Patients with cutaneous lupus erythematosus (CLE) have very poor quality of life1. When compared to those with other skin diseases, CLE patients are among those most severely affected by their condition. The psychologic aspects of quality of life in CLE are similar to, or worse than, what is experienced by patients with chronic hypertension, congestive heart failure, type 2 diabetes mellitus, and recent myocardial infarction. In considering this, we were interested in assessing whether patients who demonstrated response to treatment also experienced change to their quality of life.
Methods
This institutional review board-approved study prospectively evaluated response to systemic therapy in CLE patients using the CLE Disease Area and Severity Index (CLASI) and the Skindex-29. The CLASI is a validated clinical tool that quantifies disease activity and damage separately in patients with CLE, with higher scores indicating more severe disease2. The Skindex-29 is a validated skin-specific quality of life measure that calculates 3 subscale scores: Emotions, Functioning, Symptoms, with higher scores indicating worse quality of life4.
Eligible patients met modified Gilliam criteria for CLE and had at least 2 visits with CLASI and Skindex-29 scores measured. Patients with a diagnosis of systemic lupus erythematosus were included. Details regarding identification of eligible patients have been discussed previously5. From eligible patients, those who had been initiated on antimalarial therapy (hydroxychloroquine, hydroxychloroquine-quinacrine, chloroquine, chloroquine-quinacrine) or antimetabolite therapy (methotrexate, mycophenolate, azathioprine) were identified. Twenty-seven patients were initiated on antimalarial therapy. Hydroxychloroquine was dosed at 200 mg/day to 400 mg/day and chloroquine was dosed at 250 mg/day for 5 to 7 days/week, based on ideal body weight. Quinacrine was dosed at 100 mg/day. Twelve patients were initiated on antimetabolite therapy. Antimetabolites were initiated at a low dose with dose escalation, as tolerated, until the lowest effective dose was achieved. Median (interquartile range) methotrexate dosing was 13.8 (10.6–16.3) mg/week, mycophenolate dosing 2000 (1750–2500) mg/day, azathioprine 100 (75–125) mg/day. For 6 out of the 39 patients included in this study, data were available for initiation of two systemic therapies. In these cases, data from the first therapy that was initiated was included, and the remainder was excluded.
Consistent with previous work3, 5, response was defined as either a 4-point or 20% decrease in CLASI activity score and determined by comparing the score at the pre-treatment visit with the first post-treatment visit. The first post-treatment visit occurred at least 2 months following the pre-treatment visit. For patients with more than one post-treatment visit, the first post-treatment visit was used. All responders and non-responders were pooled together, regardless of which systemic therapy was initiated, and Skindex-29 subscale scores were compared between the pre-treatment visit and the first post-treatment visit.
Skindex-29 subscale scores were normally distributed, and paired t-tests were used. Spearman’s correlation tests were performed between change in Skindex-29 subscale score and change in CLASI activity score. Stata software, version 11.0 (StataCorp LP) and GraphPad Prism, version 5.0 (GraphPad Software Inc) were used for data analysis.
Results
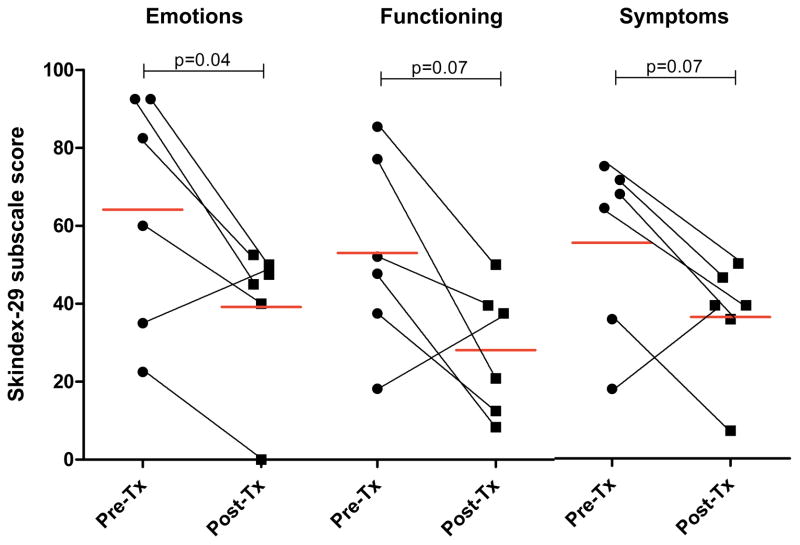
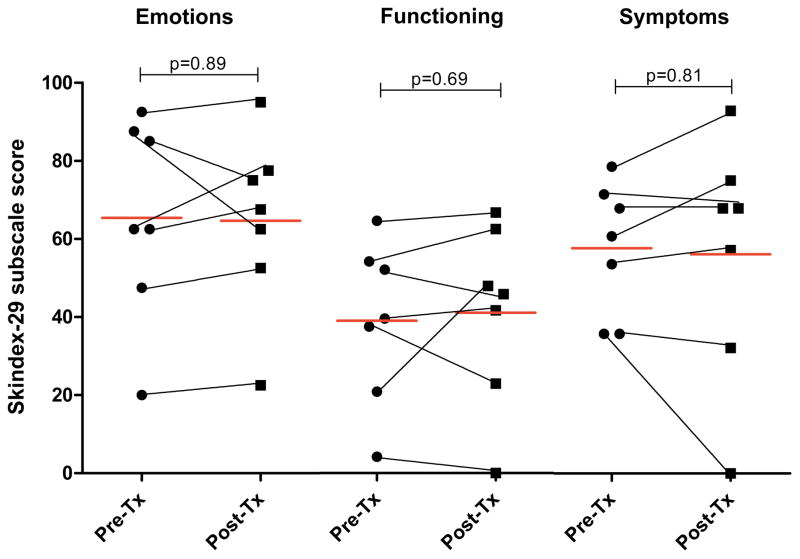
For 23 responders out of 39 patients initiated on an antimalarial or antimetabolite, the mean (95% confidence interval [CI]) Emotions score decreased from 53.9 (40.6, 67.1) to 38.8 (28.2, 49.4) (p=0.002), Functioning score decreased from 32.7 (20.5, 44.9) to 20.9 (13.1, 28.7) (p=0.01), and Symptoms score decreased from 47.2 (37.8, 56.6) to 33.7 (26.6, 40.8) (p=0.0007) (Figure 1A). For the 16 non-responders, the mean (95% CI) Emotions, Functioning, and Symptoms scores were unchanged: 48.0 (33.3, 62.7) to 53.0 (38.3, 67.6) (p=0.30); 25.1 (13.2, 37.1) to 25.1 (13.2, 37.1) (p=1.0); 44.2 (32.4, 56.0) to 43.0 (28.1, 57.8) (p=0.81), respectively (Figure 1B). Differences in pre-treatment CLASI score, age, sex, smoking status, and treatment duration between responders and non-responders were not statistically significant. Change in CLASI activity score was weakly correlated with change in Skindex-29 subscale scores: Emotions, r=0.39, p=0.01; Functioning, r=0.29, p=0.07; Symptoms, r=0.33, p=0.04.
Figure 1.
Skindex-29 subscale scores for Responders (1A) and Non-responders (1B). Pre- and post-treatment (Tx) Emotions, Functioning, and Symptoms subscale scores shown. Red lines indicate mean score. Black lines connect pre- and post-Tx data for the same individual.
Comment
Response in disease activity was accompanied by an improvement in skin-specific quality of life measures. This study is limited by its inclusion of medications from various drug classes, such that the impact of side effects on quality of life remains a possibility. Larger studies of systemic therapies in CLE that focus on both disease and quality of life outcomes are needed.
Acknowledgments
Funding/Support: This study is based upon work supported by the National Institutes of Health, including NIH K24-AR 18 02207 (Werth), and the Doris Duke Charitable Foundation (Chang).
Footnotes
Author Contributions: Ms. Chang and Dr. Werth had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chang and Werth. Acquisition of data: Chang, Ghazi, and Okawa. Analysis and interpretation of data: Chang, Ghazi, and Werth. Drafting of the manuscript: Chang. Critical revision of the manuscript for important intellectual content: Chang, Ghazi, Okawa, and Werth. Statistical analysis: Chang. Obtained funding: Chang and Werth. Administrative, technical, or material support: Okawa. Study supervision: Chang, Okawa, and Werth.
Financial Disclosure: None reported.
Contributor Information
Aileen Y. Chang, Email: aileen.ychang@gmail.com.
Elizabeth Ghazi, Email: liz.ghazi@gmail.com.
Joyce Okawa, Email: Joyce.Okawa@uphs.upenn.edu.
References
- 1.Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64:849–58. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147:203–8. doi: 10.1001/archdermatol.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133:1433–40. [PubMed] [Google Scholar]
- 5.Chang AY, Piette EW, Foering KP, Tenhave TR, Okawa J, Werth VP. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Arch Dermatol. 2011;147:1261–7. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]