varies geographically158 due to variations in genetic susceptibilities, predominance of certain histologic phenotypes (e.g., IGC is frequent in the endemic areas), and carcinogenic forces including HP. 159 Cardiac GC is more common in the West and non-cardia GC is more common in the endemic regions. Besides these differences, surgical approach is more comprehensive in Asia where a D2 dissection is a routine160 than in the West where it is D0 or D1. Other regional differences are in the adjunctive strategy for localized GC (LGC). Many of these factors may account for differences in survival of patients in different regions.159
7.2 Localized GC
Localized GC (LGC) can be cT1 or higher with or without regional nodes. Once a patient is diagnosed with LGC, a multidisciplinary evaluation (by medical oncologists, surgical oncologists, surgeons, gastroenterologists, pathologists, radiation oncologists, geneticists [if appropriate], and nutritionists) is highly recommended prior to initiating any therapy.161 Endoscopic therapy for a T1 lesion, when feasible, is recommended. For those GCs not amenable to effective endoscopic therapy, surgery should be considered. However, adjunctive therapies have contributed to the higher (~10%) cure rates than those obtained by surgery.162–165
Adjunctive Strategies
In North America and Europe, results from the INT-0116165 and MAGIC 166 trials have established specific strategies. The postoperative adjuvant chemotherapy strategy has been established in Asia.167,168
Post-operative adjuvant chemoradiation
Although, the safety of INT-0116, a phase III trial that compared observation after surgery with adjuvant chemoradiation after surgery, has been a concern; chemoradiation improved the 5-year cure rate by ~10%.165 This advantage prevailed with a longer follow-up.169 A follow-up trial coordinated by CALGB170 produced negative results as it investigated the adjuvant chemotherapy strategy that has failed many times in the West. Similarly, the ARTIST trial that used the INT-0116 strategy, but differed in two aspects: (1) the control group was treated with chemotherapy and (2) all patients had a D2 gastrectomy, failed to demonstrate benefit for chemoradiation.171 Thus the ARTIST trial raises more questions than the answers.
Postoperative Chemotherapy
The benefits of adjuvant chemotherapy after D2 gastrectomy was first established in Japan using S-1 as the adjuvant.167 The Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC)167 randomized 1,059 patients to one year of S-1 or observation. The primary analysis demonstrated a 33% improvement in overall survival (OS) for the S-1 group. The results prevailed after a longer follow-up.172 Second Asian study, the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC trial) randomized 1,035 patients post D2 gastrectomy to capecitabine plus oxaliplatin for 6 months or observation,168 and documented benefit for chemotherapy for the endpoint of disease-free survival (at 3 years; HR 0.56, 95% CI, 0.44–0.72; P < .0001). A meta-analysis based on data from 3,710 patients showed 7% improvement in OS for FU-based postoperative chemotherapy when compared to surgery164 but this evidence is soft.
Perioperative or Preoperative Chemotherapy
The MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial (that randomized 504 patients) established the evidence for perioperative chemotherapy for GC patients in the West.166 A second trial, although with differing tumor type composition (and terminated early because of the lack of interest), demonstrated benefit for preoperative chemotherapy.163 Several trials using a variety of adjunctive strategies are currently ongoing (MAGIC-B: NCT00450203, CRITICS: NCT00407186, TOPGEAR and PRODIGY: NCT01515748.173 (Table 3)
Table 3.
Major Phase III trials for gastric cancer
| Table 3a: Localized gastric cancer trials | ||||
|---|---|---|---|---|
| Trials | Treatment Arms | N | Hazard Ratio for OS; P | Primary end point comparison in months (survival rates in %) |
| INT-116165 | Surgery + CTRT (45Gy + 5FU) vs. Surgery | 556 | 1.32; 0.004 | OS (36 vs. 27) |
| MAGIC166 | ECF/Surgery/ECF vs. Surgery | 503 | 0.75; 0.009 | 5-year OS (36.3% vs. 23%) |
| CALGB-80101207 | FU/CTRT-FU/FU vs. ECF/CTRT-FU/ECF | 546 | 1.03; 0.80 | OS (37 vs. 38) |
| ARTIST171 | Surgery/XP vs. Surgery/XP/XRT/XP | 458 | 0.0862 | 3-year DFS (74.2% vs 78.2%) |
| ACTS-GC167 | Surgery vs. Surgery/S-1 | 1,059 | 0.68; 0.003 | 3-year OS (70.1% vs.
80.1%) RFS (65.4% vs. 53.1%) |
| CLASSIC168 | XELOX and Surgery vs. Surgery | 1,035 | 0.56; <0.0001 | 3-year DFS (74% vs. 59%) |
| FNLCC 163 | Perioperative Chemotherapy vs. Surgery | 224 | 0.69; 0.003 | 5-year OS (38% vs. 24%) |
| SAMIT*208 | Surgery/UFT vs. Surgery/S1 vs. Surgery/Paclitaxel/UFT vs. Surgery/Paclitaxel/S1 | 1,495 | NR | |
| ARTIST-II* | Surgery/XP vs. Surgery/XP/XRT/XP | 1,000 | NR | |
| MAGIC-B* NCT00450203 | ECX + Bevacizumab vs ECX | 1,100 | NR | |
| TOPGEAR*209 | Preoperative CT vs. Preoperative CTRT | 752 | NR | |
| CRITICS*173 | ECX/Surgery/ECX vs. ECX/Surgery/CX-CTRT | 788 | NR | |
| Table 3b: Advanced/Metastatic gastric cancer trials-First-line | ||||
|---|---|---|---|---|
| Trials | Treatment Arms | N | Hazard Ratio for OS; P | Primary end point comparison in months (survival rates in %) |
| ToGA176@ | CX/CF +trastuzumab vs. CX/CF | 584 | 0.74, 0.0046 | OS (13.8 vs 11.1) |
| AVAGAST177 | Cisplatin/Fluoropyrimidine vs. Cisplatin/Fluoropyrimidine+ Bevacizumab | 774 | 0.87, 0.1002 | OS (10.1 vs 12.2) PFS (5.3 vs 6.7) |
| EXPAND 210 NCT00678535 | CX vs. CX+ Cetuximab | 904 | 1.004, 0.9547 | OS (10.7 vs 9.4) |
| REAL-3 211 | EOC vs. mEOC–P | 574 | 1.37, 0.013 | OS (11.3 vs 8.8) |
| V325174 | DCF vs. CF | 457 | 1.47,<0.001 | TTP (5.6 vs 3.7) |
| SPIRITS175 | S-1 + Cisplatin vs. S-1 | 305 | 0.77, 0.04 | OS (13.0 vs 11.0) |
| FLAGS178 | cisplatin/S-1 vs. cisplatin/5-FU | 1,053 | 0.92, 0.20 | OS (8.6 vs. 7.9) |
| LOGIC* NCT00680901 | CapeOx plus Lapatinib vs. CapeOx plus Placebo | 545 | NR | |
| Table 3c: Advanced/Metastatic gastric cancer trials-Second-line | ||||
|---|---|---|---|---|
| Trials | Treatment Arms | N | Hazard Ratio for OS; P | Primary end point comparison in months (survival rates in %) |
| GRANITE-1 NCT00879333 | BSC Placebo vs everolimus | 648 | 0.90, 0.1244 | OS (4.3 vs 5.4) |
| REGARD180 NCT00917384 | BSC with Ramicirumab vs. BSC | 355 | 0.776, 0.0473 | OS (5.2 vs 3.8) |
| TYTAN182 | Lapatinib + paclitaxel vs. paclitaxel | 261 | 0.2441 | OS (11.0 versus 8.9) |
| Kang et al 212 | BSC vs. docetaxel or irinotecan | 202 | 0.657, 0.007 | OS (3.8 vs 5.3) |
| AIO179 | Irinotecan/BSC vs. BSC | 40 | 0.48; 0.012 | OS (4.0 vs 2.4) |
| COUGAR-02181 (Trial 13366390) | Docetaxel/ASC vs. ASC | 168 | 0.67, 0.01 | OS (5.2 vs 3.6) |
=Ongoing Trials, NR=Not Reported yet
N: Total Sample Size; P=P-value; CTRT: Chemoradiotherapy; OS: Overall Survival; RFS: Relapse Free Survival; PFS: Progression free survival; DFS: Disease free Survival; HR: Hazard Ratio; ECF: Epirubicin, Cisplatin, 5-FU; ECX: epirubicin, cisplatin capecitabine; XP, capecitabine/cisplatin, XRT: XP+ Radiotherapy; XELOX: Capecitabine plus Oxaliplatin; EOC: epirubicin, oxaliplatin and capecitabine; mEOC–P: mEOC + panitumumab; CX: cisplatin and capecitabine; CF: cisplatin, fluorouracil; D: Docetaxel; UFT: Tegafur and Uracil; CapeOx: Capecitabine, Oxaliplatin; BSC: best supportive care; ASC: Active Symptom Control; TTP=time to progression.
INT: US Intergroup Study; CALGB: Cancer and Leukemia Group B ; MAGIC: Medical Research Council Adjuvant Gastric Cancer Infusion Chemotherapy; ACTS-GC: Adjuvant Chemotherapy Trial of S1 in Gastric Cancer; CRITICS: ChemoRadiotherapy after Induction chemo Therapy in Cancer of the Stomach; ARTIST: Adjuvant Chemoradiation Therapy in Stomach Cancer; CLASSIC: Capecitabine and Oxaliplatin Adjuvant study in stomach cancer; REAL-3: Randomized ECF for Advanced and Locally Advanced Esophagogastric Cancer 3; AVAGAST: Avastin in Gastric Cancer; EXPAND: Erbitux in Combination with Xeloda and Cisplatin in Advanced Esophagogastric Cancer; GRANITE-1:Safety and efficacy of RAD001 (Everolimus) Monotherapy plus Best Supportive care in Patients with Advanced Gastric Cancer; ToGA: Trastuzumab for Gastric Cancer; LOGIC: Lapatinib Optimization Study in HER-2 Positive Gastric Cancer; SAMIT: Stomach Cancer Adjuvant Multi-institutional Trial.
the hazard ration is reduced to 0.8 on a follow up analysis ((http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm230418.htm;)
7.3 Advanced Gastric Cancer (AGC)
First line therapy
Level 1-evidence for an advantage in OS is available for only a few therapeutic agents (docetaxel,174 cisplatin,175 and trastuzumab176). Most trials have been disappointing with the exception of the trastuzumab trial investigating a biomarker-based enriched population.176 However, the longer follow up has reduced the HR for OS to 0.8 (http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm230418.htm;), suggesting that only a few patients benefit. Notable among trials with disappointing results are two randomized studies investigating the value of EGFR inhibition (REAL-3; NCT00824785 and EXPAND; NCT00678535; these references will be updated). Two other studies are worth mentioning: (1) The AVAGAST trial was conducted in 774 patients, randomized to chemotherapy with or without bevacizumab and it did not meet its primary endpoint of OS177 and (2) the First line Advanced Gastric Cancer Study (FLAGS) conducted in >1,000 patients randomized to S-1 plus cisplatin versus 5-FU plus cisplatin also failed to meet its primary endpoint of OS advantage.178
Second line therapy
A phase III AIO trial In 40 patients randomized patients to irinotecan with best supportive care (BSC) or BSC. The OS was significantly longer for the irinotecan/BSC arm but the has only 40 patients.179 The GRANITE-1 study (NCT00879333) that randomized >600 patients to everolimus or placebo in second or third line setting also did not achieve its primary endpoint of survival (will update reference). However, the REGARD trial (NCT00917384) that compared BSC with or without ramucirumab in 345 patients has demonstrated a borderline improvement in OS for ramucirumab.180 More impressively, the Cougar-02 trial randomizing approximately 186 patients to BSC or docetaxel plus BSC demonstrated a significant prolongation of OS in the docetaxel/BSC arm.181
Lapatinib, a dual inhibitor of HER2 and EGFR was investigated in a phase III study (TYTAN) of 300+ patients randomized to lapatinib versus placebo but the primary endpoint of prolongation of OS was not achieved.182
Targeting c-MET pathway is of interest. In a small study with crizotinib, 2 of 4 patients with MET copy number gain >=5 had a prolonged response.183 Rilotumumab (AMG 102), a fully human monoclonal antibody, demonstrated longer OS for patients having tumors with high total c-MET expression.184 Table 3 lists representative completed studies and important ongoing studies.
Conclusions
Considerable advances in biotechnology have improved our understanding of cancer, nevertheless immense complexities confront us. Although, GC lags behind many other tumor types, more progress is anticipated. Greater understanding of pathogenesis o IGC by HP is poised to help with more sophisticated preventive strategies. Germline susceptibility investigations have uncovered novel genes but clinical implementations have proven problematic and more work is needed. Clinically, progress has been slow but adjunctive strategies are now frequently employed for LGC around the world and this is an advance. The future progressed will be propelled by further improvements in biotechnologies that will produce better biomarkers and drugs.
Table 2.
Comparison of molecular characteristics between intestinal and diffuse subtypes of gastric cancer.
| Intestinal | Reference | Diffuse | Reference | |
|---|---|---|---|---|
| Amplification/overexpression | HER2 | 76 | c-MET | 183 |
| EGFR | 84 | TGFβ | 102 | |
| SHH/Ptch/SMO | 192 | |||
| VEGF | 105 | HER3 | 102 | |
| Notch1 | 193 | FGFR2 | 101,194 | |
| p-mTOR(47–60%) | p-mTOR (58–64%) | 195,196 | ||
| PIK3CA | 55 | |||
| MMP -1, -7(32–70%) | MMP -1, -7(62–90%) | 197,198 | ||
| Activating mutations | EGFR | 199 | PIK3CA | 55 |
| c-MET | 96 | |||
| Loss of function mutations | E-cadherin | 200 | ||
| p53(*-overall 40%) | p53(* -overall 40%) | 89,201,202 | ||
| PTEN(*) | PTEN(*) | 203 | ||
| Loss of expression | E-cadherin (69%) | E-cadherin (89%) | 197 | |
| p53(*) | P53(*) | 204 | ||
| PTEN(*) | PTEN(*) | 98 |
NS: No statistical correlation with pathological features. Other molecular alterations implicated in GC but has not been assigned to subtypes include amplification of IGF-IR 205, Ki-Ras 206.
HER2-, EGFR-, MET- and VEGF-related signaling are predominantly implicated in the intestinal subtype, while loss of E-cadherin, FGFR2-, mTOR-, HER3- and MMP-related pathways are more frequently involved in the diffuse subtype.
Acknowledgments
This work has been supported by This work was supported in part by grants from the Caporella, Cantu, Smith, Park, Fairman, Frazier, Sultan, Oaks, and Milrod families and by the Kevin Fund, Schecter Private Fund, and the Rivercreek Foundation. Also supported by the Multidisciplinary Research Grant from U. T. M. D. Anderson Cancer Center.
Footnotes
The authors have no conflict to disclose.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, et al. Recent patterns in gastric cancer: a global overview. International journal of cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Bu XL, Wang QY, Hu PJ, Chen MH. Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami E, Machado RS, Ogata SK, Langner M. Decrease in prevalence of Helicobacter pylori infection during a 10-year period in Brazilian children. Arq Gastroenterol. 2008;45:147–151. doi: 10.1590/s0004-28032008000200011. [DOI] [PubMed] [Google Scholar]
- 5.Tkachenko MA, et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J Pediatr Gastroenterol Nutr. 2007;45:428–432. doi: 10.1097/MPG.0b013e318064589f. [DOI] [PubMed] [Google Scholar]
- 6.Lee KJ, et al. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. International journal of cancer. 2006;118:2315–2321. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 8.Axon A. Review article: gastric cancer and Helicobacter pylori. Aliment Pharmacol Ther. 2002;16 (Suppl 4):83–88. doi: 10.1046/j.1365-2036.16.s4.14.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer letters. 2011;305:228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conteduca V, et al. H. pylori infection and gastric cancer: state of the art (review) International journal of oncology. 2013;42:5–18. doi: 10.3892/ijo.2012.1701. [DOI] [PubMed] [Google Scholar]
- 11.Rizzato C, et al. Risk of advanced gastric precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. International journal of cancer. 2013 doi: 10.1002/ijc.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Tsugawa H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Peek RM, Jr, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. Journal of the National Cancer Institute. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY, Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145–151. doi: 10.1046/j.1523-5378.1998.08031.x. [DOI] [PubMed] [Google Scholar]
- 16.Hatakeyama M. Helicobacter pylori CagA--a potential bacterial oncoprotein that functionally mimics the mammalian Gab family of adaptor proteins. Microbes Infect. 2003;5:143–150. doi: 10.1016/s1286-4579(02)00085-0. [DOI] [PubMed] [Google Scholar]
- 17.Mueller D, et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. The Journal of clinical investigation. 2012;122:1553–1566. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller A. Multistep activation of the Helicobacter pylori effector CagA. The Journal of clinical investigation. 2012;122:1192–1195. doi: 10.1172/JCI61578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashi H, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-ter-Vehn T, Covacci A, Kist M, Pahl HL. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. The Journal of biological chemistry. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- 21.Schirrmeister W, et al. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res. 2009;315:3500–3508. doi: 10.1016/j.yexcr.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel-Nunes P, et al. Helicobacter pylori Induces Increased Expression of Toll-Like Receptors and Decreased Toll-Interacting Protein in Gastric Mucosa that Persists Throughout Gastric Carcinogenesis. Helicobacter. 2013;18:22–32. doi: 10.1111/hel.12008. [DOI] [PubMed] [Google Scholar]
- 23.Murata-Kamiya N, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202:1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama M. Linking epithelial polarity and carcinogenesis by multitasking Helicobacter pylori virulence factor CagA. Oncogene. 2008;27:7047–7054. doi: 10.1038/onc.2008.353. [DOI] [PubMed] [Google Scholar]
- 26.Bartchewsky W, Jr, et al. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scandinavian journal of gastroenterology. 2009;44:153–161. doi: 10.1080/00365520802530853. [DOI] [PubMed] [Google Scholar]
- 27.Li CQ, Pignatelli B, Ohshima H. Coexpression of interleukin-8 and inducible nitric oxide synthase in gastric mucosa infected with cagA+ Helicobacter pylori. Digestive diseases and sciences. 2000;45:55–62. doi: 10.1023/a:1005453125433. [DOI] [PubMed] [Google Scholar]
- 28.Suganuma M, et al. TNF-alpha-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. International journal of cancer. 2008;123:117–122. doi: 10.1002/ijc.23484. [DOI] [PubMed] [Google Scholar]
- 29.Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rad R, et al. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. The Journal of infectious diseases. 2003;188:272–281. doi: 10.1086/376458. [DOI] [PubMed] [Google Scholar]
- 31.Zambon CF, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141–152. doi: 10.1016/j.cyto.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Chang YJ, et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 33.Walduck AK, et al. Identification of novel cyclooxygenase-2-dependent genes in Helicobacter pylori infection in vivo. Mol Cancer. 2009;8:22. doi: 10.1186/1476-4598-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo JH, Kim H, Kim KH. Cyclooxygenase-2 expression by transcription factors in Helicobacter pylori-infected gastric epithelial cells: comparison between HP 99 and NCTC 11637. Annals of the New York Academy of Sciences. 2002;973:477–480. doi: 10.1111/j.1749-6632.2002.tb04687.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu AQ, Ge LY, Ye XQ, Luo XL, Luo Y. Reduced FAF1 Expression and Helicobacter Infection: Correlations with Clinicopathological Features in Gastric Cancer. Gastroenterol Res Pract. 2012;2012:153219. doi: 10.1155/2012/153219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, et al. Involvement of Aquaporin 3 in Helicobacter pylori-related gastric diseases. PLoS One. 2012;7:e49104. doi: 10.1371/journal.pone.0049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura G, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. Journal of the National Cancer Institute. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- 38.Becker KF, Hofler H. Frequent somatic allelic inactivation of the E-cadherin gene in gastric carcinomas. Journal of the National Cancer Institute. 1995;87:1082–1084. doi: 10.1093/jnci/87.14.1082. [DOI] [PubMed] [Google Scholar]
- 39.Peterson AJ, et al. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology. 2010;139:2005–2017. doi: 10.1053/j.gastro.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama Y, Takahashi M, Kuwayama H. Helicobacter pylori causes runx3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochemical and biophysical research communications. 2009;388:496–500. doi: 10.1016/j.bbrc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida T, et al. Altered mucosal DNA methylation in parallel with highly active Helicobacter pylori-related gastritis. Gastric Cancer. 2013 doi: 10.1007/s10120-012-0230-x. [DOI] [PubMed] [Google Scholar]
- 42.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilford P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 44.Huntsman DG, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. The New England journal of medicine. 2001;344:1904–1909. doi: 10.1056/NEJM200106213442504. [DOI] [PubMed] [Google Scholar]
- 45.Hu Z, Ajani JA, Wei Q. Molecular epidemiology of gastric cancer: current status and future prospects. Gastrointest Cancer Res. 2007;1:12–19. [PMC free article] [PubMed] [Google Scholar]
- 46.Study Group of Millennium Genome Project for C et al . Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nature genetics. 2008;40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 47.Wang LD, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nature genetics. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 48.Abnet CC, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nature genetics. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, et al. Genetic variants at 1q22 and 10q23 reproducibly associated with gastric cancer susceptibility in a Chinese population. Carcinogenesis. 2011;32:848–852. doi: 10.1093/carcin/bgr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, et al. Potentially functional variants of PLCE1 identified by GWASs contribute to gastric adenocarcinoma susceptibility in an eastern Chinese population. PLoS One. 2012;7:e31932. doi: 10.1371/journal.pone.0031932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo D, et al. Genetic variation in PLCE1 is associated with gastric cancer survival in a Chinese population. Journal of gastroenterology. 2011;46:1260–1266. doi: 10.1007/s00535-011-0445-3. [DOI] [PubMed] [Google Scholar]
- 52.Palmer AJ, et al. Genetic variation in C20orf54, PLCE1 and MUC1 and the risk of upper gastrointestinal cancers in Caucasian populations. Eur J Cancer Prev. 2012;21:541–544. doi: 10.1097/CEJ.0b013e3283529b79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nature genetics. 2011;43:1215–1218. doi: 10.1038/ng.978. [DOI] [PubMed] [Google Scholar]
- 54.Wu KC, et al. Molecular basis of therapeutic approaches to gastric cancer. Journal of gastroenterology and hepatology. 2009;24:37–41. doi: 10.1111/j.1440-1746.2008.05753.x. [DOI] [PubMed] [Google Scholar]
- 55.Wong H, Yau T. Targeted Therapy in the Management of Advanced Gastric Cancer: Are We Making Progress in the Era of Personalized Medicine? The oncologist. 2012;17:346–358. doi: 10.1634/theoncologist.2011-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider BG, et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. International journal of cancer. 2010;127:2588–2597. doi: 10.1002/ijc.25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR Pathway - Beyond Rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annu Rev Pathol-Mech. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metast Rev. 2010;29:249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 60.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. Journal of Clinical Oncology. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan DSW, Gerlinger M, Teh BT, Swanton C. Anti-cancer drug resistance: Understanding the mechanisms through the use of integrative genomics and functional RNA interference. European Journal of Cancer. 2010;46:2166–2177. doi: 10.1016/j.ejca.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer Stem Cells: A Novel Paradigm for Cancer Prevention and Treatment. Mini-Rev Med Chem. 2010;10:359–371. doi: 10.2174/138955710791330954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. Journal of Clinical Oncology. 2008;26:2883–2889. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peinado H, Olmeda D, Cano A. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 67.Li YQ, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9951–9956. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bierie B, Moses HL. TGF beta: the molecular Jekyll and Hyde of cancer. Nature Reviews Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 70.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Research. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 71.Zhou JB, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance (vol 104, pg 16158, 2007) Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19655–19655. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer research. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh BN, Fu JS, Srivastava RK, Shankar S. Hedgehog Signaling Antagonist GDC-0449 (Vismodegib) Inhibits Pancreatic Cancer Stem Cell Characteristics: Molecular Mechanisms. Plos One. 2011;6 doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heindl S, et al. Relevance of MET activation and genetic alterations of KRAS and E-cadherin for cetuximab sensitivity of gastric cancer cell lines. Journal of cancer research and clinical oncology. 2012;138:843–858. doi: 10.1007/s00432-011-1128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takaishi S, et al. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem cells (Dayton, Ohio) 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fornaro L, et al. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastro Hepat. 2011;8:369–383. doi: 10.1038/nrgastro.2011.81. [DOI] [PubMed] [Google Scholar]
- 77.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 78.Dhanasekaran DN, Johnson GL. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. doi: 10.1038/sj.onc.1210395. [DOI] [PubMed] [Google Scholar]
- 79.Lordick F, et al. HER2 status of advanced gastric cancer is similar in europe and asia. Annals of Oncology. 2007;18:Vii95–Vii96. [Google Scholar]
- 80.Zhang XL, et al. Comparative Study on Overexpression of HER2/neu and HER3 in Gastric Cancer. World journal of surgery. 2009;33:2112–2118. doi: 10.1007/s00268-009-0142-z. [DOI] [PubMed] [Google Scholar]
- 81.Terashima M, et al. Impact of Expression of Human Epidermal Growth Factor Receptors EGFR and ERBB2 on Survival in Stage II/III Gastric Cancer. Clinical Cancer Research. 2012;18:5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 82.Browne BC, et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Annals of Oncology. 2011;22:68–73. doi: 10.1093/annonc/mdq349. [DOI] [PubMed] [Google Scholar]
- 83.Wilkinson NW, et al. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. Journal of Gastrointestinal Surgery. 2004;8:448–453. doi: 10.1016/j.gassur.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Isinger-Ekstrand A, et al. Genetic profiles of gastroesophageal cancer: combined analysis using expression array and tiling array-comparative genomic hybridization. Cancer genetics and cytogenetics. 2010;200:120–126. doi: 10.1016/j.cancergencyto.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Moutinho C, et al. Epidermal growth factor receptor structural alterations in gastric cancer. Bmc Cancer. 2008;8 doi: 10.1186/1471-2407-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu ZM, et al. Epidermal growth factor receptor mutation in gastric cancer. Pathology. 2011;43:234–238. doi: 10.1097/PAT.0b013e328344e61b. [DOI] [PubMed] [Google Scholar]
- 87.Pinto C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study) British journal of cancer. 2009;101:1261–1268. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Migliore C, Giordano S. Molecular cancer therapy: Can our expectation be MET? European Journal of Cancer. 2008;44:641–651. doi: 10.1016/j.ejca.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 89.El-Rifai W, Powell SM. Molecular biology of gastric cancer. Seminars in radiation oncology. 2002;12:128–140. doi: 10.1053/srao.2002.30815. [DOI] [PubMed] [Google Scholar]
- 90.Smolen GA, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janjigian YY, et al. MET Expression and Amplification in Patients with Localized Gastric Cancer. Cancer Epidem Biomar. 2011;20:1021–1027. doi: 10.1158/1055-9965.EPI-10-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J, et al. Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncology reports. 2011;25:1517–1524. doi: 10.3892/or.2011.1219. [DOI] [PubMed] [Google Scholar]
- 93.Guo A, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arteaga CL. HER3 and mutant EGFR meet MET. Nature medicine. 2007;13:675–677. doi: 10.1038/nm0607-675. [DOI] [PubMed] [Google Scholar]
- 95.Corso S, Comoglio PM, Giordano S. Cancer therapy: can the challenge be MET? Trends in Molecular Medicine. 2005;11:284–292. doi: 10.1016/j.molmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Corso S, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Molecular Cancer. 2010;9 doi: 10.1186/1476-4598-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bachleitner-Hofmann T, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Molecular cancer therapeutics. 2008;7:3499–3508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 98.Byun DS, et al. Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIk3CA amplification in gastric carcinoma. International journal of cancer. 2003;104:318–327. doi: 10.1002/ijc.10962. [DOI] [PubMed] [Google Scholar]
- 99.Oki E, et al. Impact of PTEN/AKT/PI3K signal pathway on the chemotherapy for gastric cancer. Journal of Clinical Oncology. 2006;24:187s–187s. [Google Scholar]
- 100.Garrett JT, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin EY, et al. Up-regulation and co-expression of fibroblast growth factor receptors in human gastric cancer. Journal of cancer research and clinical oncology. 2000;126:519–528. doi: 10.1007/s004320000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komuro A, et al. Diffuse-Type Gastric Carcinoma: Progression, Angiogenesis, and Transforming Growth Factor beta Signaling. Journal of the National Cancer Institute. 2009;101:592–604. doi: 10.1093/jnci/djp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ebi M, Kataoka H, Higashiyama S, Joh T. Tgfbeta Induces EGFR Transactivation and HB-EGF C-Terminal Fragment Nuclear Translocation Through ADAM17 Activation in Gastric Cancer Cells. Gastroenterology. 2011;140:S629–S629. [Google Scholar]
- 104.Kang MH, et al. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncology reports. 2009;22:525–534. doi: 10.3892/or_00000467. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi Y, et al. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clinical Cancer Research. 1996;2:1679–1684. [PubMed] [Google Scholar]
- 106.Sharma VK, Vasudeva R, Howden CW. Changing demographics of gastric cancer: 15 year experience. Gastroenterology. 1998;114:A40–A40. doi: 10.1111/j.1572-0241.1998.209_a.x. [DOI] [PubMed] [Google Scholar]
- 107.Zang ZJ, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nature genetics. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 108.Wang K, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nature genetics. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 109.Chen Cn, Fau-Lin JJ, et al. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J Clin Oncol. 2005;23:7286–7295. doi: 10.1200/JCO.2004.00.2253. [DOI] [PubMed] [Google Scholar]
- 110.Cho Jy, Fau-Lim JY, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deng N, Fau-Goh LK, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ivanova T, Fau-Zouridis H, et al. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut. 2013;62:22–33. doi: 10.1136/gutjnl-2011-301113. [DOI] [PubMed] [Google Scholar]
- 113.Kim Hk, Fau-Choi IJ, et al. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One. 2011;6:0016694. doi: 10.1371/journal.pone.0016694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim Hk, Fau-Choi IJ, et al. Three-gene predictor of clinical outcome for gastric cancer patients treated with chemotherapy. The pharmacogenomics journal. 2012;12:119–127. doi: 10.1038/tpj.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ooi Ch, Fau-Ivanova T, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:2. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeno A, Fau-Takemasa I, et al. Gene expression profile prospectively predicts peritoneal relapse after curative surgery of gastric cancer. Ann Surg Oncol. 2010;17:1033–1042. doi: 10.1245/s10434-009-0854-1. [DOI] [PubMed] [Google Scholar]
- 117.Tan Ib, Fau-Ivanova T, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–485. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu Y, Fau-Grabsch H, et al. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut. 2012;26:26. doi: 10.1136/gutjnl-2011-301373. [DOI] [PubMed] [Google Scholar]
- 119.Zang Zj, Fau-Cutcutache I, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nature genetics. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 120.Zouridis H, Fau-Deng N, et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci Transl Med. 2012;4:3004504. doi: 10.1126/scitranslmed.3004504. [DOI] [PubMed] [Google Scholar]
- 121.Tan IB, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–485. e411. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2013;10:109–118. doi: 10.1038/nrgastro.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li T, et al. MicroRNA-296-5p increases proliferation in gastric cancer through repression of Caudal-related homeobox 1. Oncogene. 2013 doi: 10.1038/onc.2012.637. [DOI] [PubMed] [Google Scholar]
- 124.Wang M, et al. Overexpressed miR-301a promotes cell proliferation and invasion by targeting RUNX3 in gastric cancer. Journal of gastroenterology. 2013 doi: 10.1007/s00535-012-0733-6. [DOI] [PubMed] [Google Scholar]
- 125.Wang M, et al. miR-17-5p/20a are important markers for gastric cancer and murine double minute 2 participates in their functional regulation. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 126.Wu X, et al. MicroRNA Expression Signatures during Malignant Progression from Barrett’s Esophagus to Esophageal Adenocarcinoma. Cancer Prev Res (Phila) 2013;6:196–205. doi: 10.1158/1940-6207.CAPR-12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou C, et al. microRNA-372 maintains oncogene characteristics by targeting TNFAIP1 and affects NFkappaB signaling in human gastric carcinoma cells. International journal of oncology. 2013;42:635–642. doi: 10.3892/ijo.2012.1737. [DOI] [PubMed] [Google Scholar]
- 128.Zhi Q, et al. Oncogenic miR-544 is an Important Molecular Target in Gastric Cancer. Anticancer Agents Med Chem. 2013;13:270–275. doi: 10.2174/1871520611313020013. [DOI] [PubMed] [Google Scholar]
- 129.Wang YY, et al. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013 doi: 10.1016/j.humpath.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 130.Deng H, et al. MicroRNA-195 and microRNA-378 mediate tumor growth suppression by epigenetical regulation in gastric cancer. Gene. 2013 doi: 10.1016/j.gene.2012.12.103. [DOI] [PubMed] [Google Scholar]
- 131.Wen D, et al. miR-133b acts as a tumor suppressor and negatively regulates FGFR1 in gastric cancer. Tumour Biol. 2013 doi: 10.1007/s13277-012-0609-7. [DOI] [PubMed] [Google Scholar]
- 132.Saito Y, et al. The tumor suppressor microRNA-29c is downregulated and restored by celecoxib in human gastric cancer cells. International journal of cancer. 2012 doi: 10.1002/ijc.27862. [DOI] [PubMed] [Google Scholar]
- 133.Cao W, et al. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumour Biol. 2012 doi: 10.1007/s13277-012-0632-8. [DOI] [PubMed] [Google Scholar]
- 134.Zheng L, et al. miRNA-145 Targets v-ets Erythroblastosis Virus E26 Oncogene Homolog 1 to Suppress the Invasion, Metastasis, and Angiogenesis of Gastric Cancer Cells. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-12-0534. [DOI] [PubMed] [Google Scholar]
- 135.Liu K, et al. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J Surg Oncol. 2012;10:225. doi: 10.1186/1477-7819-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Akiyoshi S, et al. Clinical significance of miR-144-ZFX axis in disseminated tumour cells in bone marrow in gastric cancer cases. British journal of cancer. 2012;107:1345–1353. doi: 10.1038/bjc.2012.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He XP, et al. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012;279:4201–4212. doi: 10.1111/febs.12013. [DOI] [PubMed] [Google Scholar]
- 138.Crone SG, et al. microRNA-146a inhibits G protein-coupled receptor-mediated activation of NF-kappaB by targeting CARD10 and COPS8 in gastric cancer. Mol Cancer. 2012;11:71. doi: 10.1186/1476-4598-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ichikawa D, Komatsu S, Konishi H, Otsuji E. Circulating microRNA in digestive tract cancers. Gastroenterology. 2012;142:1074–1078. e1071. doi: 10.1053/j.gastro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 140.Cui L, et al. Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer. 2013 doi: 10.1002/cncr.27903. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X, et al. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 2012;33:2349–2355. doi: 10.1007/s13277-012-0497-x. [DOI] [PubMed] [Google Scholar]
- 142.Cai H, et al. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med Oncol. 2013;30:452. doi: 10.1007/s12032-012-0452-0. [DOI] [PubMed] [Google Scholar]
- 143.Li C, et al. MiRNA-199a-3p in Plasma as a Potential Diagnostic Biomarker for Gastric Cancer. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2600-3. [DOI] [PubMed] [Google Scholar]
- 144.Wang M, et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Report. 2012;5:1514–1520. doi: 10.3892/mmr.2012.828. [DOI] [PubMed] [Google Scholar]
- 145.Stern HM. Improving Treatment of HER2-Positive Cancers: Opportunities and Challenges. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3001539. [DOI] [PubMed] [Google Scholar]
- 146.Nahta R. Molecular Mechanisms of Trastuzumab-Based Treatment in HER2-Overexpressing Breast Cancer. ISRN oncology. 2012;2012:428062. doi: 10.5402/2012/428062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim JW, et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer letters. 2008;272:296–306. doi: 10.1016/j.canlet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 148.Lu YH, Zi XL, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. International journal of cancer. 2004;108:334–341. doi: 10.1002/ijc.11445. [DOI] [PubMed] [Google Scholar]
- 149.Chen CT, et al. MET Activation Mediates Resistance to Lapatinib Inhibition of HER2-Amplified Gastric Cancer Cells. Molecular cancer therapeutics. 2012;11:660–669. doi: 10.1158/1535-7163.MCT-11-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shattuck DL, Miller JK, Carraway KL, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer research. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 151.Molina MA, et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer research. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 152.Nagy P, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-Expressing breast cancer cell line. Cancer research. 2005;65:473–482. [PubMed] [Google Scholar]
- 153.Gong SJ, Jin CJ, Rha SY, Chung HC. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer letters. 2004;214:215–224. doi: 10.1016/j.canlet.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 154.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: Genetic alterations and therapeutic implications. Current Genomics. 2007;8:271–306. doi: 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nature medicine. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eichhorn PJA, et al. Phosphatidylinositol 3-Kinase Hyperactivation Results in Lapatinib Resistance that Is Reversed by the mTOR/Phosphatidylinositol 3-Kinase Inhibitor NVP-BEZ235. Cancer research. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Korkaya H, et al. Activation of an IL6 Inflammatory Loop Mediates Trastuzumab Resistance in HER2+Breast Cancer by Expanding the Cancer Stem Cell Population. Molecular Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. Jama. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- 159.Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. Journal of gastric cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tamura S, Takeno A, Miki H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. International journal of surgical oncology. 2011;2011:748745. doi: 10.1155/2011/748745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ajani JA, et al. Gastric cancer. J Natl Compr Canc Netw. 2010;8:378–409. doi: 10.6004/jnccn.2010.0030. [DOI] [PubMed] [Google Scholar]
- 162.Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23:6220–6232. doi: 10.1200/JCO.2005.11.593. [DOI] [PubMed] [Google Scholar]
- 163.Ychou M, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 164.Paoletti X, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 165.Macdonald JS, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The New England journal of medicine. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 166.Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New England Journal of Medicine. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 167.Sakuramoto S, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. The New England journal of medicine. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 168.Bang YJ, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012 doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 169.Smalley SR, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fuchs CS, Tepper JE, Niedzwiecki D, Hollis D, Mamon HJ, Swanson R, Haller DG, Dragovich SR, Alberts SR, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction adenocarcinoma using epirubicin, cisplatin, infusional fluourouracil before and after infusional fluorouracil and radiotherapy compared with bolus fluorouracil/leucovorin before and after chemoradiation. Intergroup trial CALGC 80101. J Clin Oncol. 2011;29:Abstract no. 4003. [Google Scholar]
- 171.Lee J, et al. Phase III Trial Comparing Capecitabine Plus Cisplatin Versus Capecitabine Plus Cisplatin With Concurrent Capecitabine Radiotherapy in Completely Resected Gastric Cancer With D2 Lymph Node Dissection: The ARTIST Trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 172.Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 173.Dikken JL, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS) BMC Cancer. 2011;11:329. doi: 10.1186/1471-2407-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Van Cutsem E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 175.Koizumi W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. The lancet oncology. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 176.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 177.Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 178.Ajani JA, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 179.Thuss-Patience PC, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 180.Charles S, Fuchs JT, Cho Jae Yong, Dumitru Filip, Passalacqua Rodolfo, Goswami Chanchal, Safran Howard, dos Santos Lucas Vieira, Aprile Giuseppe, Ferry David R, Melichar Bohuslav, Tehfe Mustapha, Topuzov Eldar, Tabernero Josep, Zalcberg John Raymond, Chau Ian, Koshiji Minori, Hsu Yanzhi, Schwartz Jonathan D, Ajani Jaffer A. REGARD: A phase III, randomized, double-blind trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy. J Clin Oncol. 2012;30(suppl 34; abstr LBA5) [Google Scholar]
- 181.Hugo Ford AM, Wadsley Jonathan, Coxon Fareeda Y, Mansoor Wasat, Bridgewater John A, Madhusudan Srinivasan, Falk Stephen, Middleton Gary William, Swinson Daniel, Chau Ian, Thompson Joyce, Blazeby Jane M, Cunningham David, Kareclas Paula, Dunn Janet A. A randomized phase III study of docetaxel versus active symptom control in advanced esophagogastric adenocarcinoma. J Clin Oncol. 2012;30(suppl 34; abstr LBA4) [Google Scholar]
- 182.Bang Yung-Jue Seoul National University Hospital, S.N.U.C.o.M., Seoul, South Korea. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. J Clin Oncol. 2012;30(suppl 34; abstr 11) [Google Scholar]
- 183.Lennerz JK, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–4810. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kelly S, Oliner RT, Anderson Abraham, Lan Yun, Iveson Timothy, Donehower Ross C, Jiang Yizhou, Dubey Sarita, Loh Elwyn. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. J Clin Oncol. 2012;30(suppl 4; abstr 18) [Google Scholar]
- 185.Qiao L, Feng Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for Eastern Asians: a meta-analysis based on 16792 individuals. Gene. 2012;493:83–91. doi: 10.1016/j.gene.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 186.Wang T, Zhang L, Li H, Wang B, Chen K. Prostate stem cell antigen polymorphisms and susceptibility to gastric cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:843–850. doi: 10.1158/1055-9965.EPI-11-1176. [DOI] [PubMed] [Google Scholar]
- 187.Shi D, et al. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. Journal of cancer research and clinical oncology. 2012;138:1339–1345. doi: 10.1007/s00432-012-1210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang QH, et al. Association of the PSCA rs2294008 C>T polymorphism with gastric cancer risk: evidence from a meta-analysis. Asian Pac J Cancer Prev. 2012;13:2867–2871. doi: 10.7314/apjcp.2012.13.6.2867. [DOI] [PubMed] [Google Scholar]
- 189.Hwang JY, et al. Recapitulation of previous genome-wide association studies with two distinct pathophysiological entities of gastric cancer in the Korean population. J Hum Genet. 2013 doi: 10.1038/jhg.2012.158. [DOI] [PubMed] [Google Scholar]
- 190.Saeki N, et al. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology. 2011;140:892–902. doi: 10.1053/j.gastro.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 191.Gu H, et al. Replication study of PLCE1 and C20orf54 polymorphism and risk of esophageal cancer in a Chinese population. Mol Biol Rep. 2012;39:9105–9111. doi: 10.1007/s11033-012-1782-x. [DOI] [PubMed] [Google Scholar]
- 192.Fukaya M, et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 193.Hong SP, et al. Overexpression of Notch 1 signaling associates with the tumorigenesis of gastric adenorna and intestinal type of gastric cancer. Gastroenterology. 2007;132:A617–A617. [Google Scholar]
- 194.Hattori Y, et al. Immunohistochemical detection of K-sam protein in stomach cancer. Clinical Cancer Research. 1996;2:1373–1381. [PubMed] [Google Scholar]
- 195.Yu GZ, et al. Overexpression of Phosphorylated Mammalian Target of Rapamycin Predicts Lymph Node Metastasis and Prognosis of Chinese Patients with Gastric Cancer. Clinical Cancer Research. 2009;15:1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 196.Lang SA, et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. International journal of cancer. 2007;120:1803–1810. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]
- 197.Zhou YN, et al. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumor Biol. 2010;31:549–558. doi: 10.1007/s13277-010-0068-y. [DOI] [PubMed] [Google Scholar]
- 198.Kitoh T, et al. Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. Journal of gastroenterology. 2004;39:434–440. doi: 10.1007/s00535-003-1316-3. [DOI] [PubMed] [Google Scholar]
- 199.Mammano E, et al. Epidermal growth factor receptor (EGFR): Mutational and protein expression analysis in gastric cancer. Anticancer research. 2006;26:3547–3550. [PubMed] [Google Scholar]
- 200.Pereira PS, et al. E-cadherin missense mutations, associated with hereditary diffuse gastric cancer (HDGC) syndrome, display distinct invasive behaviors and genetic interactions with the Wnt and Notch pathways in Drosophila epithelia. Human molecular genetics. 2006;15:1704–1712. doi: 10.1093/hmg/ddl093. [DOI] [PubMed] [Google Scholar]
- 201.Maesawa C, et al. The Sequential Accumulation of Genetic Alterations Characteristic of the Colorectal Adenoma-Carcinoma Sequence Does Not Occur between Gastric Adenoma and Adenocarcinoma. Journal of Pathology. 1995;176:249–258. doi: 10.1002/path.1711760307. [DOI] [PubMed] [Google Scholar]
- 202.Wei JX, et al. Regulation of p53 Tumor Suppressor by Helicobacter pylori in Gastric Epithelial Cells. Gastroenterology. 2010;139:1333. doi: 10.1053/j.gastro.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo CY, Xu XF, Wu LY, Liu SF. PCR-SSCP-DNA sequencing method in detecting PTEN gene mutation and its significance in human gastric cancer. World J Gastroentero. 2008;14:3804–3811. doi: 10.3748/wjg.14.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kobayashi M, Kawashima A, Mai M, Ooi A. Analysis of chromosome 17p13 (p53 locus) alterations in gastric carcinoma cells by dual-color fluorescence in situ hybridization. American Journal of Pathology. 1996;149:1575–1584. [PMC free article] [PubMed] [Google Scholar]
- 205.Adachi Y, et al. Combined Target Therapy for Igf-I Receptor and Vegf against Human Gastric Cancer. Tumor Biol. 2010;31:S100–S100. [Google Scholar]
- 206.Cepero V, et al. MET and KRAS Gene Amplification Mediates Acquired Resistance to MET Tyrosine Kinase Inhibitors. Cancer research. 2010;70:7580–7590. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
- 207.Fuchs C, JET, Niedwiecki D, Hollis D, Haller DG, Dragovich T, Alberts SR, Bjarnason G, Mayer RJ. Postoperative adjuvant chemoradiation for gastric or gastroesophageal adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (RT): Interim toxicity results from Intergroup trial CALGB 80101. J Clin Oncol. 2011;29:Abstract no. 4003. [Google Scholar]
- 208.Tsuburaya AYK, Kobayashi M, et al. SAMIT: Preliminary safety data from a 2×2 factorial randomized phase III trial to investigate weekly paclitaxel (PTX) followed by oral fluoropyrimidines (FPs) versus FPs alone as adjuvant chemotherapy in patients (pts) with gastric cancer. J Clin Oncol. 2011;29(suppl; abstr 4017) [Google Scholar]
- 209.TOPGEAR: An international randomized phase III trial of preoperative chemoradiotherapy versus preoperative chemotherapy for resectable gastric cancer (AGITG/TROG/EORTC/NCIC CTG) J Clin Oncol. 2012;30(suppl; abstr TPS4141) [Google Scholar]
- 210.Lordick F, et al. Cetuximab in combination with capecitabine and cisplatin as first-line treatment in advanced gastric cancer: randomised controlled phase III EXPAND study. Ann Oncol. 2012;23:ixe11. (LBA3) [Google Scholar]
- 211.Waddell TS, et al. A randomized, multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3) [abstract] J Clin Oncol. 2012;30(Suppl):LBA4000. [Google Scholar]
- 212.Kang JH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]