Abstract
Introduction
CD8+ T cells were originally considered to exert a suppressive role in demyelinating disease because of bias toward the CD4+ T cell-mediated experimental autoimmune encephalomyelitis, the most common MS model. However, recent studies of human MS lesion samples and CSF provided compelling evidence about the pathogenic role of CD8+ T cells. In this review, we discuss the theoretical roles of different CD8+ T-cell subsets in MS.
Areas covered
A revised focus from CD4+ to CD8+ T cell-mediated demyelinating disease is summarized. Clonal expansion of CD8+ T cells in MS lesions and in vitro evidence that CD8+ T cells injure every CNS cell type and transect axons are discussed. The role of CD8+ T cells in two animal models of MS and of regulatory, IL-17-secreting CD8+ T cells is reviewed. Lastly, an overview about the pathogenic and/or beneficial role of various CD8+ T-cell subsets is offered.
Expert opinion
Growing evidence supports the pathogenic role of CD8+ T cells. Clonally expanded CD8+ T cells within MS lesions may damage the nervous system. Revealing the specific antigen is critical to design novel efficient treatments with minimal adverse effects. Increasing evidence exists for the role of regulatory, IL-17-secreting CD8+ T cells in MS.
Keywords: Animal Models, CD8+ T cells, Central Nervous System, Clinical Trial, MHC Class I, Multiple Sclerosis, Regulatory T cells
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) with diversity of both clinical presentations and pathological features. It represents one of the most common causes of neurological disability in young adults in the western world. There is still no definitive cause of MS and no effective cure. Knowledge accumulated during the last few decades indicates that MS may be the consequence of a complex interaction of genetic predisposition and numerous environmental factors [1]. Regardless of the underlying cause, there is consensus that the the immune system is the major culprit in MS pathogenesis. Because experimental autoimmune encephalomyelitis (EAE), the most widely used mouse model of MS, is driven by Th-1 CD4+ T cells, this subset of T cells was the main area of MS research for several decades, whereas CD8+ T cells were neglected. Therefore, most knowledge about MS etiology, pathogenesis and therapeutic trials in human MS patients was based on the EAE studies. From the standpoint of understanding autoimmunity, immune surveillance, CNS inflammation and immune-mediated tissue destruction, EAE has been very helpful. Results from EAE studies aided the development of three approved medications for treatment in MS, glatiramer acetate, mitoxantrone and natalizumab. However, there were numerous ineffective or, in some cases, even harmful novel therapeutic trials based on EAE [2, 3]. In contrast to EAE, Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease emphasizes a role for CD8+ T cells in this animal model of MS depending on viral strain and the genetic of the experimental host [4–6]. More than two decades ago, Rodriguez et al. showed that TMEV-mediated disease in mice can be abrogated by anti-CD8 treatment [7]. In addition, others showed that myelin-specific CD8+ T cells can induce severe autoimmune CNS disease in mice [8, 9].
CD4+ T cells and macrophages are present in human MS lesions [10, 11]. However, the last 15 years of research have shifted the focus toward CD8+ T cells as evidence increasingly indicates this T-cell subtype as the major immune mediator of MS pathogenesis [12–14]. Recent neuropathologic studies convincingly demonstrated that CD8+ T cells predominate and outnumber CD4+ T cells in the inflammatory infiltrate in all MS lesions, regardless of disease stage [15, 16]. Hoftberger et al. studied human autopsy material that included acute, chronic active MS, inactive MS and control cases. Using quantitative immunohistochemistry, this group discovered upregulated MHC class I expression on neurons, axons, astrocytes and oligodendrocytes in MS lesions, which correlated with disease severity and lesion activity [17]. This important study provided further evidence that cytotoxic CD8+ T cells may influence demyelination and/or axonal destruction in human MS lesions. In addition, the hypothesis that CD4+ T cells are primary culprits for pathogenic immune response in MS was seriously questioned when therapeutic depletion of this T-cell subtype did not show any therapeutic effect in MS patients [18]. Conversely, when all T cells were used as a therapeutic target, a significant reduction in MS relapses and development of new lesions was observed [19]. CD8+ T cells are generally known for their direct cytotoxic function; however, the recent growing body of literature suggests that this T-cell subtype also has a regulatory role in MS and contributes to suppressing disease progression and/or severity. Moreover, a novel subset of IL-17-secreting CD8+ T cells has been identified with a hypothesized role in worsening of MS. Genetic studies also contributed to the shift in thinking, as specific MHC class I alleles were found to have independent risk or protection from MS. These studies are consistent with the hypothesis that various CD8+ T-cell subsets not only actively contribute to MS but also protect/limit the disease. Whereas interest in the role of CD8+ T cells in MS is a relatively new trend, these cells play an important role in other proven autoimmune diseases including rheumatoid arthritis, type 1 diabetes, autoimmune thyroiditis, vitiligo, and inflammatory bowel disease [20].
2. CD8+ T Cells Are Present in MS Lesions
Besides the long-standing focus on CD4+ T cells due to use of the EAE animal model, the other reason for minimal interest in the role of CD8+ T cells was the lack of CNS tissue samples from MS patients with acute lesions [21]. Another reason was the relative scarcity of immunocytochemistry materials needed to detect CD8+ T cells on formalin-fixed CNS tissues. In one of the first studies shifting focus toward the role of CD8+ T cells, Booss et al. found that CD8+ T cells outnumber the CD4+ T-cell subset in all samples from MS patients, regardless of the MS subtype, duration and speed of disease progression [22]. Subsequently, Hauser et al. analyzed brains from 16 deceased progressive MS patients and 2 from acute MS. This group found that perivascular cuffs at the edges of active demyelinating plaques contained up to 50 times more CD8+ than CD4+ T cells [23]. Moreover, CD8+ T cells predominated in occasional perivascular cuffs in the normal-appearing white matter (NAWM). None of the analyzed brains showed predominance of CD4+ over CD8+ T cells.
There is clear evidence regarding the clonal expansion of CD8+ T cells in MS patients [24] and MS lesions [15], but the epitopes recognized by these CD8+ T cells remain elusive. Generally, peripheral blood contains approximately twice as many CD4+ T cells as CD8+ T cells. In a landmark study using single-cell polymerase chain reaction (PCR), Babbe et al. demonstrated that 1) CD8+ (not CD4+) T cells predominated in all studied MS lesions; and 2) only a few CD8+ T-cell clones were present in the infiltrate [15]. Interestingly, in one case, two CD8+ T-cell clones were detected in the peripheral blood at two different time points following brain biopsy. In a later study, several expanded clones of CD8+ T cells were present not only in biopsied MS lesions but also in the cerebrospinal fluid (CSF) and blood [16]. An independent group from Germany analyzed CD4+ and CD8+ T cells isolated from the peripheral blood and CSF of 36 MS patients for their T-cell receptor β-chain variability [25]. These authors also found significant expansion of CD8+ T cells in the CSF of most MS patients studied. Moreover, in selected patients, CD8+ T-cell expansion proved stable over several months. More recently, another group from Germany analyzed distinct MS lesions and NAWM in whole-brain autopsy specimens from four MS patients [26]. Within each patient, the authors found identical T-cell clones in at least two distant brain regions, including MS lesions and NAWM, whereas some clones were present in all analyzed brain regions. Interestingly, these patients had some identical HLA class I and class II molecules, but the observed T-cell clones were unique for each individual patient. Unfortunately, no blood or CSF samples were available, so this study could not test for the presence of expanded T-cell clones in these compartments to confirm the previous findings by Skulina et al [16]. However, this study yielded an interesting observation with significance for future studies: the authors found silent nucleotide mutations in CDR3 regions of several T-cell receptor β-chains that did not disrupt amino acid sequence. After forming in thymus, the T-cell repertoire does not mutate when T cells become activated anywhere in the body. The presence of these silent nucleotide mutations indicated that the corresponding T-cell clones were not random bystander cells but, instead, were most likely stimulated by the particular antigen and selectively recruited and accumulated in the MS brain. Finally, Jilek et al. provided evidence that highly differentiated CD8+ T cells enrich the CSF in early MS rather than in primary progressive MS or “other neurological disorders” [27]. An independent Swedish study proposed that increased CD8+ T-cell cytotoxicity in the CSF (determined by increased granzyme levels) correlates with clinical relapses in MS patients with relapsing-remitting pattern of disease [28]. Granzyme levels were normal in control subjects and patients in remission.
In summary, the repeated and independent findings regarding the clonal expansion of CD8+ T cells indicate antigen-specificity for discrete epitopes that are yet to be elucidated. Several candidate antigens recognized by these expanded CD8+ T-cell clones include myelin antigens or viruses. Myelin-specific CD8+ T cells are encephalitogenic in mice and cause severe EAE disease [8]. Several viruses have been proposed as causative factors for MS, but so far none of these agents has been unequivocally linked to MS. One of the best candidates for a viral trigger of MS is the Epstein-Bar virus (EBV). However, despite extremely intriguing results, such as strong relationship between EBV-specific CD8+ T-cell response and early MS [29], the exact viral antigen that causes MS has been difficult to prove. Further studies need to elucidate whether EBV infection indeed predisposes one to MS or simply occurs due to the same susceptibility factors that eventually lead to MS. Another herpes-type virus associated with MS is neurotropic human herpes virus-6 (HHV-6) [30]. Indeed, significantly more CD8+ T cells directed against MBP and cross-reactive to HHV-6 residues were found in MS patients than in control subjects [31]. However, solid evidence that demonstrates a definite role for HHV-6 in causing MS is missing.
3. CD8+ T Cells Cause Damage to all CNS Cell Types
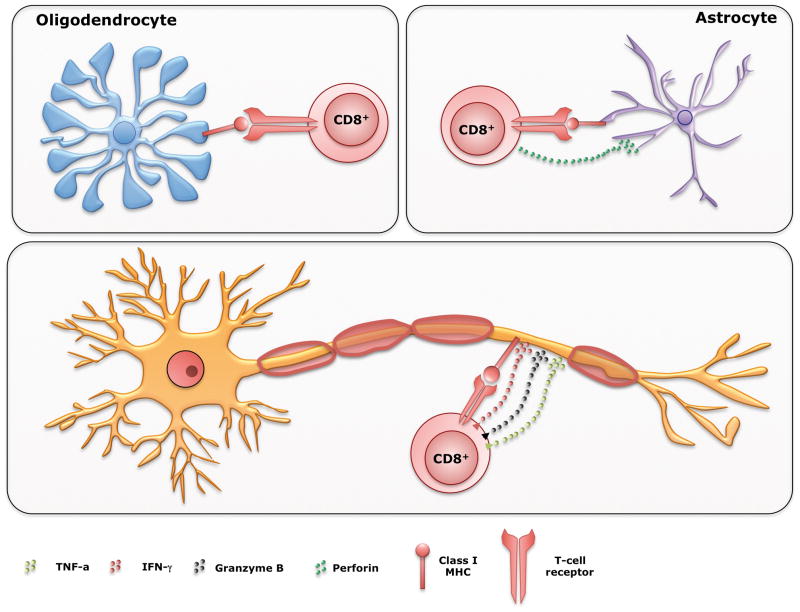
Under normal conditions, CD8+ T cells are absent or extremely scarce in the CNS tissue [32]. Also, MHC class I molecules are normally only present in vascular and meningeal cells and poorly expressed on neurons and glia [33]. However, under inflammatory conditions, all CNS cells (astrocytes, oligodendrocytes, and neurons) express MHC Class I molecules and can be recognized and lysed by cytotoxic CD8+ T cells [17, 34](Figure 1). Medana et al. demonstrated that CD8+ T cells kill astrocytes primarily by fast perforin-mediated lysis [35]. Others showed that MBP peptide-specific CD8+ T cells recognized and lysed oligodendrocytes in vitro without any exogenous antigen [36]. In addition, Pouly et al. demonstrated that treating oligodendrocytes with interferon-γ (IFN-γ) significantly upregulated expression of Fas (death receptor), which led to Fas-mediated apoptosis [37]. However, unlike astrocytes and oligodendrocytes, neurons possess some level of selective protection from CD8+ T-cell attack by cytotoxic granules or TNF and related molecules [32]. It is controversial how cytotoxic T-lymphocytes (CTLs) kill neurons. An early in vitro study concluded that CD8+ T cells kill neurons via the perforin pathway instead of the Fas ligand-mediated pathway [38]. However, Medana et al. demonstrated the opposite; neurons resisted perforin-mediated lysis but succumbed to delayed Fas ligand-mediated apoptosis [35, 39]. The resistance to perforin-induced damage supports the finding by Khanna et al. in which CD8+ T cells use perforin to suppress infection without causing cytotoxicity in a mouse model of herpes simplex virus infection of sensory neurons [40]. Until that point, all in vitro experiments focused on CTL toxicity on neuronal bodies. But, due to lack of knowledge regarding CD8+ T-cell effects on axons, the same group subsequently shifted focus. Using continuous visualization by confocal microscopy, the authors showed stable connections between CD8+ T cells and axons [41]. Following this, the first clues of axonal damage appeared within 15–20 minutes with further progression of injury, which ended in complete axonal transection within 55 minutes.
Figure 1. CD8+ T cells can kill all CNS cell types.
+ T cells can mediate pathology in MS lesions by killing all cell types in the CNS. Under inflammatory conditions, astrocytes, oligodendrocytes, and neurons/axons express MHC Class I molecules and can be recognized and killed by cytotoxic CD8+ T cells. Upon recognition of MHC Class I, CD8+ T cells in a polarized manner, release granules containing perforin and/or granzyme B, molecules that have cytolitic effect on a target cell. In addition, in the inflammatory setting IFN-γ and TNF-α are also released, affecting the homeostasis of the neurons and axons that express their corresponding receptors, leading them to apoptosis.
In pathological studies, Neumann et al. very nicely demonstrated close contact between CD8+ T cells and demyelinated axons in the human MS brain lesion as well as granzyme-B cytotoxic granules polarized towards the axons [32]. A similar observation was made in a tissue sample from Rasmussen’s encephalitis in which cytotoxic granules of closely attached CD8+ T cells were polarized towards degenerating neurons [42]. By analyzing brain biopsies from 42 MS patients, Bitsch et al. showed a statistically significant, positive correlation between the number of CD8+ T cells and amyloid precursor protein (APP) expression, which is indicative of axonal damage [43]. The authors did not observe any correlation between CD3+ T cells and APP expression. In a subsequent study, the same group found a correlation between APP expression in MS lesions and duration and course of the disease [44]. Likewise, they confirmed the previous finding of a significant correlation between the number of CD8+ T cells and the extent of axon damage. A novel finding was that the highest APP expression was observed in acute MS lesions, within one year of disease onset. The clear implication of this finding is that neuroprotective therapy should commence as early as possible and be continuous [44]. This is consistent with the recent finding from our laboratory, where mice with virus-induced demyelinating disease that received early treatment with a single dose of the human neuron-binding antibody (rHIgM12) that protects axons had more beneficial effect than mice receiving treatment later in the disease course [45].
In summary, the aforementioned literature review about in vitro killing of CNS cells provides evidence that all brain cells are potentially targets for CD8+ T cell-mediated killing in MS. Nevertheless, differences were observed in cytotoxic mechanisms and susceptibility to lysis of various CNS cells. However, the reader should note that in vivo systems are much more complex and dependent on the combination of genetic factors and extent of CNS inflammation. Human MS is a pathologically heterogeneous disease with 4 distinct patterns of lesions [46, 47], and each distinct lesion type contains both CD4+ and CD8+ T cells along with different inflammatory cytokines and mediators. In the future, it will be important to elucidate the role(s) of each individual cell type present in these distinct lesions including CD8+ T cells.
4. MHC Class I Genes Confer Risk or Protection from MS
In order to perform their function, CD8+ T cells recognize and interact with antigens presented within major histocompatibility complex (MHC) class I molecules through their T-cell receptors (TCRs). In general, certain MHC class I alleles correlate with both susceptibility and protection from MS. In one of the earliest studies, HLA-A3 [48] and HLA-B7 [49] were most convincingly associated with MS. The association of HLA-A2, HLA-A7 and W10 antigens was less convincing, although it reached statistical significance [49]. Three decades later, using larger patient series, two studies showed that, independently of MHC class II antigens, HLA-A*0301 approximately doubles the risk of MS [50, 51]. In addition, both studies confirmed that the association of HLA-B7 (B*0702) was only secondary to MHC class II HLA-DR2 alleles and due to linkage disequilibrium with these alleles. Furthermore, these studies showed the protective function of a HLA-A*0201 allele, as the risk for developing MS was up to 50% less. A more recent Swedish study confirmed protective properties of HLA-A*0201 allele not explained by linkage disequilibrium with class II alleles [52]. Interestingly, HLA-A*0301 (risk) allele and HLA-A*0201 (protective) allele work in synergy with MHC class II allele DRB1*1501, which normally confers triple risk for developing MS (Table 1).
Table 1.
| MHC Allele | Odds Ratio |
|---|---|
| HLA-A*0201 | 0.52 – 0.7 |
| HLA-A*0301 | 1.9 – 2.1 |
| HLA-B*0702 | 1.6 – 2.2 |
| HLA-DRB1*1501 | 2.9 – 3.6 |
| HLA-DRB1*1501 + HLA-A*0201 | 1.5 |
| HLA-DRB1*1501 + HLA-A*0301 | 3.7 – 5.2 |
| HLA-DRB1*1501 + HLA-B*0702 | 3.2 |
MHC – Major histocompatibility complex
All of these genetic studies support the hypothesis that MHC class I alleles and, therefore, CD8+ T cells have important roles in MS that are both protective and pathogenic. Notably, MHC class I alleles can exert their effect independently from MHC class II alleles.
5. CD8+ T Cells Induce Injury in Animal Models of MS
5.1. CD8+ T Cells Have a Role in Experimental Autoimmune Encephalomyelitis
Because MS remains a disease with an unknown etiology and no effective cure, various animal models still provide the best way to study the mechanisms of immune-mediated pathology and test novel therapeutic strategies. As mentioned in the introduction, the two most commonly studied animal models of MS are experimental autoimmune encephalomyelitis (EAE) and Theiler’s Murine Encephalomyelitis Virus (TMEV)-induced chronic demyelinating disease [53].
Generally, EAE studies have been historically biased towards CD4+ T cell, Th1-mediated pathology. This bias was corroborated by findings that CD8+ T cells have a regulatory role with only minimal effector functions [54, 55]. However, since several groups repeatedly and independently demonstrated an important role for CD8+ T cells in MS pathogenesis, focus has shifted toward the role of this T-cell subset in the EAE animal model. In the first report, Sun et al. demonstrated that adoptive transfer of MOG35–55-specific CD8+ T cells induced severe EAE disease in susceptible wild type C57BL/6 mice but not in isogeneic β2-microglobulin (β2m)-deficient mice [9]. Subsequently, by using series of truncated MOG peptides, Ford et al. revealed that CD8+ T cells specific for minimal epitope MOG37–46 induced EAE in C57BL/6 mice [56]. In another study, Huseby et al. used an MBP-primed model of EAE in C3H mice to show that MBP79–87-specific CD8+ T cells induced pronounced CNS disease characterized by scattered but focal lesions surrounding small blood vessels [8]. Interestingly, these mice presented with ataxia, spasticity and brisk reflexes, symptoms that more closely mimic human MS than traditional EAE. To study in greater detail whether autoreactive CD8+ T cells cause significant loss of oligodendrocytes, Saxena et al. generated transgenic mice in which influenza virus hemagglutinin (HA) is selectively expressed in oligodendrocytes under MOG promoter [57]. Authors demonstrated that HA-specific, pre-activated CD8+ T cells induced inflammatory lesions in the spinal cord, brain and optic nerve in these transgenic animals. Pathologically, CNS lesions in these mice were very similar to an active human MS lesion, featuring CD8+ T-cell infiltration, demyelination with axonal damage, focal loss of oligodendrocytes and microglia activation. This was the first EAE study to show in vivo CD8+ T cell-mediated oligodendrocytelysis. Using a more complex transgenic approach in which expression of cognate (self) antigen is restricted to oligodendrocytes, others proposed that myelin-specific CD8+ T cells also lead to axonal loss due to collateral bystander damage [58]. In addition, using confocal live imaging, these authors demonstrated CD8+ T-cell migration through brain tissue. Finally, in a very recent study, Ji et al. demonstrated that “Tip-dendritic cells” cross-presented MHC Class I-restricted MBP epitopes and activated naïve CD8+ T cells, which then subsequently and specifically lyse oligodendrocytes [59]. This is consistent with the previously mentioned finding that MBP-specific CD8+ T cells are able to lyse human oligodendrocytes [36]. In summary, these recent studies provide new insights about the pathogenic role of CD8+ T cells on oligodendrocytes in the EAE model of MS.
5.2. CD8+ T Cells Contribute to Axonal Injury and Demyelination in Virus-Induced Demyelinating Disease
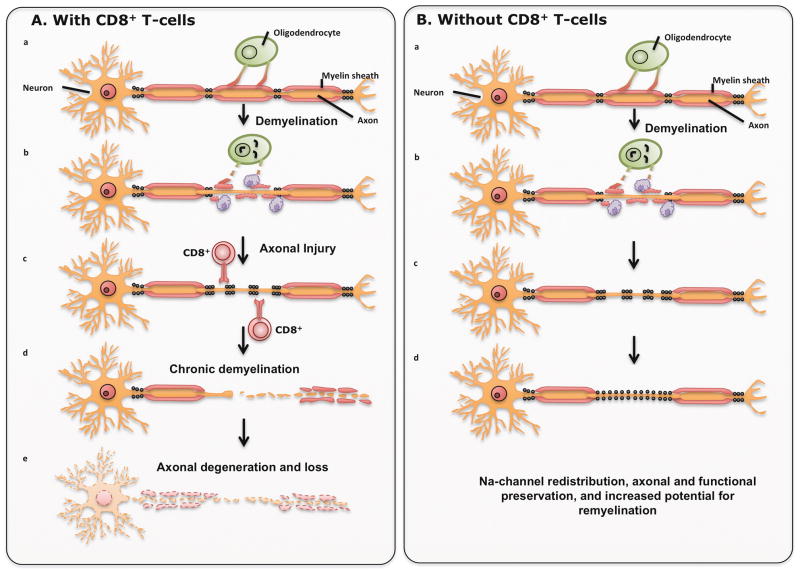
Compared to EAE, the involvement and contribution of CD8+ T cells in viral models of MS have been firmly established. In one of the first studies, Rodriguez et al. studied the individual contributions of CD4+ and CD8+ T cells on virus-induced demyelination and, as a secondary finding, observed that CD8−/− mice on a susceptible haplotype presented with less severe clinical disease than CD4−/− mice [60]. In the follow-up landmark study, Rivera-Quinones et al. clearly demonstrated the pathogenic role of CD8+ T cells in promoting neurological deficits following demyelination [4]. This study was important because it challenged the concept that demyelination was sufficient to induce clinical deficits in TMEV-infected mice. However, authors observed that, despite equivalent levels of demyelination and inflammatory cell infiltration, only β2m-deficient mice, and not β2m-competent mice, presented with normal motor function as assessed by two independent functional assays and hind-limb motor-evoked potentials. In addition, this study proposed that β2m-deficient mice remained functionally normal due to relative preservation of axons and redistribution of sodium channels within axons. In contrast, the β2m-competent mice presented with degeneration and loss of axons, which likely resulted in significantly fewer sodium channels, disrupted pattern and severe motor decline. This study provided strong direct evidence that in virus-induced mouse model of MS, neurological and electrophysiological decline absolutely requires MHC Class I and CD8+ T cells (Figure 2). Furthermore, the finding regarding sodium channels supported a study that described altered distribution of these channels in human MS lesions [61]. Subsequently, Murray et al. showed that the mechanism of neurological injury is perforin-mediated [62] as perforin-deficient mice presented with relative axonal protection and preserved motor function [5, 63]. As direct evidence that axons were preserved despite similar levels of demyelination, Ure et al. showed that β2m-deficient mice had up to 7-fold higher numbers of retrograde-labeled brainstem neurons compared to control mice [64]. In another study, Johnson et al. used a model of fulminant and fatal demyelinating disease to test contribution of virus peptide-specific CD8+ T cells [65]. Normally, as a response to TMEV infection, mice on H-2b-resistant background mount a vigorous CD8+ T-cell response against a single-virus capsid protein epitope, VP2121–130 [66]. However, despite the presence of resistant H-2b MHC haplotype, the infection of interferon-γ receptor (IFN-γR)-deficient mice leads to rapid demyelination and severe neurologic disease. One day prior to TMEV infection, authors treated these animals with VP2121–130 or control E7 peptide to induce tolerance and eliminate specific CD8+ T cells. Removal of VP2121–130-specific CD8+ T cells did not alter virus load or levels of demyelination but led to a significant preservation of motor function. Subsequently, using the same IFN-γR-deficient mice, authors demonstrated better motor performance in mice pretreated with VP2121–130 peptide, due to relative preservation of retrograde axonal transport [67].
Figure 2. CD8+ T cells are responsible for axonal loss and clinical deficits.
A) Regardless of the initial trigger that causes demyelination, CD8+ T cells induce injury to axons, lead to progressive axonal loss in the environment with bare, unprotected axons due to the chronic demyelination. B) When CD8+ T cells are absent, demyelination may still occur, but due to sodium channel redistribution there is relative preservation of axons, functional preservation and increased potential for remyelination.
Since most observations about the pathogenic role of CD8+ T cells came from experiments in animals of the resistant black (BL) background, a recent attempt was made to determine the influence of β2m deficiency on spinal cord pathology in mice with H-2q haplotype on B10 background [6]. Several important observations were made: 1) demyelination was progressive in β2m-competent mice, whereas demyelination levels in β2m-deficient mice remained steady from 45 days up to a year post infection; 2) remyelination was minimal in β2m-competent mice and occurred very late in the disease, whereas remyelination was extensive in β2m-deficient mice; 3) due to marked differences in levels of demyelination and remyelination, axonal loss was more pronounced in β2m-competent mice. These findings indicate that, in addition to their axon-damaging role, CD8+ T cells can also be responsible for increased levels of demyelination and/or suppressed repair. The concept of a remyelination-inhibiting role for CD8+ T cells is not new [68, 69]. On the other hand, because demyelination and modest axonal loss were evident in β2m-deficient mice, CD8+ T cells may not be the only mediators of the significant pathology observed in β2m-competent animals. This is supported by the study in which both T-cell subsets proved to have discrete contributions to demyelination [70]. In addition, the decreased burden of demyelination in β2m-deficient animals may, at least to some extent, be due to the absence of regulatory CD8+ T cells.
In summary, experiments in CD8-, CD4- and β2m-deficient mice provided strong evidence that demyelination and axonal damage followed by neurologic deficits are functionally separable. Apparently, demyelination creates an environment that favors the pathogenic role of CD8+ T cells in promoting axonal loss and the progression of neurological deficits.
6. CD8+ T Cells as Regulatory Cells
6.1. CD8+ T Cells Have a Regulatory Role in Animal Models of MS
Under normal conditions, regulatory T cells are necessary to maintain immunological tolerance to self-antigens and prevent disproportionate immune responses that are potentially deleterious to the host [71]. The two major functions of CD8+ T cells are cytotoxicity and suppression. Approximately two decades ago, two EAE studies supported the concept that regulatory/suppressive CD8+ T cells confer protection against disease relapses [54, 55]. It appears that the mechanism of CD8+ T cell-mediated suppression is related to Qa-1, a non-classical MHC class I molecule (mouse analog of human HLA-E) [72]. Madakamutil et al. demonstrated that expansion of regulatory CD8+ T cells induced apoptotic deletion of MBP-reactive CD4+ T cells that expressed Vβ8.2 T-cell receptor [73]. Experiments in Qa-1-deficient animals provided additional evidence. Hu et al. showed that Qa-1 is critical for immune regulation because Qa-1-deficient mice failed to prevent expansion of pathogenic, self-reactive CD4+ T cells, which therefore presented with severe PLP-mediated EAE [74]. Furthermore, another independent group revealed that antigen-specific CD8+/CD28− cells have the ability to suppress autoimmunity and prevent development of clinical deficits [75] (Table 2). Authors showed that adoptive transfer of CD8+/CD28−, but not CD8+/CD28+ cells, into CD8-deficient mice led to a significant suppression of disease, and the mechanism of suppression required direct cell-to-cell contact with antigen-presenting cells (APCs). This supports the study in which naturally occurring CD8+/CD28− regulatory T cells prevented experimental inflammatory bowel disease in mice [76]. The important function of APCs in conferring protection from autoimmune disease involves CD40-ligand, immunosuppressive IL-10 [77] and transforming growth factor β (TGF-β) [78]. More recently, Chen et al. revealed a new subset of CD8+ T cells that express latency-associated peptide (LAP) (CD8+/LAP+ T cells) and perform regulatory activity both in vivo and in vitro [79]. Utilizing IFN-γ and TGF-β these CD8+/LAP+ T cells ameliorated severity of clinical disease in EAE. A group from Japan proposed that CD8+/CD122+ regulatory T cells play an essential role during the recovery phase of EAE [80] because transferring these cells at the peak of EAE disease improved symptoms and depletion of these cells resulted in persistent clinical disease. Finally, Mangalam et al. further confirmed that CD8+/CD122+ T cells function as regulatory cells, whereas CD8+/CD122− T cells have a pathogenic role [81]. It appears that CD8+/CD122+ T cells modulate self-reactive CD4+ T cells through direct contact with APCs as well as through the release of IL-10, IFNγ and TGF-β.
Table 2.
Summary of Regulatory CD8+ T cells in EAE.
| Cell Marker | Function |
|---|---|
| CD8+/CD28− | Suppression of autoimmunity and clinical deficits [75] |
| CD8+/LAP+ | Ameliorate severity of EAE [79] |
| CD8+/CD122+ | Regulatory role during recovery of EAE [80, 81] |
EAE – Experimental autoimmune encephalomyelitis
6.2. CD8+ T Cells Have a Regulatory Role in Human MS
Treating relapsing-remitting MS patients with glatiramer acetate (GA) has provided some understanding about the role of regulatory CD8+ T cells in human MS. It was hypothesized that glatiramer-specific Th2 cells enter the brain and, following cross-reactivity with myelin antigens, induce suppression and produce anti-inflammatory effects and subsequent beneficial effects in MS patients [82]. There is consensus that GA induces CD8+ T-cell response[83] and that the up-regulation of regulatory CD8+ T cells, which suppress myelin-specific CD4+ T cells by direct cytotoxic killing [84], accounts for the beneficial effects for MS patients. In a follow-up study, Biegler et al. demonstrated the existence of oligoclonal GA-specific CD8+ T cells, among which dominant clones persisted for a long time, whereas GA-specific CD4+ T-cell responses were polyclonal and featured frequent evolution of their repertoire [85]. In addition, using quantitative real time PCR assays to assess functional evolution of CD8+ T cells, the authors observed up-regulation of several cytokines (Il-4, IL-5, IL-10 and TGF-β), which was indicative of regulatory phenotype. Another group discovered a new subset of regulatory CD8+ T cells that expresses HLA-G, has strong suppressive properties and may modulate inflammation thereby influencing the course of MS [86]. Indeed, Airas et al. demonstrated that a group of MS patients with increased postpartum disease activity had reduced numbers of both CD4+ and CD8+ HLA-G- expressing T cells [87].
Correale et al. explored the potential effects of regulatory CD8+ T cells on MS development by analyzing CSF and peripheral blood of MS patients (in remission or during exacerbations) and healthy subjects [88]. Authors determined that both CSF and blood from patients with exacerbation contained less regulatory CD8+ T cells compared with patients in remission or controls. Apparently, increased expression of the CD94/NKG2A receptor limits the suppressive activity of the CD8+ T cells. More recently, the same group investigated the role of non-cytotoxic CD8+/CD25+/FoxP3+ cells during the course of human MS [89]. Similarly, authors analyzed peripheral blood, CSF and CD8+ T-cell clones that recognize autoreactive CD4+ T cells in MS patients during exacerbation or remission, patients with other inflammatory neurological diseases and control healthy individuals. CD8+/CD25+/FoxP3+ cells suppressed secretion of IFN-γ and interleukin (IL)-17 and proliferation of autoreactive CD4+ T cells. Interestingly, this suppression was abolished if FoxP3 was silenced by small interfering RNA. An important finding of this study was that the cloning frequency of regulatory CD8+ T cells was more suppressed in MS patients during exacerbations than in patients in remission or healthy controls. Therefore, it is clear that non-selective deletion of all CD8+ T cells, including subsets of regulatory CD8+ T cells, may aggravate MS. Following the conclusions of this work, an alternative approach may be to activate regulatory CD8+/CD25+/FoxP3+ cells, which may have a beneficial effect in human MS. In the most recent article, Hu et al. discovered a unique population of CD8+/CD161−/CD56+ T cells in a normal human blood [90]. When activated, CD8+/CD161−/CD56+, but not CD8+/CD161−/CD56−, subset was able to lyse TCR-activated CD4+ T cells in vitro. This novel regulatory CD8+ T-cell subset will most likely be further explored in human MS in the hope of finding anothertherapeutic target to boost numbers of this subset. A short summary of regulatory CD8+ T cells in human MS appears in Table 3.
Table 3.
Summary of Regulatory CD8+ T cells in Human MS
| Cell Marker | Function |
|---|---|
| CD94/NKG2A | Limited suppressive activity of CD8+ T cells [88] |
| CD8+/CD25+/FoxP3+ | Suppressed secretion of IFN-γ and IL-17, and proliferation of autoreactive CD4+ T cells [89] |
| CD8+/CD161−/CD56+ | Lysed TCR-activated CD4+ T cells in vitro [90] |
MS – Multiple sclerosis
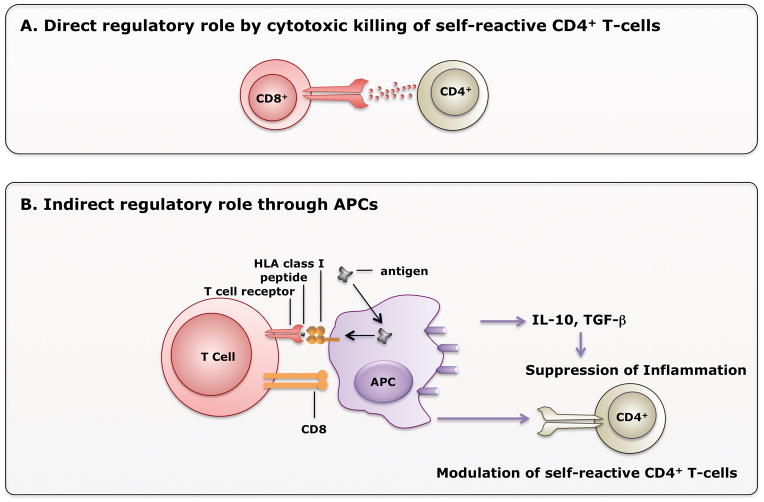
In summary, regulatory CD8+ T cells may exert their role in disease/inflammation suppression either by direct killing of self-reactive CD4+ T cells or indirectly through APCs (Figure 3).
Figure 3. Mechanisms of Regulatory CD8+ T cell-mediated suppression of disease.
A) In the direct mechanism, CD8+ T cells exert direct cytotoxic role by recognizing classical Class I molecule or non-classical HLA-E molecule on CD4+ T cells. [84] B) CD8+ T cells may indirectly suppress inflammation by acting through antigen presenting cells (APCs). APCs then secrete anti-inflammatory cytokines IL-10 or TGF-β, or modulate self-reactive CD4+ T cells.
7. CD8+ T Cells Make IL-17, which may Cause Damage in MS Lesions
Recently, there has been increased interest in the potential role of interleukin (IL)-17 in MS lesions. Compared to classic cytotoxic CD8+ T cells, IL-17-secreting CD8+ T cells (Tc-17) have much less cytotoxic functions, mainly because levels of the T-box transcription factor Eomesodermin, IFN-γ, and granzyme B are significantly reduced [91]. Initially, because of convincing results from EAE studies, CD4+ T cells were considered to be the main source of IL-17 [92, 93]. Elevated numbers of IL-17-producing mononuclear cells have been detected in peripheral blood of MS patients, especially during exacerbations [94]. The proportion of these IL-17-producing cells was higher in the CSF. However, Tzartos et al. were the first to provide evidence that CD4+ and CD8+ T cells secreted IL-17 in equivalent amounts in active MS lesions [95]. Indeed, Vanden Eijnden et al. demonstrated that in cultured human cells, IL-23 had a greater influence on polarizing naïve CD8+ T cells rather than CD4+ T cells into IL-17-secreting cells [96]. Subsequently, Tc-17 cells were assessed in peripheral blood and CSF of early MS patients and control group. Interestingly, the authors observed increased numbers of these cells in the CSF of early MS patients but not in peripheral blood. In peripheral blood from healthy human individuals, only CD4+ T cells produced IL-17 [97]. It appears that the inflammatory microenvironment of the human brain and the clonal expansion of CD8+ T cells in the disease setting initiate production of IL-17 [95]. Several pieces of evidence support this hypothesis. One is the presence of IL-23, a cytokine produced by macrophages and dendritic cells in MS lesions [98]. Additionally, a recent study suggests that pro-inflammatory cytokines IL-6 and IL-1β also polarize naïve T cells into IL-17-secreting cells [99]. Another group demonstrated that almost all circulating Tc-17 cells belonged to a subset of non-cytotoxic, pro-inflammatory CD161high CCR6+ CD8+ T cells [100]. Authors further showed that these CD161high CCR6+ CD8+ T cells comprised approximately 10% of all CD8+ T cells detected in analyzed MS lesions but were not detectable in brain samples from a control subject who passed away from cardiac arrest. Interestingly, coexisting expression of chemokine receptor 6 (CCR6) implies that these cells migrate to the brain parenchyma [101]. Together, these findings provide evidence about the pathogenic role of CD161high CCR6+ CD8+ T cells in human MS.
In summary, it is likely that Tc-17 cells contribute to human MS and contribute to worsening disease, since significant numbers of these cells appear in human MS lesions during exacerbations. Clearly, a more specific definition of IL-17-secreting CD8+ T cells represents an avenue for future research and, ideally, a new therapeutic target(s) on this CD8+ T-cell subtype.
8. Expert Opinion
Historically, most conclusions about human MS pathogenesis were based on results from CD4+ Th1 T cell-mediated EAE studies, and therefore, numerous therapeutic strategies have been developed to influence the activity of this T-cell subset. Many treatments effective in EAE were ineffective or, paradoxically, even worsened clinical disease in MS patients. The growing body of research implicating CD8+ T cells in MS pathogenesis may explain the failure of many EAE-based therapies. In addition, recent clinical trials with various monoclonal antibodies confirm previously discussed neuropathological data implicating CD8+ T cells in MS [18, 19, 102, 103].
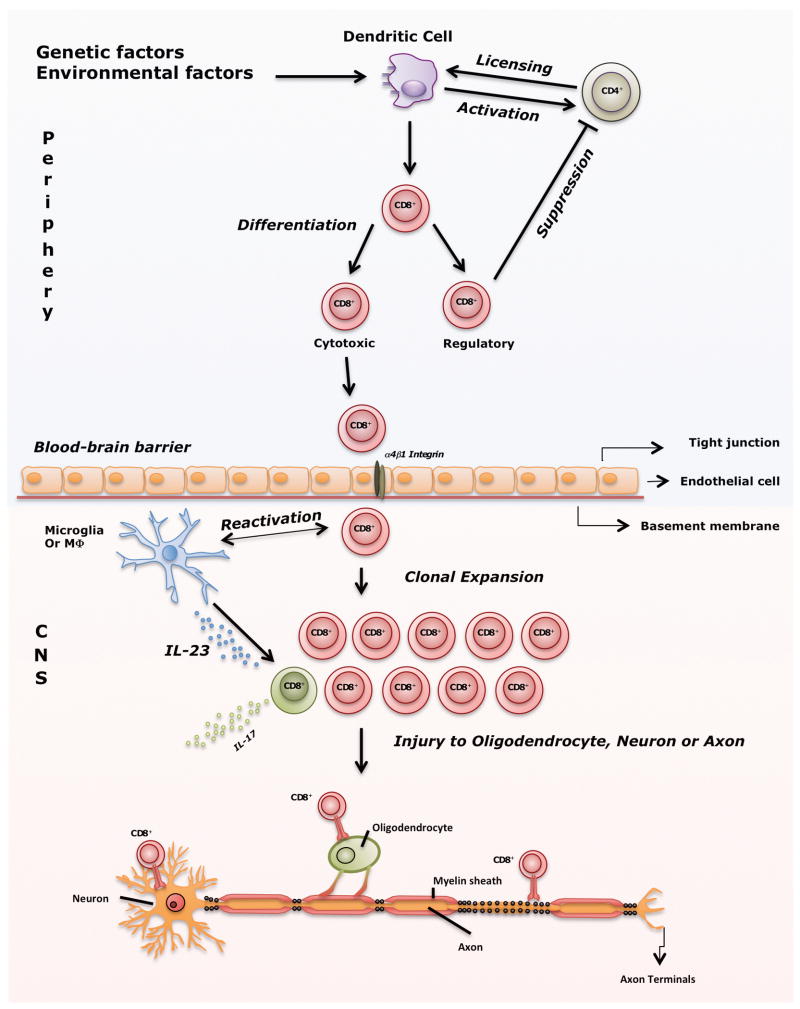
We propose that both CD4+ and CD8+ T cells have pathogenic or beneficial (regulatory/suppressive) functions in MS pathogenesis. To some extent, both functions may exist simultaneously. Recent literature indicates that CD8+ T cells are critical in MS pathogenesis. This does not diminish the role of CD4+ T cells or even B cells, which may be important in keeping CD8+ T cells continuously activated [104]. Several pieces of evidence support the hypothesis that CD8+ T cells are pathogenic: 1) clonal expansion and multifold increase of CD8+ T cells in acute and chronic MS lesions as compared to CD4+ T cells [15]; 2) up-regulation of cytotoxic mediator granzyme B in MS [28, 32]; 3) killing of neurons, transection of axons and the correlation of number of CD8+ T cells with the degree of axonal injury [32, 43]; 4) clonal expansion of CD8+ T cells, but not CD4+ T cells, evident in CSF and blood of MS patients [16, 25]; 5) higher prevalence of CNS-specific CD8+ T cells in MS patients versus healthy individuals [105, 106]; and 6) non-specific targeting of all T cells in clinical trials is beneficial in MS patients, whereas targeting only the CD4+ T-cell subset was ineffective. In addition, there is increasing evidence for the role of regulatory/suppressor CD8+ T cells, as this subset was significantly decreased in both CSF and blood in patients with exacerbated MS vs. patients in remission or controls [88]. Genetic evidence for this is MHC class I gene HLA-A2 (A*0201); it confers protection against disease, which implies that some CD8+ T cells may indeed be beneficial, whereas others are pathogenic (HLA-A3 (A*0301)). Finally, IL17-secreting CD8+ T cells shed new light on the MS pathogenesis [95, 97]. Apparently, cytotoxic and regulatory subsets of CD8+ T cells, along with CD4+ T cells, have a complex role in MS pathogenesis, and this disrupted balance may shift the disease towards remission or exacerbation. This complex interaction of immune cells with emphasis on the role of CD8+ T cells from unknown trigger to CNS injury is summarized in Figure 4. It is critical to continue investigating various CD8+ T-cell subsets to reveal their role and monitor their levels during the course of the disease. In addition, it is important to elucidate further the interactions of different T-cell subsets with each other as well as with neurons and glia in the CNS. Lastly, it is critical to determine the 9 amino-acid peptide sequence recognized by CD8+ T cells in the CNS of MS patients.
Figure 4. From unknown trigger to CNS injury; a summarized view of the role of CD8+ T cells in MS pathogenesis.
Several environmental factors in combination with genetic factors activate dendritic cells (DCs), which then present epitopes and influence priming of CD4+ T cells. Subsequently, primed CD4+ T cells license DCs to activate CD8+ T cells by cross-presenting epitopes. Activated CD8+ T cells then differentiate into cytotoxic (CTL) and regulatory cells. Under normal conditions, regulatory CD8+ T cells prevent immune response against self-antigens as shown in Figure 3. However, when this protective mechanism fails autoreactive CTLs migrate through blood-brain barrier with the help of α4β1 integrin (molecular target of natalizumab). Within the CNS, resident microglia or macrophages that express co-stimulatory and MHC class I molecules likely are involved in reactivation of CD8+ T cells. As a result of reactivation, CD8+ T cells clonally expand, meet hypothetical target antigens presented on MHC class I on neurons, axons or oligodendrocyte and induce injury. In the inflammatory milieu of an MS lesion, IL-6 and IL-1β as well as IL-23 produced by macrophages, polarize some CD8+ T cells into IL-17-secreting cells which can comprise up to 10% of all CD8+ T cells detected in MS lesions. Thus, CD8+ IL-17-secreting T cells may influence exacerbations in MS.
The clonal expansion of CD8+ T cells with apparent skewing of the CDR3 junctional regions in MS lesions is clearly established, and thus, it is extremely unlikely that these cells are just bystanders. Even though many recent studies clearly implicate CD8+ T cells in MS pathogenesis, the neurotoxicity-triggering antigen, whether it is component of a myelin, other auto-antigen or exogenous pathogen (virus), is still elusive. A possible approach to investigate specificity of clonally expanded CD8+ T cells in CSF and/or blood is to challenge them with a series of candidate antigens, as well as combinatorial peptide libraries [107]. Revealing this elusive antigen may allow targeting of only epitope-specific CD8+ T cells, as we showed previously in animal model of MS [65]. Removal of only epitope-specific CD8+ T cells may prevent further progression of MS and would also preserve the other CD8+ T cells required for protective immunity. This should eliminate the risk of opportunistic infections due to the non-selective blockade of all T cells such as PML in some of natalizumab-treated MS patients [108].
Finally, there is increased evidence that regulatory CD8+ T cells control MS. Research in this area is rapidly expanding and will hopefully bring novel molecular candidates to enhance this T-cell subset, which may be a successful therapy in human MS. In addition, given the recent insights about the possible role of IL-17-secreting CD8+ T cells (Tc-17) in early MS or during MS exacerbations, anti-IL-17 monoclonal antibodies may also treat MS. However, researchers will need to investigate and define the Tc-17 cell subset in detail to reveal its mechanism of action and identify potential therapeutic targets.
Highlights.
CD8+ T cells outnumber CD4+ T cells in MS lesions and can cause damage to all CNS cells
MHC class I genes may confer risk or protection from MS
CD8+ T cells induce injury in EAE and virus-induced animal models of demyelinating disease
CD8+ T cells have a regulatory role in animal models and human MS
CD8+ T cells can secrete IL-17, which may cause damage in MS Lesions
Acknowledgments
Financial Support
This work was supported by grants from the NIH (R01 GM092993, R01 NS048357 and R21 NS073684) and the National Multiple Sclerosis Society (CA 1060A). This work was also supported by a High-Impact Pilot and Feasibility Award (HIPFA) and Novel Methodology Award (NMDA) from the Mayo Clinic Center for Translational Science Activities (CTSA) and Mayo Clinic CTSA grant number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). We also acknowledge with thanks support from the Applebaum, Hilton, Peterson and Sanford Foundations, the Minnesota Partnership Award for Biotechnology and Medical Genomics and the McNeilus family.
References
* of interest
** of considerable interest
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Comi G, Panitch H, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med. 2000;6:1176–82. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 3.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 4**.Rivera-Quinones C, McGavern D, Schmelzer JD, et al. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–93. doi: 10.1038/nm0298-187. Important study that clearly implicates CD8+ T cells as pathogenic, leading to axonal loss and clinical deficits in a murine model of demyelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe CL, Adelson JD, Rodriguez M. Absence of perforin expression confers axonal protection despite demyelination. Neurobiol Dis. 2007;25:354–9. doi: 10.1016/j.nbd.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denic A, Pirko I, Wootla B, et al. Deletion of beta-2-microglobulin ameliorates spinal cord lesion load and promotes recovery of brainstem NAA levels in a murine model of multiple sclerosis. Brain Pathol. 2012;22:698–708. doi: 10.1111/j.1750-3639.2012.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez M, Sriram S. Successful therapy of Theiler’s virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988;140:2950–5. [PubMed] [Google Scholar]
- 8.Huseby ES, Liggitt D, Brabb T, et al. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–76. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D, Whitaker JN, Huang Z, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–87. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 10.Markovic-Plese S, McFarland HF. Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep. 2001;1:257–62. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 11.Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004;61:1613–5. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 12.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–63. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 13.Goverman J, Perchellet A, Huseby ES. The role of CD8(+) T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy. 2005;4:239–45. doi: 10.2174/1568010053586264. [DOI] [PubMed] [Google Scholar]
- 14.Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol. 2004;25:132–7. doi: 10.1016/j.it.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15**.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. One of the first studies to demonstrate clonal expansion and dominance of CD8+ T cells in the CSF of patients with early MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skulina C, Schmidt S, Dornmair K, et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428–33. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Hoftberger R, Aboul-Enein F, Brueck W, et al. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. Important study demonstrating up-regulation of MHC Class I on all CNS cell types dependent on severity of disease and lesion activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oosten BW, Lai M, Hodgkinson S, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–7. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- 19.Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 20.Liblau RS, Wong FS, Mars LT, et al. Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity. 2002;17:1–6. doi: 10.1016/s1074-7613(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 21*.Johnson AJ, Suidan GL, McDole J, et al. The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology? Int Rev Neurobiol. 2007;79:73–97. doi: 10.1016/S0074-7742(07)79004-9. First study to show that removal of virus peptide-specific CD8+ T cells led to preservation of motor function. [DOI] [PubMed] [Google Scholar]
- 22.Booss J, Esiri MM, Tourtellotte WW, et al. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci. 1983;62:219–32. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- 23.Hauser SL, Bhan AK, Gilles F, et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–87. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 24.Monteiro J, Hingorani R, Pergolizzi R, et al. Clonal dominance of CD8+ T-cell in multiple sclerosis. Ann N Y Acad Sci. 1995;756:310–2. doi: 10.1111/j.1749-6632.1995.tb44529.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen M, Cepok S, Quak E, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–50. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- 26.Junker A, Ivanidze J, Malotka J, et al. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–99. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- 27.Jilek S, Schluep M, Rossetti AO, et al. CSF enrichment of highly differentiated CD8+ T cells in early multiple sclerosis. Clin Immunol. 2007;123:105–13. doi: 10.1016/j.clim.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Malmestrom C, Lycke J, Haghighi S, et al. Relapses in multiple sclerosis are associated with increased CD8+ T-cell mediated cytotoxicity in CSF. J Neuroimmunol. 2008;196:159–65. doi: 10.1016/j.jneuroim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Jilek S, Schluep M, Meylan P, et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–21. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- 30.Fotheringham J, Jacobson S. Human herpesvirus 6 and multiple sclerosis: potential mechanisms for virus-induced disease. Herpes. 2005;12:4–9. [PubMed] [Google Scholar]
- 31.Cheng W, Ma Y, Gong F, et al. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Front Biosci. 2012;17:1648–58. doi: 10.2741/4010. [DOI] [PubMed] [Google Scholar]
- 32.Neumann H, Medana IM, Bauer J, et al. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–9. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 33.Huseby ES, Huseby PG, Shah S, et al. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol. 2012;3:64. doi: 10.3389/fimmu.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann H, Cavalie A, Jenne DE, et al. Induction of MHC class I genes in neurons. Science. 1995;269:549–52. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 35.Medana I, Li Z, Flugel A, et al. Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity. J Immunol. 2001;167:674–81. doi: 10.4049/jimmunol.167.2.674. [DOI] [PubMed] [Google Scholar]
- 36.Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–9. [PubMed] [Google Scholar]
- 37.Pouly S, Becher B, Blain M, et al. Interferon-gamma modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J Neuropathol Exp Neurol. 2000;59:280–6. doi: 10.1093/jnen/59.4.280. [DOI] [PubMed] [Google Scholar]
- 38.Rensing-Ehl A, Malipiero U, Irmler M, et al. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (APO-1/CD95) pathway. Eur J Immunol. 1996;26:2271–4. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- 39.Medana IM, Gallimore A, Oxenius A, et al. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur J Immunol. 2000;30:3623–33. doi: 10.1002/1521-4141(200012)30:12<3623::AID-IMMU3623>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Khanna KM, Bonneau RH, Kinchington PR, et al. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medana I, Martinic MA, Wekerle H, et al. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol. 2001;159:809–15. doi: 10.1016/S0002-9440(10)61755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bien CG, Bauer J, Deckwerth TL, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51:311–8. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 43.Bitsch A, Schuchardt J, Bunkowski S, et al. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation Brain. 2000;123 (Pt 6):1174–83. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- 44.Kuhlmann T, Lingfeld G, Bitsch A, et al. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–12. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- 45.Denic A, Macura SI, Warrington AE, et al. A single dose of neuron-binding human monoclonal antibody improves spontaneous activity in a murine model of demyelination. PLoS One. 2011;6:e26001. doi: 10.1371/journal.pone.0026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucchinetti CF, Bruck W, Lassmann H. Evidence for pathogenic heterogeneity in multiple sclerosis. Ann Neurol. 2004;56:308. doi: 10.1002/ana.20182. [DOI] [PubMed] [Google Scholar]
- 47.Lucchinetti C, Bruck W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Naito S, Namerow N, Mickey MR, et al. Multiple sclerosis: association with HL-A3. Tissue Antigens. 1972;2:1–4. doi: 10.1111/j.1399-0039.1972.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 49.Jersild C, Svejgaard A, Fog T. HL-A antigens and multiple sclerosis. Lancet. 1972;1:1240–1. doi: 10.1016/s0140-6736(72)90962-2. [DOI] [PubMed] [Google Scholar]
- 50.Fogdell-Hahn A, Ligers A, Gronning M, et al. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–8. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- 51.Harbo HF, Lie BA, Sawcer S, et al. Genes in the HLA class I region may contribute to the HLA class II-associated genetic susceptibility to multiple sclerosis. Tissue Antigens. 2004;63:237–47. doi: 10.1111/j.0001-2815.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 52.Brynedal B, Duvefelt K, Jonasdottir G, et al. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS One. 2007;2:e664. doi: 10.1371/journal.pone.0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denic A, Johnson AJ, Bieber AJ, et al. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–9. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–5. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 55.Koh DR, Fung-Leung WP, Ho A, et al. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–3. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 56.Ford ML, Evavold BD. Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur J Immunol. 2005;35:76–85. doi: 10.1002/eji.200425660. [DOI] [PubMed] [Google Scholar]
- 57.Saxena A, Bauer J, Scheikl T, et al. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–21. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- 58.Sobottka B, Harrer MD, Ziegler U, et al. Collateral bystander damage by myelin-directed CD8+ T cells causes axonal loss. Am J Pathol. 2009;175:1160–6. doi: 10.2353/ajpath.2009.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol. 2013;14:254–61. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez M, Rivera-Quinones C, Murray PD, et al. The role of CD4+ and CD8+ T cells in demyelinating disease following Theiler’s virus infection: a model for multiple sclerosis. J Neurovirol. 1997;3 (Suppl 1):S43–5. [PubMed] [Google Scholar]
- 61.Moll C, Mourre C, Lazdunski M, et al. Increase of sodium channels in demyelinated lesions of multiple sclerosis. Brain Res. 1991;556:311–6. doi: 10.1016/0006-8993(91)90321-l. [DOI] [PubMed] [Google Scholar]
- 62.Murray PD, McGavern DB, Lin X, et al. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998;18:7306–14. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deb C, Lafrance-Corey RG, Zoecklein L, et al. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:1037–48. doi: 10.1097/NEN.0b013e3181b5417e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ure DR, Rodriguez M. Preservation of neurologic function during inflammatory demyelination correlates with axon sparing in a mouse model of multiple sclerosis. Neuroscience. 2002;111:399–411. doi: 10.1016/s0306-4522(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AJ, Upshaw J, Pavelko KD, et al. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler’s virus infection. Faseb J. 2001;15:2760–2. doi: 10.1096/fj.01-0373fje. [DOI] [PubMed] [Google Scholar]
- 66.Johnson AJ, Njenga MK, Hansen MJ, et al. Prevalent class I-restricted T-cell response to the Theiler’s virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J Virol. 1999;73:3702–8. doi: 10.1128/jvi.73.5.3702-3708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howe CL, Ure D, Adelson JD, et al. CD8+ T cells directed against a viral peptide contribute to loss of motor function by disrupting axonal transport in a viral model of fulminant demyelination. J Neuroimmunol. 2007;188:13–21. doi: 10.1016/j.jneuroim.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez M, Lindsley MD. Immunosuppression promotes CNS remyelination in chronic virus-induced demyelinating disease. Neurology. 1992;42:348–57. doi: 10.1212/wnl.42.2.348. [DOI] [PubMed] [Google Scholar]
- 69.Miller DJ, Rivera-Quinones C, Njenga MK, et al. Spontaneous CNS remyelination in beta 2 microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15:8345–52. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray PD, Pavelko KD, Leibowitz J, et al. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998;72:7320–9. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Kumar V. Homeostatic control of immunity by TCR peptide-specific Tregs. J Clin Invest. 2004;114:1222–6. doi: 10.1172/JCI23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madakamutil LT, Maricic I, Sercarz E, et al. Regulatory T cells control autoimmunity in vivo by inducing apoptotic depletion of activated pathogenic lymphocytes. J Immunol. 2003;170:2985–92. doi: 10.4049/jimmunol.170.6.2985. [DOI] [PubMed] [Google Scholar]
- 74.Hu D, Ikizawa K, Lu L, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 75.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–48. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menager-Marcq I, Pomie C, Romagnoli P, et al. CD8+CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–85. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faunce DE, Terajewicz A, Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol. 2004;172:1991–5. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
- 79.Chen ML, Yan BS, Kozoriz D, et al. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;39:3423–35. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Lee YH, Ishida Y, Rifa’i M, et al. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–32. doi: 10.4049/jimmunol.180.2.825. Study showed essential role of regulatory CD8+ T cells during the recovery phase of EAE. [DOI] [PubMed] [Google Scholar]
- 81.Mangalam AK, Luckey D, Giri S, et al. Two discreet subsets of CD8 T cells modulate PLP(91–110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. J Autoimmun. 2012;38:344–53. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhib-Jalbut S. Glatiramer acetate (Copaxone) therapy for multiple sclerosis. Pharmacol Ther. 2003;98:245–55. doi: 10.1016/s0163-7258(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 83.Karandikar NJ, Crawford MP, Yan X, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–9. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tennakoon DK, Mehta RS, Ortega SB, et al. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–29. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 85.Biegler BW, Yan SX, Ortega SB, et al. Glatiramer acetate (GA) therapy induces a focused, oligoclonal CD8+ T-cell repertoire in multiple sclerosis. J Neuroimmunol. 2006;180:159–71. doi: 10.1016/j.jneuroim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 86.Feger U, Tolosa E, Huang YH, et al. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–77. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 87.Airas L, Nikula T, Huang YH, et al. Postpartum-activation of multiple sclerosis is associated with down-regulation of tolerogenic HLA-G. J Neuroimmunol. 2007;187:205–11. doi: 10.1016/j.jneuroim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–34. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol. 2010;67:625–38. doi: 10.1002/ana.21944. [DOI] [PubMed] [Google Scholar]
- 90.Hu D, Weiner HL, Ritz J. Identification of Cytolytic CD161(−)CD56(+) Regulatory CD8 T Cells in Human Peripheral Blood. PLoS One. 2013;8:e59545. doi: 10.1371/journal.pone.0059545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber M, Heink S, Grothe H, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–25. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 92.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 94.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 95*.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. One of the first studies to demonstrate the existence of pathogenic IL-17-secreting CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanden Eijnden S, Goriely S, De Wit D, et al. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469–75. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 97.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Chu N, Hu A, et al. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- 99.Beriou G, Costantino CM, Ashley CW, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Annibali V, Ristori G, Angelini DF, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–54. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- 101.Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–23. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 102.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 103.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 104.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–41. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 105.Crawford MP, Yan SX, Ortega SB, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–31. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 106.Zang YC, Li S, Rivera VM, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol. 2004;172:5120–7. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- 107.Hemmer B, Vergelli M, Pinilla C, et al. Probing degeneracy in T-cell recognition using peptide combinatorial libraries. Immunol Today. 1998;19:163–8. doi: 10.1016/s0167-5699(97)01217-6. [DOI] [PubMed] [Google Scholar]
- 108.Linda H, von Heijne A, Major EO, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–7. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]