Summary
A large body of evidence indicates that chronic inflammation is one of key risk factors for cancer initiation, progression, and metastasis. However, the underlying mechanisms responsible for the contribution of inflammation and inflammatory mediators to cancer remain elusive. Here we present genetic evidence showing that loss of CXCR2 dramatically suppresses colonic chronic inflammation and colitis-associated tumorigenesis through inhibiting infiltration of myeloid-derived suppressor cells (MDSCs) into colonic mucosa and tumors in a mouse model of colitis-associated cancer. CXCR2 ligands were elevated in inflamed colonic mucosa and tumors and induced MDSC chemotaxis via its receptor, CXCR2. Adoptive transfer of wild type MDSCs into Cxcr2−/− mice restored AOM/DSS-induced tumor progression. MDSCs accelerated tumor growth by inhibiting CD8+ T cell cytotoxic activity.
Introduction
Colorectal cancer (CRC) is the fourth most common malignant neoplasm and the second leading cause of cancer deaths in the USA. Although colonoscopy screening is an effective way to detect and prevent CRC by removing precancerous adenomas (Zauber et al., 2012), 70% of CRC patients present to their physician with advanced disease, resulting in an unacceptable 5 year survival rate (Yamashita and Watanabe, 2009). CRC includes hereditary, sporadic, and colitis-associated CRC. In addition to somatic mutations and epigenetic changes, epidemiologic and experimental evidence strongly implicates chronic inflammatory stimuli as a risk factor for developing CRC. Indeed, ulcerative colitis (UC), a form of inflammatory bowel disease (IBD), is associated with an increased risk for the development of CRC (Ekbom et al., 1990). More than 20% of patients with UC are reported to develop colitis-associated CRC within 30 years of diagnosis (Lakatos and Lakatos, 2008). Colitis-associated cancer often shows rapid progression, with poor response to treatment and high mortality (Feagins et al., 2009). Since there is a strong association between chronic inflammation and CRC in IBD patients, studies on colitis-associated CRC provides a “proof of concept” model to better understand how chronic inflammation and certain inflammatory mediators promote tumor initiation, growth, and metastasis.
Chronic inflammation is caused by a persistently heightened immune response following injury or exposure to foreign pathogens. For example, disruption of immune homeostasis in the intestine in response to the gut flora, which contains foreign luminal antigens from food and commensal bacteria, can result in the development of IBD. The importance of flora for IBD is evident by the observations that antibiotic treatment and/or probiotic therapy have been shown to be benefits for, at least, subsets of IBD patients (Gionchetti et al., 2003; Sutherland et al., 1991). Direct evidence for the role of luminal flora came from animal studies showing that chronic colitis is dependent on their presence (Elson et al., 2005). Antibiotic treatment and/or probiotic therapy attenuated colon chronic inflammation in different mouse models of IBD, including dextran sulfate sodium (DSS)-treated mice (Garrido-Mesa et al., 2011a; Garrido-Mesa et al., 2011b). In a mouse model of colitis-associated cancer, germ-free azoxymethane (AOM)-treated Il10−/− mice exhibited normal colon histology and did not develop colon tumors (Uronis et al., 2009). Even in a mouse model of hereditary and sporadic CRC, antibiotic treatment reduced tumor burden, indicating the luminal bacteria contributes to tumor growth (Grivennikov et al., 2012). Of note, several in vivo studies showed that pathogenic bacteria from gut flora induced expression of the inflammatory enzyme cyclooxygenase 2 (COX-2) in inflamed colonic mucosa (Abdallah Hajj Hussein et al., 2012; Cho and Chae, 2004; Lee and Kim, 2011). The levels of COX-2 and COX-2-derived prostaglandin E2 (PGE2) are known to be markedly elevated in the gastrointestinal tract of IBD patients (Lauritsen et al., 1986; Singer et al., 1998).
The main pathological feature of IBD involves a massive infiltration of neutrophils, lymphocytes, and monocytes into the inflamed intestinal tissue. Similarly, the common pathological changes associated with colitis-associated and sporadic CRC include recruitment and reprogramming of various types of dysregulated immune cells and endothelial cells to establish a tumor microenvironment (Coussens and Werb, 2002; Strober et al., 2007). Chemokines that recruit leukocytes from the circulatory system to local sites of inflammation have emerged as essential immune molecules in the pathogenesis of IBD and CRC. Chemokines exert their biological functions via binding to their cognate G-protein-coupled receptors. Elevation of pro-inflammatory chemokines and a massive infiltration of leukocytes are all observed in the intestinal mucosa of IBD patients and strongly correlates with the grade of disease activity (Fegn and Wang, 2009). Moreover, the levels of these pro-inflammatory chemokines are also higher in human sporadic colorectal carcinomas than in matched normal tissues (Fegn and Wang, 2009). However, it remains unclear how these chemokines and their receptors contribute to IBD and colitis-associated carcinogenesis.
Cancer initiation and progression also depends on escape from host immunosurveillance. Similar to other solid tumors, CRC immune evasion involves a shift of immune responses, including imbalance in Th1/Th2 responses and enhancement of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells. The number of MDSCs in the blood correlates well with clinical cancer stage and metastatic tumor burden in patients, including those with CRC (Diaz-Montero et al., 2009; Mandruzzato et al., 2009). It is widely accepted that MDSCs contribute to cancer immune evasion via suppressing functions of T and natural killer (NK) cells (Gabrilovich and Nagaraj, 2009). However, it remains unclear how MDSCs are recruited from the circulatory system to the colonic mucosa during chronic inflammation and carcinogenesis and precisely how local MDSCs contribute to CRC progression.
Our previous work showed that PGE2 directly stimulated CRC cells to produce and secrete CXCL1, which in turn resulted in induction of tumor-associated angiogenesis (Wang et al., 2006b). CXCL1 is one of the ligands that binds to the chemokine receptor CXCR2, which was originally found to be expressed on neutrophils and is crucial for the recruitment of neutrophils to sites of inflammation (Oppenheim et al., 1991). The serum level of CXCL1 is significantly elevated in the IBD patients (Alzoghaibi et al., 2008). Importantly, CXCL1 levels also correlate well with the grade of disease and are reduced after initiation of therapy (Mitsuyama et al., 2006). Similarly, our group and others showed that CXCL1 and its receptor CXCR2 are elevated in human sporadic CRC (Rubie et al., 2008; Wang et al., 2006b; Wen et al., 2006). However, the mechanisms underlying the contribution of this signaling pathway to inflammatory disease and CRC remain elusive.
RESULTS
Deletion of Cxcr2 attenuates AOM/DSS-induced colonic chronic inflammation and colitis-associated tumorigenesis
To investigate the role of CXCR2 in colitis-associated tumorigenesis, wild type (WT) or Cxcr2−/− mice were treated with AOM and DSS as indicated in Figure 1A. AOM/DSS treatment induced inflammation in the large intestine of WT mice, resulting in clear clinical signs such as bloody stools (data not shown) and shortening of colon length (Figure 1B) but did not significantly affect mouse body weight during DSS treatment (data not shown). In contrast, deletion of Cxcr2 significantly attenuated the presence of inflammation-induced bloody stools (p=0.041) and shortening of the colon (Figure 1B). Moreover, repeated administration of DSS induced chronic inflammation in WT mice but not in Cxcr2−/− mice (Figure 1C–D). Water-treated WT and Cxcr2-deficient mice showed no clinical and histologic signs of colonic chronic inflammation (data not shown). In addition, loss of Cxcr2 dramatically reduced tumor burden by inhibition of tumor cell proliferation (Figure 1E–F). The severity of chronic inflammation directly correlated with tumor multiplicity (data not shown). Histological analysis revealed that 21% of tumors were adenocarcinomas in AOM/DSS-treated WT mice, whereas only 4.6% were adenocarcinomas in AOM/DSS-treated Cxcr2-deficent mice (Figure 1G–H), suggesting that the presence of CXCR2 accelerates tumor progression. However, we did not observe sub-mucosal invasion of tumor cells. These results demonstrate that CXCR2 is required for promoting chronic inflammation, tumor formation, growth, and progression in the large intestine.
Figure 1. Deletion of Cxcr2 attenuates AOM/DSS-induced colonic chronic inflammation and colitis-associated tumor formation, growth, and progression.
(A) Schematic of mice treated with AOM and DSS (A/D). (B) The colon lengths were measured at the end of experiments. (C) Representative of H&E-stained sections from WT (top panel) and Cxcr2 null mice (bottom panel) (scale bar = 100 μm). (D) Blinded histological scoring of inflammation in colonic mucosa of mice was performed as described in Experimental Procedures. (E) Tumor number and size were measured under a dissecting microscope. (F) Left panel represents immunoreactive staining (brown) for Ki67 (scale bar = 50 μm) and the right panel represents the average numbers of Ki67+ cells in 4 fields of each slides from 8 mice for each group. (G) Representative of H&E-stained sections of colonic tissue with tumors from WT and Cxcr2 null mice are shown (scale bar = 100 μm). (H) Blinded histological scoring of average percentage of adenocarcinomas in total tumors from WT and Cxcr2-deficient mice. Data are represented as mean ± SEM (8 mice for each group). Asterisks represent statistical differences (* p<0.05, ** p<0.01).
Loss of CXCR2 diminishes DSS- or AOM/DSS-induced a massive infiltration of granulocytic MDSCs from the circulatory system to colonic inflamed mucosa and tumors
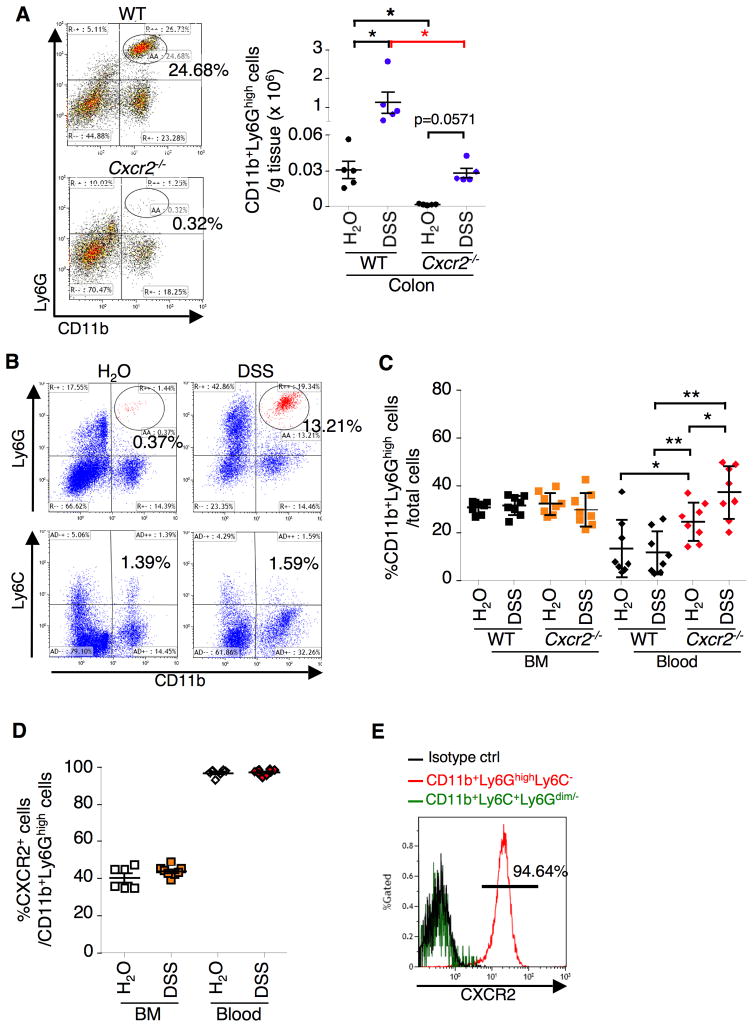
To examine whether CXCR2 is involved in immune cell infiltration, we first analyzed immunocyte profiles in the colonic mucosa of mice treated with DSS. We used cell surface markers as described in recent publications (Daley et al., 2008; Gabrilovich et al., 2012) to define mouse dendritic cells (DCs), macrophages, MDSCs, and neutrophils. Deletion of Cxcr2 did not affect repeated DSS-induced infiltration of dendritic cells (CD11c+Ly6G−F4/80−), T cells (CD3+), NK cells (CD3−CD49b+), and NKT cells (CD3+CD49b+) into the colonic mucosa but resulted in a trend toward reduction of infiltration of macrophages (CD11b+F4/80+CD11c−Ly6G−) and neutrophils (Ly6G+CD11c−F4/80−) during the chronic phase (Figure S1A), although alteration of CXCR2 clearly affected infiltration of neutrophils in the acute phase (Figure S1B). In contrast, loss of CXCR2 dramatically suppressed a massive infiltration of CD11b+Ly6GhighCD11c−F4/80− cells into the colon in both normal (water as control) and chronic inflammatory (DSS treatment) conditions (Figure 2A) and into the colon of DSS-treated with mice (Figure S1A). In mice, the immature myeloid cells, often called MDSCs, are broadly defined as CD11b+Gr-1+ cells that are further divided into 2 subsets, granulocytic (CD11b+Ly6Ghigh) and monocytic (CD11b+Ly6C+) MDSCs (Youn et al., 2008). In most experimental tumor models, granulocytic MDSCs (G-MDSCs) are more markedly expanded than monocytic MDSCs (Youn et al., 2008). Similarly, over 90% of MDSCs expressed both CD11b and Ly6G markers but not Ly6C, whereas less than 10% of MDSCs expressed both CD11b and Ly6C but not Ly6G in DSS-treated mice (Figure 2B). Furthermore, we found that the status of CXCR2 did not affect the G-MDSC populations in the bone marrow in both normal and chronic inflammatory conditions (Figure 2C). Importantly, Cxcr2−/− mice exhibited more accumulation of the G-MDSCs in the circulatory system than WT mice under both normal and chronic inflammatory conditions (Figure 2C), suggesting that Cxcr2-deficient G-MDSCs have lost their ability to migrate to local organs undergoing an inflammatory challenge. Almost all circulatory G-MDSCs expressed CXCR2 on their cell surface, whereas 40% of bone marrow G-MDSCs expressed CXCR2 (Figure 2D). Surprisingly, circulating monocytic MDSCs did not express CXCR2 on their cell surface (Figure 2E). As expected, circulating CD8+ T cells, NKs, NKT cells as well as colonic fibroblasts did not express CXCR2 on their cell surface (Figure S1C–D). However, 67% of neutrophils, 9.5% of monocytes, and 21% of DCs expressed CXCR2 in circulatory system (Figure S1C). Similarly to the circulatory system, more G-MDSCs accumulated in spleen of Cxcr2−/− mice than WT mice in both normal and chronic inflammatory conditions (Figure S1E). Since treatment of DSS did not induce inflammation in liver and lung, DSS treatment did not affect infiltration of MDSCs into those organs (Figure S1E).
Figure 2. Loss of CXCR2 inhibits DSS-induced a massive infiltration of MDSCs from circulatory system to colon.
The indicated genotypic mice aged 8 weeks were treated with 4 cycles of 1.25% DSS and the cells isolated from indicated organs were subjected to Flow Cytometry analysis. Viable granulocytes/monocytes or total cells were gated in a FSC/SSC plot. (A) The subpopulation of G-MDSCs in colonic mucosa was represented as percentage of gated granulocytes/monocytes cells (left panel) or as the numbers of G-MDSCs per gram of each mouse colon tissue (right panel). Each dot in the right panel represents the numbers of G-MDSCs in colonic mucosa taken from one mouse. (B) The profiles of G-MDSCs and monocytic MDSCs in colonic mucosa of water- or DSS-treated WT mice as mentioned above. (C) Data represents the percentage of G-MDSC in total viable cells from bone marrow (BM) and peripheral blood taken from mice. (D) Data represents the percentage of CXCR2+ MDSCs in total G-MDSCs from BM and blood in WT mice. (E) The percentage of CXCR2+ G-MDSCs and monocytic MDSCs in total G-MDSCs and monocytic MDSCs. The error bar indicates ± SEM. *p<0.05, ** p<0.01. See also Figure S1.
As expected, there was no significant difference of immune cell profiles between mice treated with DSS alone and AOM plus DSS (Figures S1A and S2A). Similar to DSS-treated mice, more G-MDSCs accumulated in the circulatory system of AOM/DSS-treated Cxcr2−/− than WT mice (Figure 3A). However, viability of circulating Cxcr2-deficent MDSCs was lower than WT MDSCs (Figure S2B–C). More intriguingly, a higher G-MDSC accumulation was observed in tumors than matched inflammatory mucosa in WT mice treated with AOM/DSS (Figure 3B). In contrast, loss of CXCR2 significantly reduced recruitment of G-MDSCs into both tumors and the matched inflamed mucosa (Figure 3B). Similar to DSS treatment, deletion of Cxcr2 also resulted in an accumulation of G-MDSCs in the spleen in both water- and AOM/DSS-treated mice (Figure S2D). However, the size and weight of the spleen of Cxcr2−/− mice was bigger and heavier than that of WT mice under both normal and chronic inflammatory conditions (Figure S2E). This could explain why the spleens of Cxcr2−/− mice have higher percentage of G-MDSCs than WT mice. Taken together, these results suggest that CXCR2 is required for homing of G-MDSCs from the circulatory system to inflamed colonic mucosa and tumors.
Figure 3. Loss of CXCR2 inhibits AOM/DSS-induced a massive infiltration of G-MDSCs from circulatory system to colonic mucosa and tumors.
(A–B) G-MDSC percentage in bone marrow and peripheral blood (panel A) and G-MDSC numbers in tumors (T) and adjacent mucosa (N) (panel B) of mice treated with AOM/DSS were analyzed by Flow Cytometry as described Figure 1A. Each dot represents the percentage of G-MDSCs in BM and peripheral blood collected from one mouse (left panel) and the numbers of G-MDSCs in colonic tumors and matched mucosa taken from one mouse (right panel). The error bar indicates SEM. See also Figure S2.
CXCR2 mediates ligand-induced G-MDSC chemotaxis
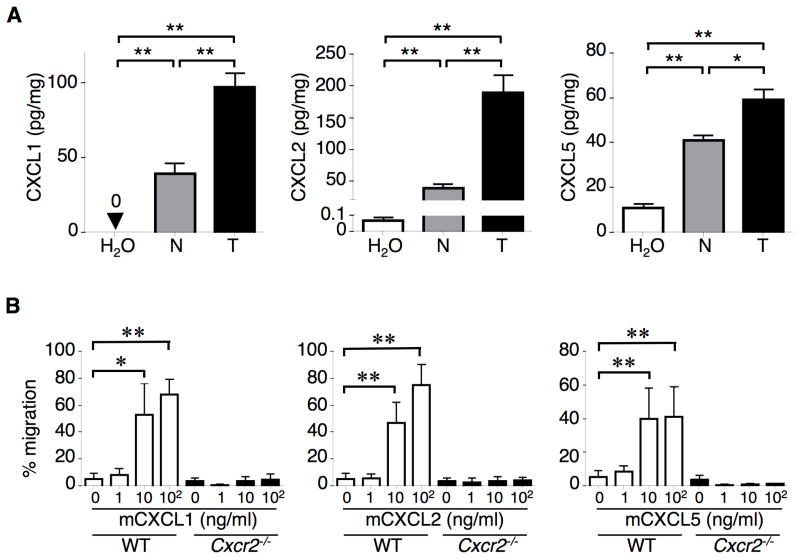
Based on our above results showing that treatment of AOM/DSS induced a massive infiltration of G-MDSCs into inflamed colonic mucosa and colitis-associated tumors in WT mice, we postulated that the levels of CXCR2 ligands, which are responsible for recruitment of CXCR2-expressing immune cells, would be elevated in colonic inflamed mucosa and tumors. Indeed, treatment of WT mice with AOM/DSS resulted in elevation of CXCL1, CXCL2, and CXCL5 in colonic tumors and adjacent inflamed mucosa as compared to the normal mucosa taken from water-treated mice (Figure 4A). Levels of these ligands are higher in tumor tissues than in adjacent inflamed mucosa (Figure 4A), which correlates with density of G-MDSCs in these tissues (Figure 3B). The results from in situ hybridization experiments revealed that Cxcl1 and Cxcl2 were mainly expressed in tumor colonic epithelial cells (Figure S3A). We then determined whether CXCR2 ligands induced MDSC chemotaxis. As shown in Fig. 4B, CXCL1, CXCL2, and CXCL5 all attracted WT MDSCs isolated from blood of WT mice but not Cxcr2-deficent MDSCs, demonstrating that CXCR2 is required for ligand-induction of MDSC chemotaxis. Since myeloid cells are a major source of IL-6 and the IL-6/Stat3 pathway plays a key role in immunosuppressive function of MDSCs (Gabrilovich and Nagaraj, 2009; Grivennikov et al., 2009), we examined IL-6 levels in the colon and blood of AOM/DSS-treated WT and Cxcr2 null mice. As expected, the pattern of IL-6 levels in colonic mucosa and tumors as well as in circulatory system (Figure S3B) is similar to that of MDSC levels in these organs (Figure 3). p-Stat3 immunostaining was observed in colonic stromal cells in WT mice treated with AOM/DSS but not in Cxcr2−/− mice (Figure S3C). These results indicate that infiltrated MDSCs in the colon have immunosuppressive functions. Collectively, our results demonstrate that CXCL1/2/5-CXCR2 signaling is responsible for recruitment of G-MDSCs into colonic inflammatory mucosa and tumors.
Figure 4. CXCR2 ligands are elevated in colitis-associated tumor and matched inflamed mucosa.
(A) CXCL1, CXCL2, and CXCL5 protein levels were measured in tumors (T) and adjacent mucosa (N) taken from mice treated with AOM/DSS as well as in normal colon tissues taken from mice fed with water only as described in Figure 1A. (B) CXCL1, CXCL2, and CXCL5 induced chemotaxis in WT MDSCs but not in Cxcr2−/− MDSCs in vitro. WT and Cxcr2−/− MDSCs were isolated from blood of AOM/DSS-treated WT and Cxcr2−/− mice, respectively. Values are reported as the mean ± SEM (6 mice for each group). *p<0.05; **p<0.01. See also Figure S3.
An inflammatory mediator, PGE2, upregulates CXCR2 ligands in colonic mucosa and tumors
Although our previous study demonstrated that PGE2 directly induced CXCL1 expression in human colorectal carcinoma cell lines and in a xenograft model (Wang et al., 2006b), it is uncertain whether this inflammatory mediator regulates CXCR2 ligands in the DSS and AOM/DSS models as well as ApcMin/+ mice. The ApcMin/+ mouse carried a point mutation at one allele of the Apc gene is used as a model for patients with familial adenomatous polyposis and a pre-malignant model for human sporadic CRC. As shown in Figures 5, PGE2 significantly induced CXCL1 and CXCL2 expression in the colon of DSS-treated mice (panel A) and in colonic tumors and adjacent mucosa of AOM/DSS-treated mice (panel B) but failed to induce CXCL5 expression. Similarly, PGE2 enhanced only CXCL1 and CXCL2 expression in colonic mucosa and tumors (Figure S4A) and all three ligands in small intestinal mucosa and tumors in ApcMin/+ mice (Figure S4B–C). Interestingly, the levels of these ligands were also elevated in intestinal tumors as compared to matched mucosa regardless PGE2 treatment (Figures 5B and S4A–C). In contrast, treatment of ApcMin/+ mice with a selective COX-2 inhibitor, celecoxib, completely suppressed CXCL1 and CXCL2 expression in intestinal mucosa and tumors (Figure S4D). These results demonstrate that an inflammatory mediator such as PGE2 is able to induce CXCR2 ligand expression in intestinal mucosa and tumors. High levels of CXCR2 ligands attract CXCR2-expressing MDSCs into colonic mucosa and tumors.
Figure 5. PGE2 treatment increased expression of CXCL1 and CXCL2 in colonic mucosa and tumors.
Eight weeks old male BALBc mice were treated with either 2 cycles of DSS alone (panel A) or AOM plus 3 cycles of DSS (panel B) with PGE2 or vehicle as described in Experimental Procedures. The protein levels of CXCL1, CXCL2 and CXCL5 in colon (panel A) and colonic tumor (T) and matched mucosa (N) (panel B) were determined by ELISA. Data are represented as mean ± SEM (5 mice for each group). *p<0.05; **p<0.01. See also Figure S4.
Adoptive transfer of WT MDSCs restores tumorigenesis in Cxcr2−/− mice
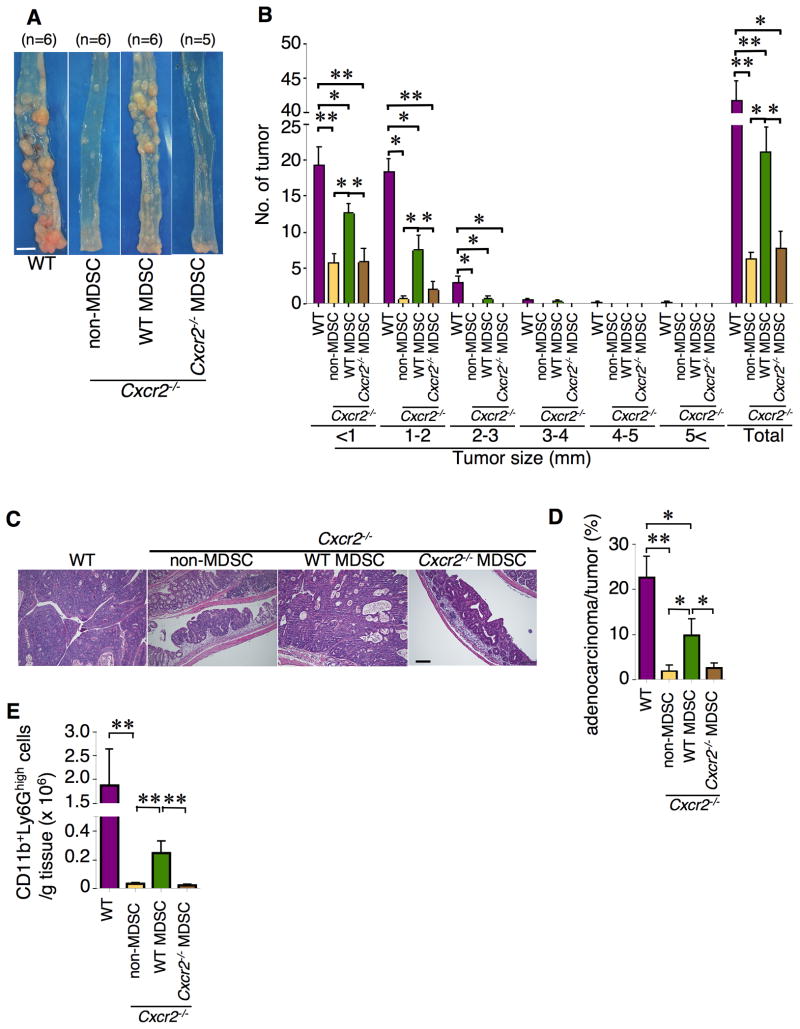
To determine whether lack of MDSC infiltration in colonic tissue directly results in reduction of colitis-associated tumor burden in Cxcr2−/− mice, adoptive transfer experiments were performed. To first examine whether the adoptive transfer of G-MDSCs works, G-MDSCs isolated from WT mice were labeled with a fluorescent dye, XenoLight DiR, and then were injected into Cxcr2 null mice. After 2 days, 73% of colonic G-MDSCs cells were detected as DiR positive, demonstrating that these DiR+ G-MDSCs cells are WT MDSCs (Figure S5A). Seventy-one percent of WT MDSCs were viable (Figure S5B). As shown in Figure 6A–C, transfer of G-MDSCs isolated from WT mice treated with AOM/DSS to Cxcr2 null mice restored the ability of AOM/DSS to induce tumor formation and growth in Cxcr2-deficent mice. Histological analysis revealed that only 2% of the tumors were adenocarcinomas in Cxcr2-deficent mice injected with non-MDSC immune cells or Cxcr2-deficient MDSCs. In contrast, 10% of the tumors were adenocarcinomas in Cxcr2-deficent mice injected with MDSCs, suggesting that MDSCs promotes tumor progression (Figure 6D). An increased accumulation of MDSCs into colonic mucosa in mice injected with MDSCs was observed as compared to mice injected with non-MDSC immune cells and Cxcr2-deficient MDSCs (Figure 6E). Moreover, immunofluorescence analysis confirmed an increased accumulation of MDSCs into colonic mucosa and tumors in the MDSC recipients (Figure S5C–D). These results demonstrate that CXCR2-expressing MDSCs contribute to colitis-associated tumor formation, growth, and progression.
Figure 6. Transfer of WT MDSCs to Cxcr2-deficent mice restores AOM/DSS-induced colitis-associated tumorigenesis.
WT G-MDSCs, Ly6G− immune cells (non-MDSC), or Cxcr2−/− G-MDSCs were intravenously (i.v.) injected into Cxcr2−/− mice as described in Experimental Procedures. The mice were treated with AOM/DSS as described in Experimental Procedures. (A) Gross view of colonic tumor of AOM/DSS-treated WT and Cxcr2−/− mice with non-MDSC immune cells, G-MDSCs, or Cxcr2-deficient G-MDSCs (Scale bar=0.5 cm). (B) Tumor number was counted based on the size. The data were represented as mean ± SEM of average of the tumor number in each size group and all groups. (C) Representative of H&E-stained sections of tumors from each group (scale bar = 100 μm). (D) Blinded histological scoring of average percentage of adenocarcinomas from WT and Cxcr2−/− mice with non-MDSC immune cells, WT MDSCs or Cxcr2−/− MDSCs. (E) The numbers of colonic MDSCs in WT mice or Cxcr2−/− mice treated with AOM and 2 cycles of DSS after transfer injection of indicated cells. Data are represented as mean ± SEM. *p<0.05; **p<0.01. See also Figure S5.
MDSCs suppress cytotoxic activity of colonic CD8+ T cells
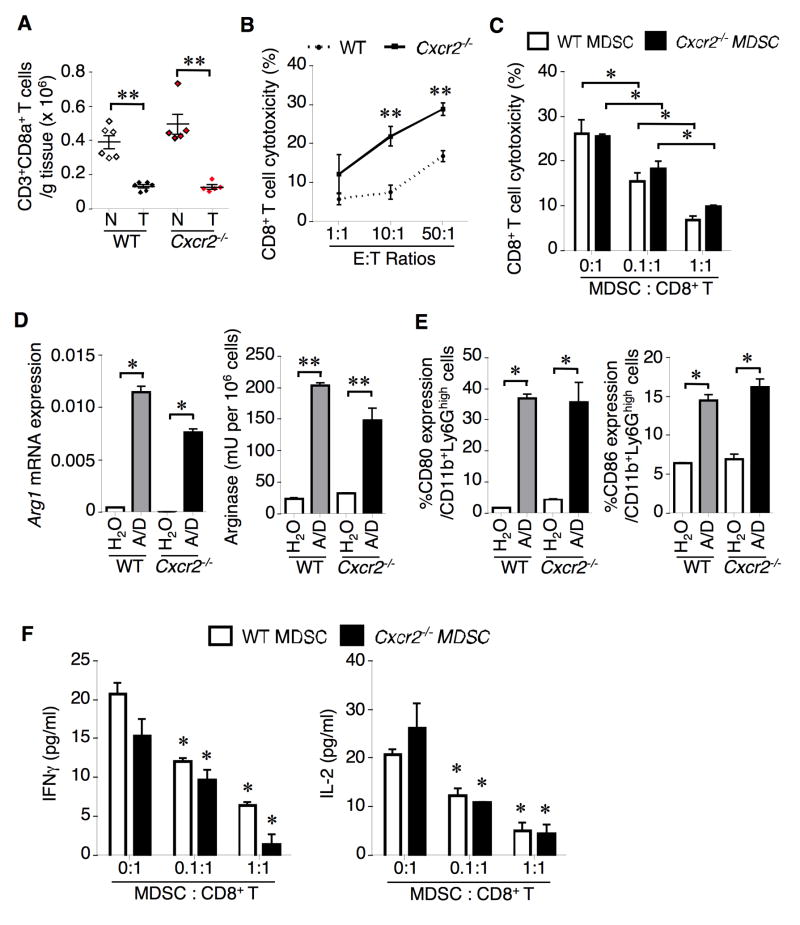
We further examined whether reduction of MDSCs in the colon of Cxcr2−/− mice resulted in induction of cytotoxic T cell number and cytotoxicity against tumor cells in our AOM/DSS model. As shown in Fig. 7A, a great reduction of the number of CD3+CD8+ T cells seen in tumors was observed when compared to adjacent mucosa in both WT and Cxcr2−/− mice. Although the status of CXCR2 did not affect the number of cytotoxic T cells in tumors, deletion of Cxcr2 led to an increase of cytotoxic T cell number in colonic mucosa but this change did not achieve statistical significance. In contrast, colonic CD8+ T cells isolated from Cxcr2−/− mice have higher cytotoxicity against epithelial tumor cells isolated from AOM/DSS-treated WT mice than colonic CD8+ T cells isolated from WT mice (Figure 7B). Importantly, CD8+ T cells isolated from tumors taken from Cxcr2−/− mice expressed high levels of CD107a, an activated CD8+ T cell marker, and produced more INFγ, Prf1, and Gzmb when compared to tumor-associated CD8+ T cells from WT mice (Figure S6A–B), indicating that activated CD8+ T cells might kill tumor cells via induction of INFγ, Prf1, and Gzmb. Moreover, colonic CD8+ T cell cytotoxicity against tumor cells was suppressed by adding circulating G-MDSCs taken from WT or Cxcr2−/− mice (Figure 7C). The MDSC inhibition of CD8+ T cell cytotoxicity was ratio-dependent. Treatment of AOM/DSS significantly induced arginase 1 expression and activity as well as other suppressor markers such as CD80 and CD86 in both colonic WT and Cxcr2−/− G-MDSCs (Figures 7D–E) and inhibited the secretion of INFγ and IL-2 from colonic CD8+ T cells in vitro (Figure 7F). Although there were 27 downregulated genes in colonic Cxcr2-deficient MDSCs as compared to WT MDSCs (Table S1), none of these genes (except Cd80) are known to be involved in MDSC immunosuppressive functions. These results demonstrate that colonic MDSCs inhibit the ability of colonic CD8+ T cells to kill tumor cells and their immunosuppressive functions are CXCR2-independent. In addition, loss of CXCR2 increased the population of Th1 (CD4+IFNγ+) cells and significantly decreased the population of Th17 (CD4+IL-17A+) cells in tumors, suggesting that CXCR2 signaling contributes to the imbalance in Th1/Th2 responses and promotes tumor-associated inflammation via enhancing Th17 cell number (Figure S6C).
Figure 7. Loss of CXCR2 does not affect CD3+CD8+ T cell number but enhances CD8+ T cell cytotoxicity against tumor cells.
(A) CD3+CD8+ cell number in tumors (T) and adjacent mucosa (N) taken from WT and Cxcr2−/− mice were determined by flow cytometry. (B) Cytotoxicity of colonic CD8+ T cells isolated from WT and Cxcr2−/− mice (E) against tumor cells (T) isolated from tumors of AOM/DSS-treated WT mice was determined as described in Experimental Procedures. (C) CD8+ T cells isolated from WT and Cxcr2−/− mice, tumor cells isolated from tumors of AOM/DSS-treated WT mice, and MDSCs isolated from blood of either WT or Cxcr2−/− mice were cultured. The ratio of CD8+ T cells and tumor cells is 50:1. (D) The levels of arginase 1 expression (left panel) and arginase activity (right panel) in colonic MDSCs isolated from indicated mice treated with water or AOM/DSS (A/D). (E) The percentage of CD80+ or CD86+ MDSCs in colonic MDSCs from indicated mice. (F) Levels of IFNγ and IL-2 secreted from WT CD8+ T cells cocultured with tumor cells (E:T=50:1) in the presence of different ratios of MDSCs from indicated mice. Data are represented as mean ± SEM (6 mice for each group). *p<0.05; **p<0.01. See also Figure S6 and Table S1.
DISCUSSION
The recognition that chronic inflammation caused by infections or autoimmune diseases as a trait of cancer has highlighted the contribution of inflamed stroma to tumor initiation, growth, progression, and metastasis. Our findings not only reveal how MDSCs are recruited to local inflamed tissues and the tumor microenvironment and how local MDSCs contribute to CRC progression, but also provide a rationale for developing therapeutic approaches to subvert chronic inflammation- and tumor-induced immunosuppression by using CXCR2 antagonists and neutralizing antibodies.
A growing body of evidence supports the important role of chemokines and their receptors in colonic inflammation and CRC (Wang et al., 2009). The levels of pro-inflammatory chemokines are positively correlated with the inflammatory state in IBD patients (Wang et al., 2009). Genetic and pharmacologic studies provide evidence showing that the activation of pro-inflammatory CCL2, CCL3, or CCL4 signaling promotes inflammation in mouse models where injurious agents are used to induce experimental colitis (Andres et al., 2000; Khan et al., 2006; Tokuyama et al., 2005). However, there are a few genetic and pharmacologic studies showing the direct evidence that CXC and CC chemokines contribute to colitis-associated cancer in mouse models. For example, genetic and pharmacologic evidence indicates that CCL2 promotes colitis-associated tumorigenesis via recruitment of macrophages (Popivanova et al., 2009). Moreover, deletion of an atypical chemokine receptor D6, a decoy and scavenger receptor, increased susceptibility to DSS-induced colitis and AOM/DSS-induced colitis-associated tumorigenesis accompanied with an increased production of chemokines and inflammatory cell recruitment via lymphatic endothelial cells (Vetrano et al., 2010). Our studies reveal that CXCR2 promotes colonic chronic inflammation and colitis-associated tumorigenesis via recruitment of G-MDSCs in the models we have tested. It has been well established that CXCR2 mediates neutrophil migration and angiogenesis after ligand binding. In humans, CXCR2 ligands include CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. Among these ligands, CXCL6 and CXCL8 also activate another receptor, CXCR1. In mice, only CXCL1, CXCL2/3, CXCL5, and CXCL7 can bind to CXCR2. These ligands and their receptor, CXCR2, have been shown to play an important role not only in activation and recruitment of neutrophils, but also in inducing exaggerated angiogenesis at sites of inflammation and tumors. For example, CXCR2 plays a key role in the recruitment of tumor-associated neutrophils, which promote tumor growth via enhancing angiogenesis (Raccosta et al., 2013). The levels of CXCR2 ligands correlate with the inflammatory state in IBD patients and are elevated sporadic CRC in humans (Fegn and Wang, 2009), indicating that the CXCR2 ligands and its receptor, CXCR2, may play a role in IBD and CRC. However, the mechanisms underlying the contribution of the CXCR2 signaling to CRC formation and progression remain unclear. Our previous study showed that CXCR2 is elevated mainly in endothelial and immune cells of human colorectal carcinomas (Wang et al., 2006b), suggesting that the CXCR2 signaling may promote tumorigenesis by influencing the biological function of stromal compartments, including immune cells, endothelial cells, or other cells. For example, Moses’ group recently reported that inhibition of CXCR2 attenuated the fibroblasts-enhanced pancreatic ductal adenocarcinoma growth in a xenograft model (Ijichi et al., 2011), suggesting that fibroblast CXCR2 contributes to tumor growth. However, we did not observe CXCR2 expression on colonic tumor-associated fibroblasts. Recently, Jamieson et al. reported that CXCR2 is required for colonic chronic inflammation and colitis-associated tumorigenesis, which is consistent with our results (Jamieson et al., 2012). We extended the scope of our research to reveal that CXCR2 is required for homing of MDSCs from the circulatory system to the colonic mucosa in both normal and chronic inflammatory conditions as well as colitis-associated tumors.
MDSCs are not only greatly expanded in tumor-bearing mice and cancer patients, but also in autoimmunity and during inflammation, including IBD (Haile et al., 2008). However, the molecular mechanism(s) underlying recruitment of MDSCs into the tumor microenvironment and inflammatory sites remain unclear. In tumor implantation models, CXCL1/2 has recently been shown to mediate mammary tumor growth and lung metastasis and CXCL1/2 knockdown in a breast cancer cell line is associated with reduction of myeloid cells (Acharyya et al., 2012). However, inhibition of CXCR2 by its antagonist does not suppress mouse mammary adenocarcinoma growth, despite partially reducing CD11b+Gr1+ cell abundance in the tumor in tumor implantation models (Yang et al., 2008). Moreover, a recent report showed that CXCL8 (IL-8)-overexpressing transgenic mice exhibited more infiltration of immature myeloid cells into colonic mucosa following DSS treatment (Asfaha et al., 2013). Since CXCL8 binds to both CXCR1 and CXCR2 receptors, it is not clear which receptor mediates the effect of CXCL8 on promoting the recruitment of immature myeloid cells. Although these studies have brought up the possibility that CXCR2 ligands are involved in recruitment of myeloid cells, the role of CXCR2 in MDSCs remains ambiguous. It has been well established that inflammatory mediators such as cytokines induce pro-inflammatory or angiogenic chemokines. Since the inflammatory COX-2-PGE2 pathway plays a key role in inflammation and cancer, our results indicate that this bioactive lipid may promote homing of MDSCs into colon via the CXCR2 ligand-CXCR2 signaling. Further studies are required to test this hypothesis. Collectively, these results explain how CXCR2-expressing MDSCs migrate to inflamed colonic mucosa and colitis-associated tumors.
Although evidence for MDSC promotion of immunosuppression is accumulating, it is still unknown whether MDSCs play a key role in colitis-associated carcinogenesis. Our results from the transfer experiments provide direct evidence demonstrating that MDSCs contribute to colitis-associated tumorgenesis. In addition to CXCR2’s functions for leukocyte recruitment and as a pro-angiogenic factor for angiogenesis, CXCR2 signaling also promotes cellular proliferation, survival, and migration of melanoma, prostate cancer, and esophageal cancer cells (Luan et al., 1997; Maxwell et al., 2007; Wang et al., 2006a). It is of great interest to determine the biological functions of CXCR2 in human CRC cells.
It is widely accepted that MDSCs have suppressive effects on both the innate immune response via NK cells and the adaptive immune response via T cells by direct cell-cell contact. In our mouse model of colitis-associated carcinogenesis, MDSCs do promote tumor growth via inhibition of CD8+ T cell function. There are several proposed mechanisms to explain how MDSCs suppress T cells (Gabrilovich and Nagaraj, 2009). One proposed mechanism indicates that MDSCs inhibit T cell proliferation by reducing L-arginine (Gabrilovich and Nagaraj, 2009). However, we did not observe a significant impact of Cxcr2 deletion on CD8+ T cell populations in both colonic inflamed mucosa and colitis-associated tumors in our mouse model. In contrast, we did find that local colonic MDSCs inhibited cytotoxic activity of colonic CD8+ T cells against tumor cells. Since arginase 1 is required for immunosuppressive effects of MDSCs on CD8+ T cell cytotoxicity (Grivennikov et al., 2009), our results show that colonic MDSCs express arginase 1 that is active and inhibit the secretion of INFγ and IL-2 from colonic CD8+ T cells, suggesting that MDSCs inhibit tumor formation, growth, and progression via induction of arginase 1, CD80, and CD86. Further studies are needed to test our hypothesis. Interestingly, CXCR2 was not required for the immunosuppressive function of MDSC in inhibiting CD8+ T cell cytotoxic activity against tumor cells in our model. In addition, it is possible that NK cells may be involved in the contribution of MDSCs to tumor growth. Moreover, emerging evidence revealed that MDSCs inhibited the binding of a specific tumor-associated peptide to tumor cell-associated MHC, resulting in a resistance of tumor cells to antigen-specific cytotoxic T cells (Lu et al., 2011). Further research is required to determine the exact mechanism(s) by which MDSCs allow tumor cells to escape from immunosurveillance. Previous studies showed that IL-6 secreted from MDSCs is essential for attenuating differentiation of tumor-specific CD4+ T cells into Th1 cells (Tsukamoto et al., 2013) and expression of TNFR-2 (Fas family member) in MDSCs is necessary for their survival (Zhao et al., 2012). Our observation that loss of CXCR2 resulted in reduction of Il-6 and Fas mRNA levels in MDSCs may explain why loss of CXCR2 increased the population of Th1 and why Cxcr2-deficient MDSCs had a shorter half-life. Collectively, these results indicate that CXCR2 is essential for G-MDSC infiltration into colonic mucosa and tumors, which promotes tumor growth and progression via inhibition of CD8+ T cell cytotoxic activity.
Th17 cells have been found to play a key role in IBD and may alter the idea of this Th1 and Th2 dichotomy in IBD (Harrington et al., 2006; Steinman, 2007). For example, in vivo studies indicated that Th17 cells promote inflammation via induction of multiple inflammatory pathways (Monteleone et al., 2012). The elevation of genes (IL17A and RORC) associated with Th17 cells is correlated with a poor prognosis in human CRC patients (Tosolini et al., 2011). Interestingly, MDSCs have been shown to enhance the differentiation of naïve CD4+ T cells into Th17 cells under Th17-polarizing conditions in vitro (Yi et al., 2012). Our in vivo results show that MDSCs positively correlated with Th17 cells in tumors of AOM/DSS-treated mice. Future studies are needed to investigate whether MDSCs promotes Th17 cell differentiation in vivo.
In summary, CXCR2 is required for G-MDSC trafficking into colonic inflamed mucosa and the tumor microenvironment and is critical for colitis-associated tumor formation, growth, and progression. An inflammatory mediator, PGE2, induces CXCR2 ligand expression in colonic mucosa and tumors. These findings provide comprehensive insights into how MDSCs are recruited to local inflamed tissues and to the tumor microenvironment and how local MDSCs contribute to CRC progression. Moreover, our work sheds light on how the inflammatory microenvironment contributes to cancer immune evasion by allowing tumor cells to escape from immunosurveillance. Our results provide a rationale to develop CXCR2 antagonists and neutralizing antibodies as therapeutic approaches subverting tumor-induced immunosuppression.
Experimental Procedures
Reagents
All reagents are provided in Supplemental Experimental Procedures.
Animal models
All animal experiments conform to our animal protocols that were reviewed and approved by the Institutional Animal Care and Use Committee of the MD Anderson Cancer Center and the Arizona State University. Cxcr2−/− in BALBc genetic background and their littermate control (WT) mice and ApcMin/+ mice were obtained from the Jackson Laboratory. Information for animal experiments is presented in Supplemental Experimental Procedures.
Isolation of immunocytes from organs
Colonic immunocytes were isolated according to the previous report (Vezys et al., 2000). Details of isolation of colonic immune cells as well as immunocytes in other organs are provided in Supplemental Experimental Procedures. 1 × 106 of immunocytes isolated from colon, blood, bone marrow, spleen, liver, and lung were subjected to the flow cytometry analysis.
Flow cytometry analysis
For multicolor flow cytometry immunotypic analysis, cells were stained with indicated monoclonal antibodies and analyzed on a Gallios flow cytometer (Beckman Coulter) as previously described (Zeng et al., 2012). The flow cytometric profiles were analyzed by counting 20,000 events using Kaluza software program (Beckman Coulter). DAPI or PI was used to exclude the dead cells during analysis of immune cell profiles. Information on antibodies and description of experimental procedures are presented in Supplemental Experimental Procedures.
Quantitative PCR and Mouse TaqMan Immune Panel
RNA was extracted from colonic MDSCs or homogenized intestinal tumor and normal mucosa using an RNeasy Mini Kit (Qiagen) and reverse-transcribed with iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad). TaqMan real-time qPCR and qPCR TaqMan mouse immune arrays were performed on a ViiA7 (Applied Biosystems). Additional information on primers, TaqMan Array Mouse Immune Cards, and description of experimental procedures are provided in Supplemental Experimental Procedures.
ELISA
Information on extraction of total proteins from colon tissues and ELISA kits are provided in Supplemental Experimental Procedures.
Chemotaxis assay
The procedure describing the chemotaxis assay is presented in Supplemental Experimental Procedures.
Immunohistochemistry and immunofluorescence staining
Immunohistochemistry and immunofluorescence staining was performed according to previous reports (Katoh et al., 2010; Katoh et al., 2012). Detailed information on antibodies and description of experimental procedures are provided in Supplemental Experimental Procedures.
CTL assay
CD8+ T cells were isolated from colonic mucosa of AOM/DSS-treated mice as effector cells (E) by CD8+ T Cell Isolation Kit II (Miltenyi) according to the manufacturer’s instructions. MDSCs were isolated from blood of AOM/DSS-treated WT mice as mentioned above. Isolation and culture of target tumor cells (T) as well as the procedures of CTL assay are described in Supplemental Experimental Procedures.
Statistical analysis
Each in vitro experiment was done at least 3 times and each in vivo experiment was conducted at least twice. Data are presented as mean ± SEM. Comparisons among multiple groups were performed by factorial analysis of variance, followed by Bonferroni test. Comparisons between two groups were performed with Student’s t-test or Mann-Whitney U test where appropriate. Fischer’s exact test was used for categorical variables. p<0.05 was considered significant.
Supplementary Material
Significance.
Up until now, CXCR2 was only thought to mediate infiltration of neutrophils to inflammatory sites. Our results here show that CXCR2 is also required for homing of MDSCs into colonic mucosa and colitis-associated tumors, revealing a role of CXCR2 in the recruitment of MDSCs from circulatory system to local tissues and tumors. Importantly, our results from experiments of adoptive transfer of MDSCs provide direct evidence that MDSCs contribute to colonic tumor formation and growth. Moreover, we also found that colonic MDSCs inhibited colonic CD8+ T cell cytotoxicity against tumor cells. Our findings provide a rationale for development of therapeutic approaches to subvert chronic inflammation- and tumor-induced immunosuppression by using CXCR2 antagonists and neutralizing antibodies.
Highlights.
Loss of CXCR2 dramatically suppressed colitis-associated tumor progression.
CXCR2 is required for infiltration of MDSCs to colonic mucosa and tumor.
MDSCs contribute to chronic inflammation-induced colitis-associated tumorigenesis.
Colonic MDSCs inhibited cytotoxicity of colonic CD8+ T cells against tumor cells.
Acknowledgments
This work is supported, in part, by the NIH MERIT award R37 DK47297, RO1 DK 62112, NCI, P01 CA77839, and CPRIT RP100960. We thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous support (R.N.D.) and the Uehara Memorial Foundation Research Fellowship (H.K.). We thank Drs. Sun-Hee Kim, Yuchen Du, and Vijaykumar R. Holla for the instruction for experiment methods. We also thank Drs. Sandra S. Ojeda and Dr. Kimberly S. Schluns for providing the protocol of colonic immunocyte extraction. We are grateful to Nalini Patel, Wendy Schober, and Duncan Mak at Flow Cytometry Core Facility of MD Anderson Cancer Center for their assistance to conduct analysis of flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah Hajj Hussein I, Freund JN, Reimund JM, Shams A, Yamine M, Leone A, Jurjus AR. Enteropathogenic e.coli sustains iodoacetamide-induced ulcerative colitis-like colitis in rats: modulation of IL-1beta, IL-6, TNF-alpha, COX-2, and apoptosisi. Journal of biological regulators and homeostatic agents. 2012;26:515–526. [PubMed] [Google Scholar]
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Neutrophil chemokines GCP-2 and GRO-alpha in patients with inflammatory bowel disease. J Dig Dis. 2008;9:144–148. doi: 10.1111/j.1751-2980.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WS, Chae C. Expression of cyclooxygenase-2 and nitric oxide synthase 2 in swine ulcerative colitis caused by Salmonella typhimurium. Veterinary pathology. 2004;41:419–423. doi: 10.1354/vp.41-4-419. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. The New England journal of medicine. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunological reviews. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nature reviews Gastroenterology & hepatology. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- Fegn L, Wang Z. Topical chemoprevention of skin cancer in mice, using combined inhibitors of 5-lipoxygenase and cyclo-oxygenase-2. J Laryngol Otol. 2009:1–5. doi: 10.1017/S0022215109004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailon E, Cueto-Sola M, Utrilla P, Nieto A, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacological research: the official journal of the Italian Pharmacological Society. 2011a;63:308–319. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochemical pharmacology. 2011b;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. 881 e871–875. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, Kamata H, Mishima T, Tamaki H, Sakagami H, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol. 2010;176:1469–1483. doi: 10.2353/ajpath.2010.090607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Yamashita K, Waraya M, Margalit O, Ooki A, Tamaki H, Sakagami H, Kokubo K, Sidransky D, Watanabe M. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia. 2012;14:559–571. doi: 10.1593/neo.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Motomura Y, Wang H, El-Sharkawy RT, Verdu EF, Verma-Gandhu M, Rollins BJ, Collins SM. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–811. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World journal of gastroenterology: WJG. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986;91:837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Lee IA, Kim DH. Klebsiella pneumoniae increases the risk of inflammation and colitis in a murine model of intestinal bowel disease. Scandinavian journal of gastroenterology. 2011;46:684–693. doi: 10.3109/00365521.2011.560678. [DOI] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- Mitsuyama K, Tsuruta O, Tomiyasu N, Takaki K, Suzuki A, Masuda J, Yamasaki H, Toyonaga A, Sata M. Increased circulating concentrations of growth-related oncogene (GRO)-alpha in patients with inflammatory bowel disease. Dig Dis Sci. 2006;51:173–177. doi: 10.1007/s10620-006-3104-4. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012;12:592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubie C, Frick VO, Wagner M, Schuld J, Graber S, Brittner B, Bohle RM, Schilling MK. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M, et al. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama H, Ueha S, Kurachi M, Matsushima K, Moriyasu F, Blumberg RS, Kakimi K. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17:1023–1034. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Nishikata R, Senju S, Nishimura Y. Myeloid-Derived Suppressor Cells Attenuate TH1 Development through IL-6 Production to Promote Tumor Progression. Cancer Immunol Res. 2013;1:64–76. doi: 10.1158/2326-6066.CIR-13-0030. [DOI] [PubMed] [Google Scholar]
- Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, et al. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006a;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN, Richmond A. The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol. 2009;9:688–696. doi: 10.1016/j.coph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006b;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Giardina SF, Hamming D, Greenman J, Zachariah E, Bacolod MD, Liu H, Shia J, Amenta PS, Barany F, et al. GROalpha is highly expressed in adenocarcinoma of the colon and down-regulates fibulin-1. Clin Cancer Res. 2006;12:5951–5959. doi: 10.1158/1078-0432.CCR-06-0736. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100:195–199. doi: 10.1111/j.1349-7006.2008.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, Liu W, Jessen K, Liu Y, Kantarjian H, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120:2679–2689. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122:4094–4104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.