Abstract
One of the mechanisms that are in place to control the activation of mature T cells that bear self-reactive antigen receptors is anergy, a long-term state of hyporesponsiveness that is established in T cells in response to suboptimal stimulation. T cells receive signals that result not only from antigen recognition and costimulation but also from other sources including cytokine receptors, inhibitory receptors or metabolic sensors. Integration of all those signals will determine T cell fate. Under conditions that induce anergy, T cells activate a program of gene expression that leads to the production of proteins that block T cell receptor signaling and inhibit cytokine gene expression. In this review we will examine those signals that determine the functional outcome following antigen encounter, review current knowledge of the factors that ensure signaling inhibition and epigenetic gene silencing in anergic cells and explore the mechanisms that lead to the reversal of anergy and the reacquisition of effector functions.
Keywords: Anergy induction, Anergy reversal, NFAT, Epigenetics
1. Introduction
Mechanisms of central and peripheral tolerance exist to eliminate or in activate self-reactive T cells and prevent dangerous responses against self-tissues. In the thymus, T cells bearing high affinity T cell receptors (TCR) that can recognize self-antigens are deleted through negative selection [1, 2]. However, cells that still carry the potential to turn into dangerous self-reactive T cells can escape negative selection and may cause autoimmune reactions. The establishment of successful mechanisms of peripheral tolerance is, therefore, necessary to prevent autoimmunity. Peripheral tolerance involves a balance of multiple control mechanisms that include T cell deletion, cell-mediated suppression and intrinsic inactivation of T cells [3–5]. Deletion of T cells bearing self-reactive TCRs can occur in the periphery by apoptosis, which is prompted by specific antigenic stimulation of self-reactive T cells. Studies using mouse models have shown that signaling through Fas regulates apoptosis of those self-reactive T cells and that proteins of the Bcl-2 family also participate in the modulation of peripheral T cell deletion [6, 7]. Active suppression of T cell activity by regulatory T cells (Treg) constitutes a major mechanism of peripheral T cell tolerance [8]. Tregs express the transcription factor Foxp3 and have the capability of suppressing the activation of other T cells in a contact-dependent manner [9–13], although studies have also shown that cytokines, such as interleukin (IL-35), transforming growth factor beta (TGFβ) or IL-10, also participates in Treg-mediated suppression of T cell responses [14–16].
This review will focus on T cell anergy, a mechanism of peripheral tolerance that defines the functional inactivation of T cells following antigen recognition under non-optimal conditions [17]. Classically, anergy is defined as the hyporesponsive state that is established in T cells when they recognized antigen (signal 1) in the absence of costimulation (signal 2), usually provided by CD28 [18–20]. Under those conditions, T cells fail to become fully activated and enter a state of unresponsiveness that prevents cell proliferation and cytokine production in response to antigen re-encounter [17, 21]. Recent studies have expanded our understanding of the context in which T cells become anergic. Together with the signals transduced by the recognition of peptide bound to Major Histocompatibility Complexes (MHC), T cells evaluate not only the presence or absence of CD28 engagement but also multiple other environmental cues that ultimately decide T cell fate [22].
2. Signal integration: T cell activation vs. T cell anergy
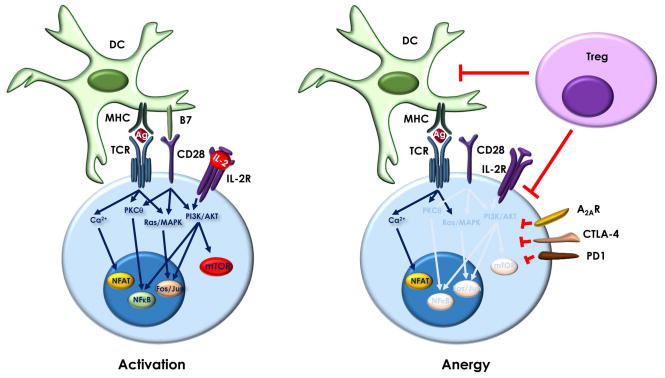
The most important variable that will determine if the fate of a T cell after encountering antigen will be to activate and engage in effector functions or to become anergic is the presence of co-stimulation provided by the binding of CD28 to its ligands, CD80 or CD86, expressed on antigen presenting cells [18, 19, 23]. However, numerous studies have made it evident that active CD28 signaling is not the only information that T cells integrate together with antigen recognition to decide their functional outcome (Fig. 1). One of the main consequences of CD28 engagement is induction of the expression and increased stabilization of the Il2 mRNA [24, 25]. Production of IL-2 constitutes one of the most important mechanisms of anergy avoidance induced by CD28 co-engagement, and signaling through the IL-2 receptor has been shown to prevent the establishment of anergy even in the absence of co-stimulation [26]. Different targets have been identified downstream of the IL-2 receptor that may explain how this signaling pathway may be responsible for the avoidance of anergy. Engagement of the IL-2 receptor activates the phosphatidylinositol 3 kinase (PI3K)/AKT axis, which, among other targets, induces the degradation of the cyclin-dependent kinase inhibitor p27kip1 [27–29]. In the absence of costimulation or IL-2 receptor signaling, p27kip1 fails to be degraded and progression through the cell cycle is halted. Consequently, T cells that lack p27kip1 become resistant to costimulation blockade-induced anergy [29]. Recently, it has been also shown that engagement of the IL-2 receptor causes repression of the expression of the histone deacetylase sirtuin 1 (Sirt 1), which by inhibiting Jun activity, plays an important role in the suppression of activation-induced responses in anergic T cells [30, 31]. This effect is also mediated by the activation of PI3K/AKT, which results in the cytosolic sequestration of FoxO3, a transcription factor required for the expression of Sirt1 in anergic cells.
Figure 1.
Signal integration determines T cell fate. Activated T cells integrate signals triggered by recognition of MHC-antigen (Ag) complexes by the TCR, together with those induced by the engagement of CD28 by B7 ligands and by binding of IL-2 to the IL-2 receptor. Those signals translate into the activation of a series of signaling pathways, including increased calcium entry and activation of PKCθ, Ras/MAPKs, PI3K/AKT and mTOR, which allow the T cell to upregulate its metabolism and induce the transcription factors (e.g. NFAT, Fos/Jun or NFκB) required to maintain an activation-induced program of gene expression. When TCR engagement occurs in the absence of costimulation and/or the presence of inhibitory signals (e.g. effects of Tregs on DCs and effector T cells or engagement of coinhibitory receptors such as CTLA-4, PD-1 or A2aR) and unbalance activation of those signaling pathways leads to the induction of an alternative program of gene expression that will result in anergy.
The importance of the mammalian target of rapamycin (mTOR) activation as a regulator of T cell fate has been brought to light recently in studies that analyzed mouse models deficient for components of the mTOR complexes in T cells [32, 33]. Activated AKT downstream of the IL-2 receptor phosphorylates tuberous sclerosis complex proteins (TSC), inhibiting the GTPase activating protein activity that TSC has on the GTP-binding protein Rheb, an mTOR activator. Consequently IL-2 receptor engagement results in increased levels of GTP-bound Rheb and mTOR activation [34, 35]. The importance of this pathway for T cell anergy was demonstrated by early studies that showed that activation of T cells in the presence of the mTOR inhibitor rapamycin induced anergy even when cells received full costimulation [36]. Though initially thought that this effect was due to the fact that mTOR was required for the T cells to undergo the G1-to-S transition, it was soon proven that inhibition of cell cycle progression through the targeting of other cell cycle regulators did not cause T cells to become anergic following full stimulation, and that transition from G1 to S did not prevent cells from becoming anergic [37, 38]. These results suggested that it was mTOR signaling itself what really was necessary to prevent anergy. In fact, it was later shown that it was the role of this kinase in the modulation of T cell metabolism what defined mTOR as a regulator of T cell fate [39]. T cell activation is a highly metabolically demanding process and activation of mTOR is necessary for T cells to adapt to this new high demand. If mTOR activity is not efficiently induced and T cells cannot increase their metabolism during activation, they become anergic. It is interesting to note once anergic T cells are not only unable to proliferate and secrete IL-2 but also to induce the metabolic machinery required to sustain activation, failing to upregulate the expression of glucose, amino acid and iron transporters [40, 41]. Inhibition of mTOR with rapamycin during activation, does not only prevent anergy avoidance but also promotes the differentiation of Tregs, cells that are also intrinsically anergic, as they do not produce IL-2 or proliferate when stimulated, unless exogenous IL-2 is provided [32].
Although the presence of costimulatory positive signals is necessary to avoid anergy, T cells also integrate signaling received through the engagement of inhibitory receptors. For instance, coinhibition through the cytotoxic T-lymphocyte-associated protein 4(CTLA-4) or the programmed cell death 1 (PD-1) receptors is also an important cue in the regulation of T cell fate, and both coinhibitory receptors have been shown to participate in the regulation of T cell anergy in different contexts [42–48]. Similarly, engagement of the adenosine receptor A2AR causes inhibition of the Ras/Mitogen activated protein kinase (MAPK) signaling pathway and induces anergy even in the presence of costimulation [49]. Recently the role of Tregs in the maintenance of T cell anergy has been proposed. Mice adoptively transferred with self-antigen specific arithrogenic CD4+T cell populations failed to induce anergy in those T cells in the absence of Tregs and could not prevent the development of arthritis [50]. This study supports that signals imparted directly or indirectly by Tregs are also key regulators of the establishment of peripheral T cell tolerance, not only through suppression of the activity of other cell populations but also by inducing anergy. All data available indicates, thus, that the induction of T cell anergy is tightly controlled process that results from the integrated balance of multiple positive and negative signals that constitute a much more complex system than the classical signal 1/signal 2 model (Fig. 1).
3. Transcriptional regulation of T cell anergy
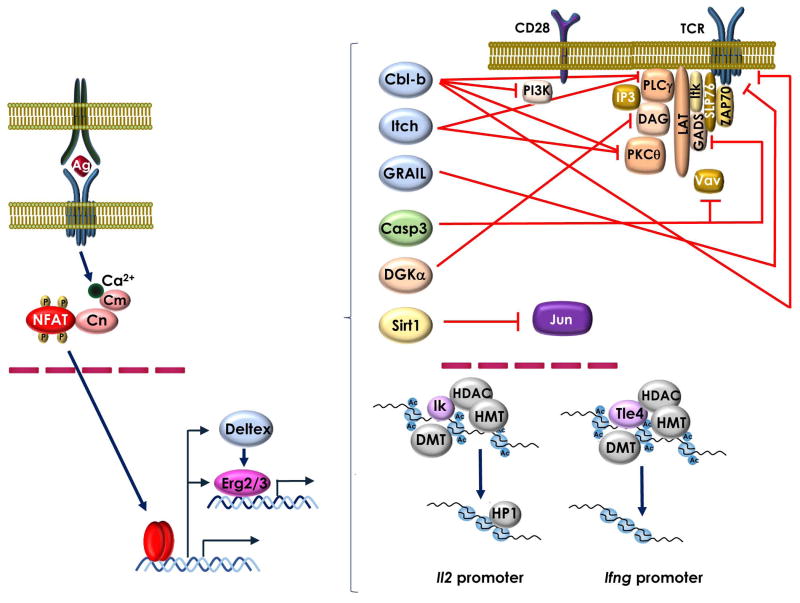
The engagement of the TCR by MHC-antigen complexes allows the CD4 or CD8 coreceptor-associated src-family lymphocyte-specific protein tyrosine kinase (Lck) to phosphorylate immunoreceptor tyrosine based activation motifs (ITAMS) on the CD3 chains of the TCR, creating docking sites for ζ-associated protein of 70 KDa (ZAP70) that is then phosphorylated by Lck [51–55]. The consequent phosphorylation of the membrane-associated linker for T-cell activation (LAT) and the SH2 domain-containing leukocyteprotein of 76 KDa (SLP-76) by Zap70, induces the recruitment of Grb2-related adapter downstream of shc (GADS) [56, 57]. The new formed docking sites allows phospholipase C-γ1 (PLCγ1) to be activated by the Tec family kinase Itk [58]. PLCγ then cleaves the membrane phospholipid phosphatidylinositol-4, 5-biphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). PKC/Ras/MAPK signaling is activated by TCR-induced DAG [59, 60], but CD28 engagement is required for efficient activation of those signaling pathways [61]. IP3 interacts with its receptor and induces the aperture of calcium channels at the endoplasmic reticulum. Depletion intracellular calcium stores is sensed by STIM proteins that then activate store-operated calcium entry (SOCE) channels at the plasma membrane [62]. This process requires only TCR engagement. When T cells are activated without the engagement of costimulation, unbalance calcium signaling in the absence of full activation of PKC/Ras-regulated pathways results in the expression of a specific set of genes encoding for proteins that are responsible for the hyporesponsive state of anergic T cells [63]. Blockade of CD28-regulated pathways by inhibitory receptors would have similar effect. The importance of calcium signaling in the induction of T cells anergy was evident form early experiments that showed that the calcineurin inhibitor cyclosporine A prevented T cells activated with anti-CD3 without CD28 engagement from becoming anergic [64]. The calcium/calmodulin-dependent phosphatase calcineurin is a major transducer of calcium signaling in T cells. Activation of calcineurin in response of increased intracellular calcium results in the dephosphorylate members of the nuclear factor of activated T cells (NFAT) family of transcription factors, which are otherwise retained in the cytosol in a highly phosphorylated state [65, 66]. Upon dephosphorylation, NFAT proteins undergo a conformational change that exposes their nuclear localization signal and allows for their translocation into the nucleus [67]. In activated T cells, NFAT partners with AP-1 and other transcription factors to induce the expression of many activation-induced genes [65, 68, 69]. Suboptimal activation of T cells would cause to the calcium/calcineurin-induced nuclear translocation of NFAT without full activation of MAPK-dependent AP-1 transcription complexes. Under these circumstances, NFAT1 forms low affinity homodimers [70, 71], instead of the higher affinity NFAT/AP-1 complexes that would assemble in fully activated cells [63, 72]. In anergic cells, NFAT homodimers form onto symmetric binding sites similar to the canonical κB sites [70, 71] to induce transcription of a specific program of anergy-associated gene expression (Fig. 2) [72, 73]. The central role of NFAT1 in the induction of T cell anergy is supported by the fact that NAFT1-deficient T cells are resistant to stimuli that induce anergy, whereas expression of a constitutively active form of this protein renders T cells hyporesponsive [73].
Figure 2.
NFAT proteins activate an anergy-inducing program of gene expression. In response to tolerizing stimuli, preferential activation of Ca2+-Calmodulin (Cm)-Calcineurin (Cn) signaling pathway leads to the expression of an NFAT-dependent program of gene expression. NFAT containing complexes, including NFAT1 homodimers, and other transcription factors, especially Egr proteins, will induce the expression of a series of proteins that will inhibit T cell activation at different levels. The ubiquitin ligases GRAIL, Itch and Cbl-b, caspase 3 and DGKα, will block TCR and CD28 signaling through targeted degradation or inactivation of multiple proteins that form part of the TCR signalosome. Sirt1 will directly deacetylate and inactivate Jun. Binding of transcriptional repressors, such as Ikaros (IK) or Tle4, to the Il2 and Ifng loci recruit chromatin remodeling proteins that together with DNA methyl transferases (DMT) will induce epigenetic modifications that will turn those genes into transcriptionally silenced loci.
In addition to NFAT1, other transcription factors also contribute in the activation of an anergy-inducing gene expression program. Expression of early growth response proteins 2 and 3 (Egr-2/Egr-3) is upregulated in an NFAT-dependent manner in anergic CD4+ T cells. Egr proteins control the expression several anergy-associated genes, including those encoding for the ubiquitin ligase Casitas B-lineage lymphoma b (Cbl-b) and diacylglycerol kinase alpha (DGKα) [74–76]. Consequently, Egr2-deficient T cells are resistant to tumor-induced anergy in vivo, which permits mice bearing those T cells to mount more efficient and potent antitumor responses [76]. The activity of Egr2 might be modulated by other proteins. Deltex-1 is also expressed in anergic T cells in an NFAT-dependent way and Deltex1-deficient T cells that are hyperactive and resistant to anergizing stimuli [77]. Deltex-1 interacts with Egr-2 to upregulate the expression of Cblb, though the mechanism through which this functional synergy occurs is still not known [77].
The hyporesponsive state in anergic T cells responds, therefore, to the activation of an anergy-associated program of gene expression that is controlled by a specific set of transcription factors including NFAT1 and Egr2. As we will discuss below, proteins encoded by those genes are responsible for the blockade of TCR signaling and the inhibition of cytokine expression that defines anergic T cells (Fig. 2).
4. Inhibitory mechanisms engaged in anergic T cells
When integration of the different positive signals and negative signals received during the interaction with cognate antigen results in anergy, T cells induce the expression of a series of proteins that eventually will prevent the cell from being activated following new encounter with antigen. Functionally, these proteins exert their inhibitory action at two different levels:blocking signaling downstream of the TCR and/or CD28, and directly inhibiting the expression of cytokine genes.
4.1. Signaling blocks in anergic T cells
Initial characterization of signaling pathways in anergic T cells identified a block in the activation of MAPK/Ras that precluded activation following re-stimulation [78, 79]. In the last 10 years many of the mediators responsible for this signaling blockade have been discovered and the mechanisms underlying their effects characterized. Several E3 ubiquitin ligases are expressed in anergic T cells that target components of the TCR- and CD28-activated signaling pathways for degradation or inactivation. The RING-type E3 ubiquitin ligase GRAIL is expressed in anergic T cells, and GRAIL-deficient cells show a hyperresponsive phenotype with resistance to tolerance induction in vitro and in vivo [80–82]. Several targets have been proposed to explain the function of this ubiquitin ligase in T cells including CD3, the Rho guanine dissociation inhibitor (Rho GDI), CD40 ligandor the actin-related protein 2/3 subunit 5 (Arp2/3–5). GRAIL-mediated ubiquitination of these proteins causes their inactivation or degradation, which leads to reduced TCR-induced signaling and impaired formation of mature immunological synapses [82–86]. Cbl-b, another RING-type ubiquitin ligase, and Itch, a HECT-type E3 ligase, are also expressed in anergic cells where they regulate the inactivation and degradation of several signaling intermediates including PKCθ, PLCγ-1, the p85 regulatory subunit of the PI3K, Jun or the zeta chain of the TCR [87–91].
Proteolytic cleavage of Vav1 and GADS also occurs in anergic CD4+ T cells but not due to the activity of the ubiquitin/proteasome system but mediated instead by the calcium-regulated activation of caspase 3. Cleavage prevents recruitment of those proteins to the plasma membrane inhibiting effective signaling through the TCR signalosome [92]. Expression of the diacylglycerol kinase alpha (DGKα) is also upregulated in CD4+ T cells in response to anergizing stimuli in an NFAT/Egr2-dependent manner [73, 76]. DGKα phosphorylates DAG to form phosphoric acid, hence blocking DAG-activated signaling pathways. Upregulation of the expression of this kinase to prevent DAG-induced PKC/Ras signaling has been shown to be acrucial inhibitory mechanism required for the induction of T cell tolerance [93, 94].
Other mechanisms that cause defective upstream signaling in anergic T cells have also been identified. Defects in LAT palmitoylation results in decreased recruitment of LAT to the plasma membrane and defective localization of this key adaptor protein into the immunological synapse, though the mechanism responsible for the changes in LAT palmitoylation remain to be identified [95].
4.2 Transcriptional regulation of cytokine expression
Although inhibition of TCR signaling should prevent the expression of cytokines, mechanisms that directly inhibit cytokine gene expression that contribute to maintain an unresponsive state are also engaged in anergic T cells. Recent studies have identified Sirtuin 1 (Sirt 1) a type III histone deacetylase as a key factor for the maintenance of T cell tolerance. Sirt 1 interacts with and deacetylates c-Jun. That modification inactivates this transcription factor that forms part of the NFAT/AP-1 complexes required to induce ll2 expression in activated T cells [31]. Furthermore, negative regulators of Il2 gene expression, including the transducer of ERBB2 1(Tob1), Smad3 and the cAMP responsive element binding protein/cAMP responsive element modulator complex (CREB/CREM) are also expressed in anergic T cells, where they bind the Il2 promoter and directly repress Il2 gene transcription [96, 97].
Inhibition of Il2 gene expression in anergic T cells is not only controlled by the transient action of transcription factors. In the last few years, several studies have shown that epigenetic imprinting occurs at this locus that silences the expression of the Il2 gene. Ikaros, the founding member of the Ikaros family of transcription factors, binds to sites in the Il2 promoter where it recruits histone deacetylases (HDAC) to remove acetyl-groups form the tails of histones 3 and 4 and induce silencing of the Il2 gene [98, 99]. It is interesting to note that two other members of the Ikaros family, Eos and Helios, have been shown to be responsible for the epigenetic silencing of the ll2 gene in natural Tregs [100, 101], suggesting that different Ikaros proteins may regulate the epigenetic silencing of Il2 transcription in distinct T cell populations that have an anergic phenotype. Histone deacetylation is however not the only chromatin modification that maintains this gene in a repressed state. Histone deacetylation-dependent recruitment of the histone methyl trasnferase Suv39H1 in anergic CD4+ T cells results in tri-methylation of histone 3 at lysine 9. This modification creates docking sites for the heterochromatin binding protein HP-1, which results in the translocation of the Il2 locus into silent heterochromatin-rich regions in the nucleus [102]. Changes in DNA methylation are also often associated with transcriptionally active or silent loci. Whereas upregulation of the Il2 gene transcription in activated T cells correlates with marked DNA demethylation of the Il2 promoter [103], in an in vivo model of superantigen induced T cell anergy, decreased Il2 expression in this cells was accompanied by increased DNA methylation of the Il2 promoter, that is likely to contribute to the imprinting of a stable silencing state of this gene in anergic cells [104]. Interestingly, this modification was not only circumscribed to the Il2 promoter but was also detected in the Ifng locus, suggesting that epigenetic silencing of cytokine expression affects not only IL-2 but may also extend to effector cytokines such as IFNγ [104]. Supporting this hypothesis, our lab has recently found that Tle4, a member of the Groucho family of transcription factors, is also expressed in anergic cells, where it binds to an Ifng enhancer, inducing epigenetic marks associated with transcriptional repression, including histone deacetylation and trimethylation of lysine 9 on histone 3 (Bandyopadhyay, S and Macian, F; unpublished observations). A recent study has suggested that alternatives check points may be also activated in anergic T cells to prevent expression of cytokines. Using an in vivo model of adaptive tolerance, TCR-transgenic CD4+ T cell transferred into a host expressing a soluble version of the cognate antigen became anergic but did not show a significant decreased in their ability to transcribe cytokine genes. These cells instead had a block in cytokine mRNA translation that resulted in greatly diminishes production of effector cytokines [105]. Though the mechanism responsible for the regulation of cytokine mRNA translation in anergic T cells have not been elucidated yet, it is tempting to speculate that post-transcriptional gene regulation by RNA-binding proteins and/or micro RNAs may constitute additional controls on the expression of cytokine in these cells.
The existence of a series of inhibitory mechanisms that target the TCR signaling pathway at multiple levels and block cytokine expression through different mechanisms should ensure that anergic T cells stay unresponsive and autoimmune reaction do not occur. It is likely, though, that distinct subsets of those mechanisms that sustain anergy might be induced in response to different tolerizing environments that deliver specific negative signals to self-reactive T cells.
5. Stability of the anergic phenotype. Plasticity in anergic T cells
Anergy is defined as a long-term state of unresponsiveness, and in many models of T cell tolerance, anergic T cells can be found long after the tolerizing stimulus has been delivered. This long-tern nature of the anergic state could respond to the epigenetic modifications on cytokine genes, that would lead to stable inhibition of gene transcription [98, 99, 102, 104]. Furthermore, it is also possible that a self-maintaining circuitry may exist. Due to the signaling blocks present in anergic cells, re-encounter with antigen would only produce a suboptimal activation that would reinforce the expression of the genetic program of anergy. This could explain why in some models continuous presence of antigen has been reported to be necessary to sustain anergy in vivo [106]. Maintenance of hyporesponsive T cell populations might respond to a need to keep an effective TCR repertoire, as elimination in the thymus or the periphery of all T cells bearing TCRs with any level of affinity for self-antigen could result in a dangerously reduced TCR diversity. If this is the case, it would be expected that plasticity should exist in the anergic T cell compartment that would allow reactivation and transformation of anergic cells into effector T cells when required. Co-stimulation alone is not able to reverse tolerance. The presence of blocks that prevent transduction of signals downstream of CD28 would explain why anergic T cells do not respond to reencounter with antigen even when costimulation is present. It has been known however for some time that signaling through the IL-2 receptor not only prevents the induction of anergy but can also reverse it [107]. Different mechanisms have been proposed to explain how IL-2 receptor engagement can break T cell tolerance. One of the main signaling defects in anergic T cells is the inhibition of the Ras/MAPK pathway, but IL-2 can directly induced activation of MAPK and AP-1, bypassing that signaling block [108]. Similarly, PI3K/AKT activation downstream of the IL-2 receptor can activate mTOR, allowing anergic T cells to upregulate their metabolic output to adapt to the demands raised by activation in otherwise metabolically anergic cells [39, 41]. Recently. It has been shown that, also as a result of the activation for the PI3K/AKT pathways downstream of the IL-2 receptor, the transcription factor FoxO3 a remains sequestered in the cytosol, preventing it from interacting with Egr proteins to induce the expression of Sirt1, a protein required for the establishment of the unresponsive state in anergic T cells [30, 31]. In any case, breaking tolerance through signaling mediated by the IL-2 receptor would allow anergic T cells to become effector cells if cross-recognition of a foreign antigen occurred in the context of an active immune response, where IL-2 would be secreted by other non-tolerant cell populations.
Well characterized in the CD8+ T cell compartment, transfer of anergic T cells into a lymphopenic host leads to homeostatic proliferation that results in a break of tolerance and the activation of effector functions in the transferred population [109]. Expansion of T cells transferred into a lymphopenic host appears to respond to interactions of T cells with MHC complexes presenting self-peptides and by homeostatic cytokines, such as IL-7 or IL-15, which by signaling through the common gamma chain could contribute to the reversal of tolerance. This process has been shown to have the potential to be a potent tool to improve anti-tumor responses by breaking tumor antigen-induced T cell anergy [110, 111]. A recent study has shown, however, that even though transfer of TCR transgenic CD8+ T cells anergic to a specific self-antigen into lymphopenic hosts results in proliferation and regaining of their ability to respond to cognate antigen, reversal of tolerance appears to be transient, and cells reacquired an anergic phenotype after a few months. Surprisingly, this occurs even in hosts that do not express the self-antigen recognized by the TCR-transgenic CD8+ T cells [112]. This study has important implications for our understanding on how T cell tolerance is maintained. The capacity of anergic T cells to regain effector functions after homeostatic proliferation is limited in time an effect which is likely due to intrinsic cell properties, as it occurs in the absence of tolerizing antigen. Available data clearly indicates that silencing of cytokine genes in anergic cells is maintained through epigenetic mechanisms, which might also include microRNAs. The “anergic” memory described in CD8+ T cells could therefore be a results of epigenetic memory, which would be temporarily overcome under specific situations (e.g. inflammatory reaction, homeostatic proliferation) but that would cause the cell to re-establish an anergic program once those stimuli that induce tolerance reversal disappear.
The development of T cells with suppressive capacity occurs concomitant with the generation of hyporesponsive cells in several models of T cell anergy, and signals that induce anergy are also involved in the generation of Tregs [32, 49, 113–115]. Furthermore, proteins that regulate the induction and maintenance of anergy have also been reported to participate in the generation of Tregs [116–119]. These results may indicate the existence of a common differentiation pathway that would lead to anergy or to the acquisition of regulatory function depending on the specific context in which antigen is encounters and the presence of specific additional signals. However, it is also possible that anergic T cells can acquire not only effector functions, as described above, but also suppressive capacity and constitute a reservoir of T cells bearing self-reactive TCRs that can become regulatory T cells.
6. Concluding remarks
In the last decade we have gained a clear insight into the molecular mechanisms that regulate the induction and maintenance of T cell anergy. T cells integrate a series of different cues that extend beyond the presence or absence of costimulation to include the activation of cytokine or coinhibitory receptors or the presence of populations of cells with suppressor activity. Under conditions that result in tolerance, NFAT and Egr transcription factors induce the activation of a program of gene expression that results in the synthesis of proteins that inhibit TCR signaling by targeting different pathways downstream of the TCR or CD28; and induce epigenetic modifications that result in the stable silencing of cytokine expression. The unresponsive phenotype in anergic cells is, however, not a differentiation end-point. IL-2 receptor signaling or forced homeostatic proliferation can break tolerance. Under these circumstances anergic T cells regain the ability to expand in response to antigen and engage in effector functions. The exact mechanisms that are responsible for anergy reversal are still not fully understood but they need to involve bypassing of signaling blocks and the removal of epigenetic repression. Recent evidence has indicated that reversal of anergy is not a stable but a transient process in CD8+ T cells. We still do not know, though, whether the same holds true for T helper cells. Tight epigenetic regulation of the transcription or translation of genes involved in the maintenance of the anergic status may account for the unstable plasticity of anergic T cells, however the mechanisms responsible for the imprinting of anergic memory in T cells are still not known. The characterization of the cell populations, signaling networks and epigenetic mechanisms that control the induction and reversal of the anergic phenotype in T cells may help us understand better the dynamic nature of anergy and identify new therapeutic targets aimed at promoting tolerance or activation to prevent autoimmune disease or graft rejection or to boost anti-tumor immune responses.
Highlights.
Anergy is a long-term state of hyporesponsiveness that is established in T cells in response to suboptimal stimulation
T cells integrate different signals to determine whether the outcome after encounter with antigen will be activation or anergy.
Anergy in T cells results from the activation of a specific program of gene expression that leads to the synthesis of proteins that block TCR signaling or directly inhibit cytokine gene expression.
In response to specific stimuli, such as IL-2 or homeostatic proliferation, anergy can be reversed and T cells can regain effector functions.
Epigenetic control of gene expression in anergic T cells might underlie the existence of “anergic memory” which may limit the extent or anergy reversal.
Acknowledgments
This work was supported by NIH grant AI059738.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 3.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 4.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 5.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 6.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 7.Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 9.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 15.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 18.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 21.Macian F, Im SH, Garcia-Cozar FJ, Rao A. T-cell anergy. Curr Opin Immunol. 2004;16:209–216. doi: 10.1016/j.coi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22:552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 25.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 26.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 27.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 28.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 29.Rowell EA, Walsh MC, Wells AD. Opposing roles for the cyclin-dependent kinase inhibitor p27kip1 in the control of CD4+ T cell proliferation and effector function. J Immunol. 2005;174:3359–3368. doi: 10.4049/jimmunol.174.6.3359. [DOI] [PubMed] [Google Scholar]
- 30.Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A. 2012;109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 37.Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–4803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 38.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J Immunol. 2002;169:6178–6186. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 40.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci U S A. 2012;109:14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 43.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 44.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 45.Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest. 2001;108:895–903. doi: 10.1172/JCI13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop KD, Harris JE, Mordes JP, Greiner DL, Rossini AA, Czech MP, et al. Depletion of the programmed death-1 receptor completely reverses established clonal anergy in CD4(+) T lymphocytes via an interleukin-2-dependent mechanism. Cell Immunol. 2009;256:86–91. doi: 10.1016/j.cellimm.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 48.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989;86:3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan AC, Irving BA, Fraser JD, Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991;88:9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 54.Isakov N, Wange RL, Burgess WH, Watts JD, Aebersold R, Samelson LE. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: the tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J Exp Med. 1995;181:375–380. doi: 10.1084/jem.181.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 56.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 58.Sommers CL, Rabin RL, Grinberg A, Tsay HC, Farber J, Love PE. A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection. J Exp Med. 1999;190:1427–1438. doi: 10.1084/jem.190.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 60.Krishna S, Zhong XP. Regulation of Lipid Signaling by Diacylglycerol Kinases during T Cell Development and Function. Front Immunol. 2013;4:178. doi: 10.3389/fimmu.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 63.Baine I, Abe BT, Macian F. Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol Rev. 2009;231:225–240. doi: 10.1111/j.1600-065X.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 65.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, et al. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 66.Loh C, Shaw KT, Carew J, Viola JP, Luo C, Perrino BA, et al. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J Biol Chem. 1996;271:10884–10891. doi: 10.1074/jbc.271.18.10884. [DOI] [PubMed] [Google Scholar]
- 67.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, et al. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 68.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 69.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giffin MJ, Stroud JC, Bates DL, von Koenig KD, Hardin J, Chen L. Structure of NFAT1 bound as a dimer to the HIV-1 LTR kappa B element. Nat Struct Biol. 2003;10:800–806. doi: 10.1038/nsb981. [DOI] [PubMed] [Google Scholar]
- 71.Jin L, Sliz P, Chen L, Macian F, Rao A, Hogan PG, et al. An asymmetric NFAT1 dimer on a pseudo-palindromic kappa B-like DNA site. Nat Struct Biol. 2003;10:807–811. doi: 10.1038/nsb975. [DOI] [PubMed] [Google Scholar]
- 72.Soto-Nieves N, Puga I, Abe BT, Bandyopadhyay S, Baine I, Rao A, et al. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J Exp Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 74.Harris JE, Bishop KD, Phillips NE, Mordes JP, Greiner DL, Rossini AA, et al. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- 75.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med. 2012;209:2157–2163. doi: 10.1084/jem.20120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsiao HW, Liu WH, Wang CJ, Lo YH, Wu YH, Jiang ST, et al. Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity. 2009;31:72–83. doi: 10.1016/j.immuni.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 78.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 80.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 81.Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc Natl Acad Sci U S A. 2009;106:16770–16775. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nurieva RI, Zheng S, Jin W, Chung Y, Zhang Y, Martinez GJ, et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 2010;32:670–680. doi: 10.1016/j.immuni.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su L, Lineberry N, Huh Y, Soares L, Fathman CG. A novel E3 ubiquitin ligase substrate screen identifies Rho guanine dissociation inhibitor as a substrate of gene related to anergy in lymphocytes. J Immunol. 2006;177:7559–7566. doi: 10.4049/jimmunol.177.11.7559. [DOI] [PubMed] [Google Scholar]
- 84.Lineberry NB, Su LL, Lin JT, Coffey GP, Seroogy CM, Fathman CG. Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy. J Immunol. 2008;181:1622–1626. doi: 10.4049/jimmunol.181.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schartner JM, Simonson WT, Wernimont SA, Nettenstrom LM, Huttenlocher A, Seroogy CM. Gene related to anergy in lymphocytes (GRAIL) expression in CD4+ T cells impairs actin cytoskeletal organization during T cell/antigen-presenting cell interactions. J Biol Chem. 2009;284:34674–34681. doi: 10.1074/jbc.M109.024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ichikawa D, Mizuno M, Yamamura T, Miyake S. GRAIL (gene related to anergy in lymphocytes) regulates cytoskeletal reorganization through ubiquitination and degradation of Arp2/3 subunit 5 and coronin 1A. J Biol Chem. 2011;286:43465–43474. doi: 10.1074/jbc.M111.222711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 88.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 89.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 90.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Huang H, Jeon MS, Liao L, Yang C, Elly C, Yates JR, 3rd, et al. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity. 2010;33:60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 94.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 95.Hundt M, Tabata H, Jeon MS, Hayashi K, Tanaka Y, Krishna R, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–522. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Berezovskaya A, et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 97.Powell JD, Lerner CG, Ewoldt GR, Schwartz RH. The -180 site of the IL-2 promoter is the target of CREB/CREM binding in T cell anergy. J Immunol. 1999;163:6631–6639. [PubMed] [Google Scholar]
- 98.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 100.Baine I, Basu S, Ames R, Sellers RS, Macian F. Helios induces epigenetic silencing of IL2 gene expression in regulatory T cells. J Immunol. 2013;190:1008–1016. doi: 10.4049/jimmunol.1200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bandyopadhyay S, Montagna C, Macian F. Silencing of the Il2 gene transcription is regulated by epigenetic changes in anergic T cells. Eur J Immunol. 2012;42:2471–2483. doi: 10.1002/eji.201142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 104.Thomas RM, Saouaf SJ, Wells AD. Superantigen-induced CD4+ T cell tolerance is associated with DNA methylation and histone hypo-acetylation at cytokine gene loci. Genes Immun. 2007;8:613–618. doi: 10.1038/sj.gene.6364415. [DOI] [PubMed] [Google Scholar]
- 105.Villarino AV, Katzman SD, Gallo E, Miller O, Jiang S, McManus MT, et al. Posttranscriptional silencing of effector cytokine mRNA underlies the anergic phenotype of self-reactive T cells. Immunity. 2011;34:50–60. doi: 10.1016/j.immuni.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 107.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 108.Dure M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Molecular Immunology. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 110.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 111.Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol. 2012;188:2630–2642. doi: 10.4049/jimmunol.1100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vendetti S, Chai JG, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol. 2000;165:1175–1181. doi: 10.4049/jimmunol.165.3.1175. [DOI] [PubMed] [Google Scholar]
- 114.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.MacKenzie DA, Schartner J, Lin J, Timmel A, Jennens-Clough M, Fathman CG, et al. GRAIL is up-regulated in CD4(+) CD25(+) T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007;282:9696–9702. doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- 117.Okamura T, Fujio K, Shibuya M, Sumitomo S, Shoda H, Sakaguchi S, et al. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci U S A. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 119.Wu YQ, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]