Abstract
Invariant natural killer T (iNKT) cells comprise a lineage of CD1d-restricted glycolipid-reactive T lymphocytes with important roles in host immunity to cancer. iNKT cells indirectly participate in antitumor responses by inducing dendritic cell maturation and producing cytokines that promote tumor clearance by CD8+ T and NK cells. Although iNKT cells thereby act as potent cellular adjuvants, it is less clear whether they directly control the growth of tumors. To gain insights into the direct contribution of iNKT cells to tumor immune surveillance, we developed in vitro and in vivo systems to selectively examine the antitumor activity of iNKT cells in the absence of other cytolytic effectors. Using the EL4 T-lymphoma cell line as a model, we find that iNKT cells exert robust and specific lysis of tumor cells in vitro in a manner that is differentially-induced by iNKT cell agonists of varying TCR affinities, such as OCH, α-galactosyl ceramide and PBS44. In vitro blockade of CD1d-mediated lipid antigen presentation, disruption of T cell receptor (TCR) signaling, or loss of perforin expression significantly reduce iNKT cell killing. Consistent with these findings, iNKT cell reconstitution of T, B, and NK cell-deficient mice slows EL4 growth in vivo via TCR-CD1d and perforin-dependent mechanisms. Together, these observations establish that iNKT cells are sufficient to control the growth of T-lymphoma in vitro and in vivo. They also suggest that the induction of iNKT cell cytotoxic responses in situ might serve as a more effective strategy to prevent and/or treat CD1d+ cancers, such as T-lymphoma.
Introduction
Cancer immune surveillance involves a complex interplay between transformed cells, tumor-supporting stromal cells and immune cells. While the contributions of CD8+ T and natural killer (NK) cells to antitumor immunity are well-appreciated, mounting evidence also implicates an important role for invariant natural killer T (iNKT) cells (1, 2). Indeed, iNKT cells are often reduced in number and/or function in the peripheral blood of patients with cancer (3-6), yet increased numbers of peripheral blood or tumor-infiltrating iNKT cells confer a more favorable therapeutic response (7-9). In mice, in vivo administration of the lipid agonist α-galactosyl ceramide (α-GalCer) induces iNKT cell activation and leads to potent antitumor activity (10-13). Finally, iNKT cell-deficient mice exhibit increased susceptibility to spontaneous (14, 15), carcinogen-induced (16) and adoptively transferred (17) tumors; however, iNKT cell reconstitution slows or prevents tumor formation.
Based on these and other findings, efforts are underway to manipulate iNKT cell functions therapeutically for cancer. To employ such iNKT cell-based therapies, it is imperative that we understand how iNKT cells recognize and respond to tumors. Currently, it is proposed that iNKT cells contribute to antitumor immunity in an indirect manner by stimulating the tumor-directed activities of other immune cells. Following TCR activation, iNKT cells produce interferon-γ (IFNγ) and up-regulate CD40 ligand, thereby inducing dendritic cell (DC) maturation and enhancing DC-mediated priming of tumor-specific T cell responses (18). iNKT cell-activated DCs also produce cytokines such as interleukin (IL)-12, which promote NK cell lysis of tumors (10, 11, 13). Such indirect modulation, however, may not entirely explain the antitumor effects of iNKT cells.
iNKT cells express perforin and granzyme B, and upon activation up-regulate the expression of Fas Ligand (FasL) (19-21). Therefore, cytotoxic and/or pro-apoptotic functions are also likely to contribute to iNKT cell protection from tumors. While published reports support this possibility, several prior studies assayed in vitro killing using whole or iNKT cell-enriched populations (12, 22-24) or examined in vivo tumor clearance in iNKT cell-deficient mice that retained NK and CD8+ T cells (25-27). As a result, it has been difficult to definitively resolve the direct effects of iNKT cells on tumors from the indirect effects of iNKT cells as inducers of NK and CD8+ T cell lysis.
To minimize such confounding factors and dissect the mechanisms by which iNKT cells directly respond to tumors, we purposefully employed systems in which NK and CD8+ T cells were lacking. Using this approach, we observed that sort-purified primary murine iNKT cells mount robust in vitro TCR- and CD1d-restricted cytotoxic responses against EL4 T-lymphoma cells, as well as several other CD1d+ targets. iNKT cell cytotoxic activity was induced by a variety of agonistic glycolipids such as α-GalCer and its analogues PBS44 and PBS57. Maximal in vitro EL4 lysis relied on iNKT cell expression of perforin and FasL, but not Tumor Necrosis Factor related apoptosis-inducing ligand (TRAIL). Finally, in immunodeficient mice that lacked NK and CD8+ T cells, iNKT cell reconstitution significantly slowed EL4 growth and prolonged overall survival. Consistent with our in vitro findings, optimal tumor immune surveillance relied upon intact TCR-CD1d interactions and iNKT cell expression of perforin. Collectively, these data demonstrate that iNKT cells alone are sufficient to control the growth of T-lymphoma. Furthermore, these findings suggest that the cytotoxic and stimulatory properties of iNKT cells constitute a unique combination of functions that might prove beneficial in preventing and/or eradicating T-lymphomas as well as other CD1d+ cancers.
Materials and Methods
Mice
Mice were housed under pathogen-free conditions according to institutional guidelines. Slp-76Fl/Fl mice (28) were a gift from Gary A. Koretzky (University of Pennsylvania). C57BL/6 (B6; WT), NOD-SCID IL2Rγ−/− (NSG), Fas ligand- (Gld) and perforin-deficient (Prf1−/−) mice were purchased from Jackson laboratories and TRAIL-deficient (Trail−/−) mice from Amgen.
Primary cells, cell lines, and reagents
To isolate hepatic iNKT cells, liver mononuclear cells were subjected to Percoll (GE Healthcare) gradient centrifugation and FACS-sorted using anti-NK1.1 and -TCRβ antibodies, yielding populations that were >97% NK1.1+TCR+. EL4, YAC-1, and J774 cells were from American Type Culture Collection and luciferase-expressing EL4 (EL4-LUCs) from Caliper Life Sciences. MBL-2, RMA, and CD1d expressing A20 (A20-mCD1d) cells were gifts from Drs. Sam T. Hwang (Medical College of Wisconsin, Milwaukee WI), Randy R. Brutkiewicz (Indiana University, Indianapolis IN), and Mitchell Kronenberg (La Jolla Institute of Allergy and Immunology, La Jolla CA), respectively. All monoclonal antibodies were from BD Biosciences, except for anti-CD1d (clone 1B1; eBioscience) and anti-NKG2D (BioLegend). NKG2D ligand expression was determined by staining with recombinant mouse NKG2D Fc chimeric molecule (R&D Systems) followed by an F(ab’)2 goat-anti-human secondary (Jackson ImmunoResearch). PBS57-loaded CD1d tetramers were obtained from the NIH Tetramer Core facility. α-GalCer was obtained from Enzo Life Sciences. PBS44, PBS57, and OCH were gifts from Dr. Paul B. Savage (Brigham Young University). PBS57 and PBS44 induced comparable EL4 lysis and were used interchangeably in our experiments.
Lipid chromatography
Lipids were extracted from EL4s and EL4-LUCs as described (29). For analysis, 100μg of polar lipid was separated on silica thin layer chromatography plates (EMD Chemicals) with a mobile phase of 65:25:4 CHCl3:CH3OH:H20 (vol/vol/vol) and carbohydrate-containing lipids were visualized with α-napthol/sulfuric acid (Sigma).
In vitro cytotoxicity assays
Unless indicated, target cells (T) were labeled with 51Cr (Perkin Elmer) and cultured in triplicate with effector cells (E) and 100ng/mL of glycolipids for 16hr. Anti-CD1d (1B1) antibody was used at 10μg/mL. Specific lysis was calculated as described (30).
In vivo tumor studies
NSG mice were injected intravenously with saline or 4×105 hepatic iNKT cells. Four days later (day 0), mice were challenged intravenously with 1×105 EL4-LUCs. To block CD1d, NSG mice were injected intraperitoneally with 50μg of anti-CD1d (3C11, BD Biosciences) or isotype control antibody (R4-22, BD Biosciences) on days 0, 2, 5, 8, and 11. To measure total body radiance, mice were injected intraperitoneally with D-luciferin (150mg/kg; Caliper) and imaged using a Xenogen Spectrum bioimager and Living Image software (Caliper). Mice were sacrificed on day 14 and organs removed. Tissues were fixed, sectioned and stained with hematoxylin and eosin. Digital images were obtained using a Zeiss AxioImager microscope, AxioCam, and AxioVision software (Carl Zeiss Microscopy). For survival studies, mice were euthanized upon loss of >20% of initial weight or development of hind-limb paralysis.
Statistical analysis
The Student’s unpaired t-test was used to calculate significance in all experiments except for survival studies in which the Log-rank test was used. Significance is reported as not significant (NS), *(p<0.05) or **(p<0.01). Unless indicated, in figures depicting in vitro cytotoxicity, asterisks indicate p-values comparing lysis in the presence versus the absence of glycolipid antigen.
Results
In vitro iNKT cell cytotoxic responses require target cell CD1d expression and the presence of agonistic glycolipid antigens (GAgs)
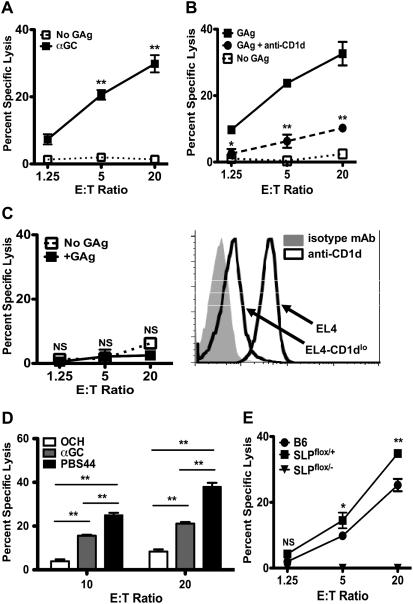
To characterize the requirements for optimal induction of in vitro iNKT cell cytotoxicity, we cultured sort-purified ex vivo hepatic iNKT cells (effectors; E) with EL4 murine CD1d+ T-lymphoma cells (targets, T) in a 51Cr-release-based killing assay. iNKT cells killed EL4s in an E:T-dependent fashion, but only when cultures were supplemented with agonistic GAgs such as α-GalCer (Figure 1A). GAg-dependent killing was observed as early as 12 hours and reached optimal levels by 16-20 hours (Figure S1 and data not shown). In comparison to IL-2 stimulated murine NK cells, GAg-stimulated iNKT cells killed EL4s with slightly delayed kinetics; however, by 16 hours the magnitude of iNKT cell-mediated cytolysis was as great as that of NK cells. iNKT cells also exhibited GAg-dependent killing of multiple other murine CD1d+ targets (Figure S2), including MBL-2 and RMA (T-lymphoma), J774 (macrophage) and A20-CD1d (a CD1d-transfected B leukemia line), demonstrating that this mode of iNKT cell killing is not restricted to EL4s. Finally, GAg-dependent iNKT cell killing of EL4s was diminished in the presence of a blocking anti-CD1d but not isotype control antibody (Figure 1B), and substantially blunted against targets expressing reduced levels of CD1d (Figure 1C). Collectively, these studies establish that iNKT cells mount potent in vitro cytolytic responses that depend on tumor cell expression of CD1d.
Figure 1. Primary iNKT cells exhibit TCR- and CD1d-dependent cytolysis.
(A) Specific lysis of EL4s by hepatic iNKT cells at 16hr in the presence (■) or absence (□) of α-GalCer. (B) Killing of EL4s in the presence (■ and ●) or absence (□) of PBS44 and anti-CD1d (●); asterisks indicate significance between lysis in the presence of PBS44 and anti-CD1d (●) versus PBS44 alone (■). (C) Killing of EL4s expressing low levels of CD1d by hepatic iNKT cells in the presence (■) or absence (□) of PBS44. (D) EL4 killing by hepatic iNKT cells at E:T ratios of 10:1 and 20:1 in the presence of OCH (white), α-GalCer (grey) or PBS57 (black). (E) Killing of EL4s in the presence of PBS44 by B6 (●), SLP76-sufficient (SLPflox/+; ■) and SLP76-deficient (SLPflox/−; ▼) iNKT cells; asterisks indicate the significance between lysis by SLP76-sufficient versus SLP76-deficient iNKT cells. Data are representative of ≥ 2 experiments/panel.
When presented by CD1d, α-GalCer displays strong affinity for the invariant TCR and it or its synthetic analogues PBS44 or PBS57 stimulate robust iNKT cell activation and IFNγ production. In contrast, the α-GalCer analogue OCH, when bound by CD1d, shows decreased TCR affinity and promotes iNKT cell IL-4 secretion (31-35). To determine whether iNKT cell cytotoxicity is modulated by GAg structure and/or its affinity for the TCR, we repeated in vitro killing assays in which cultures were supplemented with various GAgs. In these assays, α-GalCer and PBS44, but not OCH, induced robust killing of EL4s (Figure 1D). The ability of these GAgs to induce target cell lysis was independent of their capacity to stimulate IFNγ production, as IFNγ −/− iNKT cells displayed no defects in cytotoxicity (data not shown). Thus, GAg structure and/or its TCR affinity are important properties that modulate iNKT cell killing.
In vitro iNKT cell killing depends on intact TCR signaling
As CD1d/GAg complexes activate iNKT cells by engaging the TCR, we next probed the contribution of TCR signaling to iNKT cell cytotoxicity. iNKT cells were purified from mice in which the Src Homology 2 domain-containing Leukocyte Protein of 76 kDa (SLP-76) was conditionally eliminated via Cre recombinase-mediated gene deletion (28). Following TCR engagement, SLP-76 nucleates a signaling complex required for maximal phospholipase C-γ activation and release of intracellular Ca2+. Based on the known requirement for Ca2+ during cytolytic granule exocytosis, we hypothesized that the loss of SLP-76 would impair TCR-induced iNKT cell cytotoxicity. As anticipated, SLP-76-sufficient iNKT cells killed EL4s in a GAg-dependent manner comparable to WT iNKT cells. In contrast, SLP-76-deficient cells failed to mount a cytotoxic response (Figure 1E).
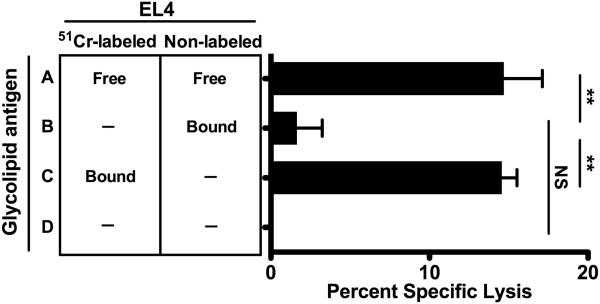
Together, these studies revealed that in vitro iNKT cell cytotoxicity required engagement of the iNKT cell TCR by CD1d/GAg complexes; however, they did not rule out additional contributions by TCR-independent mechanisms. Towards this end, we observed that hepatic iNKT cells express high levels of the activating NK receptor NKG2D and that up to half of the EL4 cells express low to moderate levels of NKG2D ligands (NKG2DLs; Figure S3). It was shown recently that the engagement of NKG2D induces human iNKT cell cytotoxicity (36). Thus, it remained possible that this mechanism of killing could also occur in our in vitro system following CD1d/GAg-mediated iNKT cell activation. To explore this possibility, we co-cultured iNKT cells with different combinations of EL4 targets that had or had not been preloaded with GAg and/or labeled with 51Cr (Figure 2). As previously observed, no 51Cr release was detected in cultures lacking GAg (row D). In contrast, iNKT cell killing was induced when free GAg was supplied in the culture medium (row A) or preloaded onto radiolabeled EL4s (row C). Exposure of iNKT cells to GAg-loaded non-radio-labeled EL4s induced a low but insignificant level of killing of non-GAg-loaded radiolabeled targets (row B). From these studies we conclude that in vitro iNKT cell killing is specific and only upon exposure of iNKT cells to GAg-bearing EL4s. Additionally, these data suggest that TCR-induced iNKT cell activation does not result in appreciable cytotoxicity as the result of NKG2D engagement or the release of soluble factors.
Figure 2. In vitro iNKT cell cytotoxicity requires GAg presentation by EL4 target cells.
Killing by hepatic iNKT cells cultured with equal numbers of 51Cr-labeled and unlabeled EL4s at a final E:T ratio of 10:1; EL4s were either pre-loaded (Bound GAg; groups B, C) or not (No GAg “—“; groups B, C, D) with PBS44. Control cultures contained soluble PBS44 (Free GAg; group A) or no PBS44 (group D). Data are representative of 2 independent experiments.
Direct in vitro cytotoxicity is a feature of multiple iNKT cell subsets
iNKT cells up-regulate the expression of perforin and granzyme B during terminal maturation in the thymus (19). To assess whether cytotoxicity is a general feature of different iNKT cell populations, we examined the killing of EL4s by TCR+NK1.1+ NKT cells sorted from various organs. Similar to hepatic cells, primary thymic TCR+NK1.1+ cells and the thymus-derived iNKT cell hybridoma DN3A4-1.2 (37) exhibited potent GAg-dependent cytotoxicity (Figures S4, S5). In contrast, splenic TCR+NK1.1+ cells displayed virtually no cytotoxicity (Figure S4B). Further examination revealed that only 50% of splenic NK1.1+TCR+ cells stained with PBS57-loaded CD1dTet (Figure S4C). Thus, unlike thymic and hepatic TCR+NK1.1+ cells, which stain uniformly with PBS57-loaded CD1dTet are therefore primarily iNKT cells, the splenic NK1.1+TCR+ fraction contains a significant proportion of cells that represent either NK1.1-expressing conventional T cells or Type II NKT cells, which are known to exhibit inhibitory properties (2, 17).
Compared to hepatic CD4+ iNKT cells, CD4−8− (DN) iNKT cells are reported to be superior at controlling the growth of CD1d− sarcomas and B16 melanomas (25). However, protection against these tumors relies on the iNKT cell-dependent activation of NK cells (25, 38, 39). In our hands, both DN and CD4+ sorted hepatic iNKT cells exhibited direct GAg-dependent EL4 lysis (Figure S6). Although CD4+ iNKT cells showed slightly greater killing, this difference was not statistically significant. These findings are concordant with the striking similarity in the transcriptional profiles of liver CD4+ and CD4− iNKT cells (40) and suggest that despite their differences in promoting indirect NK cell-mediated antitumor responses, both CD4+ and DN iNKT cell subsets are effective as direct killers of CD1d+ tumor cells.
Optimal in vitro iNKT cell killing requires perforin expression
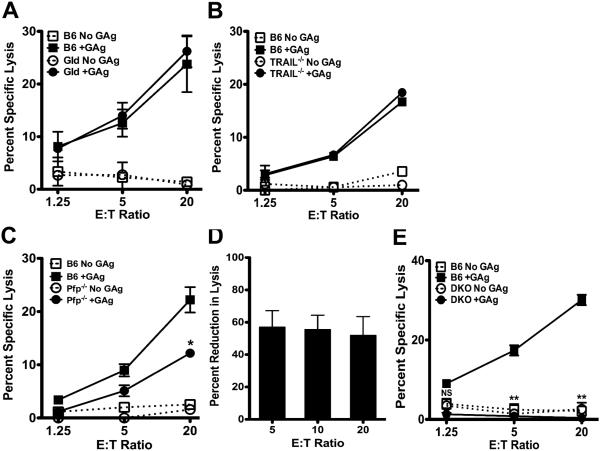
It was reported that iNKT cell control of CD1d-transfected A20 B leukemia cells relied on Fas:FasL interactions (41). To better understand the mechanisms underlying iNKT cell killing of EL4s, we performed killing assays using perforin- (Prf1−/−), FasL- (Gld) and TRAIL-deficient (Trail−/−) iNKT cells, each of which exhibited a normal surface phenotype indicative of full maturation. Gld and Trail−/− iNKT cells killed EL4 targets with magnitudes similar to WT iNKT cells (Figure 3A & B). In contrast, Prf1−/− iNKT cells exhibited reduced cytotoxicity that was on average 60% less than WT iNKT cells (Figure 3C & D). Residual cytotoxic function was fully abolished when iNKT cells lacked both perforin and FasL (double knock out; DKO) (Figure 3E). Thus, iNKT cells preferentially use perforin to kill EL4s; however, they can also utilize FasL when perforin levels are limited.
Figure 3. Optimal in vitro iNKT cell cytotoxicity requires perforin and FasL expression.
Killing of EL4s by hepatic iNKT cells from B6 (squares), or mutant (circles) Gld (A), Trail−/− (B), Prf1−/− (C), or Gld Prf1−/−(DKO, E) mice, in the presence (closed symbols) or absence (open symbols) of PBS44. Asterisks indicate significance between lysis by WT versus mutant iNKT cells in the presence of PBS44. (D) Mean reduction (± SEM) in killing by in Prf1−/− iNKT cells, averaged across 5 experiments. Data in (A-E) are representative of ≥ 2 experiments/genotype.
iNKT cells are sufficient for the perforin-dependent control of EL4 growth in vivo
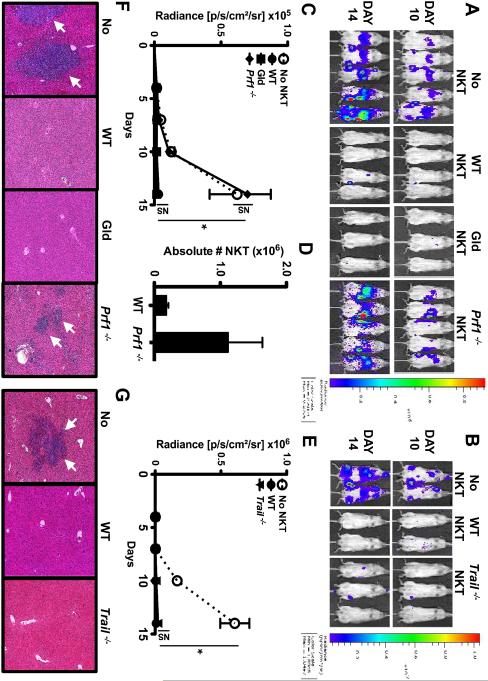
Having demonstrated that iNKT cells kill EL4s in vitro, we next assessed whether they similarly controlled EL4 growth in vivo and examined the underlying mechanisms of action. To ensure that antitumor responses were solely attributable to iNKT cells, we used NOD-SCID IL2Rγ−/− (NSG) mice which lack endogenous T, B and NK cells. We first reconstituted NSG mice with saline (No NKT) or with WT, Gld, Prf1−/− or Trail−/− iNKT cells. After 4 days, mice were challenged with luciferase-expressing EL4s (EL4-LUCs). Like parental EL4s, EL4-LUCs express CD1d and are susceptible to in vitro GAg-dependent iNKT cell-mediated lysis (not shown). Tumor burden was serially evaluated using bioluminescent imaging and mice were euthanized 14 days after EL4-LUC injection. EL4-LUCs rapidly engrafted in the bone marrow, livers, and spleens of No NKT mice, leading to readily detectible bioluminescent signals after 10 days (Figures 4A-C & E). Compared to No NKT mice, NSG mice reconstituted with WT, Gld or Trail−/− iNKT cells exhibited significantly lower bioluminescence (Figures 4A-C & E) and few to no tumor cells in the liver (Figures 4F & G). In contrast, Prf1−/− iNKT cells did not confer protection from tumors, resulting in an increased bioluminescent signal identical to that of No NKT mice (Figures 4A, C & F). We observed that Prf1−/− iNKT cells accumulated in tumor-bearing organs of NSG mice to a slightly greater extent than that seen following the adoptive transfer of WT iNKT cells (Figure 4D and data not shown). These data indicate that Prf1−/− iNKT cells can track to the site of tumors and/or expand in situ, but nonetheless they fail to limit tumor growth.
Figure 4. iNKT cells mediate perforin-dependent control of EL4 growth in vivo.
NSG mice were injected intravenously with saline (No NKT) or 4×105 sort-purified WT Gld, Prf1−/−, or Trail−/− iNKT cells. Four days later (Day 0), mice were challenged intravenously with 1×105 PBS44-loaded EL4-LUCs. (A, B) Representative total body bioluminescence on days 10 and 14. (C) Average radiance (photons/sec/cm2 ×105) ± SD among cohorts in (A). (D) Absolute number of hepatic iNKT cells (×106) from mice receiving WT or Prf1−/− iNKT cells. (E) Average radiance (photons/sec/cm2 ×106) ± SD among cohorts in (B). (F, G) H & E-stained liver sections from a representative experiment (5X; arrows indicate tumor cells). Data are representative of ≥ 6 mice/cohort across 2 independent experiments.
iNKT cells control tumor growth in vivo in the absence of exogenous GAg
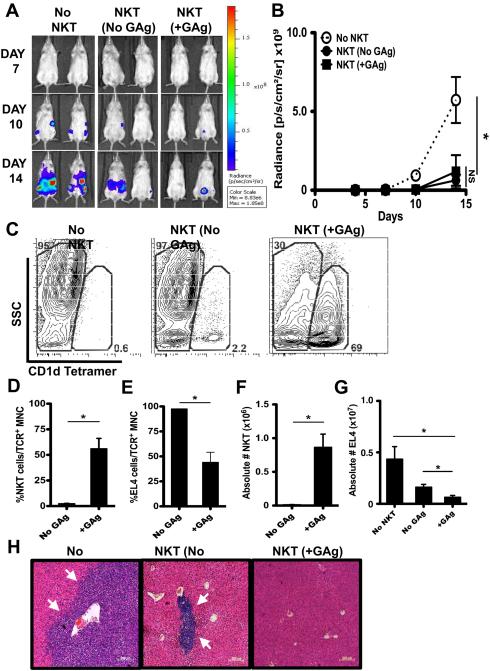
In these in vivo experiments, EL4-LUCs must undergo multiple rounds of division and it seemed unlikely that they would retain sufficient levels of exogenous GAgs to stimulate iNKT cell responses. To further examine whether tumor control relied upon the loading of EL4-LUCs with agonistic GAgs, NSG mice were reconstituted (NKT) or not (No NKT) with iNKT cells and challenged with GAg-loaded (+GAg) or unloaded (No GAg) EL4-LUCs. Consistent with our prior results, EL4-LUCs rapidly engrafted in the organs of No NKT mice (Figure 5A, B, H). To our surprise, the bioluminescent signal and distribution of tumor foci were similarly and significantly reduced in iNKT cell-reconstituted mice, regardless of whether or not the EL4-LUCs had been pre-loaded with GAg (Figure 5A, B, H), although No GAg mice did retain a higher percentage and number of EL4-LUCs (Figure 5E, G) and a lower percentage and number of iNKT cells (Figure 5D, F).
Figure 5. iNKT cells control EL4 growth in vivo in the absence of exogenous GAg.
NSG mice were injected with saline (No NKT) or 4×105 sort-purified hepatic iNKT cells (NKT). Four days later (Day 0), NKT mice were challenged with 2×105 PBS44-loaded (+GAg) or unloaded (No GAg) EL4-LUCs. No NKT mice received 2×105 GAg-unloaded EL4-LUCs. (A) Representative total body bioluminescence on days 7, 10 and 14. (B) Radiance (photons/sec/cm2 ×109) ± SD among cohorts. (C) Flow cytometric analyses of hepatic mononuclear Thy1+TCR+ cells with representative contour plots from 1 mouse within each cohort. CD1dTet− side scatter (SSC)hi cells represent EL4s while CD1dTet+ SSClo cells represent iNKT cells. Average percentage (D, E; of TCR+ cells) and absolute number (F, G) ± SEM of iNKT cells (×106) and EL4-LUCs (×107). (H) Representative H & E-stained liver sections (5X; arrows indicate tumor clusters). Results are representative of ≥ 8 mice/cohort across 3 independent experiments.
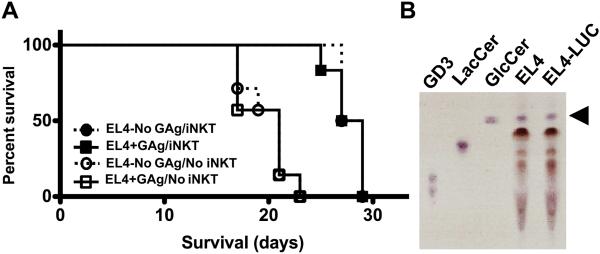
To probe the biological relevance of these findings, we examined the effects of iNKT cell adoptive transfer and tumor GAg-loading on survival. Compared to No NKT mice, which became moribund by 19-21 days after EL4 challenge (Figure 6A), iNKT cell reconstitution (NKT) significantly extended survival (p<0.001). Again, there was no statistical difference in survival between the No GAg and +GAg NKT cohorts (p>0.8). In these in vivo studies, we found few to no NK cells in the organs of NKT mice (Figure S7 and not shown), indicating that contaminating NK cells were not likely to have contributed significantly to tumor control.
Figure 6. Adoptive transfer of iNKT cells extends survival of tumor-bearing NSG mice.
NSG mice were injected with saline (No NKT; open symbols) or 4×105 sort-purified hepatic iNKT cells (NKT; closed symbols), challenged with 1×105 PBS44-loaded (+GAg; squares) or unloaded (No GAg; circles) EL4-LUCs and monitored daily. (A) Kaplan-Meier analyses showing percent survival among the cohorts. Data are from 9 mice/cohort across 2 independent experiments. (B) Thin layer chromatograph of lipids from EL4 and EL4-LUC cells with GD3 and cow’s milk lactosylceramide (LacCer) controls; the arrowhead depicts β-GlcCer.
These in vivo findings were surprising as our prior studies showed that in vitro iNKT cell cytotoxic responses were dependent strictly on the presence of exogenous GAgs. Based on these results, we questioned whether endogenous lipids might be facilitating iNKT cell clearance of GAg−unloaded tumors in vivo. To address this notion, we used thin layer chromatography to probe the lipids expressed by EL4 cells and found that they contained readily detectable levels of β-glucosylceramide (β-GlcCer) (Figure 6B), an endogenous lipid recently shown to induce iNKT cell cytokine production (29). Together, these findings suggest that iNKT cell recognition of endogenous GAgs such as β-GlcCer might be sufficient to limit the growth of EL4 tumors in vivo.
Optimal in vivo tumor control requires intact TCR-CD1d interaction
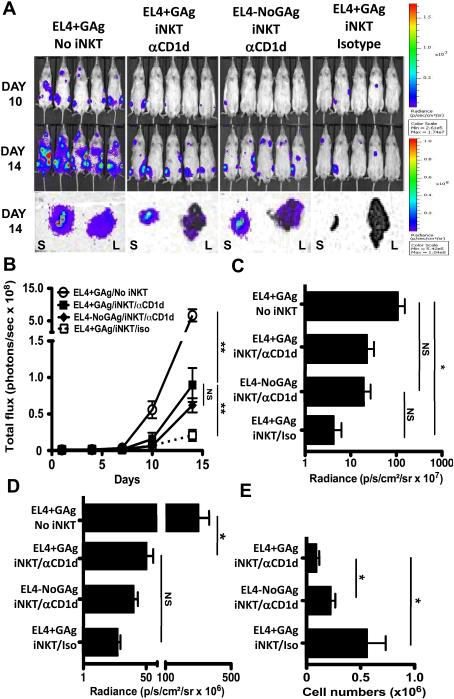
Given the comparable protection against GAg-loaded and unloaded tumors, we sought to determine whether in vivo iNKT cell responses depended on TCR-CD1d interactions. Therefore, we reconstituted NSG mice with iNKT cells (iNKT), challenged them with GAg-loaded (EL4+GAg) or unloaded (EL4-NoGAg) EL4-LUCs, and then treated mice with a blocking anti-CD1d (αCD1d) or isotype control antibody (Iso). Blockade of CD1d significantly blunted tumor protection, as evidenced by increased total body and organ-specific bioluminescence (Figures 7A-D). Of note, we observed no significant reduction in CD1d expression on EL4-LUCs in isotype or anti-CD1d antibody-treated iNKT mice (data not shown), suggesting that CD1d down-regulation is not a prevalent mechanism of iNKT cell immune evasion in this system. However, compared to Iso mice, the number of iNKTs in the livers of αCD1d mice was significantly decreased (Figure 7E). Thus, CD1d blockade may have inhibited tumor control by interfering with iNKT cell cytotoxic responses, and perhaps also iNKT cell homing, retention and/or proliferation at the tumor site.
Figure 7. Optimal control of tumor growth in vivo depends on TCR:CD1d interactions.
NSG mice were injected with saline (No iNKT) or 4×105 sort-purified hepatic iNKT cells (iNKT) and 4 days later challenged with 1×105 PBS44-loaded (+GAg) or unloaded (No Gag) EL4-LUCs. iNKT cell-injected NSG were treated with anti-CD1d (αCD1d) or an irrelevant isotype-matched control antibody (Iso). (A) Total body bioluminescence of individual mice on days 10 and 14 and organ bioluminescence of livers (L) and spleens (S) of representative mice at takedown. (B) Quantitation of total body radiance (photons/sec ×108) ± SD at indicated days. Average radiance of livers (C; photons/sec/cm2 ×107) and spleens (D; photons/sec/cm2 ×106) ± SD from each cohort. (E) Absolute numbers of CD1dTet+ SSClo hepatic iNKT cells (±SEM) in iNKT cell-injected NSG mice. Results are from 1 experiment with 5 mice per cohort.
Discussion
The contribution of iNKT cells to antitumor immunity has been appreciated for over a decade but the mechanisms by which iNKT cells control tumors have not been fully elucidated. To shed light on this issue, in this study we methodically examined the contribution of iNKT cell cytotoxic responses to the control of tumor growth in vitro and assessed the biological relevance of iNKT cell cytotoxicity to tumor immune surveillance in vivo. Using systems designed to discern the effects of iNKT cells from those of other cytolytic effectors, we definitively demonstrate that TCR-induced cytotoxicity is an inherent function shared by multiple iNKT cell subsets. We also show that iNKT cell cytotoxic activity is TCR and CD1d-dependent and serves to efficiently kill a variety of CD1d+ tumors. Although maximal in vitro lysis required exposure of iNKT cells to agonistic lipids such as α-GalCer and its analogues PBS44 and PBS57, in vivo lysis of tumors occurred even in the absence of exogenously provided lipids. From these data, we conclude that iNKT cells are potent tumor killers whose lytic as well as stimulatory properties make them attractive candidates for inclusion in novel immune-based therapies of CD1d+ tumors.
Our studies revealed that in vitro and in vivo lysis of EL4s relies on iNKT cell expression of perforin. In contrast, a previous report implicated the Fas/FasL pathway in the iNKT cell-mediated control of CD1d-transfected A20 B-lymphoma cells (41). However, A20-CD1d cells express significantly higher levels of CD1d and Fas compared to EL4s (data not shown), and thus they may induce more FasL upregulation on iNKT cells and/or be more susceptible to FasL-mediated apoptosis. In our hands, loss of perforin expression did not fully abolish EL4 lysis in vitro, while loss of perforin and FasL did so completely. Therefore, perforin and FasL both contribute to iNKT cell cytotoxicity. Taken together, results from these studies imply that the mechanism of iNKT cell-mediated tumor control may be modulated by several variables, including the levels of cytotoxic proteins and ligands expressed by the iNKT cells, the apoptosis-inducing receptors borne by the targets, and other external factors that influence iNKT:target cell interactions.
Despite the fact that in vitro iNKT cell killing is robust only when cultures are supplemented with strongly agonistic GAgs, iNKT cells controlled EL4-LUC growth in vivo in the absence of these lipids. One possible explanation for this finding is that CD1d-mediated presentation of endogenous GAgs by tumor-associated DCs might induce iNKT cell cytotoxicity in vivo. Indeed in a prior report, the melanoma-derived ganglioside GD3 was cross-presented by CD1d+ DCs to iNKT cells and induced in vitro cytokine production (42). Using thin layer chromatography, we demonstrate that EL4s synthesize β-GlcCer, an endogenous lymphoid lipid that accumulates during infection and in response to Toll-like receptor activation (29). It was shown that CD1d-mediated presentation of β-GlcCer promotes iNKT cell production of cytokines such as IFNγ (29). Based on these observations, we speculate that in vivo iNKT cell protection against tumors may result from tumor or DC-mediated presentation of β-GlcCer to iNKT cells, which then stimulates iNKT cell cytokine production and/or cytotoxic responses.
Additionally, iNKT cells might kill tumors using TCR-independent mechanisms. Arguing in favor of this possibility, hepatic iNKT cells uniformly express NKG2D and a proportion of EL4s exhibit low-level expression of NKG2DLs. However, in our in vitro assay, ex vivo iNKT cells failed to kill a variety of GAg-unloaded tumor cells, including YAC-1, which uniformly express high levels of NKG2DLs (data not shown). It was reported that human peripheral blood α-GalCer expanded iNKT cells exert NKG2D-dependent lysis of targets (36). To assess whether in vitro TCR stimulation might secondarily induce TCR-independent mechanisms of murine iNKT cell killing, we examined the cytotoxicity of iNKT cells stimulated by GAg-loaded EL4s against GAg−unloaded EL4 bystanders. When stimulated in this manner, iNKT cells did not exert a significant level of cytotoxicity against unloaded EL4s. Collectively, these observations suggest that TCR-independent mechanisms do not contribute significantly to murine iNKT cell cytotoxicity in vitro. It is important to note that antibody-mediated interference of TCR-CD1d interactions only partially abrogated in vivo tumor control. While this latter finding might reflect an incomplete level of CD1d blockade, it also remains possible that TCR-independent mechanisms such as those mediated by NKG2D, or by other stimulatory receptors and/or specific cytokines, contribute to iNKT cell-dependent mechanisms of tumor control in vivo.
By virtue of their robust cytolytic and stimulatory activities, iNKT cells hold great potential to promote innate and adaptive immune responses to tumors. Aiming to capitalize on their antitumor activities, several clinical trials have examined whether the administration of α-GalCer (43), or α-GalCer-loaded DCs with (44) or without (21, 45-47) co-administration of iNKT cells or other immunostimulatory therapies (48), might prove beneficial in the treatment or prevention of cancer. These iNKT cell-based therapies are well tolerated, yet they induce only transient clinical responses (21, 44, 47). To improve upon these results, the challenge lies in better identifying the tumors against which iNKT cells wield their greatest effects and defining the factors that influence how iNKT cells recognize and respond to such tumors. Here we demonstrate that iNKT cells exert potent cytotoxic responses towards CD1d+ T-lymphoma cells. These studies thus form a foundation to further explore the nature of iNKT:tumor cell interactions with the aim to develop novel iNKT cell-based therapies for T-lymphoma as well as other CD1d-expressing tumors, which represent a significant proportion of human cancers.
Supplementary Material
Contributions and Acknowledgements
We thank Drs. Gary A. Koretzky, Martha S. Jordan, Taku Kambayashi, Scott W. Canna, Scott M. Lieberman, and Edward M. Behrens for their thoughtful review of the manuscript, Charles H. Pletcher at the University of Pennsylvania Flow Cytometry Core for technical advice, and the NIH Tetramer Core Facility.
Financial support: This work was supported by grants from The Clinical Immunology Society, Talecris Biotherapeutics (HB) and the NIH (T32AI007634, K12HD04335, K08CA166184 [HB]; R01HL089745 [KEN]).
Footnotes
Conflicts of interest: K.N. receives partial support for research on human NKT cells from Vaccinex, Inc.. The rest of the authors declare no conflict of interest.
HB designed, performed, analyzed the experiments, and wrote the manuscript. PJB and MB provided β-GlcCers and performed TLC. RD and KEN aided in experimental design, interpretation of results, and editing of the manuscript. PG and SJW helped carry out experiments. DMB, PB, SAG, and JSO provided technical assistance and reagents for specific assays.
References
- 1.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140:119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S-I, Dhodapkar KM, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–6499. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda K, Morii T, Nieda M, Tsukaguchi N, Amano I, Tanaka H, et al. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk Res. 2005;29:147–152. doi: 10.1016/j.leukres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneiders FL, de Bruin RC, van den Eertwegh AJ, Scheper RJ, Leemans CR, Brakenhoff, et al. Circulating invariant natural killer T-cell numbers predict outcome in head and neck squamous cell carcinoma: updated analysis with 10-year follow-up. J Clin Oncol. 2012;30:567–570. doi: 10.1200/JCO.2011.38.8819. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 10.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual Review of Immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 11.Carnaud C, Lee D, Donnars O, Park SH, Beavia A, Koezuka Y, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 12.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nature Reviews Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 14.Bellone M, Ceccon M, Grioni M, Jachetti E, Calcinotto A, Napolitano A, et al. iNKT cells control mouse spontaneous carcinoma independently of tumor-specific cytotoxic T cells. PloS one. 2010;5:e8646. doi: 10.1371/journal.pone.0008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, Smyth MJ. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–6385. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dao T, Mehal WZ, Crispe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–22. [PubMed] [Google Scholar]
- 21.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 22.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, et al. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- 24.Matsumoto G, Omi Y, Lee U, Nishimura T, Shindo J, Penninger JM. Adhesion mediated by LFA-1 is required for efficient IL-12-induced NK and NKT cell cytotoxicity. European J Immunol. 2000;30:3723–3731. doi: 10.1002/1521-4141(200012)30:12<3723::AID-IMMU3723>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellici DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118:3045–3053. doi: 10.1002/ijc.21764. [DOI] [PubMed] [Google Scholar]
- 28.Maltzman JS, Kovoor L, Clements JL, Koretzky GA. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J Exp Med. 2005;202:893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orange JS, Ramesh N, Remold-O'Donnell E, Sasahara Y, Koopman L, Byrne M, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Pro Natl Acad Sci USA. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F, et al. Distinct APCs explain the cytokine bias of alpha-galactosylceramide variants in vivo. J Immunol. 2012;188:3053–61. doi: 10.4049/jimmunol.1102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 33.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Seminars in Immunology. 2010;22:68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, III, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Expl Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. 2011;41:1913–1923. doi: 10.1002/eji.200940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 38.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani J, Taniguchi M, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 40.Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, et al. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol. 2013;14:90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wingender G, Krebs P, Beutler B, Kronenberg M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J Immunol. 2010;185:2721–2729. doi: 10.4049/jimmunol.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 44.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 47.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 48.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–430. doi: 10.1182/blood-2012-06-435503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.