Abstract
Aims
Cyclophilin A (CyPA) is a pro-inflammatory mediator involved in oxidative stress-related cardiovascular diseases. It is secreted from vascular smooth muscle cell (VSMC) in response to reactive oxygen species (ROS) in a highly regulated manner. Extracellular CyPA activates VSMCs and endothelial cells (ECs) promoting inflammation, cell growth, and cell death. Recently, it was shown that acetylated CyPA (AcK-CyPA) affects its function. We investigated the role of acetylation of CyPA for its secretion and signalling in vascular cells.
Methods and results
We used angiotensin II (Ang II) to create sustained ROS and found significantly increased AcK-CyPA in VSMC. Site-directed mutagenesis showed that lysines K82 and K125 were the predominant CyPA residues acetylated in response to Ang II. Importantly, acetylation of K82 and K125 were required for Ang II-mediated CyPA secretion. ROS inhibitors, Tiron, and N-acetylcysteine inhibited Ang II-induced intracellular CyPA acetylation and also AcK-CyPA secretion. Using secreted CyPA from wild type and K82/125R mutants expressed in transduced VSMC or in vitro acetylated recombinant CyPA, we showed that extracellular AcK-CyPA significantly increased pERK1/2, matrix metalloproteinase-2 activation, and ROS production in VSMC compared with non-acetylated CyPA. Moreover, extracellular AcK-CyPA increased adhesion molecule expression (VCAM-1 and ICAM-1) in EC, which promoted monocyte adhesion.
Conclusions
ROS-dependent acetylation of CyPA is required for the generation of extracellular CyPA. Acetylated extracellular CyPA regulates VSMC and EC activation, suggesting that inhibition of acetylation of CyPA may prevent the pathogenesis of oxidative stress-related cardiovascular diseases.
Keywords: Acetylation, Cyclophilin A, Angiotensin II, Oxidative stress, Vascular smooth muscle cell, Endothelial Cell, HDAC
1. Introduction
Cyclophilin A (CyPA) is a ubiquitously expressed protein that possesses peptidyl-propyl cis–trans isomerase (PPIase) activity and also non-enzymatic scaffold function.1,2 It plays an important role in various cell functions including protein folding, intracellular trafficking, signal transduction, and transcription regulation.3 Vascular smooth muscle cells (VSMCs) secrete CyPA in response to oxidative stress, which is regulated by a Rho-dependent vesicular secretion pathway.4,5 Secreted CyPA is a pro-inflammatory mediator, which can stimulate VSMC growth via ERK activation,5 cause endothelial cell (EC) dysfunction by inducing adhesion molecule expression,6 activate monocytes, and recruit inflammatory cells.7,8 Furthermore, CyPA deficiency prevents angiotensin II (Ang II)-induced abdominal aortic aneurysm formation in ApoE−/− mice by regulating reactive oxygen species (ROS) production and matrix metalloproteinase-2 (MMP2) activation in VSMC.9
Oxidative stress regulates many cell signalling pathways by affecting post-translational modification of target proteins,10 which, in turn, affects protein function, stability, and degradation. For example, acetylation of lysine residues is a reversible post-translational process that neutralizes the amino acid's positive charge altering enzymatic activity, protein–protein interactions, and DNA binding.11–13
CyPA can be post-translationally modified by phosphorylation14 or acetylation.15–17 Proteomic analyses of spinal cord extracts from amyotrophic lateral sclerosis G93A SOD1 mice revealed that acetylated CyPA (AcK-CyPA) is associated with oxidative stress. Synthetic AcK-CyPA at lysine 125 affects CyPA's enzymatic PPIase activity, its ability to bind to cyclosporin A (CsA) and calcineurin, and also affects HIV-1 incorporation into a cell.16 Furthermore, acetylated and methylated CyPA were secreted from irradiated breast cancer cells, suggesting that post-translational modification is required for CyPA secretion.18 Although many studies have indicated that post-translational modification of CyPA is important in signal transduction and cell development, its role specifically in vascular pathology remains unclear. In this study, we hypothesized that Ang II-induced CyPA acetylation regulates vascular cell function.
2. Materials and methods
See Supplementary material online, Supplementary Data for expanded methods.
2.1. Cell isolation and culture
Animal experiments were performed using protocols approved by the Institutional Animal Care and Use Committee at the University of Rochester published by the United States National Institutes of Health. Animals (8–12 weeks old) were anaesthetized with a single intraperitoneal injection of ketamine (130 mg/kg) and xylazine (8.8 mg/kg). The depth of surgical anaesthesia was determined by toe pinch and euthanasia was by exsanguination following perfusion. Aortic smooth muscle cells from rats (RASMCs) or mice (MASMCs) from wild type (WT), Ppia−/−, or overexpressed Flag-CyPA in smooth muscle cells were isolated as described previously.7 RASMCs at passages 6–12 or MASMCs at passages 4–6 at 70–80% confluence were growth arrested by incubation in dulbecco's modified eagle medium (DMEM) containing 0.3% FBS for 24 h and stimulated with Ang II for the indicated times. Human umbilical veins were collected in accordance with the University of Rochester human subjects review board procedures that prescribe to the Declaration of Helsinki. Endothelial cells (HUVECs) were isolated as previously described19 and maintained in Medium 200. U937 monocytes were maintained in RPMI-1460 medium.
2.2. Construction of recombinant lentivirus and VSMC transduction
A complete description of the viral vector, generation of viral particles, and the method of infecting MASMCs are provided in Supplementary material online, Material and Methods section.
2.3. Preparation of conditioned medium
Conditioned medium (CM) was prepared as described previously.4 Briefly, VSMCs were growth arrested with DMEM containing 0.3% FBS for 24 h, and the medium was changed to serum-free DMEM 1 h before the experiment. The culture medium was collected and concentrated 100-fold using an Amicon Ultra Centrifuge filter-3K.
2.4. Measurement of ROS by flow cytometry
VSMCs were collected by trypsinization and incubated with 2 mM 2′,7′-dichlorodihydrofluorescein diacetate. Cells were centrifuged, washed, and incubated with CM or rhCyPA at 37°C followed by flow cytometry (Accuri® C6). The data were analysed using the FlowJo software.
2.5. Gelatin zymography for MMP2 activity
Gelatin zymography for the detection of MMP2 activity in CM was performed in 8% non-denaturing PAGE gels containing 0.1% gelatin as described in Supplementary material online, Supplementary Data.
2.6. In vitro acetylation assay
Reactions (20 µL) containing rhCyPA 50 nM, 1.2 mM acetyl-CoA, and 1 µg p300 protein in HAT buffer were incubated at 30°C for 45 min.
2.7. Immunoprecipitation and western blotting
Lysates containing equal amounts of soluble proteins were incubated with an anti-CyPA or anti-Flag antibody overnight at 4°C. Antibody complexes were collected by incubation with protein A or G agarose for 2 h at 4°C. Precipitates were washed three times in lysis buffer and then resuspended in SDS–PAGE sample buffer. Samples were separated by SDS–PAGE and analysed by western blot (WB).
2.8. Endothelial to monocyte adhesion assay
HUVECs were stimulated with CM or 50 nM rhCyPA for 6 h. Medium was removed and U937 monocytes were added and incubated for 30 min at 37°C. Unbound cells were removed by washing with phosphate-buffered saline. Adherent cells were counted in five randomly selected optical fields in each well. Phase-contrast microphotographs of cells were obtained using an inverted fluorescent microscope (IX50, Olympus) with ×20 lens.
2.9. Statistical analysis
Data are means ± SEM of at least three independent experiments. The significance between samples was determined by Student's t-test for two group comparisons or analysis of variance for more than two groups using the Graphpad Prism software. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Angiotensin II stimulates CyPA acetylation in VSMC
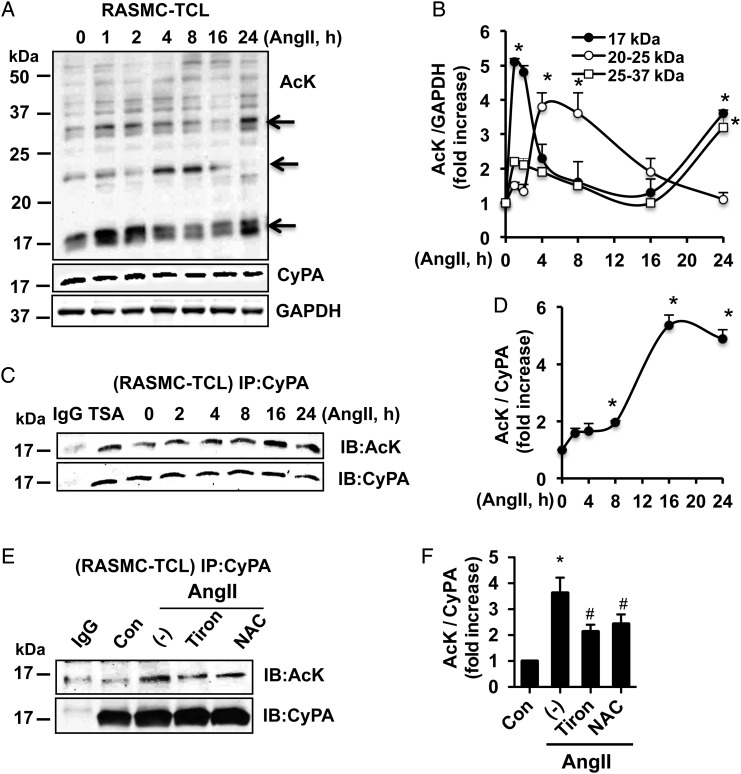
To investigate Ang II-induced acetylation of proteins in VSMCs, RASMCs were treated with Ang II and acetylation was analysed using an acetyl-lysine antibody (AcK), which detects general lysine acetylation. We observed that Ang II increased acetylation of numerous proteins in RASMCs. The time course of acetylation for different proteins varied as demonstrated in Figure 1A and B. In particular, a protein of molecular weight (MW) 17 kDa is the most heavily acetylated and exhibited a bi-phasic pattern with peak lysine acetylation at 1–2 and 24 h. Lysine acetylation for Proteins of mass 20–25 kDa peaked between 4 and 8 h, and those proteins between 25–37 kDa MW exhibited a bi-phasic pattern with peak lysine acetylation at 1–2 and 24 h (Figure 1B). Given that CyPA has a MW of 17 kDa, and that CyPA MW on the WB was coincident with the acetylated proteins, it is very plausible that CyPA is one of the acetylated proteins.
Figure 1.
Ang II induces acetylation of CyPA in VSMC. (A and B) Protein acetylation was detected by the WB from total cell lysates (RASMC-TCLs) stimulated with Ang II (300 nM). CyPA and GAPDH were used as internal loading controls. (C and D) Ang II or TSA (1 µM for 8 h)-stimulated RASMC-TCLs were immunoprecipitated with an anti-CyPA antibody, and immune complexes were immunoblotted with anti-acetyl-lysine (AcK) or CyPA antibody. (E and F) RAMSCs were pre-treated with ROS scavenger Tiron (5 mM) or N-acetylcysteine (NAC, 10 mM) for 30 min and stimulated with Ang II for 16 h followed by immunoprecipitation and WB. Data represent three independent experiments and are shown as mean ± SEM (*P < 0.05 relative to t = 0, #P < 0.05 vs. Ang II alone).
To prove that CyPA is acetylated, total cell lysates (TCLs) from Ang II or HDAC inhibitor TrichostatinA (TSA)-stimulated RASMCs were immunoprecipitated with a CyPA antibody and probed for AcK (Figure 1C and D). Importantly, Ang II-induced CyPA acetylation occurred in a time-dependent manner with a peak at 16–24 h. The time course of the acetylation of CyPA in Figure 1D differed from that observed in the acetylation of TCL, suggesting that the other proteins of MW 17 kDa are also included in the highly acetylated band. Based on this time course, we studied CyPA acetylation at 24 h, which was the peak. Similarly, we observed that TSA induced CyPA acetylation in VSMC. Similar to the RASMC, Ang II increased acetylation of exogenous Flag-CyPA in MASMC overexpressing CyPA (MASMC-FlagCyPA) with a peak at 16–24 h (see Supplementary material online, Figure S1A and B), further suggesting that CyPA is acetylated in VSMC in response to Ang II. In addition, when we compared WT (WT-MASMC) and CyPA (gene: peptidyl-prolyl isomerase) knockout mouse ASMCs (Ppia−/− MASMC), Ang II-induced acetylation of the 17 kDa protein band detected in WT-MASMC (arrow) was not observed in Ppia−/− MASMC, further providing evidence that CyPA is acetylated in VSMC (see Supplementary material online, Figure S1C and D). To understand the role of ROS in CyPA acetylation, we used the ROS scavengers Tiron and N-acetylcysteine and then assessed acetylation of CyPA. As shown in Figure 1E and F, ROS inhibition decreased Ang II-induced CyPA acetylation in VSMC.
3.2. Lysine residues K82 and K125 regulate Ang II-induced CyPA acetylation
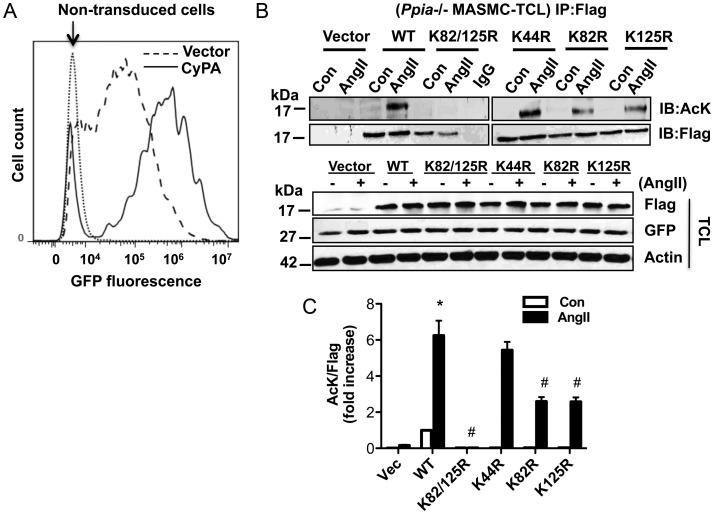
To determine lysine residues important for Ang II-induced acetylation, UniProt (http://www.uniprot.org) and PHOSIDA (www.phosida.com) were used to predict potential acetylated residues. There are 14 lysine residues in CyPA; of which, five were potential acetylation targets (K28, K44, K82, K125, and K131). Of these, five K44 is conserved in all species,20 K82 is located on the surface of CyPA and is also involved in calcineurin and CsA-CyPA complex binding,21,22 and K125, also located at the surface, is involved in CsA binding and PPIase regulation.16,23 Therefore, we mutated K44, K82, and K125 to arginine (K44R, K82R, and K125R, or K82/125R double mutant) and tested their ability to be acetylated in response to Ang II. We used lentiviral transduction to express the mutants in MASMC because plasmid transfection efficiency in MASMC was too low. The HIV-based lentiviral expression vector pLV-CMV-IRES-GFP allows simultaneous expression of Flag-CyPA cDNA (and mutants thereof) from the CMV promoter and enhanced green fluorescent protein from an internal ribosome entry sequence element. Green fluorescent protein (GFP) fluorescence measured by flow cytometry indicated 80 ± 0.3 and 86 ± 0.7% transduction efficiency in vector and WT Flag-CyPA-transduced cells, respectively (Figure 2A). To determine the acetylation of WT and mutant Flag-CyPA, Ppia−/− MASMCs were infected with virus followed by Ang II stimulation. Immunoprecipitation results showed that WT-CyPA was acetylated strongly in response to Ang II (Figure 2B, upper panel, and C). The K44R mutant was acetylated in response to Ang II to similar levels as WT-CyPA, while acetylation of the K82R and K125R mutants was decreased by ∼50% compared with WT. Ang II-induced acetylation of the K82/125R double mutant was completely inhibited compared with WT-CyPA. Importantly, GFP expression levels were the same in all viral infections and expression levels of WT and all CyPA mutants were equivalent, as demonstrated by Flag reactivity in TCLs, suggesting that the mutations do not affect protein stability (Figure 2B, lower panel). Thus, K82 and K125 are important residues for Ang II-induced CyPA acetylation in VSMC.
Figure 2.
CyPA lysine residues K82 and K125 are targets for Ang II-induced acetylation. (A) Green fluorescent protein (GFP) fluorescence was determined by flow cytometry as a measure for transduction efficiency in Ppia−/− MASMC transduced with lentiviral particles expressing FlagCyPA or vector alone. Non-transduced cells were used as a control. (B and C) Ppia−/− MASMCs were transduced with FlagCyPA with or without K/R substitutions and stimulated with 1 µM Ang II for 24 h. Cell lysates were immunoprecipitated with an anti-Flag antibody and immunoblotted with Anti-AcK or Flag. GFP and Flag expression in total cell lysates (TCLs) were analysed by western analysis. Experiments were performed three independent times and are data shown as mean ± SEM (*P < 0.05 vs. untreated WT, #P < 0.05 vs. WT-Ang II).
3.3. Ang II induces acetylated CyPA secretion in VSMC
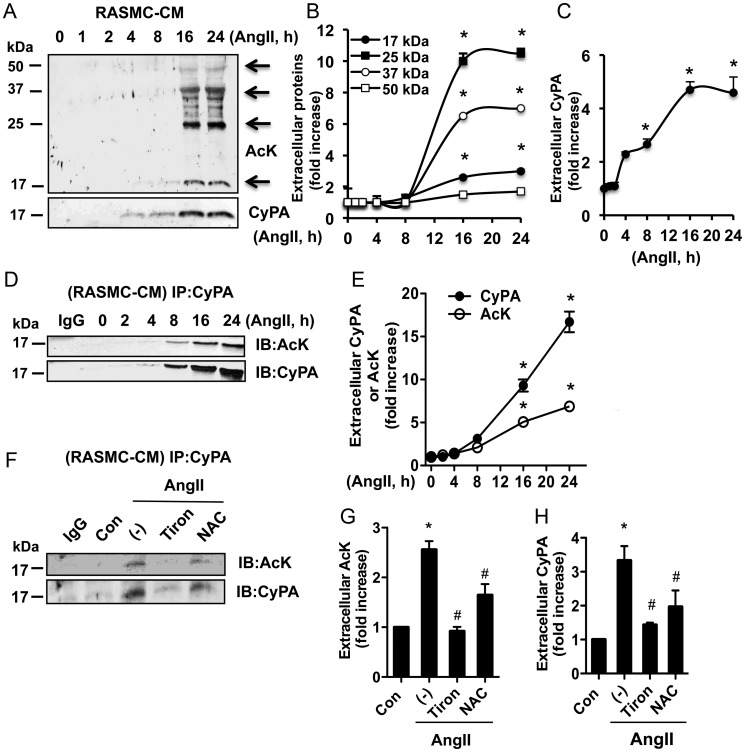
CyPA is a secreted protein involved in oxidative stress conditions.3 To determine whether AcK-CyPA can be secreted, CM from Ang II-treated RASMC was analysed by the WB. Ang II induced secretion of several acetylated proteins with molecular masses between 17 and 50 kDa in a time-dependent manner in a given WB (Figure 3A and B). When the acetylation blot was re-probed with an anti-CyPA antibody, the immunoreactivity was coincident with the 17 kDa acetylated protein (Figure 3A, lower panel), suggesting that acetylated CyPA (AcK-CyPA) was one of the proteins secreted into the CM in a time-dependent manner with a peak at 16 and 24 h (Figure 3C). There was a strong similarity in the time course of Ang II-stimulated extracellular CyPA and extracellular AcK-CyPA (Figure 3) as well as intracellular AcK-CyPA (Figure 1C and D) at 16 and 24 h. We confirmed secretion of AcK-CyPA by immunoprecipitation and immunoblot analysis. Ang II induced secretion of AcK-CyPA and total CyPA in a time-dependent manner with a peak at 24 h (Figure 3D and E). Moreover, ROS scavengers inhibited AcK-CyPA secretion, suggesting that Ang II-induced AcK-CyPA secretion is ROS dependent (Figure 3F–H). We previously showed that secretion of CyPA was dependent on Rho activity.4 To determine whether secretion of AcK-CyPA depends on the Rho-pathway, RASMCs were pre-treated with the Rho kinase inhibitor, Y27632, followed by stimulation with Ang II for 24 h. AcK-CyPA secretion was dramatically inhibited, while intracellular CyPA expression levels were unaffected by Y27632 (see Supplementary material online, Figure S2D).
Figure 3.
(A–C) Ang II-induced secretion of proteins in CM was measured by the WB using anti-AcK or CyPA antibodies. (D and E) Ang II-induced AcK-CyPA in CM was measured by immunoprecipitation and immunoblotting. (F and G) ROS scavenger Tiron (5 mM) or N-acetylcysteine (NAC, 10 mM) 30 min pre-treated RASMCs were stimulated with Ang II for 16 h and followed immunoprecipitation and immunoblotting. The results are normalized to the fluorescence intensity at the 0 time point or vehicle-treated cells, which was set to 1.0. Data represent three experiments and are shown as mean ± SEM (*P < 0.05 vs. 0 time point or vehicle, #P < 0.05 vs. Ang II).
3.4. Acetylation is required for CyPA secretion in VSMC
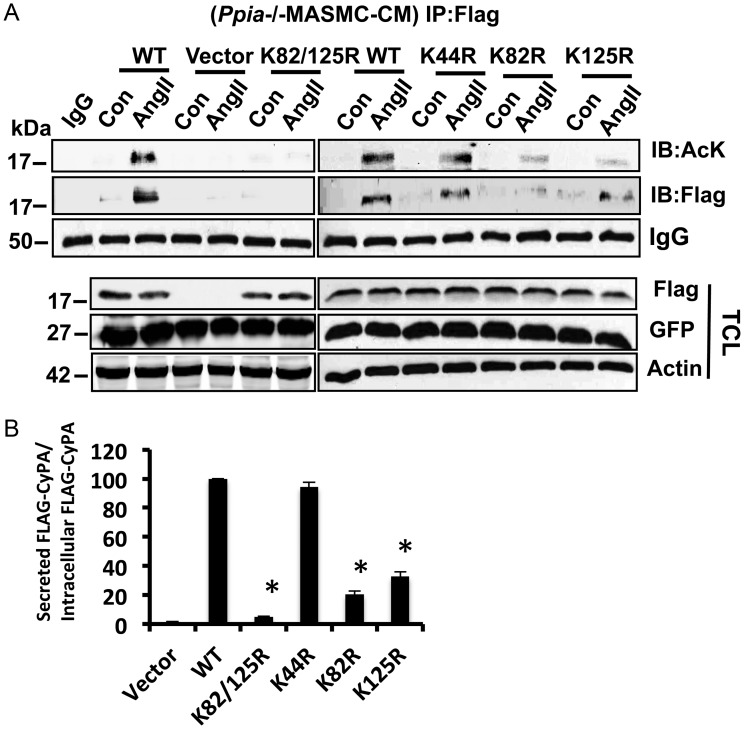
To further understand the role of CyPA acetylation on its secretion, we measured AcK-CyPA in CM from lentivirally transduced Ppia−/− MASMC (Figure 4A). We used an equal volume of CM from each sample and performed immunoprecipitation analysis using an anti-Flag antibody. These data demonstrated that Ang II induced secretion of acetylated WT-FlagCyPA. Secretion of K82R and K125R CyPA mutants was reduced compared with WT. Secretion of the CyPA K82/125R double mutant was barely detectable. In contrast, secretion of the K44R mutant, which showed a high level of intracellular AcK, did not differ from that of WT-FlagCyPA. Similar results were shown for the total level of secreted Flag-CyPA in the CM. Note that the total intracellular level of CyPA and mutants, as measured by FLAG reactivity in the TCL, was unaffected by acetylation status (Figure 4A, lower panel), suggesting that acetylation of CyPA is required for its secretion but not its stability. It is evident that, by determining the ratio of secreted Flag-CyPA to intracellular Flag-CyPA (Figure 4B), the acetylation status of CyPA affects its secretion.
Figure 4.
Acetylation is necessary for Ang II-induced CyPA secretion. (A) CM from Ang II (1 µM for 24 h) stimulated Ppia−/− MASMC transduced with lentiviral particles expressing FlagCyPA with or without K/R substitutions was immunoprecipitated with an anti-Flag antibody and immunoblotted with anti-AcK or Flag. (B) Ratio of secreted CyPA (or KtoR mutants) to total CyPA in the TCL (or KtoR mutant) was normalized to the ratio for WT CyPA, which was set to 100. Experiments were performed three independent times, and data are shown as ± SEM (*P < 0.05 vs. WT).
3.5. Acetylation promotes extracellular CyPA induced VSMC activation
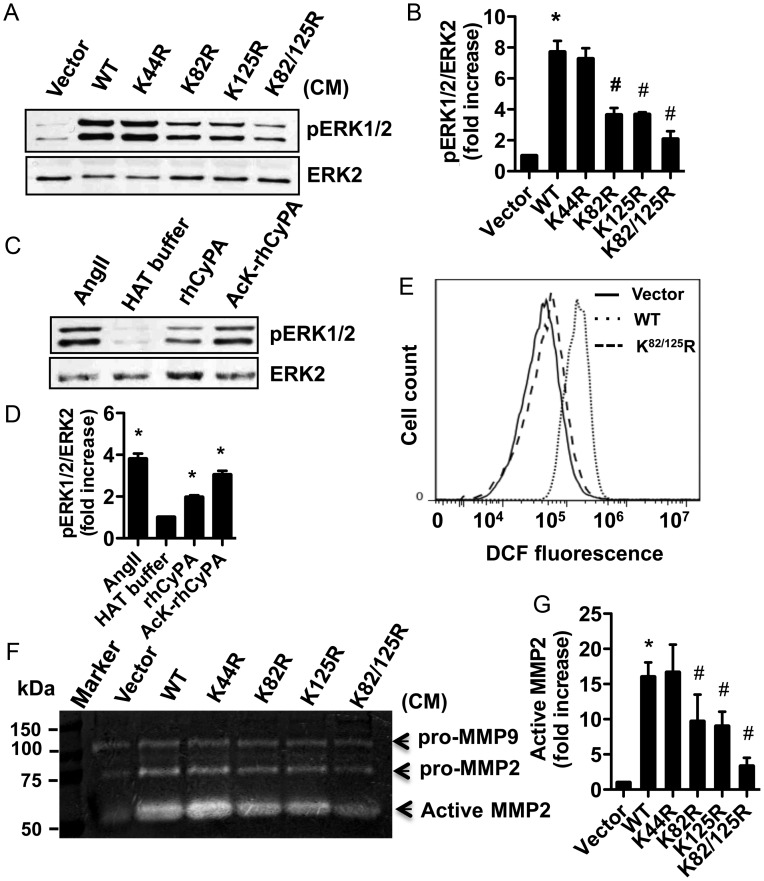
To assess whether AcK-CyPA can affect VSMC activation, RASMCs were stimulated with CM prepared from WT and mutants transduced Ppia−/− MASMC. An equal amount of CyPA (the amount of CyPA was determined from quantitation of WB reactivity from Figure 4) was used in each assay, and volumes were normalized with CM from Ang II-treated Ppia−/− MASMC. The WB in Supplementary material online, Figure S3 shows total CyPA from a series of normalizations of CM from the mutants relative to the WT CyPA. Each aliquot contains an equivalent amount of CyPA, except for the double mutant in which undetectable amounts of CyPA were secreted into the original CM. In so doing, differences between mutants can be interpreted as being the result of their altered acetylation status and not simply as the effect of different amounts of CyPA added in each assay. ERK1/2 activation measured by pERK1/2 increased significantly after stimulation with CM from WT-CyPA and the K44R mutant (Figure 5A and B). However, CM from the K82R and K125R mutants displayed decreased pERK1/2 activation, whereas the double mutant, K82/125R, exhibited dramatically reduced ERK1/2 activation. To support the role of AcK-CyPA in VSMC activation, we acetylated recombinant CyPA (rhCyPA) in vitro using recombinant histone acetyltransferase p300 and acetyl-CoA. WB analysis demonstrated the presence of acetylated recombinant CyPA (AcK-rhCyPA) (see Supplementary material online, Figure S4). RASMC treated with AcK-rhCyPA exhibited greater pERK1/2 activation compared with native rhCyPA (Figure 5C and D).
Figure 5.
ERK1/2 activity was measured in RASMC stimulated with CM titrated to assure equal amounts of extracellular CyPA (Vector, WT, or K/R substitutions) for 10 min (A and B) or 50 nM rhCyPA or AcK-rhCyPA for 10 min (C and D). HAT buffer contains no CyPA protein; rhCyPA is present in HAT buffer lacking p300 acetyltransferase and AcK-rhCyPA is present in buffer containing CyPA, p300, and acetyl-CoA (see also Supplementary material online, Figure S4). (E) ROS production was measured by flow cytometry in RASMC stimulated with CM for 4 h. (F and G) Representative gelatin zymography showed MMP2 activity in CM from Ppia−/− MASMC transduced with lentiviral particles. All experiments were repeated three different times and data are shown as mean ± SEM (*P < 0.05 vs. corresponding control, #P < 0.05 vs. WT-Ang II).
Furthermore, intracellular ROS production in RASMC was increased following stimulation with CM from WT-CyPA expressing cells compared with that from vector expressing cells. The CM prepared from the K82/125R expressing cells did not significantly stimulate ROS production compared with vector expressing cells (Figure 5E).
We previously demonstrated that Ang II-induced MMP2 activation was inhibited in Ppia−/− MASMC.9 To understand if AcK-CyPA is important in regulating MMP2 activity, we performed gelatin zymography of CM prepared from Ang II-stimulated WT and mutant CyPA expressing cells. As described above, an approximately equal amount of CyPA was used in each assay and volumes were normalized with CM from Ang II-treated Ppia−/− cells. Zymography results indicated that pro-MMP9 activity was unaffected by CyPA or its mutants. However, WT-CyPA increased MMP2 activity relative to the vector control (Figure 5F and G). Expression of the K82R and K125R mutants resulted in reduced MMP2 activation, whereas the K82/125R double mutant significantly inhibited MMP2 activity over WT-CyPA. Taken together, these data support the hypothesis that acetylation of CyPA increases its potential for VSMC activation.
3.6. Acetylated CyPA increases adhesion molecule expression and monocyte adhesion to endothelial cells
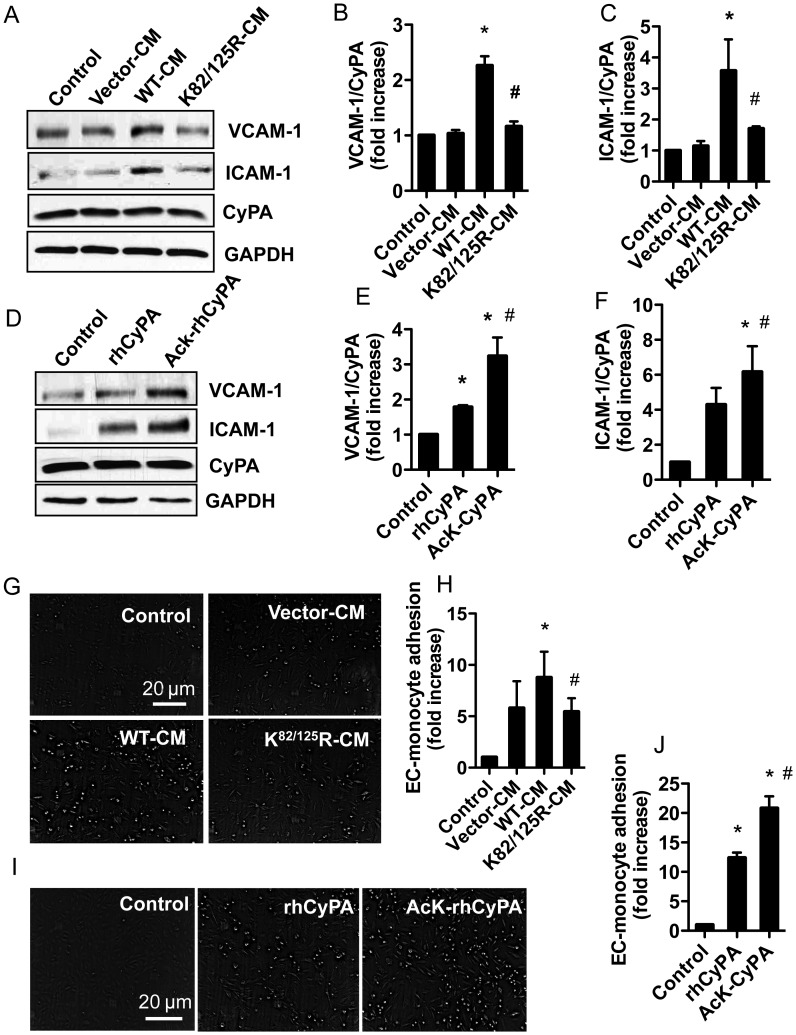
Extracellular CyPA can cause EC dysfunction by increasing adhesion molecule (VCAM-1 and ICAM-1) expression.6 To understand the role of AcK-CyPA in EC dysfunction, VCAM-1 and ICAM-1 expressions were measured from EC treated with CM prepared from Ppia−/− MASMC expressing WT or K82/125R CyPA or rhCyPA (native and acetylated form). VCAM-1 and ICAM-1 expressions were significantly (P < 0.01) decreased in EC treated with CM from K82/125R expressing cells (Figure 6A–C). In contrast, AcK-rhCyPA increased VCAM-1 and ICAM-1 expressions compared with rhCyPA (Figure 6D–F). However, total CyPA expression was not changed in any of the experiments (see lower panel of each immunoblot figure).
Figure 6.
Acetylated extracellular CyPA enhances adhesion molecule expression and EC–monocyte adhesion. HUVECs were stimulated with CM from Ppia−/− MASMC transduced with lentiviral particles (WT or K/R mutant) or 50 nM rhCyPA or AcK-rhCyPA. After 6 h incubation, VCAM-1 and ICAM-1 expressions were measured by the WB (A–F), or U937 monocytes were added to human umbilical vein endothelial cells and the adherent monocytes were counted in five different optical fields for each well (G–J). Quantified data show a fold increase of monocyte adherence to EC. Data are shown mean ± SEM of values from three independent experiments (*P < 0.05 vs. corresponding control, #P < 0.05 vs. WT or rhCyPA).
Additionally, we measured monocyte adhesion in response to CM or rhCyPA. EC treated with K82/125R-CM exhibited less monocyte adhesion compared with cells treated with WT-CM (Figure 6G and H). In contrast, AcK-rhCyPA treated EC showed augmented monocyte adhesion compared with rhCyPA (Figure 6I and J).
4. Discussion
The major findings of this study are that acetylation of CyPA is required for its secretion, and acetylated extracellular CyPA is functionally more active than the non-acetylated form. Site-directed mutagenesis identified K82 and K125 as the predominant CyPA residues acetylated in response to Ang II and required for CyPA secretion. AcK-CyPA stimulated significantly greater activation of ERK1/2 and MMP2, as well as ROS production in VSMC than non-AcK-CyPA as shown by two methods: (i) use of CM containing secreted CyPA comparing WT-CyPA vs. K82/125R-CyPA and (ii) acetylated recombinant CyPA. Moreover, AcK-CyPA increased adhesion molecule expression (VCAM-1 and ICAM-1) in EC, which promoted monocyte adhesion.
Lysine acetylation plays roles in various cardiovascular diseases.24,25 Previous research demonstrates that lysine acetylation of CyPA at K125 was important for its functions of immunity and viral infection.16 We performed a global screening of lysine acetylated proteins using Ang II as the agonist, because it is an important regulator in many cardiovascular diseases.26 Moreover, Ang II plays multiple roles in VSMC functions in which increased ROS production is one of the important mechanisms for its signalling regulation.27 We demonstrated that Ang II increased acetylation of many VSMC proteins in a biphasic manner with peaks at early and late time points following stimulation. Further analysis of these bands indicated that CyPA was one of the highly acetylated proteins.
Acetylation is a reversible process in which alterations in lysine acetyltransferase (HAT) and lysine deacetylase (HDAC) activity mediate cellular protein acetylation levels (see Supplementary material online, Figure S5B. In this study, we have used the more established terms HAT and HDAC, but there is an increasing realization that the more recent terminology of KAT/KDAC to reflect acetylation/deacetylation of non-histone proteins would also be appropriate here). ROS regulates HAT/HDAC balance by either decreasing28 or increasing 29 endogenous HDAC activity. Ang II stimulates a rapid and sustained increase in ROS generated by VSMC,30 but also directly increases HAT (e.g. p300/CBP) activation in VSMCs.31 Our data using Ang II mirrored that in which we used TSA, a Class I and Class II HDAC inhibitor suggesting that one of these HDACs is responsible for maintaining the appropriate level of CyPA acetylation. Large-scale proteomic analyses of the cellular acetylome suggest the presence of multiple deacetylases with both nuclear and cytoplasmic activities.32 The identification of the primary HATs and HDACs that exhibit the highest activity towards CyPA will be evaluated in future studies.
Many studies have reported that CyPA is a major target of redox regulation.33 CyPA is acetylated in spinal cord tissue from amyotrophic lateral sclerosis G93A SOD1 mice in which oxidative stress is highly induced.15 We showed that CyPA acetylation was increased in a time-dependent manner with a peak at later time points. Since Ang II produces a sustained increase in ROS in VSMC, we concluded that the time-dependent increase in CyPA acetylation was largely due to ongoing ROS production. Inhibition of ROS decreased CyPA acetylation, providing the evidence that ROS is involved in CyPA acetylation regulation.
Uniprot and PHOSIDA predict five predominant lysine residues in CyPA that can be acetylated. Proteomic analysis revealed that CyPA can be acetylated at many lysine residues, although K82 and K125 are the primary targets in immune cells and human cancer cells.16,17,20,23,32 This is logical because they are located on solvent accessible surfaces of CyPA.16,22 Our mutagenesis studies demonstrate that K82 and K125 are the target lysine residues involved in Ang II-induced CyPA acetylation in VSMC. Importantly, we also showed that total CyPA expression was not changed by mutation of K82 or K125, suggesting that acetylation does not affect the steady-state level of intracellular CyPA. Given the location of K82 and K125 on the surface of CyPA, these residues could be potential pharmacological targets.
CyPA is secreted in many pathological conditions such as oxidative stress, inflammation, and cardiovascular disease.5,34 In particular, it is highly secreted from VSMC in response to Ang II and oxidative stress.9 CyPA is among the most abundant intracellular proteins, consisting of 0.1–0.6% of the total cytosolic proteins.35 While it is likely that the majority of cytosolic CyPA remains in the cell, post-translational modification of CyPA, such as acetylation, can promote its secretion. For example, secretion of some acetylated proteins, such as hsp90α or sterol, is controlled by acetylation/deacetylation cycle.36,37 Our results demonstrated that Ang II induced secretion of AcK-CyPA as evident by immunoprecipitation of CyPA from CM following Ang II stimulation of VSMC. Moreover, ROS inhibition attenuated intracellular CyPA acetylation resulting in decreased AcK-CyPA secretion, further suggesting that acetylation of CyPA controls its secretion. Furthermore, we showed that AcK-CyPA secretion was mediated via a Rho-dependent pathway, which was previously described as the mechanism for CyPA secretion.4
Increasing evidence demonstrates that extracellular CyPA is an important agonist for many cell types. The present study shows that extracellular AcK-CyPA is more functionally active than native CyPA. Specifically, we showed that AcK-CyPA was secreted in response to ROS and its acetylation was integrally involved in activating ERK1/2 and ROS formation. In addition, synthetic acetylated rhCyPA dramatically increased ERK1/2 activation and ROS production. The mechanism by which extracellular CyPA activates cell signalling cascades is still unclear, although recent publications suggest at least three mechanisms. First, there may be increased binding and/or activation of the extracellular CyPA receptor, since CyPA acetylation alters its surface electrostatic charges.16 As previous studies have shown that CyPA binds to CD147 (EMMPRIN), and regulates downstream signalling in immune cells,38 it will be important to determine the effect of acetylation on CyPA binding to EMMPRIN. Secondly, a recent study demonstrated that acetylation markedly inhibited CyPA catalysis of cis–trans isomerization suggesting a plausible mechanism for altered biological activity.16 The loss of enzyme activity might stabilize interactions of acetylated CyPA with receptors or other signal mediators. Thirdly, because AcK-CyPA is more stable in acidic conditions,16 the microenvironment of cell culture and pathological conditions may make AcK-CyPA a more potent ligand.
Our previous study reported that CyPA regulates Ang II-induced MMP2 activation in VSMC and inflammatory cell accumulation.9 MMP2 is secreted as pro-MMP2, which interacts with the cell surface receptor (membrane type 1 metalloprotease and tissue inhibitor of metalloproteinase 2 complex), and subsequently undergoes cleavage and results in its activation.39 In this study, we demonstrated that the acetylated form of CyPA is integral to promoting MMP2 activity. When we used approximately the same amount of CyPA from WT-CM vs. K82R-CM or K125R-CM, WT-CM showed significant MMP2 activation, whereas K82R, K125R, and double mutant showed activation to a lesser extent. However, we did not observe a dramatic difference in pro-MMP2 or pro-MMP9 level in CyPA-transduced cells. It is possible that extracellular AcK-CyPA has increased affinity to bind with pro-MMP2, and serves as a chaperone for interaction with the cell surface receptor for MMP2.
Increased adhesion molecule expression in EC is one of the mechanisms involved in inflammatory conditions. CyPA has a pro-inflammatory effect on EC by inducing mitogen-activated protein kinases and adhesion molecule expression.6 Here, we demonstrated that extracellular AcK-CyPA enhances EC adhesion molecule (VCAM-1 and ICAM-1) expression, which promotes adhesion of inflammatory cells. It is possible that AcK-CyPA has more affinity to bind with its receptor in EC to regulate adhesion molecule expression.
In summary, we demonstrated that lysine residues K82 and K125 are targets for Ang II-induced CyPA acetylation. Acetylation of CyPA is required for its secretion and secreted AcK-CyPA plays an important role in its signalling by regulating VSMC and EC activation (see Supplementary material online, Figure S5). Our study provides rationale for designing a more targeted approach to cardiovascular diseases treatment.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health (grant HL49192 to B.C.B.).
Supplementary Material
Acknowledgements
We are grateful to Amy Mohan and Christine Christie for their expert technical assistance with animal husbandry and tissue harvests, and the Aab Cardiovascular Research Institute members for helpful suggestions.
Conflict of interest: none declared.
References
- 1.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Berk BC. Circulating smooth muscle progenitor cells: novel players in plaque stability. Cardiovasc Res. 2008;77:445–447. doi: 10.1093/cvr/cvm088. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 5.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 6.Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 7.Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan W, Ge H, He B. Pro-inflammatory activities induced by CyPA-EMMPRIN interaction in monocytes. Atherosclerosis. 2010;213:415–421. doi: 10.1016/j.atherosclerosis.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Satoh K, Nigro P, Matoba T, O'Dell MR, Cui Z, Shi X, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin ii-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol. 2012;52:550–558. doi: 10.1016/j.yjmcc.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Tian L, Popov VM, Pestell RG. Acetylation and nuclear receptor action. J Steroid Biochem Mol Biol. 2011;123:91–100. doi: 10.1016/j.jsbmb.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Guan KL. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol. 2012;198:155–164. doi: 10.1083/jcb.201202056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Vega L, Grishina I, Moreno R, Kruger M, Braun T, Schmitz ML. A redox-regulated sumo/acetylation switch of hipk2 controls the survival threshold to oxidative stress. Mol Cell. 2012;46:472–483. doi: 10.1016/j.molcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M, et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283:623–637. doi: 10.1074/jbc.M704934200. [DOI] [PubMed] [Google Scholar]
- 15.Massignan T, Casoni F, Basso M, Stefanazzi P, Biasini E, Tortarolo M, et al. Proteomic analysis of spinal cord of presymptomatic amyotrophic lateral sclerosis G93A SOD1 mouse. Biochem Biophys Res Commun. 2007;353:719–725. doi: 10.1016/j.bbrc.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 16.Lammers M, Neumann H, Chin JW, James LC. Acetylation regulates cyclophilin A catalysis, immunosuppression and HIV isomerization. Nat Chem Biol. 2010;6:331–337. doi: 10.1038/nchembio.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier F, Depagne J, Hem S, Chevillard S, Bensimon J, Bertrand P, et al. Accumulation of cyclophilin A isoforms in conditioned medium of irradiated breast cancer cells. Proteomics. 2012;12:1756–1766. doi: 10.1002/pmic.201100319. [DOI] [PubMed] [Google Scholar]
- 19.Kim GY, Nigro P, Fujiwara K, Abe J, Berk BC. P62 binding to protein kinase C zeta regulates tumor necrosis factor alpha-induced apoptotic pathway in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:2974–2980. doi: 10.1161/ATVBAHA.112.300054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masse K, Bhamra S, Haldin CE, Jones EA. Cloning and characterisation of the immunophilin X-CyPA in Xenopus laevis. Gene Expr Patterns. 2004;5:51–60. doi: 10.1016/j.modgep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Ivery MT. A proposed molecular model for the interaction of calcineurin with the cyclosporin A-cyclophilin A complex. Bioorg Med Chem. 1999;7:1389–1402. doi: 10.1016/s0968-0896(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 22.Mikol V, Kallen J, Walkinshaw MD. X-ray structure of a cyclophilin B/cyclosporin complex: comparison with cyclophilin A and delineation of its calcineurin-binding domain. Proc Natl Acad Sci USA. 1994;91:5183–5186. doi: 10.1073/pnas.91.11.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etzkorn FA, Chang ZY, Stolz LA, Walsh CT. Cyclophilin residues that affect noncompetitive inhibition of the protein serine phosphatase activity of calcineurin by the cyclophilin. Cyclosporin A complex. Biochemistry. 1994;33:2380–2388. doi: 10.1021/bi00175a005. [DOI] [PubMed] [Google Scholar]
- 24.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 25.Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res. 2009;105:830–841. doi: 10.1161/CIRCRESAHA.109.204974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh K, Nigro P, Berk BC. Oxidative stress and vascular smooth muscle cell growth: a mechanistic linkage by cyclophilin A. Antioxid Redox Signal. 2010;12:675–682. doi: 10.1089/ars.2009.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Pang J, Yan C, Natarajan K, Cavet ME, Massett MP, Yin G, et al. GIT1 mediates HDAC5 activation by angiotensin ii in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:892–898. doi: 10.1161/ATVBAHA.107.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activation in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 31.Sahar S, Reddy MA, Wong C, Meng L, Wang M, Natarajan R. Cooperation of SRC-1 and P300 with Nf-Kappab and CREb in angiotensin II-induced IL-6 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1528–1534. doi: 10.1161/ATVBAHA.107.145862. [DOI] [PubMed] [Google Scholar]
- 32.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh K, Berk BC, Shimokawa H. Vascular-derived reactive oxygen species for homeostasis and diseases. Nitric Oxide. 2011;25:211–215. doi: 10.1016/j.niox.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Nishioku T, Dohgu S, Koga M, Machida T, Watanabe T, Miura T, et al. Cyclophilin A secreted from fibroblast-like synoviocytes is involved in the induction of CD147 expression in macrophages of mice with collagen-induced arthritis. J Inflamm (Lond) 2012;9:44. doi: 10.1186/1476-9255-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, Foxwell BM. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399–404. [PMC free article] [PubMed] [Google Scholar]
- 36.Tiwari R, Koffel R, Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J. 2007;26:5109–5119. doi: 10.1038/sj.emboj.7601924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, et al. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–317. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas TL, Madri JA. Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med. 1999;9:70–77. doi: 10.1016/s1050-1738(99)00014-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.