BACKGROUND
Photosensitivity (PS) is one of the most common manifestations of systemic lupus erythematosus (SLE)1, and is 1 of only 11 criteria used to make the diagnosis of SLE2. However, the definition of photosensitivity is vague and its pathophysiology is not well understood. There is a need to better define the clinical aspects of photosensitivity in lupus, to enhance further study of this difficult problem.
While investigations into PS in lupus erythematosus (LE) have focused predominantly on CLE induction via phototesting3–6, most LE patients do not undergo phototesting as part of their clinical work-up. More commonly, clinicians apply the PS criterion to LE patients based on patient history and/or physical exam findings related to sun-induced eruptions2. Making the diagnosis of PS in LE is simple when patients report a history of LE exacerbation in the summer or after a tropical holiday. Most patients, however, describe a wide array of adverse reactions to sunlight, some of which may be related to LE and others not7–9.
On the differential diagnosis for CLE is the most common of all photodermatoses – polymorphic light eruption (PMLE). Early lesions of CLE may be difficult to distinguish from PMLE10–13, both clinically and histologically. Furthermore, PMLE has been reported to occur more frequently in LE patients than in the general population14,15. Despite these associations, studies have failed to show any convincing pathophysiologic link between PMLE and LE16–19, which suggests that the two conditions are co-morbid. An alternative explanation is that photosensitivity in LE is variable and that a PMLE-like reaction may be one of many clinical phenotypes of PS in LE.
In our tertiary referral population, we found that 70% of CLE patients reported adverse reactions to sunlight20. Patients’ descriptions of photosensitivity varied from CLE induction after sun exposure to generalized rash to PMLE-like reactions. The purpose of this study was to characterize clinical photosensitivity phenotypes among a primarily cutaneous lupus population. A secondary objective was to examine skin histology among PS phenotypes in LE and evaluate whether differences in cell type/count play a role in the pathophysiology of various PS phenotypes.
METHODS
Subject selection
Patients with LE presenting to the outpatient autoimmune skin disease clinic at the University of Pennsylvania were enrolled in an ongoing database study of prevalence and severity of lupus erythematosus. All patients over 18 years of age with clinical, histological, and/or serological evidence of cutaneous lupus and/or systemic lupus erythematosus with skin manifestations were invited to participate. Subjects were categorized according to the modified Gilliam classification into the various subtypes of CLE21. Subjects with SLE who met the American Rheumatism Association (ACR) criteria2 were included if they also had a form of CLE. The protocol for the study was approved by the Institutional Review Board of the University of Pennsylvania’s School of Medicine.
Study procedures
Study visits were completed at the time of subjects’ regularly scheduled clinic visit. Information was obtained by clinical interview, physical examination, medical record review and subject questionnaires. A complete skin examination was performed and the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) outcome measure was completed. Whenever available, recent laboratory values, including lupus serologies and/or biopsy results, were reviewed and documented in the study chart.
Clinical interview using the photosensitivity survey
The photosensitivity survey provided a framework for characterizing subject’s experience of sun sensitivity or lack thereof (Table 1). The PS survey was based on information gathered over nine months, during which patients in the autoimmune skin disease clinic were interviewed about their experience with sunlight. Recurring themes of self-reported photosensitivity, relating to sun-induced reactions, morphology, characteristics and timing, were identified and incorporated into a brief PS survey.
Table 1.
Photosensitivity Survey
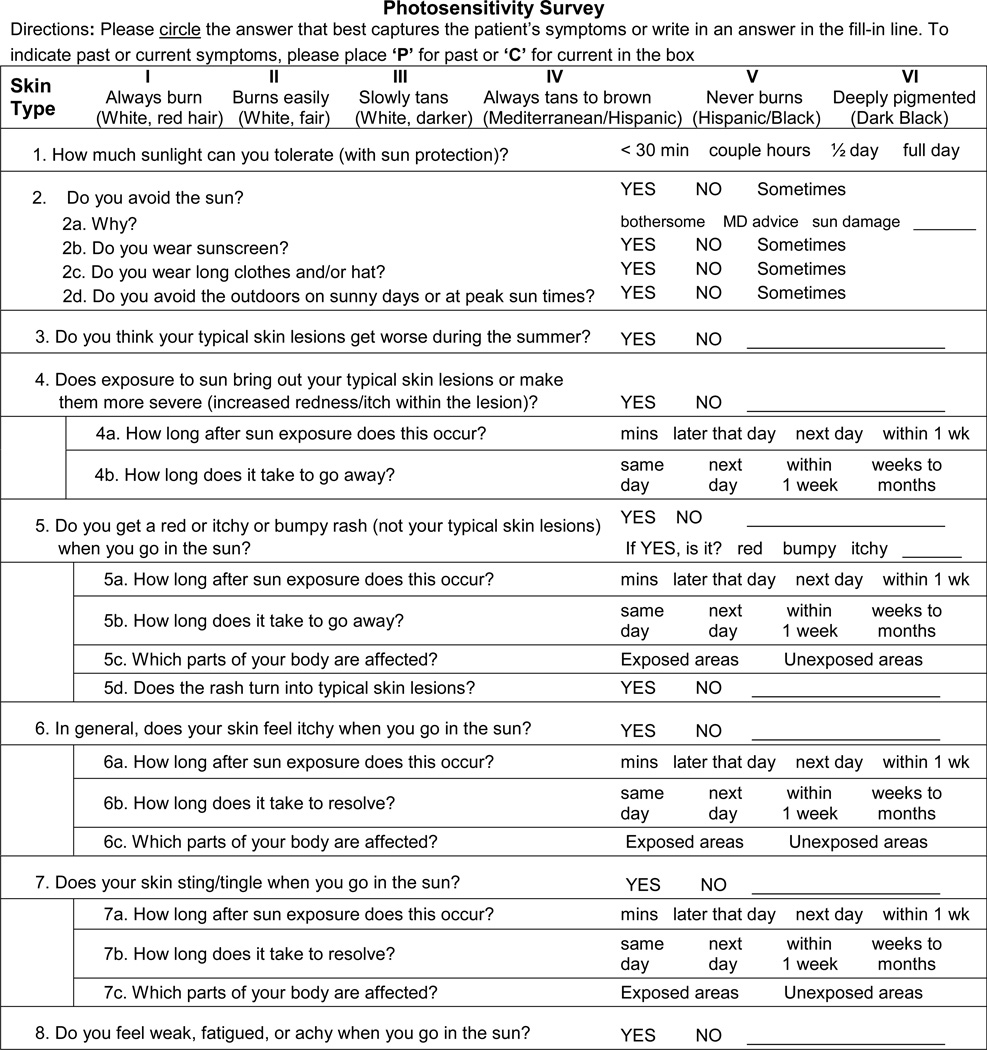 |
Subjects were instructed to: Tell me about what happens when you go in the sun. Study personnel completed the PS survey using the subject’s free-form answer. Only after the subject was allowed to speak freely did study personnel ask questions from the PS survey to limit information bias. Any adverse reaction to sunlight described by the subject was accounted for and classified into a photosensitivity phenotype. Data collection took place from November 2009 – January 2011.
Photosensitivity phenotypes
Subject reported adverse reactions to sunlight were classified into 1 of 5 categories based on answers to the survey (Table 2). In general, question 4 corresponded with directCLE, question 3 with genCLE, question 5 with genSkin, questions 6 and 7 with genRxn, and question 8 with genSys. If a subject’s report did not correspond with the answer options provided, the study personnel could write answers in the blanks provided. This occurred almost exclusively for the genCLE phenotype. Thus, subjects reporting “yes” to question 3 or those necessitating a write-in answer, suggestive of a link between CLE and sun exposure, were classified as the genCLE phenotype. Finally, the directCLE and genCLE phenotypes were mutually exclusive, but all other photosensitivity phenotypes were not and patients could be classified as multiple PS phenotype.
Table 2.
Clinical photosensitivity phenotypes
| Photosensitivity phenotypes |
Definition |
|---|---|
| Direct CLE reaction [directCLE] | A sun-induced reaction that is specific for cutaneous lupus lesions
|
| General association between CLE & Sun [genCLE] | An observation that CLE is worse in the summer months or that sun exposure is somehow related to CLE flares.
|
| General Skin Eruption [genSkin] | A PMLE-like eruption that is dissimilar to CLE skin disease
|
| Pruritus/Paresthesia [genRxn] | A generalized sensation of itching or stinging or burning of skin
|
| Systemic Symptoms [genSys] | Any sun-induced systemic symptom including not limited to: arthralgia, weakness, fatigue, headache |
Timing of PS reactions
Timing of 3 PS phenotypes was ascertained for: directCLE, genSkin, and genRxn. Subjects were asked about onset of reactions and time until resolution of cutaneous reactions after sun exposure. Reactions were labeled early, transient; early, lasting; or late, lasting.
| Onset | Time | Resolution | Time |
|---|---|---|---|
| Early | within minutes to next day | Transient | same day to within 1 week |
| Late | within 1 week | Lasting | weeks to months |
Systemic disease activity in lupus: SLEDAI and PGA
Disease activity in SLE was assessed by the Safety of estrogens in Lupus Erythematosus National Assessment SLE Disease Activity Index (SELENA-SLEDAI), a validated instrument used in the SELENA trials22. This SLEDAI, uses a weighting system to evaluate disease activity in 9 organ systems. The total SLEDAI score ranges from 0 (no activity) to 105 (maximum activity). Overall systemic disease activity is further assessed by the clinician via the Physicians Global Assessment (PGA) score, a 0–3 scale with 0 = none to 3 = severe systemic disease activity.
Cutaneous lupus erythematosus disease area and severity index (CLASI)
The CLASI is a validated tool to assess disease severity in cutaneous lupus erythematosus23–25. It quantifies disease activity (erythema, scale) and damage (dyspigmentation, scar) over 13 distinct areas of the body. Activity and damage scores range from 0–70 and 0–56 respectively, with higher scores representing more severe disease. Disease activity is classified into mild (0–9) and moderate-to-severe (≥ 10) by CLASI activity score.
Immunohistochemistry
Preliminary investigation into potential mediators of photosensitivity phenotype was undertaken by examining skin biopsies from 11 subjects and 5 controls (age-, and location-balanced). The goal of this exploratory observation study was to generate, rather than test hypotheses; so, power analysis to justify sample size is not presented. Four-mm punch biopsies were taken from sun-exposed, extensor, non-lesion, forearm skin of photosensitive lupus patients. The biopsies were formalin-fixed, paraffin-embedded, 4 um cut sections with three tissue sections placed on each slide.
After slide deparaffinization and hydration, antigen retrieval was performed in Target Retrieval Solution, High pH (S3308; Dako) for 30 min using a water bath. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 10 min and then protein-blocked was performed using serum-free protein blocking solution (Dako; X0909) for 1 hour. Tissue sections were incubated overnight at 4°C with either anti-CD3 mouse monoclonal antibody (1:50, Clone LN10; Novocastra, Newcastle-upon-Tyne, UK) or anti-CD11c rabbit monoclonal antibody (1:50, clone EP1347Y; ABCAM). Slides were then incubated at 25°C for 40 min with universal biotinylated linker secondary antibody (Dako; K0690) for CD3 or secondary antibody specific for rabbit primary (Dako; K4010) for CD11c. After, streptavidin-HRP from the Universal LSAB+ Visualization System (Dako) was applied to tissue sections for 30 min. Finally sections were developed with freshly prepared NovoRed (Vector) for 8 min for CD3 or with DAB chromogen (Dako) for 8 min for CD11c. Slides were counterstained with hematoxylin. 1% BSA in PBS was applied to 1 tissue section of each slide to serve as a negative control.
Cell quantification was performed for CD3+ (T-cells) and CD11c+ (myeloid DC) cells. For each specimen, 5 consecutive fixed fields in the papillary dermis and the reticular dermis were photographed using 20× objective and 10× eyepiece and Nikon microscopy camera. Cells were counted using ImageJ software (NIH, Bethesda, MD). The mean number of cells per high power field (hpf) averaged across 10 hpf (200×) was used for analyses.
Statistical Analysis
For data that were assumed normally distributed, frequencies and means ± standard deviations were reported. Pearson’s chi-square analysis was employed to determine associations of gender or race with PS phenotypes. Simple and multivariable logistic regression analyses were performed to determine relationships between PS phenotypes (dependent variable) and CLE subtype, SLE diagnosis, systemic lupus activity (measured by PGA), and cutaneous lupus activity (measured by CLASI activity score). Non Gaussian response variables were reported as frequencies and medians ± interquartile ranges. Group differences were assessed by either Kruskal-Wallis or Mann-Whitney U tests. Reported indices of association were calculated as two-tailed p-values. S
RESULTS
Subject Characteristics
A total of 91 subjects were enrolled with mean age ± standard deviation of 46 ± 13 yrs. Gender, race, diagnosis, and SLE manifestations are presented in Table 3. The >1 CLE subtype category was comprised of three subjects with DLE and SCLE, three with DLE and ACLE, one with LET and SCLE and one with LET and DLE. Forty-two percent of subjects had CLE and met criteria for SLE.
Table 3.
Subject Characteristics
| Characteristics | N | |
|---|---|---|
| Diagnosis | ||
| DLE | 45 | |
| SCLE | 21 | |
| LET | 9 | |
| > 1 CLE subtype | 8 | |
| ACLE | 5 | |
| CCLE other | 3 | |
| Subjects with CLE & SLE | 38 | |
| Systemic Manifestations in Subjects with CLE & SLE | ||
| Arthritis | 14 | |
| Renal | 4 | |
| Neurologic | 1 | |
| Hematologic | 6 | |
| Race | ||
| African American | 29 | |
| Asian | 7 | |
| Hispanic | 2 | |
| Other | 1 | |
| White | 52 | |
| Gender | ||
| Male | 18 | |
| Female | 73 | |
| Total | 91 | |
Prevalence photosensitivity phenotypes in lupus
Clinical interview utilizing the PS survey revealed that 81% of subjects ascribed to at least one PS phenotype. There were no significant (p<0.05) relationships between gender, race, SLE diagnosis, CLE diagnosis, PGA or CLASI activity and absence of photosensitivity.
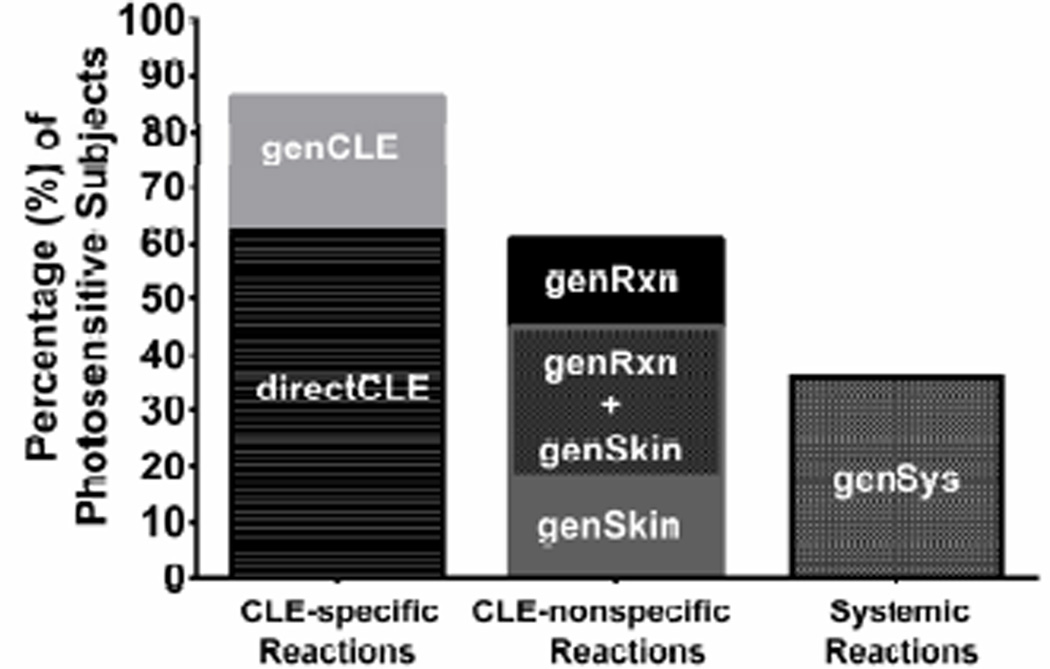
Of those reporting photosensitivity (N=74), 86% (64/74) reported photosensitivity as worsening of CLE after sun exposure: 46 subjects described specific occurrences of sun-induced CLE exacerbation [directCLE] and 18 reported a general association between sun exposure and CLE [genCLE]. 60% of subjects (44/74) experienced cutaneous reactions that were not typical for lupus: 13 subjects had a PMLE-like eruption [genSkin], 12 experienced general pruritus/paresthesia of sun-exposed skin [genRxn], and 19 experienced both genSkin and genRxn (Figure 1). Rarely did subjects experience these lupus non-specific cutaneous reactions in the absence of CLE specific photosensitivity – only 5/32 had genSkin and 2/31 of genRxn subjects reported these reactions in the absence directCLE or genCLE phenotypes. 36% (27/74) of subjects reported sunlight-induced systemic symptoms [genSys] and in all but 3, these reactions co-occurred with CLE-specific (directCLE or genCLE) and/or general cutaneous reactions (genSkin and/or genRxn). 52% of subjects reporting genSys met criteria for SLE.
Figure 1.
Cutaneous Lupus Erythematosus. Percentage (%) of photosensitive subjects (N=83) reporting each photosensitivity phenotype as captured by the photosensitivity survey. Note: Since 23 subjects reported both genSkin and genRxn (concomitantly), this overlap is listed on the graph to allow for accurate % calculation.
Timing of photosensitivity phenotypes
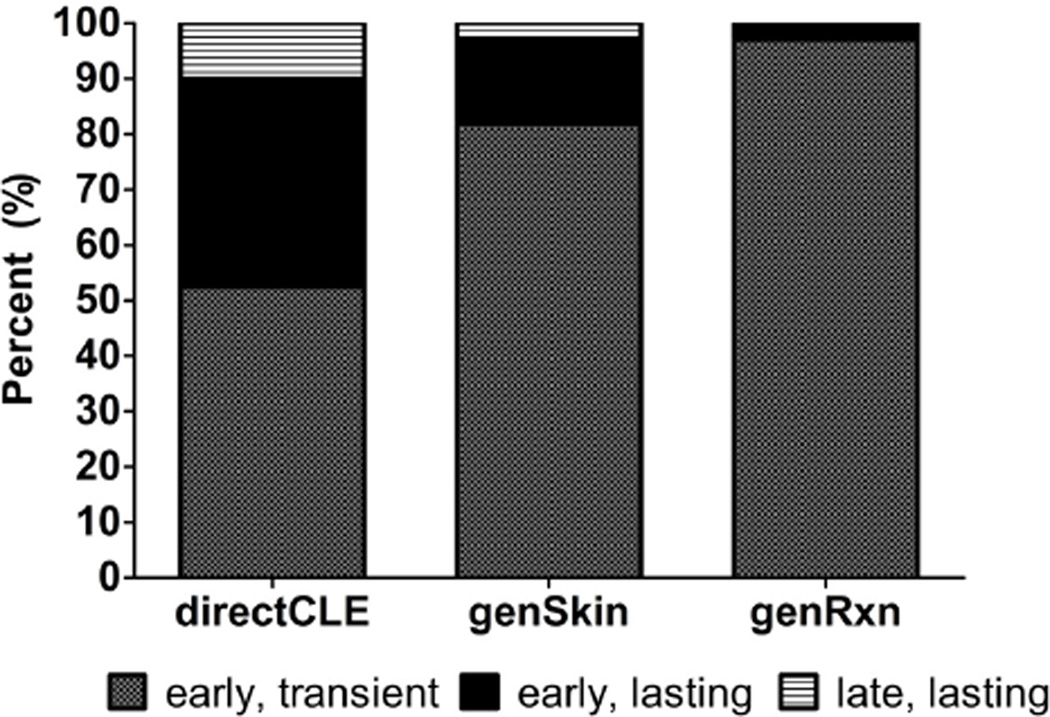
The time course of three PS phenotypes was investigated: directCLE, genSkin, and genRxn. Of those with directCLE, 90% experienced CLE worsening, soon after sun exposure; half of these subjects reported early (within 1 week) resolution, while others ascribed to lasting skin reactions. Only 4/39 subjects described late-onset sun-induced CLE-specific skin reactions. GenSkin and genRxn groups nearly always experienced early onset, transient (resolving within 1 week) reactions to sunlight (Figure 2).
Figure 2.
Cutaneous Lupus Erythematosus. Percentage (%) of time courses for photosensitivity reactions among subjects experiencing directCLE, genSkin, and genRxn. Early – PS symptoms occur within minutes to next day; Transient – PS symptoms resolve the same day to within 1 week; Lasting – PS symptoms last for weeks to months.
Associations among gender, race, and PS phenotypes
Gender
Gender was significantly associated with two PS phenotypes: genSkin (PMLE-like reaction, p = 0.01) and genSys (sun-induced systemic symptoms, p = 0.03), but not significantly associated with any other PS phenotype, with more females reporting these PS phenotypes than expected.
Race
There were no significant associations between race and any PS phenotype.
Relationships among CLE subtypes, SLE diagnosis, CLASI activity, systemic disease activity, and PS phenotypes
directCLE phenotype
There was a statistically suggestive (p=0.094) trend for CLASI activity scores to predict experiencing the directCLE phenotype with more subjects with moderate-severe compared to mild CLASI activity to experience direct CLE exacerbation after sun exposure. While CLE subtype, SLE diagnosis, and systemic activity (measured by PGA) were not significantly related to directCLE, the association between CLASI activity and directCLE remained statistically suggestive (p=0.093) in the multivariable model (Table 4 and Table 5).
Table 4.
P-values for Simple Logistic Regression Analyses with PS Phenotypes as Dependent Variables and Predictive Variables: CLE subtype (DLE, SCLE, ACLE, LET, CCLE other, >1 CLE subtype), SLE diagnosis (CLE alone vs. CLE and SLE), systemic activity (PGA = 0 none vs. PGA ≥ 1 mild-severe) and cutaneous activity (CLASI activity < 9 mild vs. activity ≥ 10 moderate-severe)
| PS phenotypes | CLE subtype | SLE diagnosis | PGA | CLASI |
|---|---|---|---|---|
| directCLE | 0.438 | 0.971 | 0.542 | 0.094 |
| genCLE | 0.077 | 0.831 | 0.398 | 0.949 |
| genSkin | 0.843 | 0.356 | 0.017 | 0.488 |
| genRxn | 0.098 | 0.003 | 0.051 | 0.712 |
| genSys | 0.359 | 0.135 | 0.021 | 0.754 |
Table 5.
P-values for Multivariable Logistic Regression Analyses with PS Phenotypes as Dependent Variables and Predictive Variables: CLE subtype (DLE, SCLE, ACLE, LET, CCLE other, > 1 CLE subtype), SLE diagnosis (CLE alone vs. CLE and SLE), systemic activity (PGA = 0 none vs. PGA ≥ 1 mild-severe) and cutaneous activity (CLASI activity < 9 mild vs. activity ≥ 10 moderate-severe)
| PS phenotypes | CLE subtype | SLE diagnosis | PGA | CLASI |
|---|---|---|---|---|
| directCLE | 0.454 | 0.331 | 0.695 | 0.093 |
| genCLE | 0.099 | 0.229 | 0.499 | 0.994 |
| genSkin | 0.755 | 0.740 | 0.051 | 0.227 |
| genRxn | 0.367 | 0.042 | 0.125 | 0.707 |
| genSys | 0.511 | 0.619 | 0.064 | 0.419 |
genCLE phenotype
In both the simple (p=0.077) and multivariable (p=0.099) models, there was a statistically suggestive trend for subjects with tumid LE (LET) compared to other CLE subtypes to experience a general link between CLE flares and sun exposure.
genSkin phenotype
Systemic disease activity as measured by PGA was predictive of the genSkin phenotype with more subjects with PGA≥1 (mild-severe, p=0.02) experiencing PMLE-like reactions compared to subjects with no systemic disease activity (PGA=0, p=0.05).
genRxn phenotype
In both the simple and multivariable model, SLE diagnosis was predictive of the genRxn phenotype such that subjects with both SLE and CLE were more likely (p=0.003) to experience PMLE-like eruptions compared to those with CLE alone (p=0.04). PGA scores were predictive of genRxn in the simple model, but failed to reach significance in the multivariable analysis.
genSys phenotype
Sun-induced systemic reactions were predicted by PGA scores in the simple model (p=0.021) and trended toward predictive in the multivariable model (p=0.064); such that, subjects with more active systemic disease (PGA ≥ 1) experienced the genSys phenotype; whereas, those with no systemic activity (PGA = 0) did not.
Immunohistochemistry
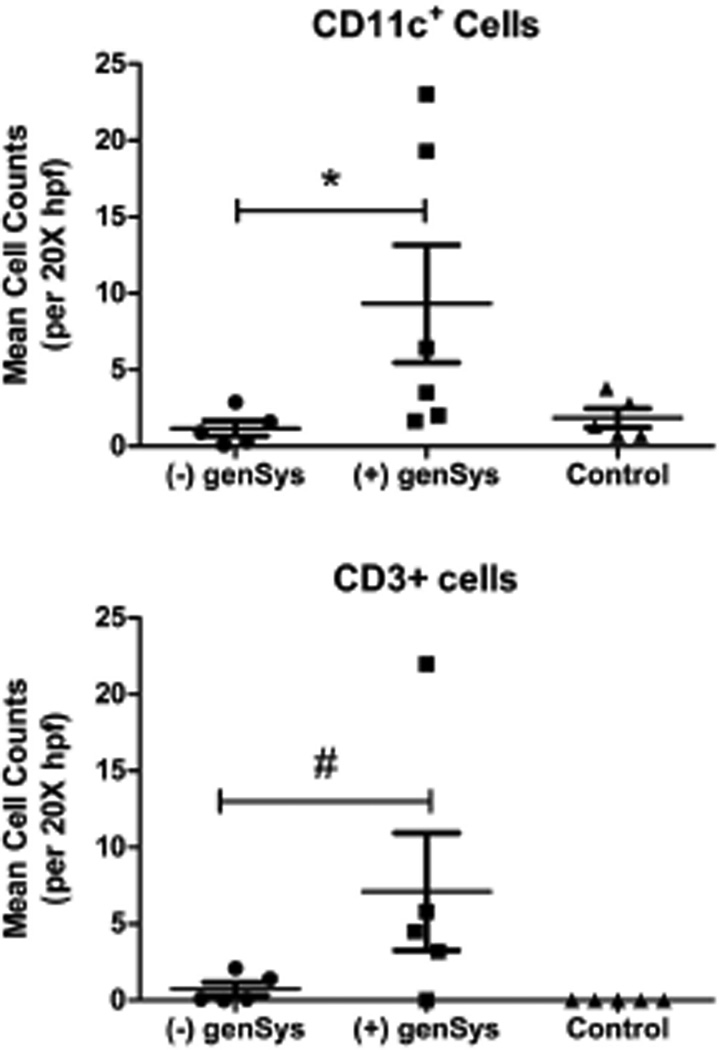
Immunohistochemistry for mDCs and T-cells was conducted using anti-CD11c and anti-CD3 monoclonal antibody, respectively. The Mann-Whitney test indicated a significant difference in myeloid dendritic cell (CD11c) counts between subjects with compared to those without sun-induced systemic symptoms [genSys] (p=0.04) and a statistically suggestive trend (p=0.06) toward subjects with systemic symptoms having more resident (CD3) T-cells (Figure 3). There were no significant associations between genSys and SLE diagnosis; nor were there significant differences in mDC or T-cell counts between subjects with and without SLE. Subjects with genSys tended to have lower CLASI activity scores compared to subjects denying sun-induced systemic symptoms [median ± IQR: 5 ± 12 vs 16 ± 13] and SLEDAI scores were not significantly different.
Figure 3.
Cutaneous Lupus Erythematosus. Median (± interquartile range) of cell counts for myeloid dendritic cells (CD11c +) and T-cells (CD3+) in the dermis of photosensitive lupus patients with and without sun-induced systemic reactions and in age- and skin-type matched controls. Mann-Whitney tests of cell counts of subjects with genSys versus without genSys; * p = 0.04, # p = 0.06.
DISCUSSION
Clinical interviews utilizing the PS survey allowed us to carefully characterize self-reported photosensitivity among a primarily CLE population. There was tremendous variability in how patients with lupus experience photosensitivity. Overall, we found that 81% of subjects ascribe photosensitivity. Unlike previous reports suggesting that photosensitivity occurs more commonly in Caucasians compared to other racial groups, we found no associations between any PS phenotype and race1,26–27.
Not surprisingly, most photosensitivity reactions fell in the category of CLE specific. The most common PS phenotype was directCLE with 62% of photosensitive subjects reporting specific examples of sun-induced CLE exacerbation. In contrast to reports describing a delay between sun exposure and CLE induction, the majority of subjects reported sun-induced CLE flares occurring early after sun exposure4. Exacerbations were commonly described to be transient as opposed to lasting (for weeks to months). Interestingly, there was a trend for subjects with higher CLASI activity scores to report directCLE phenotype. We have shown previously that higher CLASI activity scores were correlated with photosensitivity in lupus20. It would be interesting to investigate whether patients with more active CLE disease have a greater degree of photosensitivity or whether sun-induced reactions lead to more active CLE disease.
CLE nonspecific photosensitivity reactions were related to systemic disease activity and SLE diagnosis. More active systemic disease (as measured by PGA) but not SLE diagnosis predicted PMLE-like reactions and systemic reactions; whereas, SLE diagnosis and PGA predicted the genRxn phenotype of stinging/itching sensation. Though these reactions nearly always occurred in association with a CLE-specific phenotype (i.e., directCLE or genCLE), experiencing a general cutaneous reaction to sunlight may suggest more active systemic disease.
A PMLE-like eruption was reported by 43% of patients, which is consistent with prior reports that suggest an increased prevalence of this form of eruption in lupus patients, compared to the general population13, 14. These reactions, however, often occurred immediately after sun exposure, resolved within one day and rarely occurred in the absence of CLE-specific PS reactions. Because these reactions differ from PMLE in timing and setting, these findings suggest that PMLE-like reactions may occur as part of a photosensitivity spectrum in lupus16 rather than PMLE as a co-occurring disorder28, 29.
Over one third of patients reported systemic reactions to sunlight, only 50% met criteria for SLE and analysis indicated that higher PGA scores were predictive of the genSys phenotype. Furthermore, immunohistochemical analysis of sun-exposed skin of a subset of patients with genSys was associated with an increased number of myeloid dendritic cells (mDC) and a trend toward more T-cells compared to photosensitive patients without sun-induced systemic symptoms. Skin resident T-cell and mDC populations have been described, recently30, 31, and greater prevalence of immunologically-active cells were found resident in the skin of CLE patients with systemic lupus features. These results highlight the complexity of UVR effects in lupus and suggest that resident inflammatory cells in the skin may play a role in systemic reactions of photosensitivity in lupus.
This study had several limitations. First, study participants were treated at the autoimmune skin disease clinic of the University of Pennsylvania, which is a referral-only center. Second, photosensitivity reactions were inferred and were not directly observed. Third, study staff made every effort to use open-ended questions in the clinical interview pertaining to photosensitivity to minimize patient recall bias, however, some element of recall bias is likely present which could artificially inflate the prevalence of photosensitivity phenotypes in the sample. Further, data collection occurred across seasons which may contribute to recall bias. Finally, investigation of the pathomechanism of self-reported photosensitivity was hypothesis-generating in nature. With only a small number of subject biopsies for immunohistochemistry, our analyses were not powered to detect differences that might truly exist in resident cell populations among the various cutaneous PS phenotypes or specific CLE diagnoses.
CONCLUSION
Characterization of self-reported photosensitivity in lupus reveals that patients experience combinations of CLE-specific, -nonspecific, and systemic reactions to sunlight. Sun-induced CLE flares are associated with more active CLE disease. PMLE-like, generalized stinging/itching, and systemic reactions are associated with more active systemic disease regardless of SLE diagnosis. Though the pathomechanism of these varied PS phenotypes is far from understood, these data suggest that resident immune cells in the skin might contribute to both systemic and cutaneous lupus activity. Future studies, examining immunologically active cells in non-lesional skin both pre- and post-UVR exposure, could help elucidate the contribution of resident skin cells on various PS phenotypes and explain how photosensitivity contributes to both CLE-specific and systemic disease activity.
-
▪
According to the American College of Rheumatology (ACR), photosensitivity in lupus is determined by clinical examination or by patient history of unusual reaction to sunlight.
-
▪
Self-reported photosensitivity in CLE comprises CLE-specific, non-specific cutaneous eruptions, paresthesias, and systemic symptoms.
-
▪
Physicians should recognize the varied manifestations of photosensitivity in lupus because these phenotypes are associated with both systemic and cutaneous disease activity.
Acknowledgments
This material is based upon work supported by the National Institutes of Health, including NIH K24-AR 18 02207 (Werth), National Center for Advancing Translational Research TL1RR024133 which is now National Center for Advancing Translational Sciene TL1TR00138 (Foering and Cucchiara), and by the Department of Veterans Affairs, Veteran Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- 1.Scheinfeld N, Deleo VA. Photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. 2004 Oct;20(5):272–279. doi: 10.1111/j.1600-0781.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 3.Kind P, Lehmann P, Plewig G. Phototesting in lupus erythematosus. J Invest Dermatol. 1993 Jan;100(1):53S–57S. doi: 10.1111/1523-1747.ep12355594. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn A, Sonntag M, Richter-Hintz D, Oslislo C, Megahed M, Ruzicka T, Lehmann P. Phototesting in lupus erythematosus: A 15-year experience. J Am Acad Dermatol. 2001;45(1):86–95. doi: 10.1067/mjd.2001.114589. [DOI] [PubMed] [Google Scholar]
- 5.Schornagel IJ, Knol EF, van Weelden H, Guikers CL, Bruijnzeel-Koomen CA, Sigurdsson V. Diagnostic phototesting in polymorphous light eruption: The optimal number of irradiations. Br J Dermatol. 2005 Dec;153(6):1234–1236. doi: 10.1111/j.1365-2133.2005.06954.x. [DOI] [PubMed] [Google Scholar]
- 6.Lokitz ML, Billet S, Patel P, Kwon EJ, Sayre RM, Sullivan KE, Werth VP. Failure of physiologic doses of pure UVA or UVB to induce lesions in photosensitive cutaneous lupus erythematosus: Implications for phototesting. Photodermatol Photoimmunol Photomed. 2006;22(6):290–296. doi: 10.1111/j.1600-0781.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jong CT, Finlay AY, Pearse AD, Kerr AC, Ferguson J, Benton EC, Hawk JL, Sarkany RP, McMullen E, Rhodes LE, et al. The quality of life of 790 patients with photodermatoses. Br J Dermatol. 2008 Jul;159(1):192–197. doi: 10.1111/j.1365-2133.2008.08581.x. [DOI] [PubMed] [Google Scholar]
- 8.Morison WL. Clinical practice. photosensitivity. N Engl J Med. 2004 Mar 11;350(11):1111–1117. doi: 10.1056/NEJMcp022558. [DOI] [PubMed] [Google Scholar]
- 9.Millard TP, Hawk JL. Photosensitivity disorders: Cause, effect and management. Am J Clin Dermatol. 2002;3(4):239–246. doi: 10.2165/00128071-200203040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Pincus LB, LeBoit PE, Goddard DS, Cho RJ, McCalmont TH. Marked papillary dermal edema--an unreliable discriminator between polymorphous light eruption and lupus erythematosus or dermatomyositis. J Cutan Pathol. 2010 Apr;37(4):416–425. doi: 10.1111/j.1600-0560.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JH. Polymorphous light eruption. Dermatol Clin. 1986 Apr;4(2):243–251. [PubMed] [Google Scholar]
- 12.Holzle E, Plewig G, von Kries R, Lehmann P. Polymorphous light eruption. J Invest Dermatol. 1987 Mar;88(3 Suppl):32s–38s. doi: 10.1111/1523-1747.ep12468916. [DOI] [PubMed] [Google Scholar]
- 13.Hasan T, Nyberg F, Stephansson E, Puska P, Hakkinen M, Sarna S, Ros AM, Ranki A. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings. Br J Dermatol. 1997 May;136(5):699–705. [PubMed] [Google Scholar]
- 14.Nyberg F, Hasan T, Puska P, Stephansson E, Hakkinen M, Ranki A, Ros AM. Occurrence of polymorphous light eruption in lupus erythematosus. Br J Dermatol. 1997 Feb;136(2):217–221. [PubMed] [Google Scholar]
- 15.Gronhagen C, Gunnarsson I, Svenungsson E, Nyberg F. Cutaneous manifestations and serological findings in 260 patients with systemic lupus erythematosus. Lupus. 2010;19(10):1187–1194. doi: 10.1177/0961203310367656. [DOI] [PubMed] [Google Scholar]
- 16.Tzaneva S, Volc-Platzer B, Kittler H, Hönigsmann H, Tanew A. Antinuclear antibodies in patients with polymorphic light eruption: A long-term follow-up study. Br J Dermatol. 2008;158(5):1050–1054. doi: 10.1111/j.1365-2133.2008.08500.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn A, Herrmann M, Kleber S, Beckmann-Welle M, Fehsel K, Martin-Villalba A, Lehmann P, Ruzicka T, Krammer PH, Kolb-Bachofen V. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis & Rheumatism. 2006;54(3):939–950. doi: 10.1002/art.21658. [DOI] [PubMed] [Google Scholar]
- 18.Hasan T, Ranki A, Jansen CT, Karvonen J. Disease associations in polymorphous light eruption. A long-term follow-up study of 94 patients. Arch Dermatol. 1998 Sep;134(9):1081–1085. doi: 10.1001/archderm.134.9.1081. [DOI] [PubMed] [Google Scholar]
- 19.Hasan T, Stephansson E, Ranki A. Distribution of naive and memory T-cells in photoprovoked and spontaneous skin lesions of discoid lupus erythematosus and polymorphous light eruption. Acta Derm Venereol. 1999 Nov;79(6):437–442. doi: 10.1080/000155599750009861. [DOI] [PubMed] [Google Scholar]
- 20.Foering K, Okawa J, Rose M, Goreshi R, LoMonico J, Werth V. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(S1–149):S10. [Google Scholar]
- 21.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981 Apr;4(4):471–475. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 22.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcon GS, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann Intern Med. 2005 Jun 21;142(12):953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 23.Krathen MS, Dunham J, Gaines E, Junkins-Hopkins J, Kim E, Kolasinski SL, Kovarik C, Kwan-Morley J, Okawa J, Propert K, et al. The cutaneous lupus erythematosus disease activity and severity index: Expansion for rheumatology and dermatology. Arthritis Rheum. 2008 Mar 15;59(3):338–344. doi: 10.1002/art.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonilla-Martinez ZL, Albrecht J, Troxel AB, Taylor L, Okawa J, Dulay S, Werth VP. The cutaneous lupus erythematosus disease area and severity index: A responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008 Feb;144(2):173–180. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albrecht J, Werth VP. Development of the CLASI as an outcome instrument for cutaneous lupus erythematosus. Dermatol Ther. 2007 Mar-Apr;20(2):93–101. doi: 10.1111/j.1529-8019.2007.00117.x. [DOI] [PubMed] [Google Scholar]
- 26.Smikle MF, Barton EN, Morgan OS, Deceulaer K. Photosensitivity and antinuclear antibodies in black patients with systemic lupus erythematosus. J Assoc Acad Minor Phys. 1996;7(2):53–55. [PubMed] [Google Scholar]
- 27.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus. identication of racial and socioeconomic influences. Arch Intern Med. 1990;150(4):849–853. [PubMed] [Google Scholar]
- 28.Millard TP, Lewis CM, Khamashta MA, Hughes GR, Hawk JL, McGregor JM. Familial clustering of polymorphic light eruption in relatives of patients with lupus erythematosus: Evidence of a shared pathogenesis. Br J Dermatol. 2001 Feb;144(2):334–338. doi: 10.1046/j.1365-2133.2001.03897.x. [DOI] [PubMed] [Google Scholar]
- 29.Millard TP, Kondeatis E, Vaughan RW, Lewis CM, Khamashta MA, Hughes GR, Hawk JL, McGregor JM. Polymorphic light eruption and the HLA DRB1*0301 extended haplotype are independent risk factors for cutaneous lupus erythematosus. Lupus. 2001;10(7):473–479. doi: 10.1191/096120301678416024. [DOI] [PubMed] [Google Scholar]
- 30.Clark RA. Skin-resident T cells: The ups and downs of on site immunity. J Invest Dermatol. 2010 Feb;130(2):362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaba LC, Krueger JG, Lowes MA. Resident and "inflammatory" dendritic cells in human skin. J Invest Dermatol. 2009 Feb;129(2):302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]