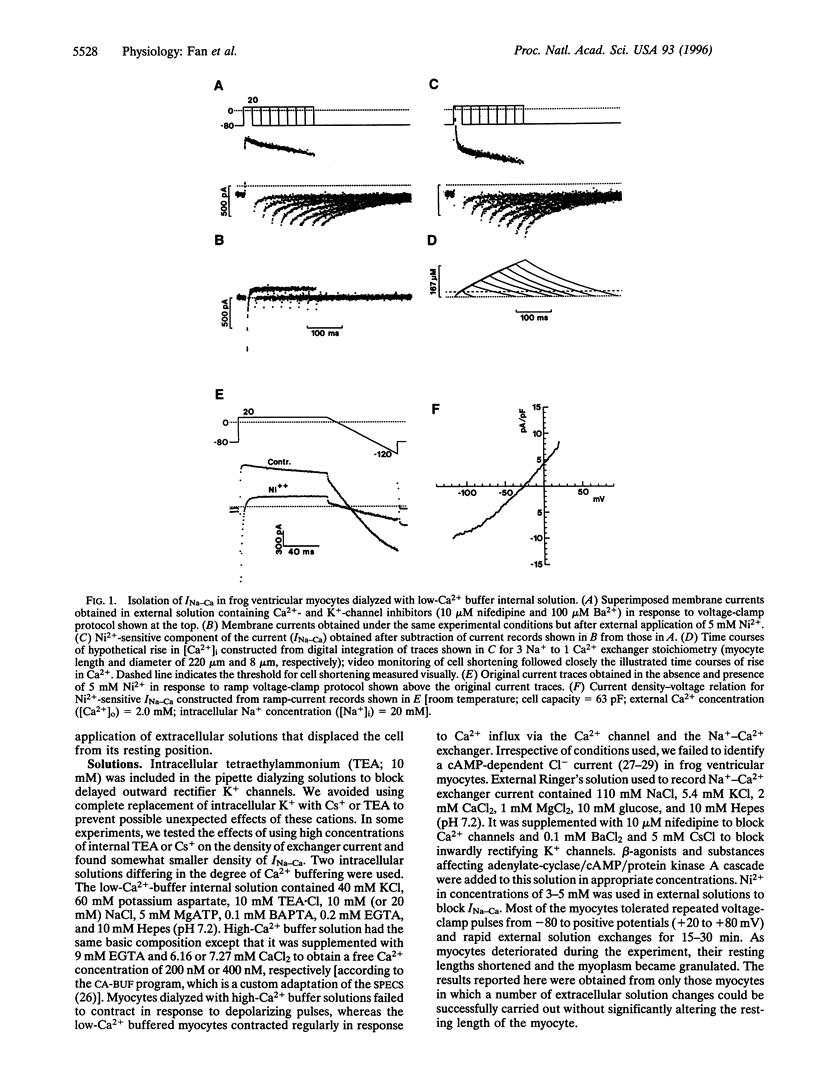
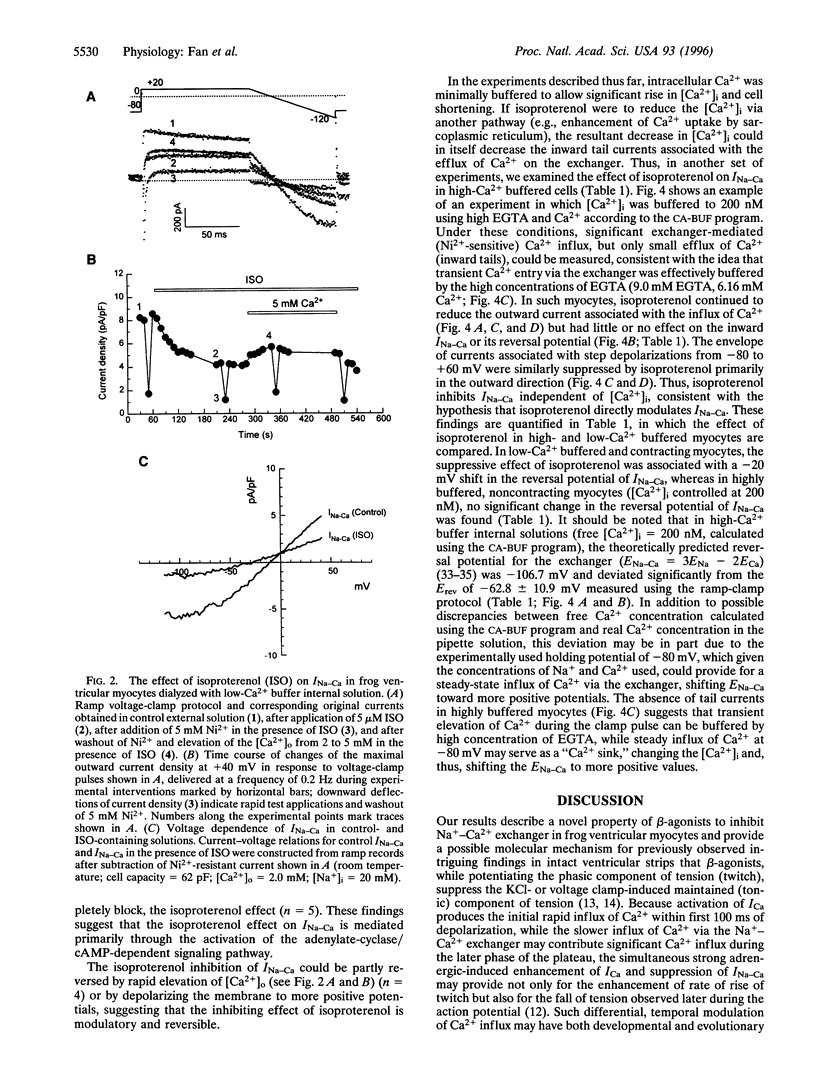
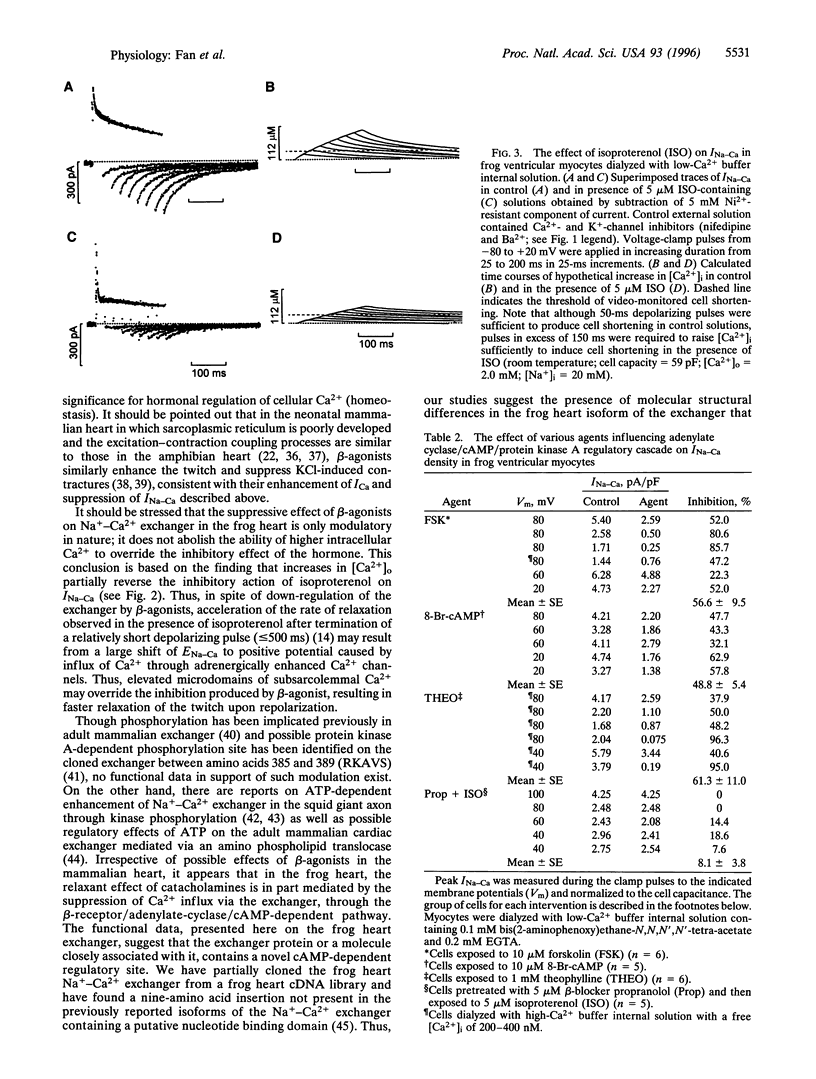
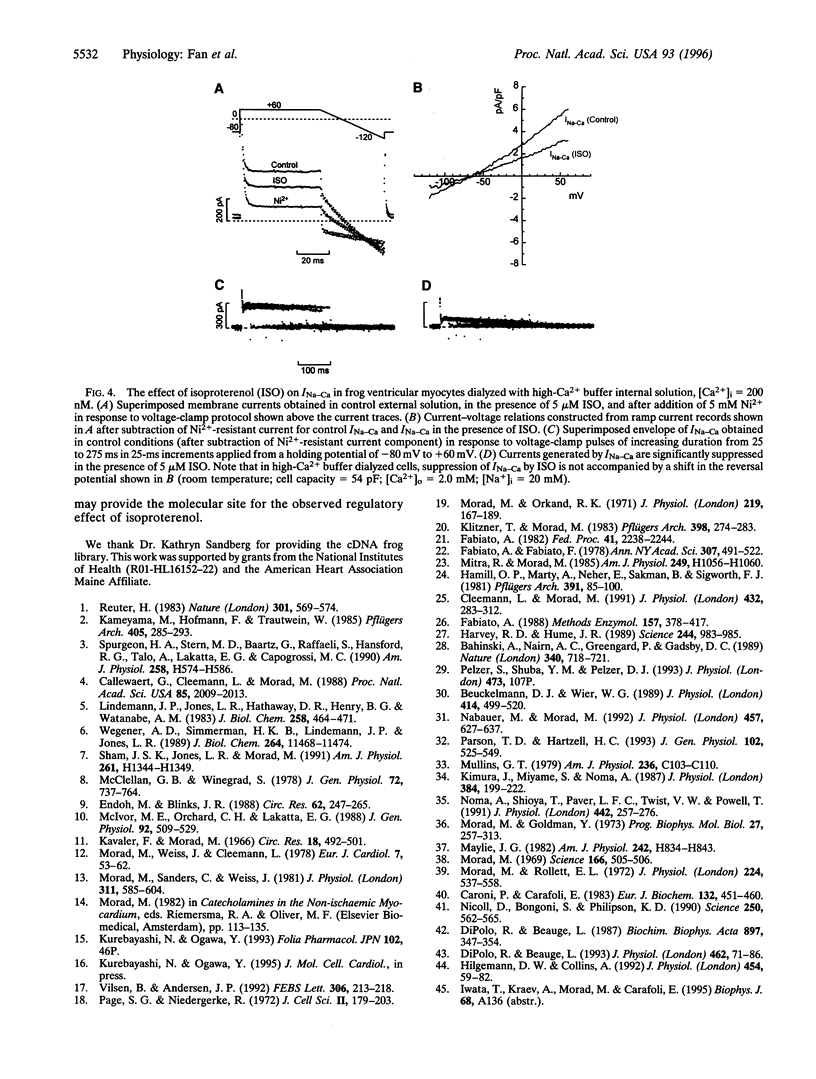
Abstract
Na+-Ca2+ exchanger and Ca2+ channel are two major sarcolemmal Ca2+-transporting proteins of cardiac myocytes. Although the Ca2+ channel is effectively regulated by protein kinase A-dependent phosphorylation, no enzymatic regulation of the exchanger protein has been identified as yet. Here we report that in frog ventricular myocytes, isoproterenol down-regulates the Na+-Ca2+ exchanger, independent of intracellular Ca2+ and membrane potential, by activation of the beta-receptor/adenylate-cyclase/cAMP-dependent cascade, resulting in suppression of transmembrane Ca2+ transport via the exchanger and providing for the well-documented contracture-suppressant effect of the hormone on frog heart. The beta-blocker propranolol blocks the isoproterenol effect, whereas forskolin, cAMP, and theophylline mimic it. In the frog heart where contractile Ca2+ is transported primarily by the Na+-Ca2+ exchanger, the beta-agonists' simultaneous enhancement of Ca2+ current, ICa, and suppression of Na+-Ca2+ exchanger current, INa-Ca would enable the myocyte to develop force rapidly at the onset of depolarization (enhancement of ICa) and to decrease Ca2+ influx (suppression of INa-Ca) later in the action potential. This unique adrenergically induced shift in the Ca2+ influx pathways may have evolved in response to paucity of the sarcoplasmic reticulum Ca2+-ATPase/phospholamban complex and absence of significant intracellular Ca2+ release pools in the frog heart.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Effects of some metal-ATP complexes on Na(+)-Ca2+ exchange in internally dialysed squid axons. J Physiol. 1993 Mar;462:71–86. doi: 10.1113/jphysiol.1993.sp019544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. In squid axons, ATP modulates Na+-Ca2+ exchange by a Ca2+i-dependent phosphorylation. Biochim Biophys Acta. 1987 Mar 12;897(3):347–354. doi: 10.1016/0005-2736(87)90432-9. [DOI] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed Proc. 1982 May;41(7):2238–2244. [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Collins A. Mechanism of cardiac Na(+)-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol. 1992 Aug;454:59–82. doi: 10.1113/jphysiol.1992.sp019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kavaler F., Morad M. Paradoxical effects of epinephrine on excitation-contraction coupling in cardiac muscle. Circ Res. 1966 May;18(5):492–501. doi: 10.1161/01.res.18.5.492. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzner T., Morad M. Excitation-contraction coupling in frog ventricle. Possible Ca2+ transport mechanisms. Pflugers Arch. 1983 Sep;398(4):274–283. doi: 10.1007/BF00657237. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Maylie J. G. Excitation-contraction coupling in neonatal and adult myocardium of cat. Am J Physiol. 1982 May;242(5):H834–H843. doi: 10.1152/ajpheart.1982.242.5.H834. [DOI] [PubMed] [Google Scholar]
- McClellan G. B., Winegrad S. The regulation of the calcium sensitivity of the contractile system in mammalian cardiac muscle. J Gen Physiol. 1978 Dec;72(6):737–764. doi: 10.1085/jgp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor M. E., Orchard C. H., Lakatta E. G. Dissociation of changes in apparent myofibrillar Ca2+ sensitivity and twitch relaxation induced by adrenergic and cholinergic stimulation in isolated ferret cardiac muscle. J Gen Physiol. 1988 Oct;92(4):509–529. doi: 10.1085/jgp.92.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Morad M. Contracture and catecholamines in mammalian myocardium. Science. 1969 Oct 24;166(3904):505–506. doi: 10.1126/science.166.3904.505. [DOI] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Sanders C., Weiss J. The inotropic actions of adrenaline on frog ventricular muscle: relaxing versus potentiating effects. J Physiol. 1981 Feb;311:585–604. doi: 10.1113/jphysiol.1981.sp013606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Weiss J., Cleemann L. The inotropic action of adrenaline on cardiac muscle: does it relax or potentiate tension? Eur J Cardiol. 1978 Jun;7 (Suppl):53–62. [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Longoni S., Philipson K. D. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science. 1990 Oct 26;250(4980):562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Noma A., Shioya T., Paver L. F., Twist V. W., Powell T. Cytosolic free Ca2+ during operation of sodium-calcium exchange in guinea-pig heart cells. J Physiol. 1991 Oct;442:257–276. doi: 10.1113/jphysiol.1991.sp018792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näbauer M., Morad M. Modulation of contraction by intracellular Na+ via Na(+)-Ca2+ exchange in single shark (Squalus acanthias) ventricular myocytes. J Physiol. 1992 Nov;457:627–637. doi: 10.1113/jphysiol.1992.sp019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Hartzell H. C. Regulation of Ca2+ current in frog ventricular cardiomyocytes by guanosine 5'-triphosphate analogues and isoproterenol. J Gen Physiol. 1993 Sep;102(3):525–549. doi: 10.1085/jgp.102.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Sham J. S., Jones L. R., Morad M. Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1344–H1349. doi: 10.1152/ajpheart.1991.261.4.H1344. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Vilsen B., Andersen J. P. Deduced amino acid sequence and E1-E2 equilibrium of the sarcoplasmic reticulum Ca(2+)-ATPase of frog skeletal muscle. Comparison with the Ca(2+)-ATPase of rabbit fast twitch muscle. FEBS Lett. 1992 Jul 20;306(2-3):213–218. doi: 10.1016/0014-5793(92)81003-5. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Lindemann J. P., Jones L. R. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989 Jul 5;264(19):11468–11474. [PubMed] [Google Scholar]



