Abstract
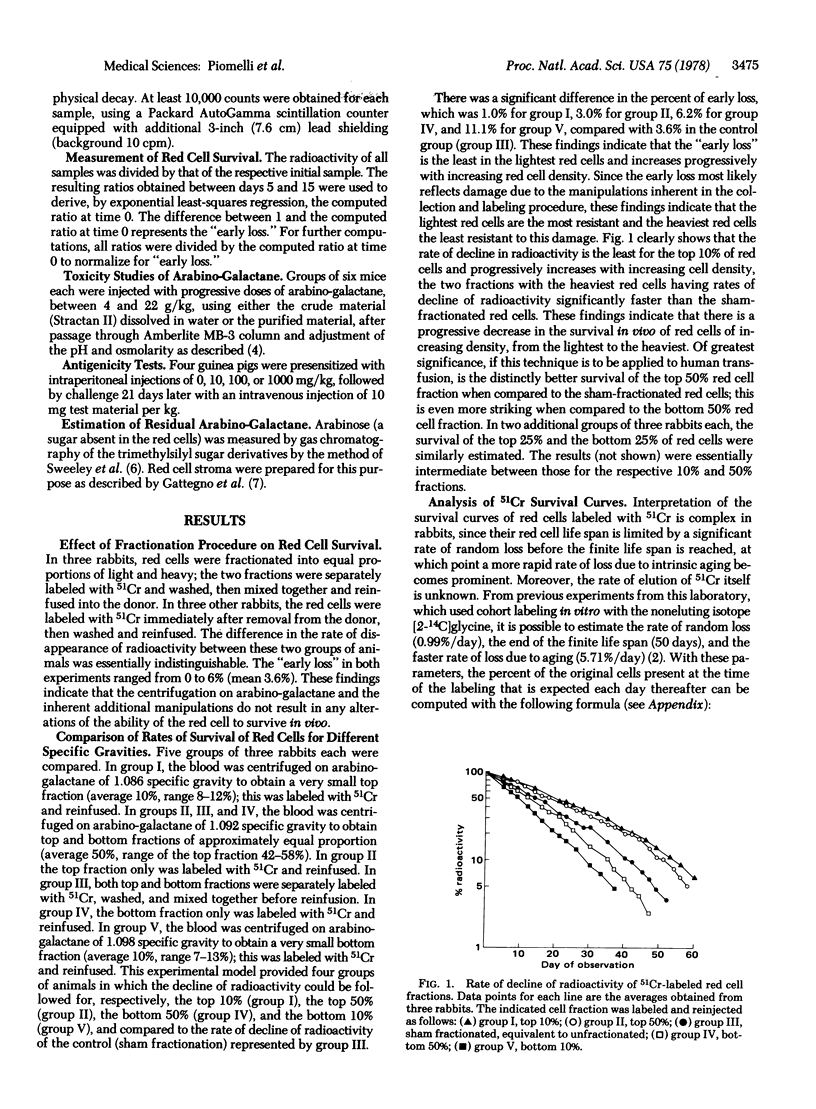
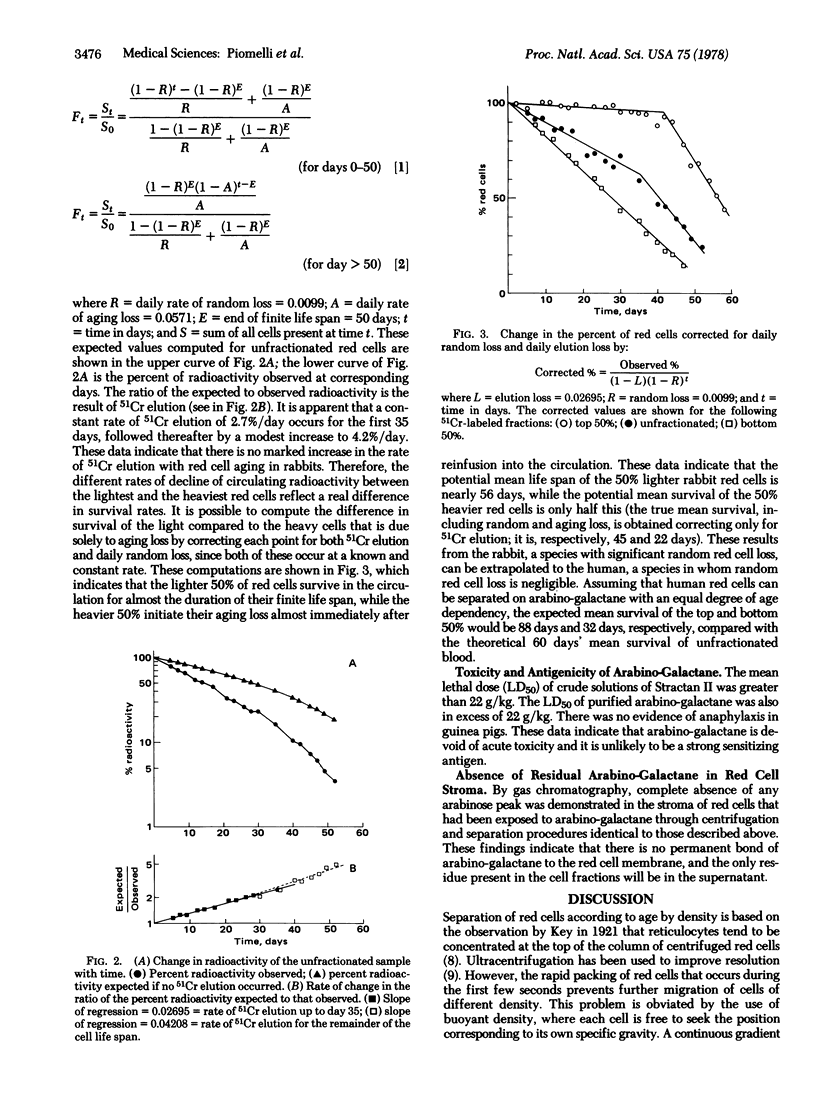
Transfusion of donor blood containing predominantly younger red cells with prolonged survival in vivo could significantly reduce the iron overload of patients requiring chronic transfusion. Age-dependent separation of red cells can be obtained by buoyant density centrifugation on isotonic solutions of arabino-galactane. By this technique, rabbit red cells were separated on a single layer of arabino-galactane and the appropriate fraction, after being labeled with 51Cr, was reinfused into the donor. The survival in vivo was calculated by a mathematical model which corrects for both 51Cr elution and random loss. There was a significant difference in survival in vivo between the light young red cells and the heavy old red cells. The potential survival in vivo of the 50% lightest red cells was 56 days, compared to 28 days for the heaviest red cells. Arabino-galactane appeared to be devoid of acute toxicity and of strong antigenicity and it did not appear to adhere to the red cell stroma. These data extrapolated to humans indicate that it may be feasible and advantageous to use red cells fractionated by this technique for transfusion. The 50% lightest human red cells can be expected to have a mean survival of 88 days, compared with 60 days for unfractionated blood. Transfusion of young red cells could significantly reduce the iron overload for patients requiring chronic transfusion, by avoiding infusion of the oldest red cells, which contribute equally to iron overload yet offer only transient survival in vivo.
Keywords: thalassemia, iron overload, buoyant density
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORUN E. R., FIGUEROA W. G., PERRY S. M. The distribution of Fe59 tagged human erythrocytes in centrifuged specimens as a function of cell age. J Clin Invest. 1957 May;36(5):676–679. doi: 10.1172/JCI103468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A. Editorial: "Propper" use of desferrioxamine. N Engl J Med. 1976 Jun 24;294(26):1456–1457. doi: 10.1056/NEJM197606242942613. [DOI] [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- GARBY L., HJELM M. ULTRACENTRIFUGAL FRACTIONATION OF HUMAN ERYTHROCYTES WITH RESPECT TO CELL AGE. Blut. 1963 Aug;9:284–291. doi: 10.1007/BF01678992. [DOI] [PubMed] [Google Scholar]
- Gattegno L., Bladier D., Garnier M., Cornillot P. Changes in carbohydrate content of surface membranes of human erythrocytes during ageing. Carbohydr Res. 1976 Dec;52:197–208. doi: 10.1016/s0008-6215(00)85960-1. [DOI] [PubMed] [Google Scholar]
- LEIF R. C., VINOGRAD J. THE DISTRIBUTION OF BUOYANT DENSITY OF HUMAN ERYTHROCYTES IN BOVINE ALBUMIN SOLUTIONS. Proc Natl Acad Sci U S A. 1964 Mar;51:520–528. doi: 10.1073/pnas.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli S., Corash L. Hereditary hemolytic anemia due to enzyme defects of glycolysis. Adv Hum Genet. 1976;6:165–240. doi: 10.1007/978-1-4615-8264-9_3. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Lamola A. A., Poh-Fitzpatrick M. F., Seaman C., Harber L. C. Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. I. Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J Clin Invest. 1975 Dec;56(6):1519–1527. doi: 10.1172/JCI108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli S., Lurinsky G., Wasserman L. R. The mechanism of red cell aging. I. Relationship between cell age and specific gravity evaluated by ultracentrifugation in a discontinuous density gradient. J Lab Clin Med. 1967 Apr;69(4):659–674. [PubMed] [Google Scholar]
- Rubin C. S., Balis M. E., Piomelli S., Berman P. H., Dancis J. Elevated AMP pyrophosphorylase activity in congenital IMP pyrophosphorylase deficiencey (Lesch-Nyhan disease). J Lab Clin Med. 1969 Nov;74(5):732–741. [PubMed] [Google Scholar]
- Sanderson R. J., Bird K. E., Palmer N. F., Brenman J. Design principles for a counterflow centrifugation cell separation chamber. Anal Biochem. 1976 Apr;71(2):615–622. doi: 10.1016/s0003-2697(76)80036-x. [DOI] [PubMed] [Google Scholar]
- Stockman J. A., Oski F. A. Editorial: Thalassemia major: a problem of iron overload. Ann Intern Med. 1974 Aug;81(2):262–263. doi: 10.7326/0003-4819-81-2-262. [DOI] [PubMed] [Google Scholar]
- Weiner M., Karpatkin M., Hart D., Seaman C., Vora S. K., Henry W. L., Piomelli S. Cooley anemia: high transfusion regimen and chelation therapy, results, and perspective. J Pediatr. 1978 Apr;92(4):653–658. doi: 10.1016/s0022-3476(78)80316-3. [DOI] [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]


