1. Introduction
Daily rhythms are a common feature of living systems. Generally, these rhythms are not just passive consequences of cyclic fluctuations in the environment, but instead originate within the organism. In mammals, including humans, the master pacemaker controlling 24-hour rhythms is localized in the suprachiasmatic nuclei of the hypothalamus (SCN). This circadian clock is responsible for the temporal organization of a wide variety of functions, ranging from sleep and food intake, to physiological measures such as body temperature, heart rate and hormone release.
Experimental evidence has shown that the mammalian retina contains a complete circadian clock system –biochemical machinery that generates temperature-compensated 24 hour oscillations, an input pathway by which light synchronizes the cycling of the retinal clock to the environmental light/dark cycle, and neurochemical output pathways that transmit the clock’s influence throughout the retina and into the rest of the brain. The retinal circadian clock drives many processes within the retina including gene expression, synaptic communication and metabolism, which reconfigure retinal circuits and shape the functioning of the retina according to time of day. The circadian clock system in the retina allows the anticipation of the normal cycle of photopic and scotopic visual conditions that alternate with the cycling of solar day and night. Another important function of the circadian clock resides in the capability to act as the gate for sensory information, and this function is a fundamental property of sensory organs and systems in a wide variety of species. Here, we focus on the mammalian retinal circadian clock and recent advances in its understanding, while previous reviews have covered studies of circadian rhythms in non-mammalian retinas, where this line of research began (Besharse, 1982; Besharse, Iuvone and Pierce, 1988; Cahill and Besharse, 1995).
Several studies have clearly established that many aspects of mammalian retinal physiology and function are under the control of a retinal circadian clock (Figure 1). Melatonin release (Besharse and Iuvone, 1983; Tosini and Menaker 1996; Tosini and Menaker 1998), dopamine synthesis (Nir et al. 2000; Doyle et al. 2002), gamma-aminobutyric acid (GABA) turnover rate and release (Jaliffa et al. 2001), extracellular pH (Dmitriev and Mangel 2001), electroretinogram (ERG) b-wave amplitude (Barnard et al., 2006; Storch et al. 2007), rod disk shedding Teirstein et al. 1980), and UV opsin and rhodopsin gene expression (von Schantz et al., 1999,), are all regulated in a circadian manner. In addition, the mammalian retinal clock and its outputs influence cell survival and growth processes in the eye including the susceptibility of photoreceptors to degeneration from light damage (Organisciak et al. 2000; Grewal et al. 2006), photoreceptor survival in animal models of retinal degeneration (Ogilvie and Speck 2002), photoreceptor and retinal ganglion cell viability during in aging (Baba et al. 2009), and the degree of refractive errors in primate models of myopia (Iuvone et al. 1991). In addition, circadian signals originating in the retina drive rhythms in the hypothalamic biological clock, even in the absence of light/dark cycles (Lee et al. 2003).
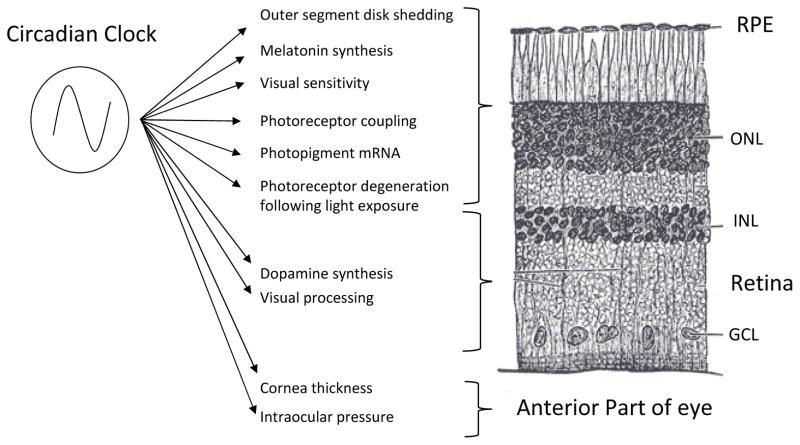
Figure 1. The circadian clock system in the mammalian retina controls several functions.
Many studies have shown that rhythms in the eye are under direct control of the retinal circadian clock system. Recent studies have also indicated that the many different cell types within the eye contain circadian clocks that interact to modulate many ocular functions. Shown here are several known circadian processes in the retina and the eye, with their approximate location identified by retinal layer. RPE = retinal pigment epithelium, ONL = outer nuclear layer, INL = inner nuclear layer, GCL = ganglion cell layer.
Additional experimental evidence also suggests that other ocular structures (e.g., retinal pigment epithelium and cornea) possess functional circadian clocks that control important functions such a phagocytosis, disk shedding and cornea thickness (Yoo et al., 2005; Baba et al., 2010). Interestingly, as seen in the SCN, it appears that the neural retina communicates the photic information to the other ocular structures via humoral signals (e.g., melatonin and dopamine) since these structures are not capable of direct light transduction (Baba et al., 2010).
2. Molecular Organization of the Retinal Circadian Clock
2.1 Molecular basis of mammalian circadian rhythms
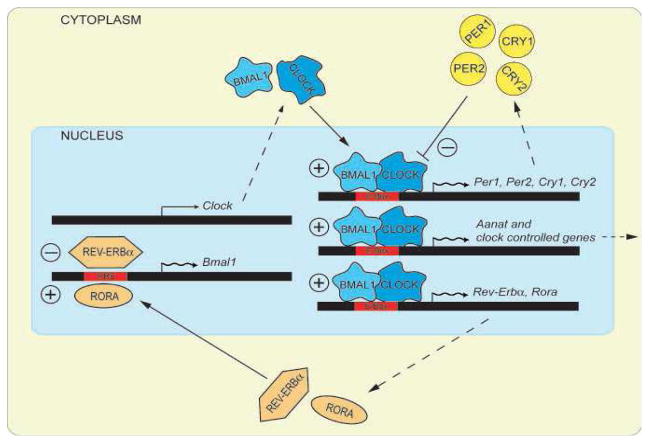
Circadian rhythms are generated by autoregulatory transcription/translation feedback-loops that generate near-24 hour cycles in gene expression and protein abundance (Takahashi et al. 2008). In mammals, while more than a dozen genes contribute to this network, the core functional elements are six genes that directly participate in the negative feedback loop of the clock mechanism (Figure 2). These genes are: the Period genes Per1 and Per2, the Crypotochrome genes Cry1 and Cry2, and the transcription factors Clock and Bmal1. CLOCK and BMAL1 are basic helix-loop-helix (bHLH) PAS domain transcription factors that form a heterodimeric transcription complex that periodically drives the expression of two Period genes (Per1, 2) and two Cryptochrome genes (Cry1-2) by binding to circadian E-box (CACGTG) enhancer elements in their promoters. Once, transcribed and translated, and the resulting PER and CRY proteins also form heteromeric complexes that translocate back into the nucleus to suppress their own transcription by inhibiting the action of CLOCK and BMAL1, forming the fundamental negative feedback loop at the center the molecular circadian clock (Takahashi et al. 2008). A second feedback loop involves the transactivation of the Rev-Erbα and Rora genes by CLOCK/BMAL1. The protein products of these genes compete for binding to RRE elements in the Bmal1 promoter, driving a daily rhythm of Bmal1 transcription and closing the second feedback loop (Figure 2).
Figure 2. The molecular mechanism generating the circadian oscillation is composed of two feedback loops.
In the positive feedback loop the transcription factors Bmal1 and Clock activate the transcription of Period (Per) and Cryptochrome (Cry) genes, as well as that of clock-controlled genes (CCGs). In the cytoplasm, PERIOD and CRYPTOCHROME proteins heterodimerize, enter the nucleus and interact with BMAL1/CLOCK, inhibiting the transactivation of their own promoters (negative feedback). BMAL1 and CLOCK also stimulate the transcription of Rev-Erba and Rora (Adapted from Tosini et al., BioEssays, 2008).
Several studies also support the notion that there is some redundancy among the Per genes and Cry genes and an absolute requirement for Bmal1, when assayed using disruption of behavioral activity/rest cycles as an endpoint (van der Horst et al. 1999; Vitaterna et al. 1999; Bunger et al. 2000; Bae et al. 2001). In addition, the clock gene network drives extensive, tissue-specific transcriptional networks of clock-controlled genes (CCGs) that regulate rhythmic physiology and metabolism (Takahashi et al. 2008).
2.2. Expression of clock genes in the retina
Clock genes are widely expressed in the retina and early studies mostly focused on the overall expression of these important molecular components of circadian oscillators in the retina (Peirson et al., 2006, Khampuis et al., 2005; Tosini et al., 2007a; Sandu et al., 2011; Schneider et al., 2010). These studies indicated that the core genes were expressed in the neural retina with a profile not different from those reported from other tissues. However, it is important to mention that this type of approach has not provided much insight into the functioning of retinal circadian clocks because the retina is a highly heterogeneous tissue with circadian clocks in several types of cells. In situ hybridization studies have shown that clock genes may be expressed in different cell types and possibly with different phases (Tosini et al., 2008). Therefore, measuring mRNA levels from the whole retina can only provide an overall average picture of what is likely a tissue with multiple circadian oscillators organized in cellular networks.
With regard to the cell-specific expression patterns, in rat retina Period1 (Per1) mRNA levels are low in photoreceptors and more abundant in the inner retinal neurons (Namihira et al., 2001). A similar pattern has been observed with Bmal1 and Clock mRNAs (Namihira et al., 1999). Two recent studies using laser capture microdissection (LCM) combined with RT-PCR support these early findings by demonstrating that low levels of most of the clock genes are present in rat photoreceptors (Tosini et al., 2007b). In one study it was reported that the mRNA levels for Clock, Bmal1, Per1, Per3, Cry2 and Casein kinaseIε (CKIe)variations during 24 hrs in rats maintained in 12light:12dark cycle, whereas after 36 hours in DD only Clock and Per3 showed a significant variation during the 24-hr period (Schneider et al., 2010). An additional study using the same experimental approach mostly confirmed previous work (Sandu et al., 2011). Interestingly, they also reported that the mRNA levels in two CCGs in the photoreceptor layers (i.e., Aanat and c-Fos) were still rhythmic after 36 hrs of DD (Sandu et al., 2011). Such results suggested that although the core clock genes are rhythmically expressed in the photoreceptors of rat maintained in light:dark cycles, these genes were not rhythmically transcribed in constant darkness. Although these results question the presence of a circadian clock in the rat photoreceptors, it must be mentioned that the cultured photoreceptor layer can express circadian rhythms in Per1-luc bioluminescence and melatonin synthesis (Tosini et al., 2007b). However, since in this preparation some inner retinal neurons are still present (less than 5%) we cannot completely exclude that the circadian rhythms in these photoreceptor layers could be driven by these surviving inner retinal neurons.
In the mouse retina, the situation is much more complicated and the results obtained are rather contradictory. Initially, it was reported that Per1, Clock, and Bmal1 mRNAs were present and co-localize in photoreceptors and the inner retinal neurons (Gekakis et al., 1998). Additional studies reported that photoreceptors do (Yujnovsky et al., 2006, Dinet et al., 2007) or do not express (Witkovsky et al., 2003, Storch et al., 2007) Per1 transcripts. More recently Ruan et al. (Ruan et al., 2006), using single cell RT-PCR, detected expression of circadian clock gene transcripts in a small percentage of photoreceptors. However, co-expression of the six core clock genes (Per1, Per2, Cry1, Cry2, Clock, Bmal1) within the same photoreceptor cells was not detected. In contrast, inner retinal neurons, and in particular DA neurons, co-express all of these clock genes (Witkovsky et al., 2003, Gustincich et al., 2004, Ruan et al., 2006, Dorenbos et al., 2007). Whether the failure to detect the full complement of clock genes in photoreceptors represents limits of detection or a lack expression is currently unknown.
A recently published article, in which LCM and qRT-PRC was also used, reported that all of the core clock genes are rhythmically expressed in the photoreceptors but not in the inner retina of the mouse (Dkhissi-Benyahya et al., 2013); unexpectedly the circadian rhythm in gene expression was lost in mice lacking melanopsin (Opn4 knock-out) and the photic induction of Per1 and Per2 in the outer retina was also lost (Dkhissi-Benyahya et al., 2013). Thus melanopsin and its signaling is somehow involved in the functioning of the clockwork in the photoreceptors.
A recent study has investigated the expression of circadian clock proteins in the mouse retina (Liu et al., 2012). In this paper the authors report that the core circadian clock proteins (CLOCK, BMAL1, NPAS2, PERIOD1, PERIOD2 and CRYPTOCHROME 2) are expressed in the outer and inner retina, but only in the cone photoreceptors do these proteins show a diurnal and circadian variation. Hence it is possible to speculate that a circadian clock is in the cone photoreceptors.
In conclusion although several studies have investigated the presence of circadian clock genes (mRNA and protein) in the mammalian retina the results are contradictory and not conclusive about the type(s) of retinal cells that contain the circadian clock. Interpretation of these studies is complicated by the fact that they have used techniques with widely differing limits of detection (e.g. in situ vs. RT-PCR, vs. LCM RT-PCR, vs single cell RT-PCR, vs. immunocytochemistry), and different methods of assessing the independence of cell populations (pharmacology vs. physical lesions vs. genetic lesions). Resolution of the existing ambiguities may lie in assays of individual cell rhythms in isolated cell culture to establish not just which retina cell types express clock genes, but which can act as functional circadian pacemakers.
2.3. Clock gene reporters as tools to understand retinal circadian organization
Additional studies have used rats or mice in which clock gene promoters or chimeric clock proteins are linked with luminescence or fluorescence reporters that provide an optic signal to measure the abundance of the gene. These new animal models, have been especially successful as real-time read-outs of circadian clock cycling in living tissues (Hastings et al. 2005). These “clock gene reporters” have the advantage of monitoring components of the clock mechanism itself, rather than downstream neurochemical or physiological rhythms. In particular, real-time gene expression assays of the retina using transgene reporters that use bio-luminescent fire-fly luciferase have proven successful, as well as one using short half-life green fluorescent protein (GFP). The three reporter transgenics used most for experiments with mammalian retina are the Per1::Luc rat (Yamazaki et al., 2000), PER2::LUC mouse (Yoo et al., 2004), and Per1::GFP mouse Kuhlman et al., 2000). Cultured retinas, isolated from transgenic mice and rats harboring luciferase-based clock gene reporters (Per1::LUC; PER2::LUC), demonstrate robust, persistent circadian cycles in bio-luminesce (Ruan et al. 2006; Ruan et al. 2008; Ruan et al. 2012, Figure 3), both confirming the endogenous nature of the retinal circadian clock, as well as providing the means to examine the organization of the retinal circadian clock at the molecular level. Using this new technique, it has been possible to demonstrate that a circadian clock is present in the outer retina of the rat (Tosini et al., 2007) and in the inner retina of the mouse (Ruan et al., 2006; 2008). In addition, this approach has been successfully used to demonstrate the presence of circadian clock in the RPE and cornea (Yoo et al., 2005; Baba et al., 2010). These extra-retinal circadian clocks are also independent from the SCN, and the RPE clock requires retinal input to remain entrained to the light:dark cycle (Baba et al.., 2010). An advantage of these real-time reporters is that they assay functional gene rhythms, rather than just the presence of clock genes.
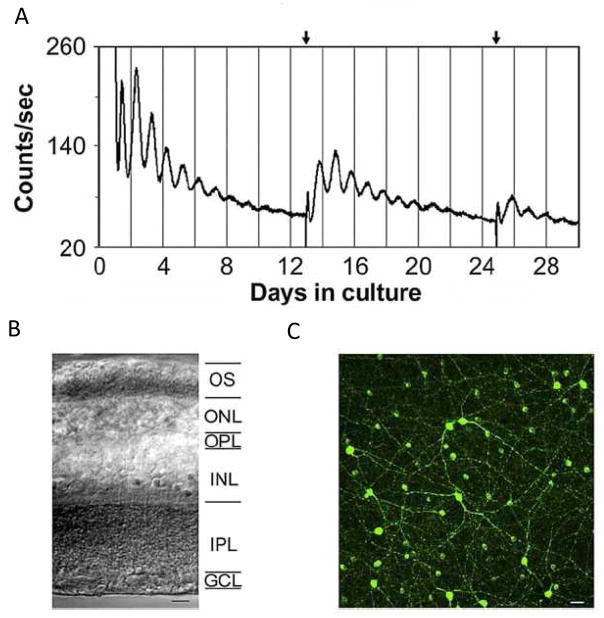
Figure 3. Bioluminescence Rhythms from mPer2Luc Mouse Retinal Explants.
(A) Long-term culture of an intact mouse retinal explant showing persistent circadian rhythms in PER2::LUC expression. Arrows indicate times of media changes. (B) Representative DIC image of vertical retinal sections. Retinal explants were cultured, and vertical retinal slices were cut with a tissue slicer at 150 μm. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, photoreceptor outer segments. (C) Flat-mount view showing tyrosine hydroxylase immunoreactivity in cultured retinal explants. The immunoreactive cells with relatively large somata and two to three thick primary processes that arise from the cell body are dopaminergic amacrine cells, whereas the immunoreactive cells with relatively small cell body and very few processes are type 2 catecholamine amacrine cells. (adapted from Ruan et al., , Plos Biol. 2008).
While most circadian oscillators utilize CLOCK/BMAL1 heterodimers to drive the expression of clock genes and CCGs, neuronal PAS domain protein 2 (NPAS2; also known as MOP4), is a CLOCK homolog that is also capable of heterodimerizing with BMAL1 and activating circadian E-box promoter elements (Reick et al., 2001). NPAS2 has overlapping roles with CLOCK in the suprachiasmatic nucleus and some peripheral clocks (DeBruyne et al., 2007). An NPAS2 reporter mouse has been generated in which the bHLH domain has been replaced with lacZ; the mouse produces an NPAS2-lacZ fusion protein that is a functional null because it lacks the DNA binding domain (Garcia et al., 2000). Using lacZ histochemistry and β-galactosidase immunohistochistry this fusion protein has been localized to a subset of retinal ganglion cells in the mouse retina, with no detectable expression in any other retinal cell type (Hwang et al., 2013). This suggests that NPAS2 may play a unique role in retinal ganglion cell circadian clocks.
2. 4 Genetic disruption of the clock gene network disrupts retinal circadian rhythms
The molecular organization of circadian clocks has been approached primarily by knockout of clock genes in mice followed by assays of behavioral or tissue rhythmicity. Individual clock genes have been defined as essential for clock function if circadian rhythmicity was lost following knockout. Functional redundancy was also explored by combining knockout mouse lines to produce double or even triple gene knockouts. In general, these types of experiments have demonstrated that the roles of individual clock genes varies across tissues, suggesting that there may be tissue-specific variants of the core circadian clockworks (Liu et al. 2007; Pendergast et al. 2010).
Which clock genes are essential for expression of molecular circadian rhythms by the retina? How does the molecular organization of other neural and non-neural clocks compare with the retinal circadian clock? Using the electroretinogram (ERG) circadian rhythm as a functional assay, initial studies found that knockout of Bmal1, or of the two Cryptochrome genes, Cry1 and Cry2, each ablated rhythmicity in the photopic ERG (Storch et al. 2007; Cameron et al. 2008). In addition, loss of Bmal1 disrupted circadian rhythms of retinal gene expression, consistent with its role as a core clock gene (Storch et al. 2007). Further studies, using retinas explanted from PER2::LUC and Per1::LUC mouse retinas, showed that in addition to Bmal1, the Per1, Cry1 and Clock genes are each required individually in order for the retina to express molecular circadian rhythms; whereas Per2, Per3 and Cry2 are dispensable (Ruan et al. 2012). Interestingly, the suprachiasmatic nuclei explanted from the same animals as the retinal explants, showed qualitatively similar, but quantitatively different effects of the same gene knockout. Knockout of Per1, Cry1 or Clock decreased robustness of molecular SCN rhythms, but the disruption was less dramatic than the effect of those same gene knockouts on retinal molecular rhythms – only knockout of Bmal1 alone can abrogate SCN molecular rhythms. While, the effects of gene knockout on the robustness of these two neural circadian clocks differed only in degree, the effects on the intrinsic period of molecular rhythms were divergent. Knockout of Per1 or Per3 shortened the circadian rhythm of retinas, whereas knockout of Clock lengthened retinal period. In contrast, Per1 knockout in the SCN lengthened circadian period, while period length of Per3 and Clock knockout SCN was unaffected. Thus, the retinal neural clock has a unique clock gene dependence, with the amplitude and robustness of molecular rhythms more vulnerable to gene knockout than the SCN clock, and with divergent regulation of rhythmic period by the Period genes (Figure 4).
Figure 4. Differential Clock Gene dependence of Retinal and SCN Neural Circadian Clocks.
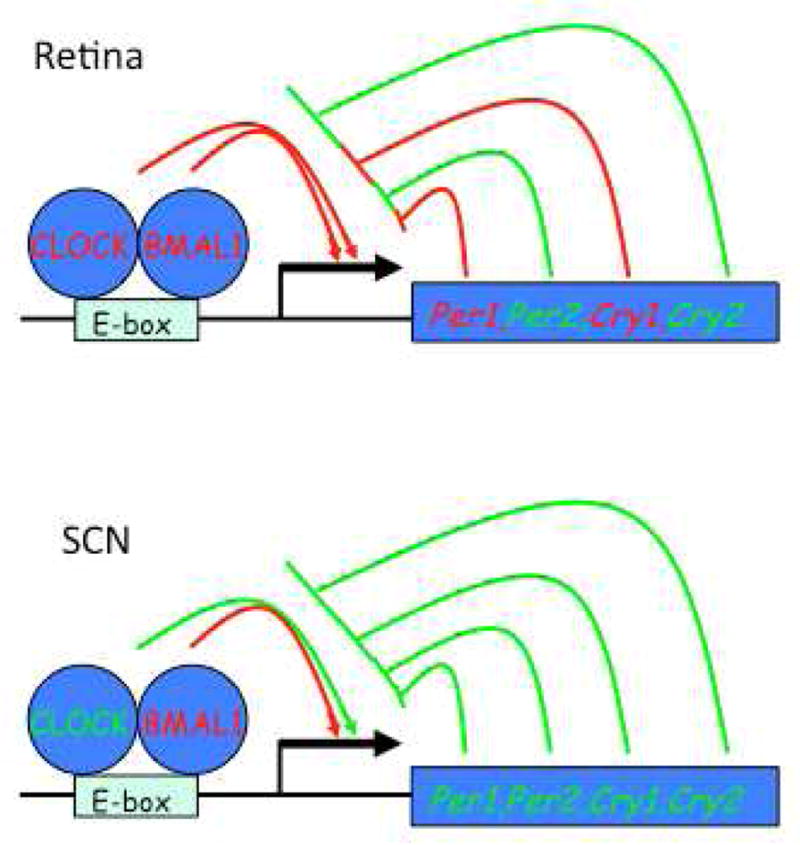
Simplified core clock gene diagrams as in Figure 1. Genes and action lines shown in red depict genes which when knocked out individually severely attenuate clock function in retina or SCN, while knockout of those shown in green has minimal effect. Note that the retinal clock is more vulnerable to single gen knockouts than the SCN.
One explanation for the increased effects of clock gene knockouts in the retina could be that neurochemical coupling of neurons is weaker in the retina than in the SCN, where it has been suggested that coupling buffers against genetic perturbation of the clock (Liu et al. 2007). This suggests that the retinal clock may in general be more vulnerable to gene mutations, and that there may be disturbances in retinal rhythms in human or animal genotypes in which SCN-driven rhythms appear normal. It should be noted however, that this tissue-level analysis leaves open the question of whether loss of rhythmicity in knockout retinas is due to cell-autonomous effects on cellular clocks or to the desynchronization of clock cells.
3. Cellular Organization of the Retinal Circadian Clock
3.1.Generation of retinal circadian rhythms is distributed among many cell types
The forgoing experiments measured gene expression in whole tissue retinal explants – treating the retina as a homogenous tissue with regard to clock function. Of course, the retina is not at all homogeneous but consists of 6 classes of neurons – rods, cones, horizontal cells, bipolar cells, amacrine cells and ganglion cells, each with multiple subtypes – exquisitely ordered in 3 nuclear and two synaptic layers. Which retinal cell types then generate circadian rhythms, i.e. which cells are the clock cells of the retina?
To address this question, two general approaches have been used: the cell-specific distribution of clock gene expression in the retina was mapped to determine which cell types expressed the genetic components of the circadian clock, and the ability of cell-types or retinal layers to generate circadian rhythms in isolation was examined. In non-mammalian vertebrates, circadian clock gene expression is concentrated in the outer nuclear layer where the photoreceptors are located, as typified by the African Clawed frog Xenopus laevis, in which isolated retinal photoreceptor layers continue to express circadian rhythms in melatonin synthesis (Cahill and Besharse 1993), and in chicken photoreceptors, which express rhythms in opsin gene synthesis (Pierce et al. 1993) in ion channel activity (Ko et al. 2004), and cyclic AMP accumulation (Ivanova and Iuvone, 2003; Chaurasia et al., 2006). The retinas of many species of fish exhibit circadian rhythms in retinomotor movements of cones (Burnside, 2001), a phenomenon absent from mammalian retinas. Photoreceptor-specific expression of a dominant negative form of the Clock gene, which breaks the circadian transcription/translation feed back loop, blocks the rhythms of melatonin synthesis in frog retinas (Hayasaka et al. 2002), with most of this effect apparently due to abrogating clock cycling in rods (Hayasaka et al. 2010). Thus, in non-mammalian retinas, there is compelling experimental evidence that photoreceptor cells contain an endogenous circadian clock that drives circadian rhythms within these cells.
The circadian organization of the mammalian retina is more complex and is still unclear which retinal cells harbor a circadian clock. As seen in Xenopus and chicken retinas, rhythmic melatonin synthesis occurs in the photoreceptors in vivo (Liu et al., 2004) and in vitro (Tosini et al., 2007b) hence suggesting that photoreceptors are putative clock cells in the mammal as well. However, clock gene mRNAs and proteins seem to be predominantly concentrated in the inner nuclear layer (Gekakis et al. 1998; Miyamoto and Sancar 1998; Ruan et al. 2006; Liu et al. 2012) and their levels in the photoreceptors are low or absent (Ruan et al., 2006, Dorenbos et al., 2007).
Several inner retina cell types (i.e., horizontal cells, bipolar cells, dopaminergic amacrine cells, and ganglion cells) express most of the core clock genes (Ruan et al. 2006). The cell type with the highest rate of clock gene expression is the dopaminergic amacrine cell (Ruan et al. 2006; Dorenbos et al. 2007) and since dopamine is secreted with a circadian rhythm it has been proposed that these cells are the loci of the retinal circadian clock (Ruan et al., 2006, Dorendos et al., 2007). This hypothesis is supported by strong experimental data demonstrating that they express key clock components at the protein level (Witkovsky et al. 2003; Gustincich et al. 2004; Dorenbos et al. 2007). Although this experimental evidence would suggest that these dopaminergic neurons contain a circadian clock that via dopamine release regulates additional circadian rhythms within the retina, it is important to mention that in melatonin deficient mice the daily rhythm in dopamine, or its metabolites, does not persist in constant darkness (DD) whereas it does in melatonin-synthesizing mice (Doyle et al., 2002a). Also, the Aanat mRNA circadian rhythm, which in part drives the rhythm of melatonin synthesis, is still present in rat retinas in which dopaminergic neurons have been destroyed and dopamine levels are low and not rhythmic (Sakamoto et al., 2006). The independence of the circadian rhythm in melatonin synthesis from dopamine has been also observed in chicken (Zalwiska and Iuvone, 1992; Thomas et al., 1993). Thus these results indicate that melatonin is required for the circadian regulation of DA release, but DA is not required for the circadian regulation of melatonin synthesis. It is important to point out that although circadian rhythms in dopamine content are absent from mouse retinas lacking melatonin (Doyle et al., 2002a), a circadian rhythm in dopamine signaling is still likely maintained in these retinas due to rhythmic expression of the gene coding for the dopamine D4 receptor (Jackson et al., 2011).
Another possible explanation for the somewhat contradictory results relating melatonin and dopamine in retinal circadian organization can be found in the observation that other inner retinal neurons may be the loci for circadian clock and drive circadian rhythmicity in the retina. Among other identified amacrine cell types, the nitric oxide synthase (NOS) expressing amacrine cells also express the Per1::GFP reporter rhythmically, as do the broad class of GABA secreting amacrine cells, which include the dopamine neurons (Zhang et al. 2005). In contrast, the glycinergic amacrine cell class does not express the Per1::GFP clock gene reporter rhythmically (Zhang et al., 2005). Thus, there is substantial evidence that GABAergic retinal amacrine cells, which comprise the majority of amacrine cells in the mammalian retina including the dopamine neurons, express circadian rhythms in clock gene abundance in the intact retina.
The other inner nuclear layer neuronal cell types, horizontal cells and bipolar cells, also express the core circadian clock genes (Ruan et al., 2006; Liu et al. 2012). Whether the gene transcripts cycle rhythmically in these cells as well has not been tested. However, imaging of a bioluminescent reporter of PER2 has shown that the circadian cycling of gene abundance occurs across the entire depth of the inner nuclear layer, and is therefore likely to be produced by all three neuronal cell types of that layer – horizontal cells, bipolar cells, and amacrine cells, and possibly by Müller glial cells.
Retinas obtained from mice in which the photoreceptors have degenerated (rd/rd), but with an intact inner nuclear layer, continue to generate molecular circadian rhythms of the bioluminescent PER2 reporter gene, and other clock genes as well (Ruan et al. 2006). In addition, retinas from a mouse strain in which melatonin synthesis is genetically disrupted also show robust circadian gene expression rhythms similar to strains that can synthesize melatonin (Ruan et al. 2008). Thus, neither the physical presence of the photoreceptors, nor their secreted circadian neuro-hormone melatonin, is necessary for the inner retinal expression of molecular rhythms.
The clock genes are also expressed in some ganglion cells in the mammalian retina, including the intrinsically photoreceptive retinal ganglion cells (ipRGCs) that communicate the photic information to the SCN. These ganglion cell photoreceptors exhibit daily rhythms in the expression of the melanopsin pigment gene that are influenced by light and dopamine (Sakamoto et al. 2004; Sakamoto et al. 2005; Mathes et al. 2007), suggesting that they may be clock neurons generating endogenous circadian rhythms. Indeed, there are daily rhythms in the responsiveness of ipRGCs to light (Weng et al. 2009), and in the amplitude of the pupillary light response driven by these cells (Zele et al. 2011), indicating circadian modulation in the function of these retinal ganglion cells. However, there have been no direct measurements of clock gene rhythms in these or other ganglion cell types to address the question endogenous vs. exogenous generation of these rhythms.
A recently published study has reported that removal of the melanopsin induces significant changes in the expression of clock genes in the photoreceptors layer and that the presence of melanopsin is required for the photic induction of Per1 and Per2 mRNA in the photoreceptors (Dkhissi-Benyahya et al., 2013). These authors also reported that the light-induced (480 nm, 15 min) increase of tyrosine hydroxylase (TH) mRNA and of dopamine requires melanopsin, and that in mouse retinas lacking the melanopsin gene, DA and DOPAC levels during the early part of the subjective day were elevated compared to wild type controls. These results suggest that DA is an important factor in the regulation of retinal circadian rhythms and ipRGGs may play a still unappreciated role in the cellular network responsible for the synchronization of retinal daily and circadian rhythms.
To summarize the findings on clock genes and cells in the retina - in non-mammalian vertebrate retinas the photoreceptors contain endogenous clocks that synthesize melatonin rhythmically and control photoreceptor function through circadian expression of ion channels and visual pigments. Both rods and cones can generate endogenous circadian rhythms, with the rods contributing the majority of melatonin synthesis. Clock genes are expressed at lower levels in the inner retina and it is not clear if cell types in those layers can generate endogenous rhythms.
In contrast, in the mammalian retina there are likely multiple sites of molecular circadian rhythm generation within the retina. There are low levels of clock gene expression in the photoreceptor layer, perhaps primarily limited to cones, which make up a small percentage of the cells. However, the cones themselves, and the photoreceptor layer in isolation, may express molecular circadian rhythms. In addition, clock gene expression is concentrated within the inner nuclear layer, where the GABAergic class of amacrine cells, including in particular the dopaminergic amacrine cells, as well as the retinal Müller glia, can express circadian rhythms in clock gene expression. This inner retinal circadian clock generates molecular circadian rhythms independent of photoreceptors and melatonin, and likely influences the photoreceptors and other retinal neurons through the rhythmic secretion of dopamine. Finally, also it appears that the ipRGCs are involved in the generation and entrainment of retinal circadian rhythms.
4. Neurochemical Organization of the Retinal Circadian Clock
4.1. Intercellular coupling may not be necessary for rhythms generation
Studies of the molecular organization of the retinal circadian clock revealed that multiple cell types may be genetically capable of generating and expressing circadian rhythms. How are these multiple cell type oscillators then organized into a tissue-level clock? Is neurochemical communication between cell types required for the retinal rhythms generation? Of the major neurotransmitters and neuromodulators in the retina – glutamate, GABA, glycine, acetylcholine, dopamine and melatonin – melatonin, dopamine and GABA are known to be secreted with a circadian rhythm (Tosini and Menaker 1996; Jaliffa et al. 2001; Doyle et al. 2002a; Doyle et al. 2002b) and could serve as synchronizing signals throughout the retina. Cellular coupling through gap junctions is also prominent in retinal circuits, and serves to couple clock cell populations in invertebrate retinal clocks (Block and McMahon 1984). In addition, in the SCN neural clock spike-mediated neurotransmission and the neuropeptide vasoactive intestinal polypeptide (VIP) are required to maintain cellular synchronization and tissue-level rhythms (Yamaguchi et al. 2003; Aton et al. 2005; Maywood et al., 2006). Comprehensive testing of the necessity for retinal molecular rhythms generation of each of the major neurotransmitters, plus spike mediated transmission and gap junctions with pharmacological agonists, antagonists and genetic knockouts revealed that individually none of these mechanisms of neurotransmission was necessary for tissue level retinal molecular rhythms, as read out by the PER2 bioluminescence reporter gene (Ruan et al. 2008). Blockade or saturation of neurotransmission through glutamate, GABA, glycine, acetylcholine, dopamine, melatonin, gap junctions or spikes did not block the production of molecular rhythms in explanted whole retinas.
Taken together, these results suggest that unlike the SCN neural clock in which cellular coupling is critical for maintenance of cellular and tissue level rhythms, the cellular coupling among clock cells in the retina may be minimal. The model for retinal rhythms generation most consonant with these data is that retinal clock cells are distributed throughout the retinal layers and generate molecular rhythms in a cell-autonomous manner. Consistent with this notion, the damping rate of the retinal clock in vitro is about 5-fold faster than that of the SCN clock (Ruan et al. 2008), possibly due to individual cell clocks becoming desynchronized much more quickly. How does the retinal clock as tissue then maintain cell synchronization? One possibility is that since the retina is normally exposed directly to the daily light-dark cycle, that this strong external synchronizing input is sufficient to maintain rhythmic organization of the individual cell oscillators in the absence of strong cellular coupling mechanisms.
4.2. Entrainment of the retinal clock involves intra-retinal signaling by dopamine
While none of the major neurotransmitters of the retina were found to be necessary for rhythms generation, two did have striking effects on other fundamental properties of the retinal circadian clock – altering dopamine levels resets the retinal circadian clock, while changing GABA levels changes the amplitude of the PER2 bioluminescence rhythms. Dopamine is secreted in the retina with a circadian rhythm, with elevated levels in the day-time portion of the circadian cycle, and it is also released in the retina in response to light in both mammalian and non-mammalian retinas. The sole source of dopamine in the retina is the dopaminergic amacrine cells of the inner nuclear layer, which receive light input from rods and cones through bipolar cells, and from ipRGCs through retrograde intra-retinal transmission (Zhang et al. 2008; Zhang et al. 2012). Dopamine released from amacrine cells has widespread effects throughout the entire retina – with dopaminergic cells in many species elaborating interplexiform processes that innervate horizontal cells, bipolar cells and photoreceptor terminals of the outer retinal layers, and there is evidence for volume transmission of dopamine by diffusion as well (Witkovsky 2004).
The receptors for dopamine are also widespread in retinal circuits – of particular relevance here are the expression of D2 family dopamine receptors (D2 or D4) on photoreceptors in a range of species (Cahill and Besharse 1991; Cohen et al. 1992). A role for retinal dopamine in light resetting of the retinal clock was first shown in Xenopus, in which blockade of dopaminergic transmission through D2-like receptors was shown to block light –induced phase shifts of melatonin secretion (Cahill and Besharse 1991). Since melatonin is primarily synthesized in photoreceptors, this suggests a somewhat puzzling mechanism in which the clock within photoreceptors is not reset directly by the light signal transduced by the photoreceptors, but instead is reset indirectly by feedback from dopaminergic amacrine cells. Indeed, light-induced dopamine release causes the induction of the Period2 clock gene in Xenopus photoreceptors (Besharse et al. 2004), a putative mechanism of clock resetting. Similarly, in mouse retina, blockade of dopaminergic signaling blunts light effects on Per2 bioluminescence rhythms, but in this species D1 dopamine receptors play a more prominent role (Ruan et al. 2008). In addition, light induced dopamine acutely stimulates the expression of Period clock genes in the retina, an effect that is blunted by knockout of D2 receptors (Yujnovsky et al. 2006).
Although it seems odd that rod and cone photoreceptors might, in a sense, ignore their own phototransduction for resetting of their clocks, one speculation is that the retrograde drive of the ipRGC’s on dopamine cells (Zhang et al. 2012), which provides a much more sustained drive to dopamine neurons than rod/cone input, is a key factor in circadian entrainment of the retinal clock. This speculation is attractive as the ipRGCs would then serve entrainment of both the retinal and brain clocks, ensuring synchronization of these two neural oscillators (Zhang et al. 2008). Indeed, light-induced dopamine responses and dopamine rhythms have generally been found to be preserved in rod/cone degenerate retinas in which melanopsin ganglion cells could serve as the remaining photoreceptors (Morgan and Kamp 1980; Nir and Iuvone, 1994; Doyle et al. 2002b; Vugler et al. 2007), presumably due to melanopsin ipRGC drive to dopaminergic neurons, (but see (Cameron et al. 2009)). In addition, it has been demonstrated in Xenopus that phototransduction in rods drives the contraction of red cones, again indirectly through dopamine (Besharse and Witkovsky, 1992). In any case, though the precise circuitry remains to be established, it is clear that dopamine acts as a critical input to the clock neurons in the retina, mediating the effects of light on clock phase.
Earlier studies have reported the presence of at least two photopigments in the retinal pigment epithelium (RPE), including melanopsin (Peirson et al., 2004), and it has been suggested that the RPE is directly photosensitive (Peirson et al., 2004). Indeed, in neonatal rat until postnatal day 3, Per1 and c-fos expression in the RPE can be induced by a pulse of light (Mateju et al., 2007). However, Baba et al., (2010) reported that PER2::LUC bioluminescence circadian rhythm in isolated RPE-choroid were not affected by a light pulse, suggesting that the circadian clock in the RPE-choroid is not directly capable of light perception. They suggested the light entrainment of the circadian rhythm of the PER2::LUC bioluminescence rhythm in the RPE-choroid may depend on the retinal photoreceptor. The mechanism by which this information is transmitted from the photoreceptor to the RPE is unknown, but it may involve diffusible factors such as melatonin and dopamine, two well-known modulators of retinal circadian rhythms (Tosini et al., 2008). Indeed, in a follow up study they reported that dopamine, but not melatonin, can phase-shift the circadian rhythm of PER2::LUC bioluminescence in the RPE thus suggesting that dopamine may be the signaling molecule that the retina uses to entrain the circadian clock located in the RPE.
4.3 GABA signaling influences the amplitude of retinal circadian rhythms
GABA is the principal fast inhibitory neurotransmitter in the retina, and the primary sources of GABA secretion are horizontal cells and amacrine cells. There are two major classes of GABA receptors GABAA receptors, which are ionotropic Clion channels, and GABAB receptors, which are metabotropic G-protein coupled receptors. Isoforms of these two receptor classes are widely expressed in retinal circuits and the retina is particularly rich in the rho isoform of GABAA receptor subunits, which is sometimes called the GABAC receptor (Qian and Dowling 1993). GABA levels fluctuate in the retina in a circadian rhythm, with elevated levels in the night portion of the circadian cycle (Jaliffa et al. 2001).
Pharmacological blockade of GABAA and GABAC receptors significantly increases the amplitude of PER2 bioluminescence rhythms by retinal explants, indicating that endogenous retinal GABAA neurotransmission, including GABAA rho-containing receptors, partially dampens the amplitude of gene cycling in the retinal circadian clock (Ruan et al. 2008). Indeed, increased stimulation of GABAA receptors with agonists greatly dampens the amplitude of the retinal circadian clock, and at high doses actually stops the motion of the molecular clock. These effects can be partially reversed by counteracting the hyperpolarizing effects of GABA-induced Clflux on cell membrane potential, and fully reversed when casein kinase epsilon (CKIe), an enzyme that promotes the degradation of PERIOD proteins through regulating their phosporylation, is also inhibited (Ruan et al. 2008).
The function of the GABAergic effects on retinal clock gene cycling is less clear than that of dopamine, but one speculation is that whereas dopamine is a circadian signal for the day phase in the retina, GABA may be a circadian signal that reinforces the night phase. The night-time peak in retinal GABA levels could be due to prolonged depolarization of GABA-secreting horizontal cells in the dark, resulting in prolonged secretion of GABA. Whereas, daytime circadian and light drive on dopamine secretion serves to reinforce the rise in the expression of Period clock genes during the day portion of the molecular circadian cycle, night-time GABA-mediated stimulation of PERIOD protein degradation may reinforce the falling phase of the clock gene cycle that occurs at night, removing these negative feedback complexes and preparing retinal clock neurons for initiation of the next cycle of Period gene transcription at dawn. Interestingly, GABAA receptors have been found to physically associate with CKIe in SCN clock neurons, although no function has been ascribed (Ning et al. 2004). Thus, elevated GABAA signaling at night may reinforce the degradative portion of the Period clock gene cycle, in counter point to dopamine’s reinforcement of the synthetic portion of the cycle during the day.
5. Outputs of the Retinal Circadian Clock
5.1 Melatonin and Dopamine are key elements of the ocular circadian system
Many studies have focused on the function of melatonin and dopamine in the retina and the available data indicate that these two neuro-hormones are involved in many aspects of retinal physiology and the pathology (Tosini et al, 2008). The roles played by these two neuromodulators within the eye is now well understood due to the use of transgenic animal models in which the retinal melatonergic or dopaminergic system has been disrupted (Figure 5).
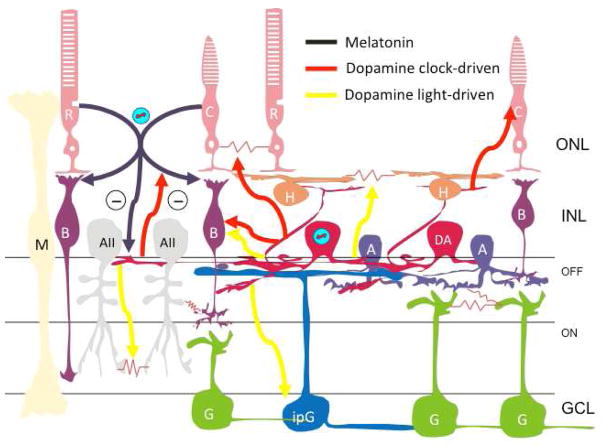
Figure 5. Neurochemical Outputs of the Retinal Circadian Clock.
Melatonin secreted from photoreceptors at night (black) modulates both the scotopic (dark-adapted) and photopic (light-adapted) ERG b-wave. This is denoted as arrows to rod and cone bipolar cells, which are the primary site of production of the scotopic and photopic b-waves, respectively, although the sites of action for these effects may include other cells. Melatonin also antagonizes dopamine release from dopaminergic amacrine cells. Clock-driven dopamine release during the day phase of the retinal circadian rhythm (red) acts to uncouple the electrical synapses between rods and cones, to increase the amplitude of the photoptic ERG b-wave, and to antagonize melatonin release. Light-driven dopamine release (yellow) has the additional effects of uncoupling AII amacrine cells and horizontal cells, as well as increasing the amplitude of the ERG b-wave and decreasing the amplitude intrinsically photoreceptive ganglion cell responses.
In mammals, retinal melatonin synthesis is under control of the circadian clock and removal of the suprachiasmatic nucleus (SCN) of the hypothalamus clock does not abolish the circadian rhythm of Aanat transcription in the rat retina (Sakamoto et al, 2000). Additional studies have also shown that a circadian rhythm in melatonin release could be recorded for several days when the retinae were maintained in culture (Tosini and Menaker 1996; 1998a); these retinal explants could be entrained by light in vitro (Tosini and Menaker 1996; 1998a) and the circadian rhythm in melatonin release is temperature compensated (Tosini and Menaker 1998b). In addition, a genetic mutation that affects the period of the circadian oscillation (i.,e. the tau mutation in the hamster) affects the period of the retinal clock controlling melatonin synthesis and of disk shedding in the same manner that this gene influences the period of the circadian pacemaker located in the brain (Tosini and Menaker 1996; Grace et al., 1996). Thus, the molecular clockwork in the retina is broadly similar to the molecular clockwork in the SCN., but with specific differences in its dependence on the individual clock genes (see Section 2.4).
Compelling experimental evidence also indicates that circadian clock driving melatonin synthesis is located within the outer nuclear layer since in Xenopus, chicken and rat; rhythmic melatonin synthesis persists in retinae in which the inner retina has been destroyed (Cahill and Besharse 1993; Zawilska and Iuvone 1992; Thomas et al, 1993; Sakamoto et al, 2006: Tosini et al., 2007b). Moreover in mice lacking rod photoreceptors (i.e., rd/rd mice) melatonin synthesis is not rhythmic (Tosini and Menaker 1998a). However it is still unclear whether the circadian clock that drives the rhythm of melatonin synthesis is intrinsic to the rod photoreceptors (where melatonin is mostly synthetized, Liu et al., 2004) or whether interactions among rods and cones are necessary to generate this circadian rhythm. Interestingly, immunocytochemical (Liu et al., 2012) and physiological evidence (Barnard et al., 2006; Storch et al., 2007) supports the idea that, at least in the mouse, the cones contain a circadian clock.
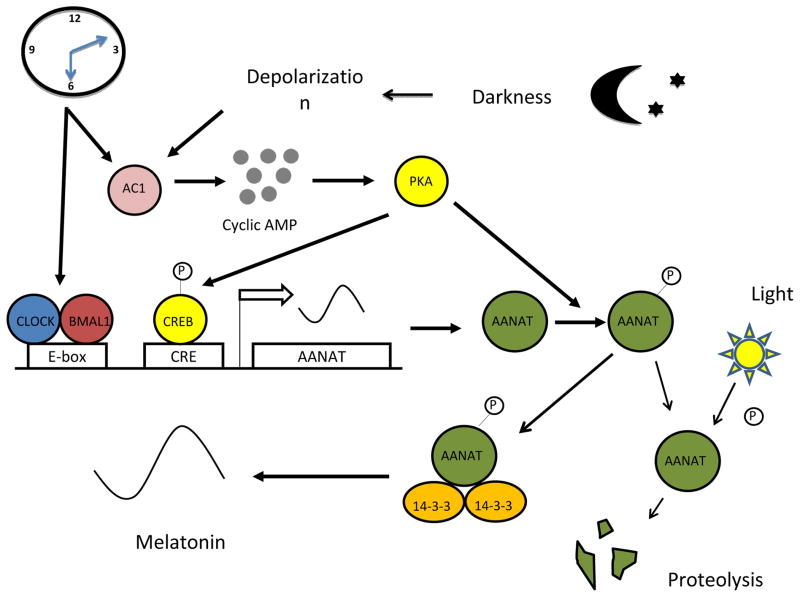
In the rat and chicken retina the circadian rhythm in melatonin synthesis is generated by changes in the transcriptional and post-transcriptional regulation of arylalkylamine N-acetyltransferase (AANAT) (Iuvone et al, 2005, Figure 6). The transcription of the Aanat gene in photoreceptor cells is under the direct control of the circadian clock via the action of BMAL1:CLOCK heterodimer on the E-box located on Aanat promoter (Chen and Baler 2000; Fukuhara et al, 2004; Haque et al., 2010).
Figure 6. Regulation of retinal melatonin levels by light and the circadian clock.
During the night cAMP levels in the photoreceptors are elevated thus activating PKA and Aanat gene transcription. Phosphorylated AANAT (pAANAT) associates with 14-3-3 proteins, which activate and stabilize the enzyme resulting in increased conversion of serotonin to N-acetylserotonin, and ultimately to melatonin. Light at night exposure decreases cAMP levels resulting in dephosphorylation of AANAT and its subsequent degradation by proteasomal degradation. The circadian clock controls melatonin levels by directly regulating Aanat transcription and by gating the cAMP signaling cascade (adapted from Tosini et al., Exp. Eye. Res. 2013).
Dopamine (DA) is the primary catecholamine in the retina. This neuromodulator is synthetized and released from unique populations of cells in the inner nuclear layer that are either amacrine or interplexiform neurons (Witkovsky 2004). Although the number of dopaminergic neurons in the retina is small (about 500 in the mouse retina, Sengupta et al, 2011), dopamine has widespread effects within the retina. The daily changes in dopamine synthesis and release depend from the interaction between the photoreceptors and the dopaminergic neurons, and dopamine release is stimulated by light (Iuvone et al, 1978; Nir et al, 2000).
Melatonin and dopamine exert their influence by binding to G-protein coupled receptors (GPCRs) named melatonin receptor type 1 (MT1) and type 2 (MT2) and dopamine receptors 1–5 (D1–D5). MT1 and MT2 receptors are both present in the vertebrate retina (reviewed in: Wiechmann and Summers 2008). In rodents MT1 receptors are found on photoreceptors, horizontal cells, and amacrine cells, in the inner plexiform layer, on retinal ganglion cells (RGCs), and the retinal pigment epithelium (RPE) (Fujieda et al, 1999, Baba et al., 2009; Sengupta et al., 2011). Dopaminergic neurons may also express MT1 receptors (Fujieda et al, 2000), suggesting that melatonin can directly modulate the activity of these cells. In humans, melatonin receptors (MT1 and MT2) have been located on the rod photoreceptors and on RGCs (Savaskan et al, 2002; Scher et al, 2002; Meyer et al, 2002; Savaskan et al, 2007). The colocalization of MT1 and MT2 receptors in photoreceptors suggests that these receptors may form heteromeric complexes in vivo, as demonstrated previously in vitro (Ayoub et al., 2004). Indeed a recent paper has demonstrated that in the mouse photoreceptors melatonin receptors can form functional heteromers that mediate the action of melatonin in these cells (Baba et al. 2013). The fact that melatonin receptors are expressed on the same cells responsible for its synthesis also suggests that melatonin may feedback on the photoreceptors to regulate its own levels or to produce other autocrine effects.
Dopamine receptors (D1–D5) are organized in two different families (D1- and D2- like). The D1 family includes the D1 and D5 receptors that are positively coupled with adenylyl cyclase (i. e., their activation increases the intracellular levels of cAMP). Although the D1 and D5 receptors have similar pharmacologies with respect to selective agonists and antagonists, the D5 receptor has a much higher affinity for DA than does the D1 receptor. The D2 family includes the D2, D3 and D4 subtypes and these receptors are negatively coupled with adenylyl cyclase (i.e., their activation decreases the intracellular levels of cAMP).
As seen for the melatonin receptors, dopamine receptors also have a widespread distribution in the mammalian retina and therefore is not surprising that dopamine can influence many retinal functions. D1 receptors have been localized in the outer and inner plexiform layers and within the cells bodies of horizontal cells, some cone bipolar cells, amacrine cells and perhaps in the ganglion cells (Nguyen-Legros et al, 1999). D5 receptor mRNA appears to be present in the RPE (Versaux-Botteri et al, 1995), but is expressed at relatively low levels in the neural retina (Jackson et al, 2009).
D2 receptors (D2) are widely expressed in the retina and function as both postsynaptic receptors and autoreceptors that inhibits dopamine release. D2 receptors are expressed by amacrine, bipolar, and ganglion cells (Derouiche and Asan 1999; Nguyen-Legros et al, 1999) and possibly on the intrinsically photosesensitive RGCs (ipRGCs, Sakamoto et al, 2005). D2-like immunoreactivity has been found in mouse photoreceptors (Doi et al., 2006), but these cells do not appear to express the Drd2 transcript, which encodes the receptor (Cohen et al., 1992). D4 receptors transcripts have been detected in the photoreceptors, interplexiform layer (IPL) and ganglion cells (Cohen et al., 1992; Kay et al, 2011), but expression appears to be highest in photoreceptors. D3 receptors seem to be absent from the retina (Jackson et al, 2009).
Several studies have shown that melatonin and dopamine play opposing roles in the regulation of retinal adaptive physiology (reviewed in: Iuvone et al, 2005; Tosini et al, 2008, Figure 5). Dopamine functions as a humoral signal for light, producing light adaptive physiology, whereas melatonin produces dark-adaptive effects.
Melatonin inhibits the release of dopamine through an action on melatonin receptors (Dubocovich 1983; Boatright et al, 1994; Ribelayga et al, 2004), whereas dopamine inhibits melatonin synthesis and release from photoreceptor cells by acting on D2-like dopamine receptors, presumably the D4 subtype (Zawilska and luvone 1992; Zawilska et al., 1994; Nguyen-Legros et al, 1996; Tosini and Dirden 2000). Therefore, these two neuromodulators form a cellular feedback loop functioning to regulate circadian retinal physiology (Figure 5). Although experimental data indicate that retinal dopaminergic cells in the retina contains the core molecular component of the circadian clock (Ruan et al, 2006), it appears that the circadian rhythm of dopamine release depends on the presence of the circadian rhythm in melatonin levels.
Dopamine appears to play a highly conserved role in retinal clock entrainment. Dopamine is required to synchronize circadian rhythms of rhodopsin promoter activity in Zebrafish rods (Yu et al., 2007), and stimulates Per2 induction and shifts the phase of the circadian clock in Xenopus photoreceptors (Cahill and Besharse, 1993; Steenhard and Besharse, 2000). Emerging experimental evidence also indicates that DA signaling plays an important role in the regulation of circadian rhythms in the mouse retina (Ruan et al, 2008; Pozdeyev et al, 2008; Jackson et al, 2011; Jackson et al, 2012). Ruan et al (2008) demonstrated that the circadian rhythm in PER2::LUC bioluminescence observed in inner retina is phase-shifted by of D1R agonist, whereas D4R may be involved in the entrainment of the photoreceptor circadian clock (Pozdeyev et al, 2008; Jackson et al, 2011). Dopamine can phase shift rhythms of gene expression and regulates protein phosphorylation in photoreceptors via the D4R. Furthermore, D2R knock-out mice showed a significant reduction in the light induction of Per1 in the retina (Doi et al, 2006) and CLOCK:BMAL1 transcriptional activity is augmented by activation of D2R (Yujnovsky et al, 2006). D2R signaling also contributes to the stability of BMAL1 protein (Sahar et al, 2010). The precise molecular mechanisms by which dopamine may drive the entrainment of the circadian clock is still unknown, but is believed to involved the induction of the Period1 (Per1) and/or Period2 (Per2) mRNA to drive the resetting process (Akiyama et al, 1999; Albrecht et al, 2001). However, it is important to note that the induction of Per1mRNA is positively coupled with activation of cAMP response element-binding protein (CREB) located on the promoter region of the Per1 gene (Obrietan et al, 1998, 1999) whereas D2-like receptors are negatively coupled with adenylyl cyclase and thereby lead to a decrease of cAMP levels. Thus it is unlikely that D2R activation induces Per1 mRNA via activation of the cAMP signaling pathway. Per1 mRNA induction in the retina may be regulated by cAMP-independent signaling pathways since photic induction of Per1 mRNA is dramatically decreased in D2R deficient mice (Doi et al, 2006) and a functional CRE is not required for its induction (Yujnovsky et al, 2006).
Melatonin may also influence the circadian clock in the retina since the amplitude and the phase of Per1 and Cry1 mRNA and protein in the mouse retina is different between melatonin proficient and melatonin deficient mice (Dinet et al, 2007; Dinet and Korf 2007).
5.2. Regulation of Gene Expression by the Circadian Clock
One of the fundamental functions of the circadian clock gene network is to drive tissue-specific networks of clock-controlled genes to underpin circadian physiology. Early work in the retina demonstrated light-induced as well as circadian clock control of opsin gene expression in the photoreceptors of non-mammalian vertebrates (Korenbrot and Fernald 1989; Pierce, et al. 1993), and recently this issue has been examined on a genomic scale to define the circadian transcriptome of the mammalian retina (Storch et al. 2007). Using gene microarrays to characterize global retinal gene expression across multiple time points in LD and in DD, Storch et al., (Storch et al. 2007) found that ca. 3000 genes cycled in a light-driven manner in LD and ca. 300 retinal genes continued to cycle under clock control in DD. The circadian clock-controlled retinal genes came from classes that contributed to many basic neural and cellular functions such as synaptic transmission, photoreceptor signaling, intercellular communication, and regulation of the cytoskeleton and chromatin. Thus, the transcriptional regulation of retinal gene expression by the retinal clock is a critical mechanism for its widespread control of retinal function and metabolism. Interestingly, the retinal clock molecular mechanism also contributes to light-regulation of gene expression, as knockout of the molecular clockworks also disrupted rhythmic gene expression in LD cycles (Storch et al. 2007).
A similar approach has been also recently used to identify genes that are involved in the circadian regulation of the photoreceptor phagocytosis (Mustafi et al., 2013). The authors of this study identified 365 protein-coding transcripts that show a circadian oscillation, interestingly several of these oscillating proteins implicate the phosphoinositide lipid signaling pathway in the circadian regulation of photoreceptor phagocytosis (Mustafi et al., 2013).
Finally, a study has also investigated the circadian proteomics of the mouse retina and identified several proteins that showed a circadian pattern of expression (Tsuji et al., 2007). These proteins were involved in vesicular transport, calcium-binding, protein degradation, metabolism, RNA-binding and proteins folding. In conclusion, the widespread transcriptional and post-transcriptional control by the retinal circadian clock suggests that disruption of the retinal molecular clockworks is likely to be involved in the pathogenesis of eye diseases.
5.3. Signaling by the Retinal Clock to the Brain and from the Brain Clock to Retina
The retina signals the daily light dark cycle to the hypothalamic SCN circadian clock through a specialized subset of intrinsically photosensitive retinal ganglion cells that express the photopigment melanopsin (Berson 2003). However, in addition to these light-driven daily signals, there is evidence that retinal circadian signaling may occur without light:dark stimulation. For example, in constant darkness, there are sub-regions of the SCN clock nuclei that express retina-driven circadian rhythms (Lee et al. 2003). The retinorecipient zone of the SCN clock in hamsters shows circadian rhythms in the phosphorylation of the signaling kinase ERK, a molecule normally activated by retinal ganglion cell input to the SCN during light-resetting of the clock. The SCN ERK activation rhythms in DD were ablated upon enucleation, suggesting that they originate in the retina, rather than in the SCN itself. In addition, retinal signals in DD also appear to contribute to the maturation and functional organization of the brain’s biological clock, as enucleation in DD during the first few weeks of life causes changes in the free-running period of locomotor rhythms in hamsters (Yamazaki et al. 2002). Interestingly, ipRGC’s have been shown to express clock genes (Liu et al. 2012) and their light responses are modulated with a circadian rhythm and by dopamine (Weng et al., 2009; Van Hook et al., 2012), suggesting the possibility that the retina-driven rhythms observed in the SCN originate in the ipRGCs themselves, or in retinal neurons with synaptic input to the melanopsin-expressing ipRGCs (e.g. dopamine amacrine cells). In addition, it has been also reported that ipRGCs are the first cells in the retina to respond to light during development (Sekaran et al., 2005) and light pulses can induce c-fos in the ganglion cells at postnatal day 1 (Mateju et al., 2010). Therefore ipRGGs may play a role in the entrainment of the circadian rhythm in the first days of postnatal life.
Finally compelling experimental evidence indicates that ocular rhythms do not depend from the presence of the SCN. Nevertheless, it is worth mentioning that although the rhythmic disk shedding persists after SCN removal (Teirstein et al., 1980), this rhythm could not be entrained to a new light:dark schedule, suggesting that the SCN –or other brain area - may be involved in the regulation of this rhythm.
6. The Retinal Clock’s in regulation of eye functions
6.1. Processing of Visual Information
Moving beyond the effects of the clock on individual cells and synapses, what is the global effect of the retinal circadian clock on visual function? The most consistent findings are that the retinal clock acts to bias the retina toward enhanced cone vision in the daytime and enhanced rod function in the nighttime. An extreme example of this effect, which illustrates the more general point, is the case of young zebrafish fry in which the cone synaptic ribbons are actually disassembled at night, preventing cone-mediated neurotransmission (Emran et al. 2010). Since these newly-hatched fish have not yet developed a significant number of rod photoreceptors they are functionally blind at night during the first few days of free-swimming life. Synaptic transmission from their cones is turned off at night by circadian remodeling to the transmitter release apparatus, and they do not yet have sufficient functioning rods. Across a number of vertebrate species including fish, amphibians, birds and mammals, the retinal circadian clock has been shown to drive shifts in the rod-cone balance of vision such that cone vision is favored in the day phase and rod vision is favored at night (Wang and Mangel 1996; Krizaj et al. 1998; Manglapus et al. 1999; Ribelayga et al. 2008).
Several investigations have demonstrated that the light-adapted (photopic) ERG is under control of the circadian clock (Barnard et al., 2006; Storch et al., 2007; Cameron et al., 2008; Jackson et al., 2012), with higher B-wave amplitudes during the subjective day than the subjective night. In a very elegant study Storch et al. (2007) using retinal specific Bmal1 knock-out reported that these mice do not show any circadian rhythmicity of the light-adapted ERG. Since these mice possess a functional SCN (Storch et al. 2007) they concluded that circadian rhythmicity in the ERGs depends on the circadian clocks located within the retina. A similar result has been obtained in mice lacking Cryptochrome 1 and 2 (Cameron et al. 2008). An intriguing result was also reported in mice lacking melanopsin (Opn4−/−). It appears that the circadian rhythmicity of the cone isolated photopic ERG was lost in these animals with ERGs resembling a middle ground between ‘day-time’ and ‘night-time’- like (Barnard et al. 2006). This result suggests that ipRGCs are required to maintain ERG rhythmicity under circadian conditions.
It also appears that melatonin controls the daily rhythms of the scotopic and photopic ERGs in C3H-f+/+ mice (Baba et al, 2009). The circadian regulation in the photopic ERG was abolished by targeted disruption of the MT1 receptor, indicating that in melatonin-proficient mice MT1 receptor signaling is required for the circadian regulation of the photic ERGs (Sengupta et al, 2011). Interestingly, removal of the MT1 receptors did not affect the circadian regulation of DA and DOPAC. Therefore, the loss of circadian regulation in the photopic ERGs observed in MT1−/− mice is not a consequence of the loss in the circadian regulation of DA and DOPAC. A possible explanation for these results may be found in a previous study that demonstrated altered photopic ERG responses in mice lacking melanopsin (Barnard et al., 2006). Since MT1 receptors are present on the ipRGCs (Sengupta et al, 2011) it possible that the lack of MT1 signaling may affect the circadian regulation of the photic ERG by acting on MT1 receptors present on the ipRGCs
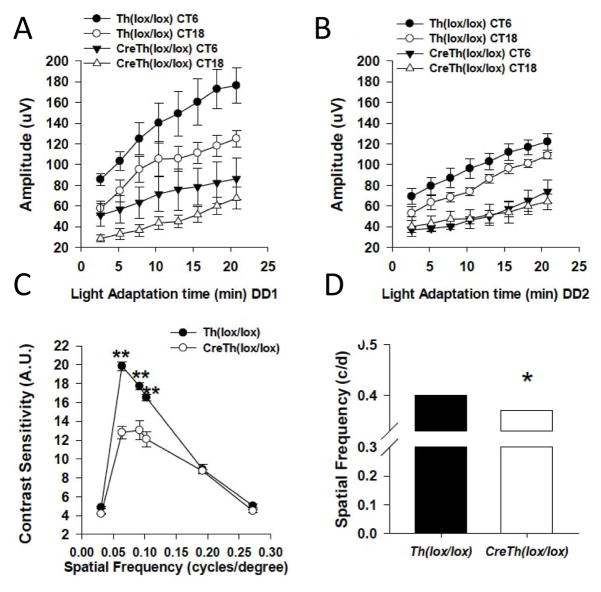
A recent study investigated the effects of genetic depletion of retinal dopamine, by retina-specific knockout of the key dopamine synthesizing enzyme tyrosine hydroxylase (rTHKO). Depletion of retinal dopamine blunts the circadian rhythm in the light-adapted ERG, reducing the normal daytime elevation of this cone-driven response (Jackson et al. 2012, Figure 7). The regulation of the circadian ERG rhythm is mediated by dopamine D4 receptors. The effect of dopamine depletion can be rescued by a specific D4 receptor agonist and is phencopied in D4R knockout mice. Interestingly, a similar effect is observed on behaviorally measured contrast sensitivity in rTHKO mice, with a reduction in contrast sensitivity function during the daytime that is rescued by the D4R agonist and phenocopied in D4R knockout mice. This finding suggested that this aspect of vision is likely under circadian control as well, and a recent study demonstrated that contrast sensitivity, measured under photopic conditions, is indeed circadian with much higher sensitivity during the subjective daytime that the subjective night (Hwang et al., 2013). In these mice with greatly reduced retinal dopamine, the retina appears to be perpetually locked in the nighttime state (e.g. low amplitude photopic ERG and reduced contrast sensitivity). Human patients with parkinsonian degeneration of dopaminergic neurons exhibit similar visual deficits in ERG and contrast sensitivity, suggesting that depletion of retinal dopamine may play a role in the visual symptoms of this neurodegenerative disease (Haug et al. 1994; Ikeda et al. 1994; Ingster-Moatiet al. 1996). Thus, the overall retinal sensitivity to photopic light, contrast sensitivity, and the function of specific classes of ganglion cells is under circadian control by dopamine signaling.
Figure 7. Dopamine modulates the circadian rhythm in visual processing.
Light-adapted ERG b-wave amplitudes at CT 6 (filled circles and triangles) or CT 18 (open circles and triangles) plotted as a function of light adaptation time in Ctrl or rTHKO mice during DD1 (A) or DD2 (B). (A) There are significant day/night difference during DD1 for both genotypes (B) On the second day in DD, no significant day/night differences are observed in rTHKO mice, for Ctrl mice; however, day/night differences persist in Ctrl mice. (C) Contrast sensitivity is significantly reduced in rTHKO mice at three of the six tested spatial frequencies. (D) Acuity threshold is significantly lower in rTHKO mice compared with control mice. All data are represented as means ± SEM. (Adapted from Jackson et al., J. Neurosci. 2012).
Results with retina-specific dopamine knockout indicate that dopamine reconfigures the retina for enhanced responses to light in the presence of photopic backgrounds and enhanced contrast sensitivity during the day, as well as high-spatial resolution (Jackson et al., 2012). Following genetic depletion of dopamine, the retina is essentially configured in its night-time state in which photopic light responses, resolution and contrast sensitivity are low. Dark-adapted scotopic ERGs were normal in retinal dopamine depleted mice, while light-adapted photopic ERGs were reduced in amplitude and their circadian regulation disrupted by reduction of day-time b-wave amplitudes to night-time levels. Contrast sensitivity and visual acuity thresholds are also degraded in mice that cannot synthesize retinal dopamine. Thus, dopamine regulates the ability of the retina to respond in the presence of photopic background light, while modulating its sensitivity to contrast and spatial resolution. Retinal dopamine signaling is elevated in the circadian day, either as a consequence of rhythmic dopamine secretion driven by the retinal circadian clock, or as a result of diurnal or circadian rhythms in dopamine D4 receptor gene expression (Storch et al., 2007, Jackson et al., 2011; Bai et al. 2008; Klitten et al., 2008). In the light/dark cycles experienced in the natural environment, light-induced dopamine release would also enhance dopamine signaling during the day (Witkovsky 2004). Interestingly, while the studies of the rTHKO mouse model found effects primarily on cone-mediated vision, dopamine also acts in a similar way to extend the operating range of rod signaling in the presence of background illumination (Herrmann et al., 2011). Thus, from the most general perspective the dopamine signaling acts to enhance both rod and cone system performance in the presence of significant background light.
A second empirical generalization that has emerged regarding dopamine signaling in the retina, is that circadian dopamine signaling is mediated by D2 family receptors, whereas light-adapted dopamine signaling is mediated through D1 receptors (Jackson et al 2012). Circadian retinal functions lost due to genetic depletion of retinal dopamine (photopic ERG amplitude and contrast sensitivity) are restored by D4 agonists, but not D1 agonists, while non-circadian aspects of dopamine mediated retinal function (acuity) are restored by D1 agonists, but not D4 (Jackson et al., 2012). In addition, circadian changes in rod-cone coupling are mediated by D2-family receptors, whereas modulation of horizontal cell coupling, which is light-driven, not circadian, is mediated by D1 receptors. Rybelayga and Mangel (2008), using tracer labeling in the mouse retina, were able to show that the retinal circadian clock also controls the extent and strength of rod-cone coupling by activating dopamine D2-like receptors during the day, so that rod-cone coupling is weak during the day but remarkably robust at night. In a separate study they showed that horizontal cell coupling was constant in circadian profile, but could be greatly reduced by light adaptation through dopamine D1 receptor signaling (Ribelayga and Mangel, 2003). Thus, D2 family receptors, and in particular D4 receptors, can be considered as the “circadian signaling channel” for dopamine in the retina, and D1 receptors as the “light-adaptation signaling channel”. This dichotomy likely originates from the higher sensitivity of D4 receptors and the fact that their gene expression is driven by the circadian clock, whereas D1 receptor expression is not (Jackson et al., 2011; Bai et al., 2008; Klitten et al., 2008).
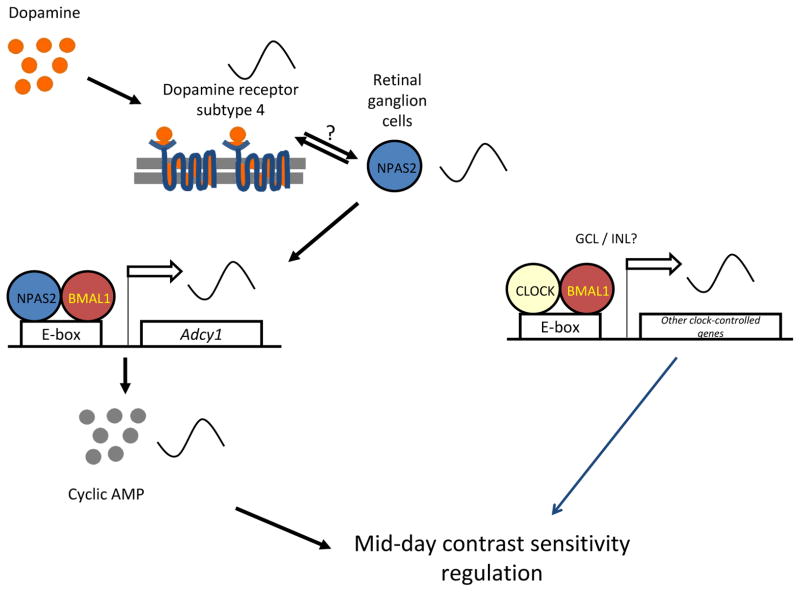
The circadian regulation of contrast sensitivity appears to involve a D4R ⇒ NPAS2 ⇒ cAMP signaling pathway in a subset of retinal ganglion cells (Hwang et al., 2013). NPAS2 is a CLOCK homolog that heterodimerizes with BMAL1 and activates circadian E-box promoter elements. Using an NPAS2-lacZ reporter mouse, NPAS2 expression was localized to a subset of Brn3a-positive retinal ganglion cells, and Npas2 mRNA expression was robustly rhythmic in the ganglion cell layer (GCL), isolated by laser capture microdissection. The amplitude of the circadian rhythm of contrast sensitivity was significantly reduced in Npas2−/− mice, by selectively reducing daytime contrast sensitivity. A remarkably similar reduction in the contrast sensitivity rhythm was observed in D4R knockout mice, in which the rhythmic expression of Npas2 was abolished. D4Rs regulate the circadian expression of Adcy1 (Jackson et al., 2011), the gene that encodes the type 1 Ca2+/calmodulin-sensitive adenylyl cyclase (AC1). Mice with targeted deletion of Adcy1 also have a nearly identical reduction in the amplitude of the contrast sensitivity rhythm as in Drd4−/− and Npas2−/− mice, and the circadian rhythm of Adcy1 expression is abolished in the GCL of both Drd4 and Npas2 knockout mice (Hwang et al., 2013). Moreover, NPAS2/BMAL1 was shown to activate the E-box in the Adcy1 promoter in a heterologous expression system. These studies identify a subset of retinal cells and a molecular mechanism for the circadian regulation of contrast sensitivity (Figure 8). However, deletion of D4Rs, NPAS2, and AC1 only reduce the amplitude of the rhythm and do not abolish it. This indicates that other circadian clock mechanisms, possibly involving CLOCK/BMAL1 and other cell types yet to be determined, are involved in driving the circadian rhythm of contrast sensitivity (Figure 8).
Figure 8.
A model for mid-day contrast sensitivity regulation. Dopamine, through a D4 receptor pathway, regulates the rhythmic expression of Npas2 in the GCL. NPAS2 in the retinal ganglion cells regulates the rhythmic expression of the Adcy1 gene in the GCL, which ultimately modulates day-time contrast sensitivity. CLOCK, in part through an Adcy1 independent mechanism, also regulates the circadian rhythm of contrast sensitivity. (Adapted from Hwang et al., J. Neurosci. 2013)
6.2. Disk Shedding and Phagocytosis
The RPE is involved in many physiological functions that are key to maintain the health of the photoreceptor (Bok, 1993). One of the most important and fascinating of the roles played by the RPE is the phagocytosis of the disks that are shed by photoreceptor outer segments. Rod photoreceptor outer segments (ROS) are continuously renewed by the assembly of new membranous disks at the base of the ROS and by displacing older disks at the top of the outer segment. These old disks are then shed from the apical tip of the ROS (Young, 1967). The shed ROS fragments are then engulfed, phagocytized, and degraded by the RPE. Disk shedding and phagocytosis occur every day in a synchronized fashion shortly after the onset of light for rod photoreceptors (Besharse et al., 1977), and at the onset of night for cones (Young, 1978). The rhythms of disk shedding and phagocytosis persist in constant darkness, indicating that they are under control of circadian clocks (LaVail, 1976; Teirstein et al., 1980; Terman et al., 1993; Grace et al., 1996). Experimental evidence indicates that the circadian clock controlling disk shedding is located in the eye, possibly in photoreceptors (Teirstein et al., 1980, Terman et al., 1993). However, it must be noted that the photoreceptors are not the sole controller of disk shedding since it has been reported that close approximation of photoreceptors and RPE is necessary before the disk shedding can occur (Matsumoto et al., 1987; Williams and Fisher, 1987) and lysosomal fusion in the RPE can be triggered by light and circadian signals (Bosch et al., 1993). A recently published paper has shown that the diurnal rhythm in exposure of phosphatidylserine by rod outer segments, which appears to play role in disk shedding and phagocytosis, is not entirely controlled by the photoreceptors, but RPE cells participate in the synchronization of this process (Ruggiero et al., 2012). The mechanism by which the circadian clock controls the circadian rhythm in disk shedding and phagocytosis is not fully understood but it is believed to involve the activation of the of αvβ5 integrin receptors, the FAK-MerTK signaling pathway (Finneman et al., 2003,Nandrot et al., 2004) and – possibly - phosphoinositide signaling (Mustafi et al., 2013).
Finally, emerging evidence has shown that the RPE contains an autonomous circadian clock that controls the circadian rhythms in PER2::LUC bioluminescence (Baba et al, 2010, Figure 9). In conclusion, further studies are required to fully understand the mechanisms by which the circadian rhythms disk shedding and phagocytosis are regulated.
Figure 9. Representative examples of PER2::LUC bioluminescence rhythm in RPE.
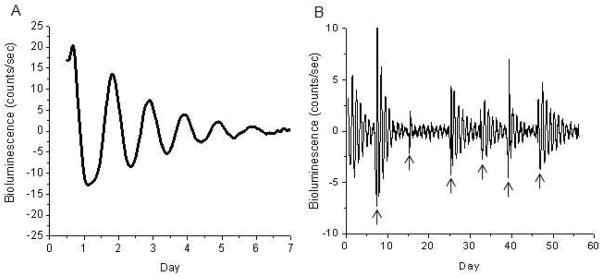
(A) A clear circadian rhythm IN PER2::LUC bioluminescence can be recorded in the RPE. The circadian rhythm in the bioluminescence damp-out after 6–7 days. (B) A medium exchange will reinitiate the circadian rhythm in these cultures thus allowing the recording for several weeks. The arrows indicated the day when the old medium in the culture was replaced with new one (Adapted from Baba et al., Molecular Vision, 2010).
6.3. Intraocular Pressure
A large number of studies have demonstrated that intraocular pressure (IOP) of rabbits, rats, chicks, marmosets, and mice show a daily pattern when the animals are maintained in a 12-hour light–dark cycle (Rowland et al., 1981; Moore et al., 1996; Nickla et al., 1998; 2002; Aihara et al., 2003) and in rabbits, rats and mice this daily pattern persists when the animals are maintained in constant darkness (Rowland et al., 1981; Moore et al., 1996). The daily and circadian variation in IOP has been also reported in humans (Liu et al., 1998).
Several factors are involved in the regulation of 24-hour rhythm in IOP. Sympathetic nerves are thought to play an important role because neuronal impairment abolishes day–night variations in IOP (Braslow and Gregory, 1987; Liu et al., 1991; Yoshitomi et al., 1991). Melatonin is also involved in the regulation of IOP (Samples et al., 1988; Osborne and Chidlow, 1994; Pintor et al., 2001; Wiechmann and Wirsig-Wiechmann, 2001).
To investigate the relationship between IOP rhythm and the circadian clock, a study investigated 24-hour profiles of IOP in Cry1/Cry2-double knockout mice, which are behaviorally arrhythmic (van der Horst et al. 1999). The authors reported that IOP measurements of Cry-deficient mice were constant throughout the day regardless of environmental light conditions (Maeda et al., 2006). Although this study provided evidence that a functional circadian clock is required for the generation of the daily and the circadian rhythm of IOP, it does not provide any indication whether the SCN or others ocular clocks (i.e., the retina or the ciliary body clock) control this rhythm. Another study in the mouse reported that the 24- hour IOP rhythm is abolished under constant exposure to light (Aihara et al., 2003). Since exposure to LL induces arrhythmicty in the SCN, this study also does not provide any insight on the location of the circadian clock controlling the rhythm IOP.
Finally, a recent study has shown that the mean IOP levels were higher during the night in mice lacking MT1 receptors (MT1−/−) than in the wild-type mice, but not during the day (Alcantara-Contreras et al., 2011). In addition, administration of exogenous melatonin in wild-type mice reduced IOP at night but not during the day further suggesting that the action of melatonin on the IOP may be gated by the circadian clock.
In conclusion, the available data do not provide any insight whether the circadian rhythm in IOP is driven by the retinal circadian clock of by a circadian clock located elsewhere.
7. The Retinal Clock’s Potential Role in Eye Disease
The widespread control of retinal signaling, metabolism, and gene expression exerted by the retinal circadian clock suggests that the retinal molecular clockworks, or its output signals, may contribute to retinal disease and pathology, as well as normal retinal function. This is an area just beginning to be explored at the molecular level, but previous studies have shown that there is circadian modulation of photoreceptor vulnerability to light-induced degeneration (Organisciak et al. 2000), and melatonin signaling has been shown to affect photoreceptor viability (Wiechmann and O’ Steen, 1992; Sugawara et al., 1998; Baba et al., 2009), and to promote ganglion cell survival during aging (Baba et al. 2009). Trophic signaling by the retinal clock and its outputs seem to play a role in the regulation of eye growth and refractive errors. Disruption of retinal dopamine rhythms causes myopic changes in the chicken eye (Stone et al., 1989; Rohrer et al. 1993; Weiss and Schaeffel 1993; Bartmann et al. 1994; Guo et al. 1995; Feldkaemper et al. 1999). Visual form deprivation, which induces myopia, decreases retinal dopamine (Stone et al., 1989) and disrupts daily rhythms of eye growth (Weiss and Schaeffel, 1993). Expression profiling in chicken retina/RPE has also shown altered circadian clock gene and melatonin receptor expression in lens-induced myopia (Stone et al., 2011). Similar dopamine- and circadian clock-dependent eye growth regulatory mechanisms likely operate in the mammalian retina (Iuvove et al., 1989; Iuvone et al., 1991; Pardue et al., 2013; Stone et al. 2013). Emerging evidence also suggests that Period genes may play a role in the regulation of vascularization signals in the retina in disease models of diabetic retinopathy (Bhatwadekar et al. 2013). Given that retinal clock gene expression is disrupted in proliferative neovascularizing diseases (Busik et al. 2009), this link may prove to be an important pathway for therapeutic intervention in these conditions. In this regard, decreases in retinal dopamine have been shown to contribute to visual dysfunction in early diabetes in mice and rats, and progression of the visual dysfunction can be retarded by treating with the dopamine precursor, L-DOPA (Aung et al., 2013).
Two recent studies have implicated clock genes (i.e, Rev-erb alpha and Rora) in retinal functioning (Mollena et al., 2011) and age-related macular degeneration (Jun et al., 2011). Mice lacking Period1 and Period2 show significant alteration in the distribution of cone photoreceptors and a significant reduction in the levels of cone opsin mRNA and protein (Ait-Hmyed et al., 2013). These observations - together with the previously mentioned study on the expression of clock proteins in mouse cones (Liu et al., 2012) and the regulation of the photopic ERGs (Barnard et al., 2006; Storch et al., 2007; Sengupta et al., 2011) – suggest that in the mammalian retina cone photoreceptors may indeed be major contributors to the retinal circadian clock network.
The lack of circadian ROS disk shedding and RPE phagocytosis impairs retinal and RPE functions. In young mice lacking the β5 integrin subunit in the RPE, retinal morphology and the ERG are no different from those in wild-type mice. However during the aging process β5−/− mice lose cone and rod photoreceptors and the functioning of these cells is significantly impaired (Nandrot et al., 2004). Thus, loss of the daily rhythm in RPE phagocytosis leads to reduced viability of photoreceptors during aging.
Although it is unclear if circadian clocks are involved, we should mention that in mice lacking Clock or Bmal1 gene there is a significant increase in the rate of cataract development during aging (Kondratov et al., 2006; Dubrovsky et al., 2010).
8. Future Directions
Among vertebrate neural circadian clocks the retina represent a unique structure. It contains a complete circadian system – mechanisms for circadian phototransduction, rhythm generation and rhythmic outputs to signal circadian time both within and downstream of the retina. Thus, the retina represents an ideal model to study fundamental questions of how neural circadian systems are organized and what signaling pathways are used to maintain synchrony with the different structures in the system.
Furthermore, several studies have shown that multiple sites within the retina are capable of generating circadian oscillations. The strength of circadian clock gene expression, and the emphasis of rhythm expression is divergent across vertebrate retinas, with photoreceptors as the primary locus of rhythm generation in amphibians, while in mammals clock activity is most robust in the inner nuclear layer. Melatonin and dopamine serve as signaling molecules to entrain circadian rhythms in the retina and also in other ocular structures. Recent studies have also suggested GABA as an important component of the system that regulates retinal circadian rhythms. These transmitter-driven influences on clock molecules apparently reinforce the autonomous transcription-translation cycling of clock genes.
The molecular organization of the retinal clock is similar to what has been reported for the SCN although inter-neural communication among retinal neurons that form the circadian network is apparently weaker than those present in the SCN, suggesting that it is more sensitive to genetic disruption than the central brain clock. Thus, the retinal circadian clock is similar in its molecular constituents to other tissue circadian clocks, but lacks the genetic and cellular buffering against perturbation that is characteristic of the SCN.
A large number of studies over the last twenty years have demonstrated that the retinal circadian clock extends its influence on many retinal functions. The melatonin-dopamine system is the signaling pathway that allows the retinal circadian clock to reconfigure retinal circuits to enhance light-adapted cone-mediated visual function during the day and dark-adapted rod-mediated visual signaling at night. Additionally, the retinal circadian clock also controls circadian rhythms in disk shedding and phagocytosis, and possibly IOP.
The circadian clock is also implicated in the pathogenesis of eye disease and compelling experimental data indicate that dysfunction of the retinal circadian system negatively impacts photoreceptors, RPE, RGCs, and, possibly, the cornea and the lens. The available data indicate that the negative impact of circadian rhythm dysfunctions – either by genetic or environmental cause - is likely to emerge during aging of the retina and its circadian clock.
We believe that there are still several questions that need to be addressed about the functioning of this fascinating circadian system. These questions include determining which of the several genetically competent cell populations in the retina are true clock cells, if and how their rhythms are coordinated, and the action of the circadian clock on specific retinal circuits and functional modules by which it modulates vision. Finally more research is needed on the impact of the retinal clock dysfunction on ocular health.
Acknowledgments
Research in the authors’ laboratories is supported in part by grants from the NIH (R01 EY004864, EY022216, EY015815, P30-EY008126, P30 EY006360, T32 EY007092, U54 NS083932, G12-RR03034), the Katz Foundation, and an unrestricted departmental grant from Research to Prevent Blindness, Inc. (RPB). PMI is a recipient of an RPB Senior Scientific Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–686. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, et al. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013:371048–60. doi: 10.1111/ejn.12103. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, et al. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–21. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–4. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Alcantara-Contreras S, Baba K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neuroscience Letters. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Han MK, Park H, Abey J, Thule, et al. Role of Dopamine Deficiency in Visual Dysfunction in Early-stage Diabetic Retinopathy. Arvo abstract 2013 [Google Scholar]
- Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptors heterodimers with distint ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–21. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, Guillaume JL, et al. Heteromeric MT1/MT2 Melatonin receptor modulate photoreceptor function. Sci Signal. 2013;6(296):ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, Contreras-Alcantara S, Tosini G. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–11. [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zimmer S, Rickes O, Rohleder N, Holthues H, et al. Daily oscillation of gene expression in the retina is phase-advanced with respect to the pineal gland. Brain Res. 2008;1203:89–96. doi: 10.1016/j.brainres.2008.01.073. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Zhuang M, Freeman K, Fogerty J. Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci. 2004;20:167–174. doi: 10.1111/j.1460-9568.2004.03479.x. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Witkovsky P. Light-evoked contraction of red absorbing cones in the Xenopus retina is maximally sensitive to green light. Vis Neurosci. 1992;8:243–249. doi: 10.1017/s0952523800002893. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, Pierce ME. Regulation of rhythmic photoreceptor metabolism: A role for post-receptoral neurons. Progress in Retinal Research. 1998;7:21–61. [Google Scholar]
- Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–5. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Besharse JC. The daily light-dark cycle and rhythmic metabolism in the photoreceptor—Pigment epithelial complex. Progress in Retinal Research. 1982;1:81–124. [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments: II. Membrane addition and loss in relationship to light. J Cell Biol. 1977;75:507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatwadekar AD, Yan Y, Qi X, Thinschmidt JS, Neu MB, et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GD, McMahon DG. Cellular analysis of the Bulla ocular circadian pacemaker system III. Localization of the circadian pacemaker. J Comp Physiol [A] 1984;155:387–395. [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994;11: 1013–8. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile patner in vision. J Cell Science. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993;41:253–263. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- Braslow RA, Gregory DS. Adrenergic decentralization modifies the circadian rhythm of intraocular pressure. Invest Ophthalmol Vis Sci. 1987;28:1730–1732. [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. Journal of Experimental Medicine. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Progress in Retinal and Eye Research. 1995;14:267–291. [Google Scholar]
- Cameron MA, Barnard AR, Hut RA, Bonnefont X, van der Horst GT, et al. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23:489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, et al. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci. 2009;29:761–767. doi: 10.1111/j.1460-9568.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR, Iuvone PM. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J Neurochem. 2006;99:1142–50. doi: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res Mol Brain Res. 2000;81: 43–0. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007;17(14):R538–9. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Asan E. The dopamine D2 receptor subfamily in rat retina: ultrastructural immunogold and in situ hybridization studies. Eur J Neurosci. 1999;11:1391–02. doi: 10.1046/j.1460-9568.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- Dinet V, Ansari N, Torres-Farfan C, Korf H-W. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonindeficient (C57BL) mice. J Pineal Res. 2007;42:83–1. doi: 10.1111/j.1600-079X.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Dinet V, Korf H-W. Impact of melatonin receptors on pCREB and clock-gene protein levels in the murine retina. Cell Tissue Res. 2007;330: 29–4. doi: 10.1007/s00441-007-0468-5. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70(18):3435–47. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Circadian clock regulation of pH in the rabbit retina. J Neurosci. 2001;21:2897–2902. doi: 10.1523/JNEUROSCI.21-08-02897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, et al. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9:732–4. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002a;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002b;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983 Dec 22–1984 Jan 4;306(5945):782–4. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging. 2010;2(12):936–44. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Dowling JE. Zebrafish larvae lose vision at night. Proc Natl Acad Sci USA. 2010;107:6034–6039. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkaemper M, Diether S, Kleine G, Schaeffel F. Interactions of spatial and luminance information in the retina of chickens during myopia development. Exp Eye Res. 1999;68:105–115. doi: 10.1006/exer.1998.0590. [DOI] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–54. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SFM, et al. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17: 63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Expression of mt1 melatonin receptor in rat retina: evidence for multiple cell targets for melatonin. Neuroscience. 1999;93:793–799. doi: 10.1016/s0306-4522(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC-K, Storm DR, et al. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neuroscience. 2004;24:1803–11. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288(5474):2226–30. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Grace MS, Chiba A, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999;16: 909–18. doi: 10.1017/s0952523899165106. [DOI] [PubMed] [Google Scholar]
- Grace MS, Wang LA, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735: 93–100. doi: 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- Grewal R, Organisciak D, Wong P. Factors underlying circadian dependent susceptibility to light induced retinal damage. Adv Exp Med Biol. 2006;572:411–416. doi: 10.1007/0-387-32442-9_58. [DOI] [PubMed] [Google Scholar]
- Guo SS, Sivak JG, Callender MG, Diehl-Jones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995;14:385–389. doi: 10.3109/02713689508999936. [DOI] [PubMed] [Google Scholar]
- Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, et al. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci U S A. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, et al. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113(5):1296–306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, McMahon DG, Maywood ES. Analysis of circadian mechanisms in the suprachiasmatic nucleus by transgenesis and biolistic transfection. Methods Enzymol. 2005;393:579–592. doi: 10.1016/S0076-6879(05)93030-9. [DOI] [PubMed] [Google Scholar]
- Haug BA, Trenkwalder C, Arden GB, Oertel WH, Paulus W. Visual thresholds to low-contrast pattern displacement, color contrast, and luminance contrast stimuli in Parkinson’s disease. Mov Disord. 1994;9:563–570. doi: 10.1002/mds.870090510. [DOI] [PubMed] [Google Scholar]
- Hayasaka N, LaRue SI, Green CB. In vivo disruption of Xenopus CLOCK in the retinal photoreceptor cells abolishes circadian melatonin rhythmicity without affecting its production levels. J Neurosci. 2002;22:1600–1607. doi: 10.1523/JNEUROSCI.22-05-01600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka N, LaRue SI, Green CB. Differential contribution of rod and cone circadian clocks in driving retinal melatonin rhythms in Xenopus. PLoS ONE. 2010;5:e15599. doi: 10.1371/journal.pone.0015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, et al. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011 Oct 6;72(1):101–10. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Chaurasia SS, Jackson CR, Chan GC, Storm DR, Iuvone PM. Circadian Rhythm of Contrast Sensitivity Is Regulated by a Dopamine-Neuronal PAS-Domain Protein 2-Adenylyl Cyclase 1 Signaling Pathway in Retinal Ganglion Cells. J Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Head GM, Ellis CJ. Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vision Res. 1994;34:2629–2638. doi: 10.1016/0042-6989(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Ingster-Moati I, Le Coz P, Albuisson E, Fromont G, Pierron C, et al. Static contrast sensitivity in idiopathic Parkinson disease. Rev Neurol (Paris) 1996;152:738–743. [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Haque R, Klein DC, Chaurasia SS. Circadian Clocks, Clock-Controlled Genes and Melatonin Biosynthesis in the Retina. Prog Retin Eye Res. 2005;24: 433–56. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–2. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–03. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: Development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal Dopamine Mediates Multiple Dimensions of Light-Adapted Vision. J Neurosci. 2012;32:9359–9368. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Hwang CK, Iuvone PM. Dopamine D4 receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur J Neurosci. 2011;34:57–64. doi: 10.1111/j.1460-9568.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, et al. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009;109(1):148–57. doi: 10.1111/j.1471-4159.2009.05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaliffa CO, Saenz D, Resnik E, Keller Sarmiento MI, Rosenstein RE. Circadian activity of the GABAergic system in the golden hamster retina. Brain Res. 2001;912:195–202. doi: 10.1016/s0006-8993(01)02736-6. [DOI] [PubMed] [Google Scholar]
- Jun G, Nicolaou M, Morrison MA, Buros J, Morgan DJ, et al. Influence of ROBO1 and RORA on risk of age-related macular degeneration reveals genetically distinct phenotypes in disease pathophysiology. PLoS One. 2011;6(10):e25775. doi: 10.1371/journal.pone.0025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Biophys Res Commun. 2005;330(1):18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–62. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitten LL, Rath MF, Coon SL, Kim J-S, Klein DC, Møller M. Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Experimental Eye Research. 2008 Nov 1;87(5):471–7. doi: 10.1016/j.exer.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport. 2000;11: 1479–1482. [PubMed] [Google Scholar]
- La Vail MM. Rod outer segment disk shedding in the rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1073. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS ONE. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aanat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315: 197–1. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- Liu JH. Circadian rhythm of intraocular pressure. J Glaucoma. 1998;7:141–147. [PubMed] [Google Scholar]
- Liu JH, Dacus AC. Endogenous hormonal changes and circadian elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 1991;32:496–500. [PubMed] [Google Scholar]
- Maeda A, Tsujiya S, Higashide T, Toida K, Todo T, et al. Circadian intraocular pressure rhythm is generated by clock genes. Invest Ophthalmol Vis Sci. 2006;47(9):4050–2. doi: 10.1167/iovs.06-0183. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejů K, Sumová A, Bendová Z. Expression and light sensitivity of clock genes Per1 and Per2 and immediate-early gene c-fos within the retina of early postnatal Wistar rats. J Comp Neurol. 2010;518(17):3630–44. doi: 10.1002/cne.22421. [DOI] [PubMed] [Google Scholar]
- Mathes A, Engel L, Holthues H, Wolloscheck T, Spessert R. Daily profile in melanopsin transcripts depends on seasonal lighting conditions in the rat retina. J Neuroendocrinol. 2007;19:952–957. doi: 10.1111/j.1365-2826.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto B, Defoe DM, Besharse JC. Membrane turnover in rod photoreceptors: ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc Royal Soc Lond B. 1987;230:339–354. doi: 10.1098/rspb.1987.0023. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86: 1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A. 1998;95:6097–102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollema NJ, Yuan Y, Jelcick AS, Sachs AJ, von Alpen D, et al. Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One. 2011;6(3):e17494. doi: 10.1371/journal.pone.0017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- Morgan WW, Kamp CW. Dopaminergic amacrine neurons of rat retinas with photoreceptor degeneration continue to respond to light. Life Sci. 1980;26:1619–1626. doi: 10.1016/0024-3205(80)90365-3. [DOI] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Genoud C, Bai X, Palczewski K. Photoreceptor phagocytosis is mediated by phosphoinositide signaling. FASEB J. 2013 doi: 10.1096/fj.13-237537. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namihira M, Honma S, Abe H, Masubuchi S, Ikeda M, et al. Circadian pattern, light responsiveness and localization of rPer1 and rPer2 gene expression in the rat retina. Neuroreport. 2001;12:471–5. doi: 10.1097/00001756-200103050-00010. [DOI] [PubMed] [Google Scholar]
- Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, et al. Circadian rhythms and light responsiveness of mammalian clock gene, Clock and BMAL1, transcripts in the rat retina. Neurosci Lett. 1999;271(1):1–4. doi: 10.1016/s0304-3940(99)00407-3. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking avB5 integrin. J Exp Med. 2004;200: 1536–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- Ning K, Li L, Liao M, Liu B, Mielke JG, et al. Circadian regulation of GABAA receptor function by CKI epsilon-CKI delta in the rat suprachiasmatic nuclei. Nat Neurosci. 2004;27:489–90. doi: 10.1038/nn1236. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Nir I, Iuvone PM. Alterations in light-evoked dopamine metabolism in dystrophic retinas of mutant rds mice. Brain Res. 1994;649(1–2):85–94. doi: 10.1016/0006-8993(94)91051-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol. 1999;19: 181–04. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996;67: 2514–20. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274: 17748–6. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1: 693–00. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Ogilvie JM, Speck JD. Dopamine has a critical role in photoreceptor degeneration in the rd mouse. Neurobiol Dis. 2002;10:33–40. doi: 10.1006/nbdi.2002.0489. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- Osborne NN, Chidlow G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye, Exp. Eye Res. 1994;59: 3–9. doi: 10.1006/exer.1994.1076. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013;114:96–105. doi: 10.1016/j.exer.2012.12.014. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, et al. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun. 2006;351(4):800–7. doi: 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Bovee-Geurts PH, Lupi D, Jeffery G, et al. Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res Mol Brain Res. 2004;123(1–2):132–5. doi: 10.1016/j.molbrainres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Friday RC, Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS ONE. 2010;5:e8552. doi: 10.1371/journal.pone.0008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, et al. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits, Eur. J Pharmacol. 2001;416: 251–4. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–9. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004;554: 467–82. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol. 2003;467:243–53. doi: 10.1002/cne.10927. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Gamble KL, Risner ML, Young LA, McMahon DG. Divergent Roles of Clock Genes in Retinal and Suprachiasmatic Nucleus Circadian Oscillators. PLoS ONE. 2012;7:e38985. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci USA. 2012;109:8145–8. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Krause G, Lewy AJ. Effect of melatonin on intraocular pressure, Curr. Eye Res. 1988;7: 649–3. doi: 10.3109/02713688809033192. [DOI] [PubMed] [Google Scholar]
- Sandu C, Hicks D, Felder-Schmittbuhl M-P. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34:507–16. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G. Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine Nacetyltransferase mRNA in rat photoreceptors. Mol Vis. 2006;12:117–4. [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, et al. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Oishi K, Shiraishi M, Hamano S, Otsuka H, et al. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport. 2000;11:3995–7. doi: 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Krächi K, et al. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–6. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, et al. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Curr Alzheimer Res. 2007;4: 47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT1 melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–7. [PubMed] [Google Scholar]
- Schneider K, Tippmann S, Spiwoks-Becker I, Holthues H, Wolloscheck T, et al. Unique clockwork in photoreceptor of rat. J Neurochem. 2010;115(3):585–94. doi: 10.1111/j.1471-4159.2010.06953.x. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS ONE. 2011 Jan 1;6(9):e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhard BM, Besharse JC. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci. 2000;20: 8572–7. doi: 10.1523/JNEUROSCI.20-23-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, et al. Melanops-independent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15(12):1099–107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, McGlinn AM, Baldwin DA, Tobias JW, Iuvone PM, et al. Image Defocus and Altered Retinal Gene Expression in Chick: Clues to the Pathogenesis of Ametropia. Invest Ophthal Visual Sci. 2011;52 (8):5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Sieving PA, luvone PM, Bush RA. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Invest Ophthalmol Vis Sci. 1998;39: 2458–65. [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teirstein PS, Goldman AI, O’Brien PJ. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest Ophthalmol Vis Sci. 1980;19:1268–1273. [PubMed] [Google Scholar]
- Terman JS, Remé CE, Wirz-Justice A. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605: 256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601: 303–7. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The Circadian Clock System in Mammalian Retina. BioEssays. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Kasamatsu M, Sakamoto K. Clock gene expression in the rat retina: effects of lighting conditions and photoreceptor degeneration. Brain Res. 2007a;1159:134–40. doi: 10.1016/j.brainres.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. Faseb J. 2007b Dec;21(14):3866–71. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Dirden JC. Dopamine Inhibits melatonin release in the mammalian retina: in vitro evidence. Neuroscience Lett. 2000;286: 119–2. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998a;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Temperature Compensation of retinal circadian oscillators in wild-type and tau mutant hamsters. NeuroReport. 1998b;9:1001–5. doi: 10.1097/00001756-199804200-00009. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Hirota T, Takemori N, Komori N, Yoshitane H, et al. Circadian proteomics of the mouse retina. Proteomics. 2007;7(19):3500–8. doi: 10.1002/pmic.200700272. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci. 2012;35:507–518. doi: 10.1111/j.1460-9568.2011.07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaux-Botteri C, Giber JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neurosci Lett. 1995;237:9–2. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96(21):12114–9. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schantz M, Lucas RJ, Foster RG. Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res Mol Brain Res. 1999;72:108–114. doi: 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Hewson-Stoate NJ, Greenwood J, Coffey PJ. Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. J Chem Neuroanat. 2007;33:9–22. doi: 10.1016/j.jchemneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol [A] 1993;172:263–270. doi: 10.1007/BF00216608. [DOI] [PubMed] [Google Scholar]
- Weng S, Wong KY, Berson DM. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. Journal of Biological Rhythms. 2009;24:391–402. doi: 10.1177/0748730409343767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–60. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Wirsig-Wiechmann CR. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells, Exp. Eye Res. 2001;73: 617–3. doi: 10.1006/exer.2001.1073. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O’Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 1992;33: 1894–2. [PubMed] [Google Scholar]
- Williams DS, Fisher SK. Prevention of rod disk shedding by detachment from retinal pigment ephitelium. Invest Ophthalmol Visual Sci. 1987;28:184–187. [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, et al. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23:7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Alones V, Menaker M. Interaction of the retina with suprachiasmatic pacemakers in the control of circadian behavior. J Biol Rhythms. 2002;17:315–329. doi: 10.1177/074873040201700405. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, et al. A noncanonical Ebox enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102(7):2608–13. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi T, Gregory DS. Ocular adrenergic nerves contribute to control of the circadian rhythm of aqueous flow in rabbits. Invest Ophthalmol Vis Sci. 1991;32:523–528. [PubMed] [Google Scholar]
- Young RW. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol. 1978;17:105–116. [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-J, Gao Y, Li P, Li L. Synchronizing multiphasic circadian rhythms of rhodopsin promoter expression in rod photoreceptor cells. J Exp Biol. 2007;210:676–684. doi: 10.1242/jeb.02694. [DOI] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci U S A. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska JB, Derbiszewska T, Nowak JZ. Clozapine and other neuroleptic drugs antagonize the light-evoked suppression of melatonin biosynthesis in chick retina: involvement of the D4-like dopamine receptor. J Neural Transm Gen Sect. 1994;97:107–7. doi: 10.1007/BF01277947. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Iuvone PM. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci Lett. 1992;135:71–4. doi: 10.1016/0304-3940(92)90138-w. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS ONE. 2011;6:e17860. doi: 10.1371/journal.pone.0017860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS ONE. 2012;7:e42647. doi: 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, et al. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou T, Ruan GX, McMahon DG. Circadian rhythm of Period1 clock gene expression in NOS amacrine cells of the mouse retina. Brain Res. 2005;1050:101–109. doi: 10.1016/j.brainres.2005.05.042. [DOI] [PubMed] [Google Scholar]