Abstract
Aims
Diabetes mellitus (DM) is associated with poor clinical outcomes in humans with peripheral arterial disease (PAD) and in pre-clinical models of PAD, but the effects of glycaemic control are poorly understood. We investigated the effect of glycaemic control on experimental PAD in mice with Type 1 DM and explored the effects of hyperglycaemia on vascular endothelial growth factor receptor 2 (VEGFR2) expression in ischaemia.
Methods and results
Hind limb ischaemia was induced in non-diabetic, untreated Type 1 DM, and treated Type 1 DM mice. We assessed perfusion recovery, capillary density, VEGFR2 levels, and VEGFR2 ubiquitination in ischaemic hind limbs. We found that untreated Type 1 DM mice showed impaired perfusion recovery, lower hind limb capillary density 5 weeks post-ischaemia, and lower VEGFR2 protein in Day 3 post-ischaemic hind limbs when compared with non-DM controls. Treated Type 1 DM mice had perfusion recovery, capillary density, and VEGFR2 protein levels comparable with that of non-diabetic mice at the same time points. Treatment with anti-VEGFR2 antibody negated that the improved perfusion recovery displayed by treated Type 1 DM mice. In ischaemic Type 1 DM hind limbs and endothelial cells exposed to simulated ischaemia, high glucose impaired VEGFR2 expression and was associated with increased VEGFR2 ubiquitination. Inhibition of the ubiquitin–proteasome complex restored normal endothelial VEGFR2 expression in simulated ischaemia.
Conclusion
Hyperglycaemia in Type 1 DM impairs VEGFR2 protein expression in ischaemic hind limbs, likely due to increased ubiquitination and degradation by the proteasome complex. Glycaemic control allows normal levels of VEGFR2 in ischaemia and improved perfusion recovery.
Keywords: VEGF receptor, Glycaemic control, Diabetes, Peripheral arterial disease, Proteasome, Hyperglycaemia
1. Introduction
Peripheral arterial disease (PAD) refers to atherosclerosis occurring in vascular beds outside the heart, and the lower extremities are the most common sites of PAD. Both Type 1 and Type 2 diabetic mellitus (DM) patients are at an increased risk of developing PAD.1,2 Compared with patients with PAD but no DM, individuals with PAD with concurrent DM have a seven-fold higher risk of critical limb ischaemia and a five-fold higher risk of amputation.3,4 While individuals with diabetes may develop PAD at an earlier age and thus have had PAD for a longer time than PAD patients without DM, it is more likely that DM results in impairment in the adaptation to the vascular occlusions resulting in a greater severity of disease. Despite the well-established importance of glycaemic control in improving outcomes of certain diabetes complications (e.g. retinopathy and nephropathy),5 the role of glycaemic control in improving PAD outcomes is poorly understood.
The mouse hind limb ischaemia (HLI) model that involves surgical ligation and excision of the femoral artery has been used extensively to study processes that are involved in perfusion recovery following vessel occlusion.6 Using this model, several studies, including ones from our laboratory, have shown that perfusion recovery is impaired in mice with DM (Table 1). This pre-clinical model ‘creates’ PAD at the same time and to the same extent across different mouse groups, which allows the investigation of mechanisms that are involved in perfusion recovery.
Table 1.
Studies showing impaired perfusion recovery in DM1 and DM2 mice following HLI surgery
| References | Primary focus | DM model | VEGF/VEGF receptor analysed | Therapeutic intervention |
|---|---|---|---|---|
| Hazarika et al.7 | Impaired angiogenesis in DM2 | DM2 = HFD | VEGFA, R1, and R2 | None |
| Rivard et al.8 | Rescue impairment of angiogenesis by VEGFA | DM1 = NOD | VEGFA | Yes (VEGFA) |
| Tamarat et al.9 | Elevated advanced glycation end products as a mechanism for impaired angiogenesis in DM | DM1 = STZ | Not evaluated | Yes (amino guanidine) |
| Ebrahimian et al.10 | ROS as a mechanism for impaired perfusion recovery in DM1 | DM1 = STZ | VEGFA | Yes (N acetyl-1-cysteine) |
| Li et al.11 | VEGFA activating transcription factor can improve perfusion recovery DM2 | DM2 = HFD | VEGFA, R1, R2 (mRNA only) | Transcription factor activating VEGFA |
| Thangarajah12 | Impaired HIF-1 alpha expression in DM2 | DM1 = STZ DM2 = db/db |
Not evaluated | Deferoxamine |
| Moriya et al.13 | Impaired PDGF-C, Nrp1 and Nrp2 in DM1 | DM1 = STZ | Not evaluated | PDGF-C and VEGFA |
Summary of recent studies showing impaired perfusion recovery and potential mechanisms of impairment in Type 1 and Type 2 DM mice following HLI. All studies show impaired perfusion recovery in DM and some showed impaired expression of VEGFA. None addressed the effects of controlling hyperglycaemia on perfusion recovery or effects of hyperglycaemia or DM1 on VEGFR2 protein expression.
DM1, type 1 diabetes mellitus; DM2, type 2 diabetes mellitus; Nrp1, neuropilin 1; Nrp2, neuropilin 2; PDGF-C, platelet derived growth factor C; ROS, reactive oxygen species; STZ, streptozotocin; VEGFA, vascular endothelial growth factor A.
Angiogenesis is the growth of new vessels from pre-existing vascular structures. Vascular endothelial growth factor (VEGF) is a well-known pro-angiogenic agent involved in blood vessel growth during development and post-natal angiogenesis in the adult.14 The biological effects of VEGF are mediated through binding to its two main receptors, such as VEGF receptors 1 and 2 (VEGFR1 and VEGFR2),14–16 which are high-affinity receptor tyrosine kinases. VEGFR1 has a 10-fold higher binding affinity for VEGF than VEGFR2 but a 10-fold lower tyrosine kinase activity.14–16 The VEGF/VEGFR pathway plays a critical role in perfusion recovery following HLI, but VEGFR2 is considered the dominant receptor that mediates post-natal angiogenesis.14–16 Prior studies investigating the role of the VEGF/VEGFR in impaired perfusion recovery in diabetes have focused primarily on impaired vascular endothelial growth factor A (VEGFA) expression in Type 1 DM (summarized in Table 1). We are not aware of any study that has explored the possibility that impaired VEGFR2 expression in ischaemic hind limbs of Type 1 DM mice may be associated with impaired perfusion recovery. Additionally, normalizing hyperglycaemia in mice with Type 1 DM and exploring the impact on perfusion recovery following experimental PAD have not been studied.
2. Methods
For more details, see Supplementary material online.
2.1. Mice and model of Type 1 diabetes
All mice (C57BL/6 and C57BL/6J-Ins2Akita) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) either directly or bred internally from parental strains obtained from the Jackson Laboratory (more details in Supplementary material online). The C57BL/6J-Ins2Akita is the Ins2Akita strain on a C57BL/6 background. The Ins2Akita is a previously described mouse model of Type 1 diabetes17 (see Supplementary material online, Methods for details of blood glucose assessment).
2.2. Experimental PAD/HLI and perfusion recovery
HLI was achieved by unilateral femoral artery ligation and excision as described previously.6 Blood flow in the ischaemic and contralateral non-ischaemic limbs were measured by laser Doppler perfusion imaging as described previously.11 Controls were strain, age, and sex matched.
2.3. RNA, quantitative PCR, and protein analysis
Total RNA was isolated and used for real-time quantitative RT–PCR as previously described.18 Total hind limb muscle protein lysates were obtained as previously described.11 Levels of VEGFR2 were analysed by ELISA following the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA) and were normalized to the total protein from each hind limb muscle lysate. A fold increase in expression reflects the normalized expression in the ischaemic hind limb compared with that in the non-ischaemic hind limb. VEGFR2 in human umbilical vein endothelial cells (HUVECS, no higher than Passage 8) was analysed by western blotting as previously described11 using an anti-VEGFR2 antibody 55B11 (Cell Signaling, Danvers, MA, USA). The extent of VEGFR2 ubiquitination was analysed by immunoprecipitation (IP) of VEGFR2 from cells and muscle lysates followed by western blotting with anti-ubiquitin antibody FK2 (Enzo Life Sciences, Farmingdale NY, USA; see Supplementary material online, Methods). To inhibit the ubiquitin–proteasome degradation pathway, cells were pre-incubated with 5 nmol/L of epoxomycin (Cayman Chemical, Ann Arbor, MI, USA) 24 h prior to simulated ischaemia.
2.4. Cell line and culture
HUVEC and endothelial cell growth medium (ECGM) were obtained from Cell Applications, Inc. (San Diego, CA, USA). ECGM was supplemented with 10% fetal bovine serum. In vitro simulation of ischaemia was achieved as previously described,19 with slight modifications (see Supplementary material online, Methods).
2.5. Capillary density and immunohistochemistry
In the ischaemic and non-ischaemic hind limb muscles, capillaries were identified using CD31 staining or by endogenous alkaline phosphatase as previously described.20
2.6. Statistical analysis
All measurements were expressed as mean ± SEM. Statistical comparisons between two groups (e.g. treated vs. untreated) at a specific time point was performed with the independent Student's t-test or χ2 where appropriate. Comparison of more than two groups at a time was performed with analysis of variance. In all cases, a P-value of <0.05 was considered statistically significant.
3. Results
3.1. Effects of hyperglycaemia on perfusion recovery, limb necrosis, and capillary density following experimental PAD
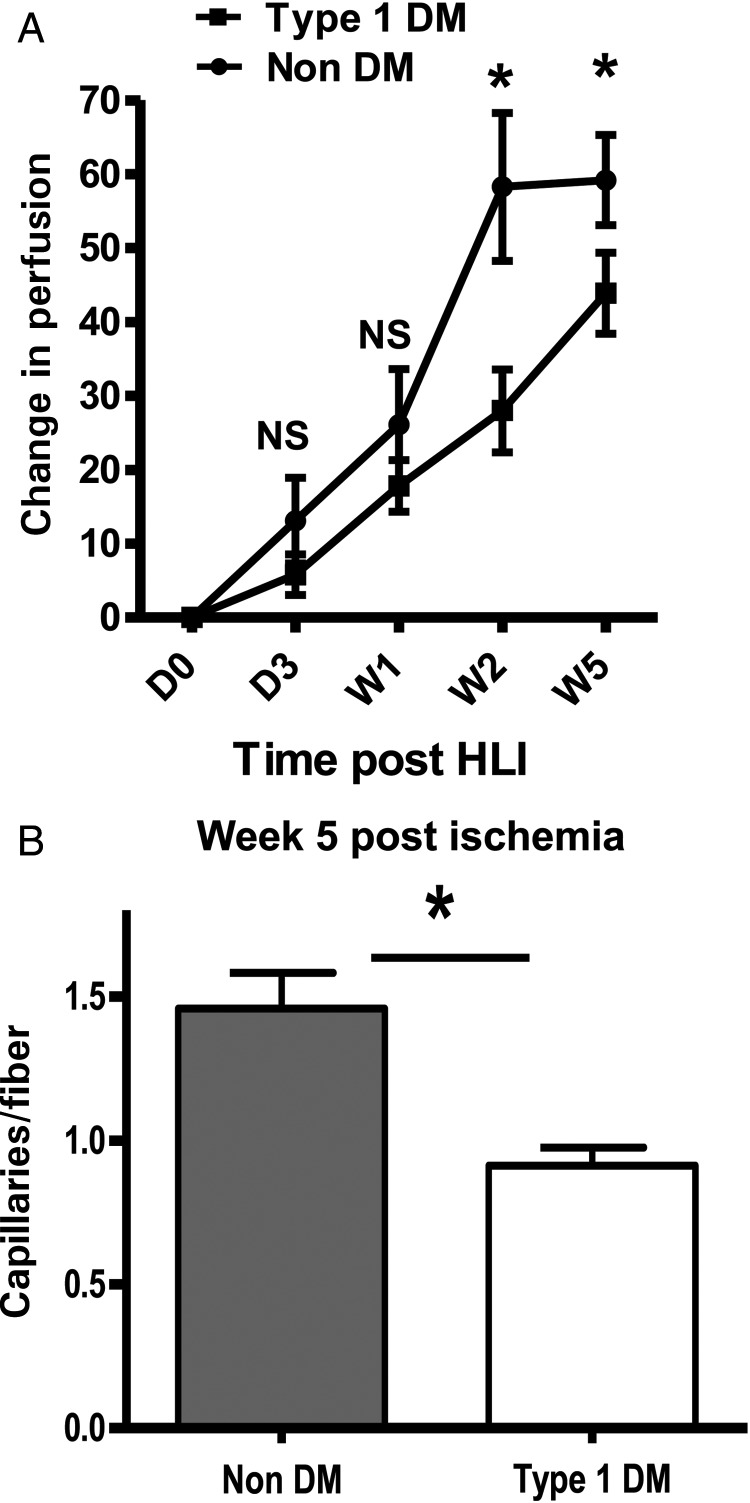
Untreated Type 1 DM mice were allowed 12 weeks of hyperglycaemia exposure, resulting in significantly higher HbA1c levels (10.5 ± 1.2, n = 62) than non-diabetic controls (4.4 ± 0.4, n = 56). Following HLI in Type 1 DM mice, 12.5% of the mice showed Stage 2 necrosis compared with 0% in non-DM controls (n = 16 and 8, respectively, not statistically significant using a χ2 test). Additionally, perfusion recovery over time was impaired in the untreated Type 1 DM mice (Figure 1A). This difference was detectable at Week 2 following HLI (28 ± 5.6 vs. 58.3 ± 10.0, P = 0.006, Figure 1A) and persisted until Week 5 (43.9 ± 5.5 vs. 59.2 ± 6.1, P = 0.02, Figure 1A). At 5-week post-HLI, untreated Type 1 DM mice showed a lower capillary density compared with non-diabetic controls (0.93 ± 0.06 vs. 1.46 ± 0.12, n = 5 and 7, respectively; P = 0.006, Figure 1B). We obtained a similar result with alkaline phosphatase staining (0.55 ± 0.1 vs. 0.86 ± 0.14, n = 4 and 5, respectively, P = 0.02). These results are consistent with prior studies using different models of Type 1 diabetes.10
Figure 1.
Perfusion recovery is impaired in untreated Type 1 DM mice and associated with decreased capillary density in Week 5 post-ischaemic (A) impaired perfusion recovery in untreated type 1 diabetes mellitus (DM1). The Y-axis shows perfusion ischaemic to non-ischaemic limb normalized to values immediately post-surgery, while the X-axis shows time post-surgery (Type 1 DM n = 16–17 and ND control, n = 8, NS = not significant, P > 0.05, *P = 0.006 at W2 and 0.02 at W5). (B) Since maximum perfusion is already achieved at Week 5 post-ischaemia, we compared capillary density in Week 5 post-ischaemic hind limbs from untreated DM1 mice (n = 5) with that from non-DM controls (n = 7) and found lower capillary density (*P = 0.006) in untreated DM1 mice at Week 5.
3.2. Impaired VEGFR2 expression in non-ischaemic and Day 3 post-ischaemic mouse hind limb muscles despite similar capillary density in Type 1 DM compared with non-DM controls
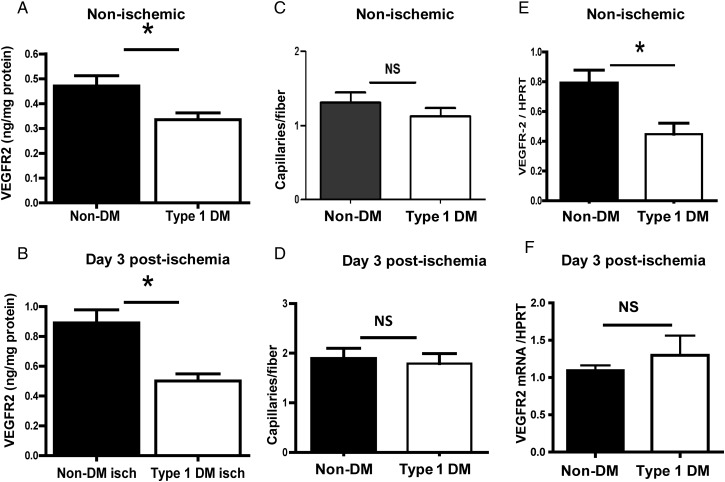
We analysed the expression of VEGFR2 in non-ischaemic and Day 3 post-ischaemic (a time point where the perfusion recovery was comparable between the groups) hind limb muscles of untreated Type 1 DM mice and non-diabetic controls. Our results showed ∼30% less VEGFR2 protein expression in untreated Type 1 DM non-ischaemic hind limbs compared with non-diabetic controls (0.34 ± 0.02 vs. 0.47 ± 0.04 ng/mg total protein, P = 0.02, n = 6 and 7, respectively, Figure 2A). We also found ∼50% less VEGFR2 expression in Day 3 post-ischaemic hind limbs of untreated Type 1 DM mice compared with non-diabetic controls (0.50 ± 0.05 vs. 0.89 ± 0.09 ng/mg of total protein, n = 5 and 6, respectively, P = 0.01, Figure 2B). Next, we assessed whether the difference in VEGFR2 expression was due to a difference in capillary density between non-ischaemic hind limbs of untreated Type 1 DM compared with non-diabetic controls. Our results showed no difference in capillary density between the two groups (Figure 2C). We also found no difference in capillary density when we compared Day 3 post-ischaemic hind limbs from untreated Type 1 DM and non-diabetic controls (Figure 2D). Next, we analysed whether there was lower VEGFR2 mRNA expression in the non-ischaemic and ischaemic hind limb muscles. We found that, in the non-ischaemic hind limbs from untreated Type 1 DM mice, VEGFR2 mRNA expression was lower compared with that from non-diabetic mice (Figure 2E). In the ischaemic hind limbs, there was no difference in VEGFR2 mRNA expression in untreated Type 1 DM mice when compared with non-diabetic controls (Figure 2F). Therefore, in non-ischaemic hind limb muscles, untreated Type 1 DM mouse showed lower expression of VEGFR2 compared with non-diabetic controls, likely due to decreased mRNA transcription, while in the ischaemic hind limbs decreased VEGFR2 expression does not appear to be from impaired mRNA expression.
Figure 2.
VEGFR2 expression is impaired in non-ischaemic and Day 3 ischaemic hind limbs of untreated Type 1 DM mice. (A) In non-ischaemic hind limbs, VEGFR2 protein expression is ∼30% less compared with non-DM controls (n = 7, *P = 0.02). (B) In Day 3 ischaemic hind limbs, VEGFR2 protein expression is ∼50% less in untreated Type 1 DM (n = 6) compared with non-DM controls (n = 5, *P = 0.01). Capillary density was comparable between untreated Type 1 DM and non-DM controls in non-ischaemic (C) (Type 1 DM n = 5, non-DM, n = 7, NS, P = 0.33) and ischaemic (D) hind limbs (Type 1 DM n = 5, non-DM n = 7, NS = P = 0.73). (E) In non-ischaemic hind limbs, VEGFR2 mRNA expression was l in untreated Type 1 DM compared with non-DM controls (n = 5/grp, P = 0.004) and may account for the decreased VEGFR2 protein. (F) VEGFR2 mRNA in Day 3 ischaemic hind limbs (n = 4) was not impaired, but comparable with non-DM controls (n = 5, NS, P = 0.44).
3.3. Treatment of Type 1 DM mice with insulin results in enhanced perfusion recovery and increased capillary density in the ischaemic limb
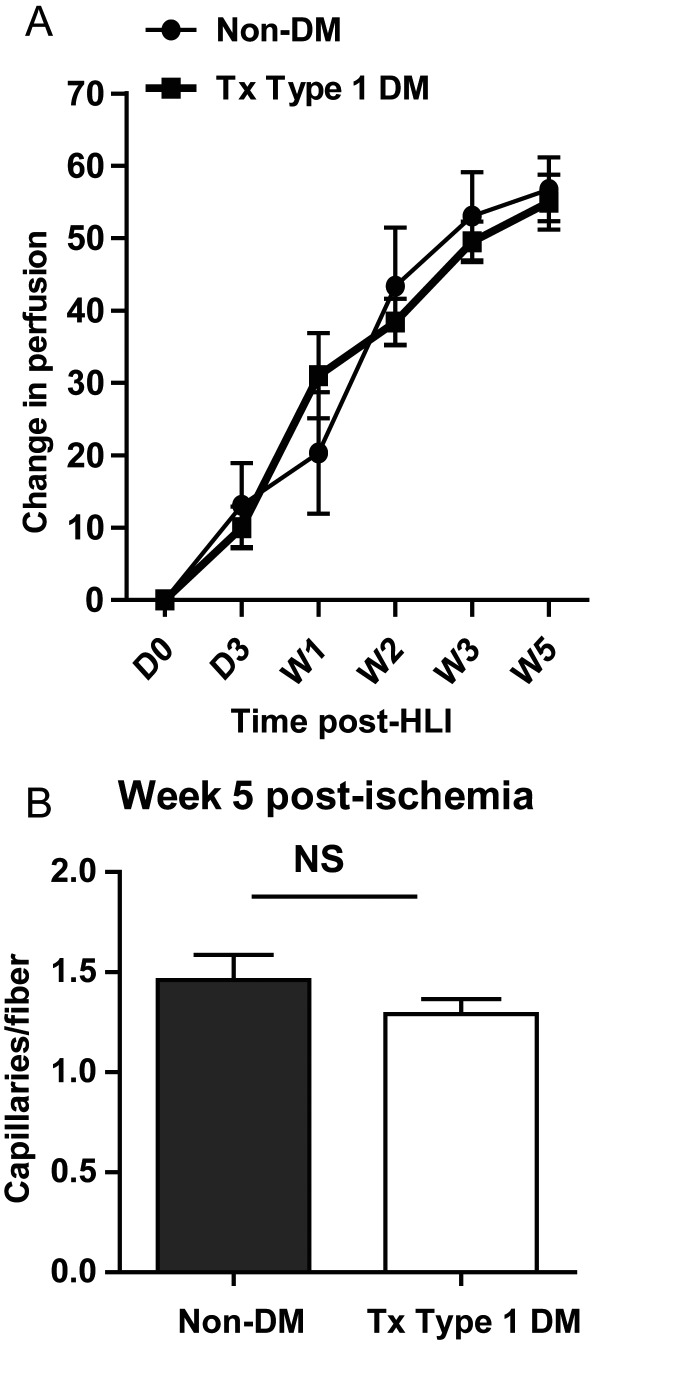
Mice with Type 1 DM were treated with insulin achieving an Hb1ac of 5.6 ± 0.1 (n = 56), which is consistent with current recommended treatment targets (i.e. Hba1c of ≤ 6.5) for individual with diabetes.21 We found no necrosis in the control non-DM and treated Type 1 DM mice (n = 12 and 21, respectively). Insulin-treated Type 1 DM mice showed perfusion recovery that was comparable with non-diabetic controls (change in perfusion at Week 5 for treated Type 1 DM = 55.0 ± 3.8 vs. non-DM = 56.7 ± 4.4, n = 21 and 8–12, respectively, P = 0.6, Figure 3A). Additionally, treated Type 1 DM mouse hind limb muscle showed comparable capillary density (by CD31 staining) with non-diabetic controls at Week 5 post-experimental PAD (1.28 ± 0.1 vs. 1.46 ± 0.12, n = 10 and 7, respectively, P = 0.3, Figure 3B). The results were similar, when capillary density was assessed by the alkaline phosphatase method (treated DM = 0.7 ± 0.04 vs. non-DM control = 0.9 ± 0.14, n = 6 and 5, respectively, P = 0.2). Hence, treating hyperglycaemia in Type 1 DM mice improves perfusion recovery and hind limb capillary density following HLI.
Figure 3.
(A) Perfusion recovery in treated Type 1 DM mice (n = 21) is comparable with that of non-diabetic controls (n = 8–12). The Y-axis shows the perfusion ratio (ischaemic-to-non-ischaemic limb) normalized to values immediately post-surgery. The X-axis shows the time point at which perfusion was assessed (P > 0.05) at all time points. (B) Capillary density in Week 5 post-ischaemic hind limb muscles of treated Type 1 DM mice (n = 10) is comparable with that of non-diabetic controls (n = 7, NS, P > 0.05).
3.4. Effect of treatment with insulin on VEGFR 2 expression in ischaemic hind limb muscle
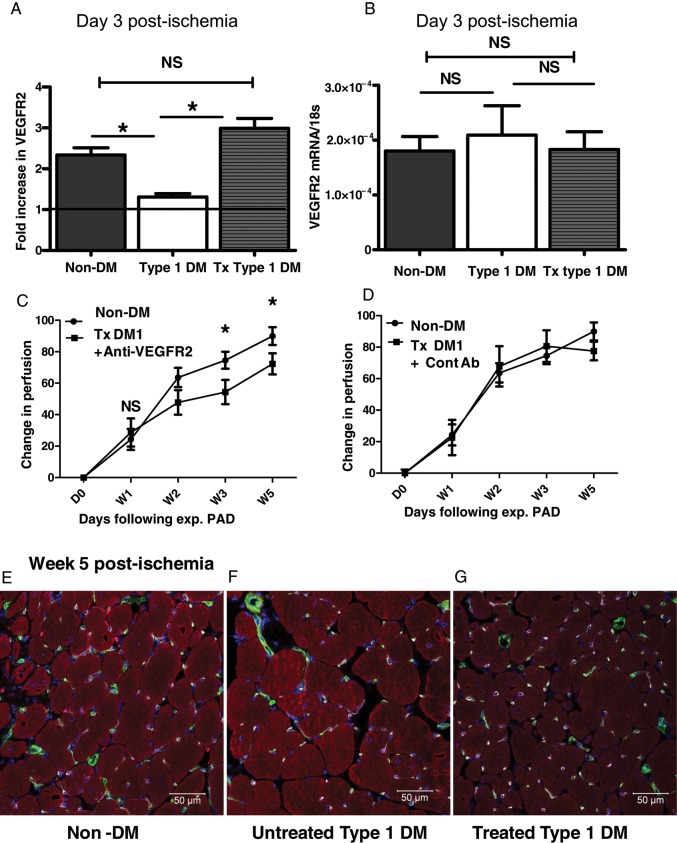
Muscle lysates from Day 3 post-ischaemic hind limbs of treated Type 1 DM, untreated Type 1 DM, and non-diabetic control mice were analysed for levels of VEGFR2 protein. We found that VEGFR2 protein was approximately two-fold higher in the treated Type 1 DM mice compared with the untreated Type 1 DM mice and comparable with the levels in the non-diabetic control (treated = 2.99 ± 0.20 vs. untreated = 1.31 ± 0.08, non-diabetic control = 2.34 ± 0.18, n = 7, 6, and 5, respectively. P < 0.0001 for treated vs. untreated and P = 0.07 for treated vs. non-diabetic controls, Figure 4A). In contrast, the level of VEGFA protein expression in treated Type 1 DM hind limb muscle increased compared with that in untreated Type 1 DM, but was not comparable with that in non-diabetic controls (non-diabetic control = 30.7 ± 1.1, n = 10, untreated = 17.2 ± 0.5, n = 8, and treated = 22.4 ± 1.2, n = 11, VEGFA pg/mg total protein, P < 0.01 in all, see Supplementary material online, Figure S1D). We compared VEGFR2 mRNA levels in Day 3 post-ischaemic hind limb muscles of treated Type 1 DM, untreated Type 1 DM, and non-diabetic control mice. We found no difference in VEGFR2 mRNA expression among the three groups compared (Figure 4B). This suggests that the increased VEGFR2 protein expression in Day 3 post-ischaemic hind limbs of treated Type 1 DM mice likely lies at the post-transcription level.
Figure 4.
Treatment of hyperglycaemia improves VEGFR2 expression in ischaemic hind limb muscles of Type 1 DM mice, and neutralizing VEGFR2 abolishes the treatment-related improved perfusion recovery. (A) A fold increase in VEGFR2 protein is comparable in treated Type 1 DM and non-DM controls in Day 3 post-ischaemic hind limb muscles, but impaired in untreated Type 1 DM mice (treated Type 1 DM, n = 7; non-DM controls n = 5; untreated Type 1 DM n = 6, *P < 0.05, NS = not significant, P > 0.05). (B) VEGFR2 mRNA expression by quantitative PCR showed no difference in the level of expression between non-DM, untreated Type 1 DM, and treated Type 1 DM in Day 3 post-ischaemic hind limbs (n = 6, 7, and 5, respectively, NS, P > 0.05). (C) Treatment with a VEGFR2-neutralizing antibody results in impaired perfusion recovery in treated Type 1 DM. The Y-axis shows the perfusion ratio (ischaemic-to-non-ischaemic limb) normalized to values immediately post-surgery, while the X-axis shows time post-surgery [non-DM controls n = 6–7, treated (Tx) type 1 DM n = 8–10, *P = 0.008 at W3 and 0.01 at W, NS, P = 0.99]. (D) Treatment with isotype matched control antibody had no effect on perfusion recovery in treated Type 1 DM mice [non-DM controls n = 7, treated (Tx) Type 1 DM n = 5]. (E, F, and G) Immunostaining of sections from Week 5 post-ischaemic hind limb muscles of non-diabetic control (E), untreated Type 1 DM (F), and Treated Type 1 DM mice (G). It shows co-localization of VEGFR2 expression with CD31-expressing cells (CD31 = green, VEGFR2 = pink, actin = red, blue = nuclear staining, green + pink = white) consistent with endothelial cell expression.
3.5. Role of VEGFR2 in perfusion recovery in insulin-treated DM1 mice
We administered a VEGFR2-neutralizing antibody22 into treated Type 1 DM mice and followed perfusion after HLI. Our results showed that administering a VEGFR2-neutralizing antibody resulted in impaired perfusion recovery in treated Type 1 DM mice. This effect was present starting at Week 2 post-HLI and persisted until the end of the experiment at Week 5 (Figure 4C). Of note, treated Type 1 DM mice that received control antibody showed no impairment in perfusion recovery (Figure 4D). Therefore, these findings show that improved perfusion recovery in treated type 1 diabetes mellitus (DM1) mice is mediated at least in part through VEGFR2. Next, we assessed which cell types in the mouse ischaemic hind limbs were expressing VEGFR2 by immunostaining of frozen sections of the mouse hind limbs. We found that positive VEGFR2 staining was found primarily in endothelial (CD31+) cells (Figure 4E).
3.6. High glucose is sufficient to impair human endothelial cell VEGFR2 expression in simulated ischaemia
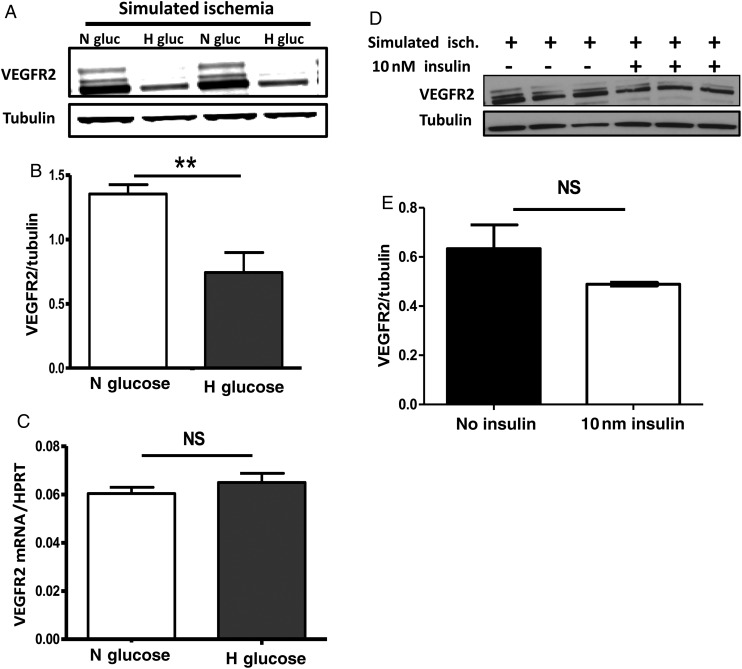
Next, we sought to determine whether high glucose is sufficient to impair endothelial cell VEGFR2 expression in simulated ischaemia. We cultured HUVECS at the same passage number in normal (5 mmol/L) glucose, in increasing concentrations of d-glucose, and in l-glucose (at 15, 25, and 50 mmol/L) then exposed the cells to simulated ischaemia.19 l-glucose provides the same osmolarity, but is not metabolized by the cells. Our results show glucose concentration-dependent impairment in VEGFR2 expression with peak impairment at 25 mmol/L of glucose (see Supplementary material online, Figure S1). Next, we assessed whether high glucose impaired VEGFR2 expression in ischaemia by impairing its mRNA synthesis. HUVECS were cultured in normal (5 mmol/l) or high glucose (25 mmol/l), then exposed to 24 h of simulated ischaemia; followed by assessment of VEGFR2 protein and mRNA expression. We found that VEGFR2 protein expression was impaired in HUVECS cultured in high glucose (Figure 5A and B) compared with those cultured in normal glucose. However, culture in high glucose did not impair VEGFR2 mRNA expression in HUVECS, as mRNA levels were similar between cells cultured in high and normal glucose (Figure 5C). Therefore, high glucose is sufficient to impair VEGFR2 expression in simulated ischaemia, and this was not due to impaired mRNA expression but is likely to be at the post-translational level.
Figure 5.
Hyperglycaemia impairs VEGFR2 expression in simulated ischaemia (hypoxia and nutrient deprivation) without altering its mRNA expression. (A) In simulated ischaemia, VEGFR2 protein expression is impaired in HUVECS pre-cultured in high glucose (25 mM or H Gluc) compared with HUVECS cultured in normal glucose (5 mM or N Gluc). (B) Quantization of the bands in A, n = 5/grp, **P < 0.01. (E) In simulated ischaemia, VEGFR2 mRNA by quantitative RT-PCR showed normal expression in HUVECS cultured in high glucose prior to simulated ischaemia exposure (n = 6/grp, NS = not significant, P = 0.33). (D) Insulin treatment did not increase VEGFR2 expression in HUVECs. Cells were cultured in the presence or absence of insulin, and then exposed to simulated ischaemia for 12, 24, 48, and 72 h following an initial overnight starvation in insulin and growth factor-free medium. A representative western blot of VEGFR2 expression following treatment with 10 nM of insulin at 72 h is shown. (E) Quantization of the blot in D (n = 3/grp, NS = not significant, P > 0.05).
3.7. Effect of insulin on endothelial cell VEGFR2 expression
In vivo, control of hyperglycaemia with insulin corrected the impaired VEGFR2 expression in ischaemic hind limbs of mice with Type 1 DM. This could be due to a direct effect of insulin on endothelial VEGFR2 expression, or alternatively it could be due to correcting hyperglycaemia.
Therefore, we sought to determine the effects of insulin on endothelial cell VEGFR2 expression in simulated ischaemia. HUVECS, cultured in media containing different doses of insulin (1, 10, and 100 nmol/L), prior to exposure to simulated ischaemia for 24–72 h were analysed for VEGFR2 expression. We found that insulin did not increase VEGFR2 protein expression at any of the doses or time points assessed (data shown for 10 nmol/L of insulin at 72 h, Figure 5D and E). To determine whether insulin treatment can protect HUVECS grown in high glucose from impaired VEGFR2 expression in ischaemia, the above experiment was repeated with HUVECS cultured in 15 and 25 mM glucose. Insulin was not protective, as VEGFR2 expression was still impaired in a dose-dependent manner in the presence of insulin (see Supplementary material online, Figure S1C, data shown for 10 nm insulin but identical results observed with 100 nM).
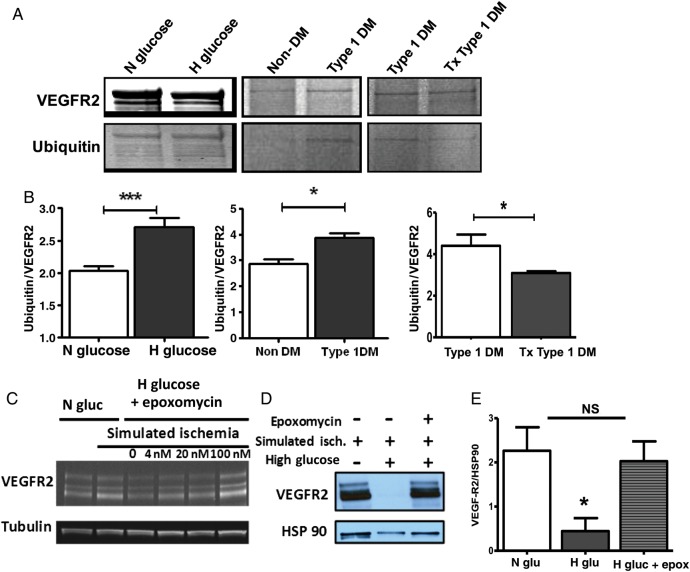
3.8. High glucose increases VEGFR2 ubiquitination and degradation via the ubiquitin–proteasome complex
Studies have implicated the ubiquitin–proteasome system in the regulation of VEGFR2 expression following VEGFA binding.23 Additionally, there is evidence that hyperglycaemia may increase proteasome-mediated degradation of proteins.24 We therefore hypothesized that increased degradation of VEGFR2 via the ubiquitin–proteasome system may account for decreased endothelial cell expression of VEGFR2 in hyperglycaemia and ischaemia. We analysed the extent of VEGFR2 ubiquitination in HUVECS cultured in either normal or high glucose before exposure to simulated ischaemia. We found higher levels of VEGFR2 ubiquitination in HUVECS exposed to high glucose compared with those exposed to normal glucose (Figure 6A and B, left panel). In vivo, comparison of VEGFR2 ubiquitination in ischaemic hind limbs of non-DM and untreated Type 1 DM mice showed higher ubiquitination in the untreated Type 1 DM (Figure 6A and B, middle panel). Additionally, comparison of VEGFR2 ubiquitination in ischaemic hind limbs of treated and untreated Type 1 DM mice showed higher ubiquitination in the untreated Type 1 DM (Figure 6A and B, right panel). As positive and negative control, western blotting of lysates of mouse ischaemic hind limbs was performed and probed with either the secondary antibody alone or the same anti-poly-ubiquitin antibody used to probe VEGFR2 IPs. The blot showed a smear consistent with prior reports23 (see Supplementary material online, Figure S1G, top panel), while the secondary antibody alone showed no signal (see Supplementary material online, Figure S1G, bottom panel).
Figure 6.
(A) VEGFR2 immunoprecipitation followed by anti-ubiquitin western blotting reveals higher VEGFR2 ubiquitination in HUVECs exposed to simulated ischaemia in the setting of high glucose compared with those in the setting of normal glucose (left panel). In vivo, VEGFR2 ubiquitination is higher in ischaemic hind limbs from Type 1 DM mice (middle panel). Insulin treatment results in reduced VEGFR2 ubiquitination in ischaemic hind limbs of Type 1 DM (Tx Type 1 DM, right panel). (B) Ubiquitin-to-VEGFR2 ratio from quantization of VEGFR2 band represented in A (left panel, n = 3 for N glucose and 4 for H glucose, ***P = 0.006; middle panel, n = 3 for non-DM and 4 for Type 1 DM, *P = 0.01; right panel, n = 5 for Type 1 DM and 4 for Tx Type 1 DM, *P = 0.04). (C) Blocking ubiquitin–proteasome degradation with epoxomycin restores VEGFR2 expression in simulated ischaemia in a dose-dependent manner. (D) Representative blot showing VEGFR2 expression in simulated ischaemia restored with epoxomycin treatment. (E) Quantification of the bands in D (N Gluc = normal glucose, H Gluc = high glucose, Epo = epoxomycin, n = 3, *P < 0.05, NS = not significant). Each blot is representative of at least three experiments.
Taken together, our results show that, in hyperglycaemia or high glucose, there is increased ubiquitination of VEGFR2, and this may lead to increased proteasome-mediated degradation of VEGFR2. To assess whether the ubiquitin–proteasome system is involved in the degradation of VEGFR2, HUVECs were pre-treated with different concentrations of the proteasome inhibitor, epoxomycin, and then exposed to simulated ischaemia. We found inhibition of the proteasome complex with epoxomycin protected against VEGFR2 degradation in a dose-dependent manner (Figure 6C). The level of VEGFR2 expression in HUVECS exposed to high glucose but pre-treated with epoxomycin was similar to those from HUVECS cultured in normal glucose (Figure 6D and E). Thus, impaired VEGFR2 protein expression in ischaemia in the setting of high glucose is likely due to increased degradation of VEGFR2 via the ubiquitin–proteasome system.
4. Discussion
Diabetes is well known to have adverse effects on the clinical course of patients with PAD.4 In pre-clinical models of PAD, the presence of DM was associated with worse outcomes (Table 1). However, the mechanism involved in the impaired perfusion recovery and the effects of glycaemic control with insulin was poorly understood. To our knowledge, our study provides the first evidence that in mice with Type 1 DM controlling hyperglycaemia to current clinically recommended Hb1ac targets normalizes perfusion recovery following HLI and this was associated with modulation of VEGFR2 expression in ischaemic endothelium.
In the previous studies, proposed mechanisms of impaired perfusion recovery following HLI in mice with DM include: accumulation of advanced glycation end products, generation of reactive oxygen species, decreased bone marrow mononuclear cells, impaired endothelial progenitor cell mobilization, increased inflammation, impaired HIF-1 alpha expression, and alterations in the expression of VEGF, impaired platelet derived growth factor C (PDGF-C), and neuropilin 1 and neuropilin 2 expression (summarized in Table 1). While impaired VEGFA expression in Type 1 DM mice has been shown to contribute to poor perfusion recovery following HLI,8,10,11 less is known about the role of VEGF receptors. VEGFR2 is the dominant VEGF receptor involved in post-natal angiogenesis,15,16 and hence its modulation by hyperglycaemia in Type 1 DM is a likely candidate to impair perfusion recovery. Here, we show that VEGFR2 expression is impaired in non-ischaemic and ischaemic hind limb muscles of mice with Type 1 DM. In the ischaemic hind limbs, despite comparable capillary densities at Day 3 following HLI, VEGFR2 expression was decreased in untreated Type 1 DM hind limbs compared with non-diabetic controls. This shows lower expression of VEGFR2 even when adjusting for capillary content. Recent studies by Moriya et al.13 showed that PDGF-C but not VEGFA vector injection improved perfusion recovery in streptozotocin-induced Type 1 DM following HLI. The role of VEGFR2 in the lack of VEGFA efficacy was not assessed in that study and although the lack of efficacy of VEGFA could be due to a number of factors, impaired VEGFR2 expression as shown in our study is likely to be a contributing factor.
Improved VEGFR2 expression in hind limb muscles of insulin-treated Type 1 DM mice may have been due to the glucose-lowering effect of insulin or due to a direct effect of insulin on VEGFR2 expressing cells. As expected, we showed that VEGFR2 expression was primarily on endothelial cells in muscle in vivo. To more easily separate the effects of insulin and glucose, we moved to an in vitro system. In vitro, we showed that high glucose was sufficient to impair endothelial VEGFR2 expression in simulated ischaemia. Insulin treatment of endothelial cells did not increase VEGFR2 expression nor did it protect against impaired VEGFR2 expression in high glucose in the setting of simulated ischaemia. Although we cannot exclude the possibility that insulin has other effects that contribute to improved perfusion recovery in vivo, our data clearly show that insulin has no effect on improving endothelial cell VEGFR2 expression. Therefore, it is more likely that the improved VEGFR2 expression was due to the glucose-lowering effect of insulin rather than a direct effect of insulin on endothelial VEGFR2 expression in ischaemia. Finally, in vivo, systemic delivery of a VEGFR2-neutralizing antibody was sufficient to block perfusion recovery following HLI in the treated Type 1 DM mice, thus confirming a role of VEGFR2 expression in perfusion recovery in the insulin-treated Type 1 DM mice. A recent study by Villalta et al.25 nicely showed that insulitis in the NOD model of Type 1 DM can be abrogated by blocking VEGFR2 and consequently delay onset of Type 1 DM. However, the Type 1 DM model (Akita mice) used in our study develops diabetes as a result of progressive loss of beta cells due to accumulation of mis-folded Ins 2 protein within the beta cells rather than through insulitis as seen in NOD mice.26 Additionally, the Akita mice in our study were already treated with insulin to near-normal glycaemia (Hba1c 5.6 ± 0.1) prior to injection of VEGFR2 antibody. Therefore, it is very unlikely that the effect of VEGFR2 blocking antibody used in our study is through modulation of diabetes.
Meyer et al.23 established a role for ubiquitination and proteasome degradation in the regulation of VEGFR2 expression. Here, we show that VEGFR2 is more highly ubiquitinated in untreated Type 1 DM ischaemic hind limbs and endothelial cells cultured in high glucose and subjected to simulated ischaemia when compared with controls. Moreover, inhibition of the ubiquitin–proteasome complex restored endothelial cell VEGFR2 levels in simulated ischaemia and high glucose. Hence, our current studies extend prior findings, by showing that impaired VEGFR2 expression in ischaemia and high glucose is regulated by the ubiquitin–proteasome complex and links this to impaired perfusion recovery in Type 1 DM following HLI. While it is likely that other proteins involved in ischaemic vascular remodelling are also affected by increased ubiquitination in high glucose, the current study was not meant to be exhaustive in evaluating all possible proteins affected by increased ubiquitination. Instead, the goal was to define the effect of high glucose on VEGFR2, since it is one of the proteins that play a pivotal role in post-natal angiogenesis.
In conclusion, while glycaemic control is likely to affect more than one process involved in perfusion recovery following HLI, our current study provides a framework from which we can begin to understand the potential benefits of glucose normalization as a therapeutic target in PAD.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
A.O.D. was supported by a Robert Wood Johnson Foundation, Harold Amos Medical Faculty Development Program award, and NIH/NHBLI, 3 R01 HL101200-01S1. The work was also supported by R01 HL101200-01 to B.H.A.
Supplementary Material
Acknowledgements
We acknowledge technical assistance provided by Natasha Duggan.
Conflict of interest: none declared.
References
- 1.Melton LJ, Macken KM, Palumbo PJ, Elveback LR. Incidence and prevalence of clinical peripheral vascular disease in a population-based cohort of diabetic patients. Diabetes Care. 1980;3:650–654. doi: 10.2337/diacare.3.6.650. [DOI] [PubMed] [Google Scholar]
- 2.Welborn TA, Knuiman M, McCann V, Stanton K, Constable IJ. Clinical macrovascular disease in Caucasoid diabetic subjects: logistic regression analysis of risk variables. Diabetologia. 1984;27:568–573. doi: 10.1007/BF00276969. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR. HbA1c and peripheral arterial disease in diabetes. Diabetes Care. 2006;29:877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- 4.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes—a review. Diabetic Med. 2010;27:4–14. doi: 10.1111/j.1464-5491.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- 5.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 6.Couffinhal T, Silver M, Zheng L, Kearney M, Witzenbichler B, Isner J. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 7.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 8.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamarat R, Silvestre J-Sb, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, et al. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci. 2003;100:8555–8560. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Hazarika S, Xie D, Pippen AM, Kontos CD, Annex BH. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007;56:656–665. doi: 10.2337/db06-0999. [DOI] [PubMed] [Google Scholar]
- 12.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci. 2009;106:13505–13510. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N. PDGF-C promotes revascularization in ischemic limbs of diabetic mice. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2013.04.053. http://dx.doi.org/10.1016/j.jvs.2013.04.053 . [DOI] [PubMed] [Google Scholar]
- 14.Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 15.Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Q, Huang J, Klitzman B, Dong C, Goldschmidt-Clermont PJ, March KL, et al. Engineered zinc finger-activating vascular endothelial growth factor transcription factor plasmid DNA induces therapeutic angiogenesis in rabbits with hindlimb ischemia. Circulation. 2004;110:2467–2475. doi: 10.1161/01.CIR.0000145139.53840.49. [DOI] [PubMed] [Google Scholar]
- 19.McClung JM, McCord TJ, Keum S, Johnson S, Annex BH, Marchuk DA, et al. Skeletal muscle specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol. 2012;180:2156–2169. doi: 10.1016/j.ajpath.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg. 2006;44:166–175. doi: 10.1016/j.jvs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Handelsman Y, Mechanick J, Blonde L, Grunberger G, Bloomgarden Z, Bray G, et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocrine Pract. 2011;17:1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann RC, Hartman T, Bohlen P, Sauer MV, Kitajewski J. Preovulatory treatment of mice with anti-VEGF receptor 2 antibody inhibits angiogenesis in corpora lutea. Microvasc Res. 2001;62:15–25. doi: 10.1006/mvre.2001.2312. [DOI] [PubMed] [Google Scholar]
- 23.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol. 2011;31:2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasubramanyam M, Sampathkumar R, Mohan V. Is insulin signaling molecules misguided in diabetes for ubiquitin–proteasome mediated degradation? Mol Cell Biochem. 2005;275:117–125. doi: 10.1007/s11010-005-1083-y. [DOI] [PubMed] [Google Scholar]
- 25.Villalta SA, Lang J, Kubeck S, Kabre B, Szot GL, Calderon B, et al. Inhibition of VEGFR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function. Diabetes. 2013;62:2870–2878. doi: 10.2337/db12-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.